Abstract
Introduction:
Configuration and size of the foramen magnum and posterior fossa plays an important role in the pathophysiology of the posterior fossa and craniovertebral junction disorders. This study is aimed to find out various dimensions of the foramen magnum and posterior fossa.
Materials and Methods:
This is a prospective study of 100 consecutive normal computerized tomography (CT) scans of posterior fossa and 100 dry adult skulls without any bony abnormality. The posterior fossa volume was calculated by abc/2 in method 1 and by advanced work station of CT scan in method 2. Various dimensions of posterior fossa and foramen magnum were also studied.
Results:
Age ranged from 16 to 89 years with a mean of 51.3 years. Mean height of posterior fossa were 3.01 cm (±0.22) and 3.52 (±0.43) cm in dry skull and CT scan group, respectively (P < 0.0001). Mean volume of posterior fossa were 157.88 (±27.94) cm3 and 159.58 (±25.73) cm3 by method 1 and method 2, respectively (P > 0.05). All the dimensions of posterior fossa and foramen magnum were larger in male as compared to female. Mean anteroposterior (AP), transverse diameter and surface area of the foramen magnum were 3.31 (±0.35) cm, 2.76 (±0.31) cm, and 729.15 (±124.87) mm2, respectively, in CT scan group as compared to 3.41 (±0.29) cm, 2.75 (±0.25) cm, and 747.67 (±108.60) mm2, respectively, in dry skull group.
Conclusion:
Normal values of posterior fossa and foramen magnum could serve as a future reference. Dry skull dimensions could be different from CT scan measurement. More studies are needed as there could be variations in dimensions in different regions in India
Keywords: Foramen magnum, posterior cranial fossa, skull
Introduction
Configuration and size of the foramen magnum and posterior fossa (PF) plays an important role in the pathophysiology of various disorders of the PF and craniovertebral junction. Stenosis of foramen magnum causes brainstem compression manifested by respiratory complications, lower cranial nerve dysfunctions, upper and lower extremity paresis, hypo- or hypertonia, hyperreflexia, or clonus.[1–3] Thus, a fundamental knowledge of normal anatomy of this region is important to the clinician for diagnosis and treatment. The aim of our study was to find out various measurements of the foramen magnum and PF in Indian population. These results could help in the better diagnosis, classification, and treatment of diseases related to this region and serve as a future reference defining an anatomic range. Although we agree that India is a vast country and the dimensions may vary in different regions. Although alignment of upper and lower cervical spine has been described,[4] there is no anatomical study about the dimensions of PF and foramen magnum for the Indian population to the best of our knowledge.
Materials and Methods
The study was conducted in a tertiary referral hospital from July 2010 to October 2011 in both adult human and adult human dry skulls. Hundred consecutive head injury patients with normal computerized tomography (CT) scans (without any bony or soft-tissue abnormality) admitted to Neurosurgery Unit were included in the study. Those with fracture or hematoma of PF were excluded from the study. Hundred dry adult skulls without any gross bony abnormality were also studied. The dry skulls were obtained from the Department of Anatomy from the bone bank. Dry skulls were not taken directly from dead bodies.
Inferior basal view was observed for the measurement of foramen magnum in the dry skulls. The anteroposterior (AP) and the transverse diameter were measured using a vernier caliper. The area of the foramen magnum was calculated using formula πr2, where r is average radius calculated from AP and transverse diameter. The normal ranges of dimensions of the foramen magnum were also noted. The height of the PF was measured as the perpendicular distance between twinning's line and McRae line [Figure 1]. PF volume (PFV) was measured by abc/2 where a is the height, b is anteroposterior diameter, and c is transverse diameter of the PF.[5]
Figure 1.
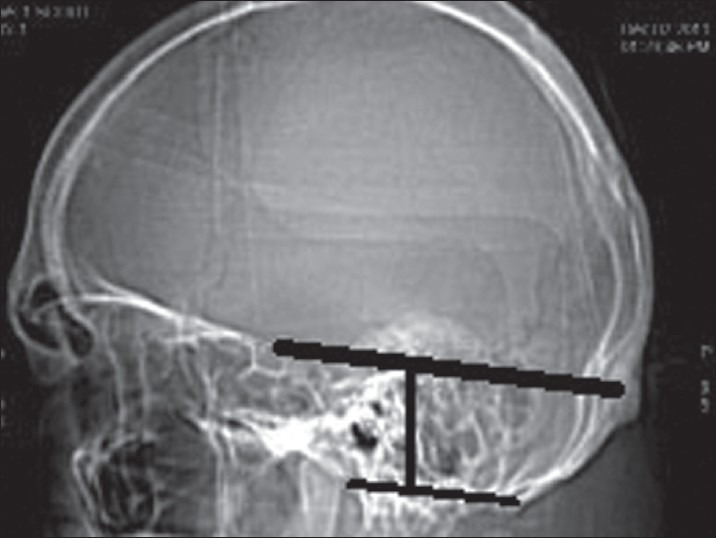
Height of posterior fossa
In adult human PFV along with both the measurements (foramen magnum dimensions and PF height) were studied by performing a CT of the skull. The CT scans of human subjects were performed on a 16-row bright speed CT scan. The PFV was measured by two methods. In first method, volume was calculated by abc/2, where a is the height, b is AP diameter, and c is transverse diameter of the PF.[5] In second method, PFV was calculated by an advanced work station of 16-row bright speed CT scan. The data obtained by the CT scan of foramen magnum and PF were compared with the dry skull. Data were entered in Microsoft Excel 2007 worksheet and statistical analysis was performed by IBM SPSS statistics 19 version. Independent sample t test and ANOVA (analysis of variance) test were used. P < 0.05 was considered as significant.
Results
Volume and the height of the PF and the dimensions of the foramen magnum were recorded in 100 head injury patients without any pathology of PF and 100 dry skulls. Age ranged from 16 to 89 years with a mean of 51.3 years. There were 64 male patients of head injury. There was no difference in dimensions of the PF and foramen magnum in various age groups, as shown in Tables 1 and 2. All the dimensions of PF and foramen magnum were larger in male as compared to female [Table 3]. These differences were statistically significant in all except PF height, as shown in Table 3. The mean height of PF was 3.52 cm (±0.43) in head injury patients [Table 4]. The mean surface area, AP, and transverse diameter of foramen magnum were 729.15 mm 2 (±124.87), 3.31 cm (±0.35) and 2.76 cm (±0.31), respectively, Table 4 in head injury patients. The mean value of PFV were 157.88 (±27.94) cm3 (range 98.75-216.88 cm3) and 159.58 (±25.73) cm3 (range 116.03-252.99 cm3) measured by method 1 and method 2, respectively. However, the difference was not significant statistically (P > 0.05).
Table 1.
Posterior fossa dimensions according to various age groups in head injury
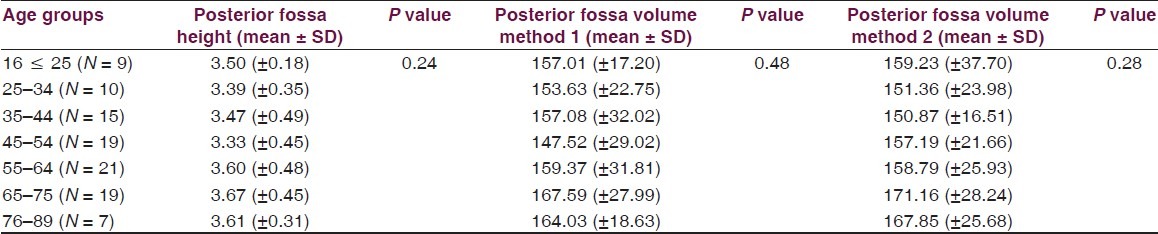
Table 2.
Foramen magnum dimensions according to age groups in head injury
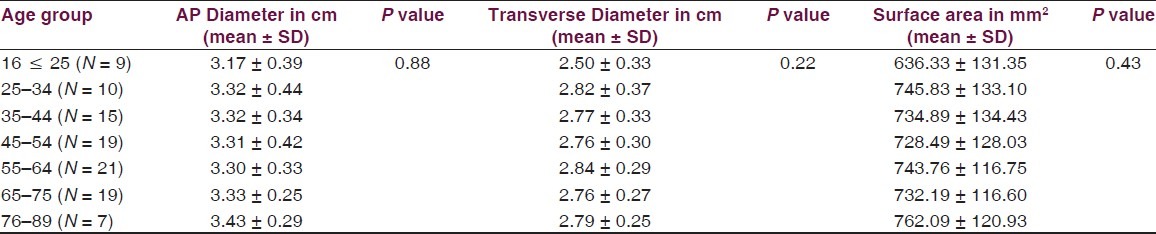
Table 3.
Posterior fossa and foramen magnum dimensions in head injury patients in relation to gender
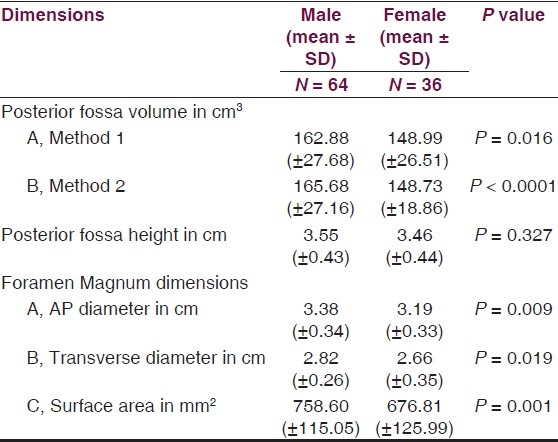
Table 4.
Posterior fossa and foramen dimensions in CT scan of head injury patients and dry skull
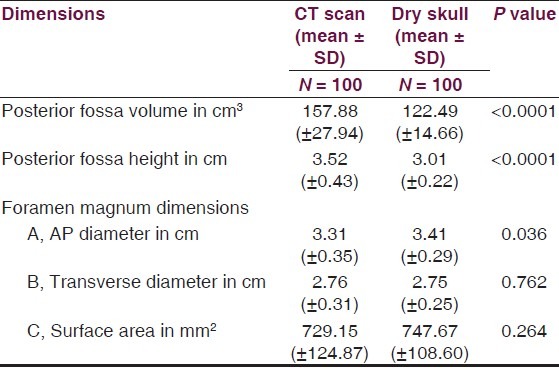
The mean height and volume of PF were 3.01 cm (±0.22) (range 2.5–4.0 cm) and 122.49 (±14.66), respectively, in dry skull [Table 4]. Both these dimensions in dry skull were significantly smaller than in the CT scan group (P < 0.0001). The mean surface area, AP, and transverse diameter of foramen magnum were 747.67 mm2 (±108.60), 3.41 cm (±0.29), and 2.75 cm (±0.25), respectively, in dry skull [Table 4]. AP diameter of dry skull was larger than CT scan group (P = 0.036), while there was no significant difference in transverse diameter and surface area of the two groups [Table 4]. A Whisker-Boxplot for various dimensions of PF and foramen magnum by gender and comparison of CT scan and dry skull were also done [Graphs 1–5].
Graph 1.
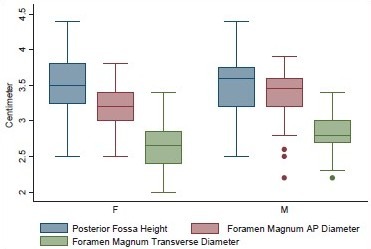
Dimensions of posterior fossa and foramen magnum in head injury patients in different genders
Graph 5.
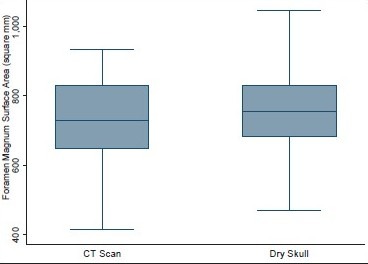
Surface area of foramen magnum in head injury patients and dry skull
Graph 2.
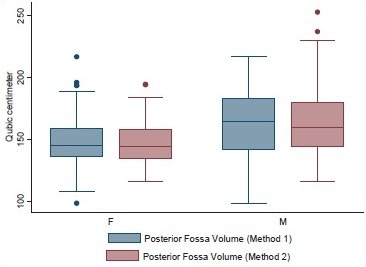
Posterior fossa volume by methods 1 and 2 in male and female head injury patients
Graph 3.
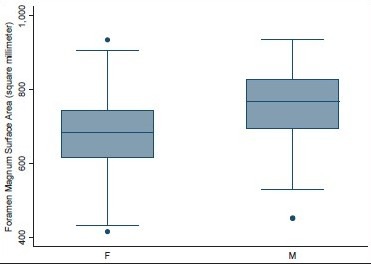
Surface area of foramen magnum in male and female head injury patients
Graph 4.
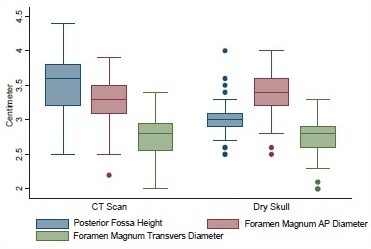
Posterior fossa height, AP, and transverse diameters of foramen magnum in head injury patients and dry skull
Discussion
Pathologies of the PF and craniovertebral junctions are very common. Knowledge of anatomy of this region and the normal range is important in the proper planning of the management. We all know that there are normal variations amongst various races, religions, body habitus, and gender, geographical, and genetic factors. Although there are studies on normal dimensions of the PF and foramen magnum, there is no study in the Indian population.
PF dimensions
The mean height of PF in dry skull was smaller as compared to CT scan (P = < 0.0001). Lower range of dimensions in dry skull could be due to the shrinkage.[6] Height of PF can be measured by MRI also, using soft-tissue landmarks from splenium of corpus callosum to the McRae Line.[7] The height of PF measured by MRI was more[7] and seems to be more useful clinically.
The mean volume of PF by method 1 was smaller as compared to method 2, although the difference was statistically not significant (t = 0.448 and P = 0.655). Calculation by method 1 is simple and it is already in use to calculate the volume of intracranial hematoma and abscess,[5,8] while the calculation by CT scan using in built software could give more accurate value.
Clinical implications of posterior cranial fossa height and volume
The overcrowding of posterior cranial fossa (PCF) can be determined if the normal range of the volume and height is available. Patients with hypoplasia of the bony structures could present at early age. The severity of presentation could be more with more overcrowding in congenital anomalies like Chiari Malformation 1 (CM 1). Short height of PF and underdeveloped bony structures could lead to downward herniation of the contents in adult[9–14] and in pediatric patients.[15,16] This could result in syringomyelia due to obstruction of the normal CSF circulation.[17–20] Grant et al. reported that the myelomeningocele was associated with tonsillar herniation and a smaller PF as compared to control fetuses. Antenatal surgical repair corrected both abnormalities.[21] Noudel et al. observed that the occipital hypoplasia was the main cause of overcrowding within the PCF. Basioccipital shortness was a cardinal feature of the shallow PCF. This could proceed from a congenital disorder of the cephalic mesoderm of the parachordal plate or occur later in the infancy because of premature stenosis of the sphenooccipital synchondrosis.[22] Badie et al.[23] reported that the ratio of PFV to supratentorial volume (PF ratio) [PFR]) was smaller in most patients with CM 1. They concluded that a smaller PF may be a primary cause of tonsillar herniation. Patients with CM I who have smaller PFRs tend to develop symptoms earlier than those with normal values. They also observed that the patients with smaller PFRs tend to respond better to suboccipital decompression. Sgouros et al.[24] reported that the children with isolated CM 1 did not have a smaller PFV than normal, whereas children with both CM 1 and syringomyelia had a significantly smaller PFV than normal. They suggested that the two subgroups may represent different phenotypic expression or even a different pathogenesis. Trigylidas et al.[25] observed that the mean PFV/ intra cranial volume (ICV) ratio for all the CM 1 patients was statistically smaller than that of the control patients. On the other hand Tubbs et al.[26] performed volumetric analysis in a family of CM 1, documented in 4 generations. They observed that it is not necessary that patients with a CM 1 will have a smaller PCF volume. Goel et al.[27] reported that small PFV were associated with Chiari malformation in basilar invasion while patients with only basilar invasion without Chiari malformation had normal PFV. They recommended decompression of the foramen magnum in small PFV group patients.
Foramen magnum dimensions
The mean AP diameter of foramen magnum was slightly larger in dry skull as compared to CT Scans. Mean transverse diameter was same in dry skull and CT Scans. The mean surface area of foramen magnum was more in dry skull as compared to CT scan. Higher value of surface area in dry skull could be due to higher range of AP diameter in dry skull. This difference was not statistically significant (t = 1.119, P = 0.264). Higher range of AP diameter in dry skulls could be due to demineralization of dry skulls. AP, transverse diameter, and surface area in Tubbs et al. series were 3.1 (2.5–3.7 cm), 2.7 cm (2.4–3.5 cm), and 558 mm2 (385–779 mm2), respectively.[6] The variation in our results and in Tubbs et al. series could be due to the difference in races, habitus, geographical, and genetic factors. Lang[28] classified the shapes of foramen magnum into five groups: (a) two semicircles, (b) an elongated circle, (c) egg-shaped, (d) rhomboidal, and (e) rounded. He found that the average AP and transverse diameter of the foramen were 3.5 and 3 cm, respectively. Our findings were consistence with the Lang et al. series.
Clinical implications of dimensions of foramen magnum
The foramen magnum is a fundamental component in the complex interaction of bony, ligamentous, and muscular structures composing the craniovertebral junction. Shape and size of the foramen is critical parameters for the manifestation of clinical signs and symptoms in craniocervical pathology. The AP and transverse diameters of the foramen magnum have been found to be independent risk factors in patients with craniocervical anomalies.[1,29–31]
The configuration of the foramen magnum in patients with Chiari I and Chiari II malformations has been found to be larger than in the normal population.[19] Another analysis based on semiaxial cranial x-rays demonstrated a significantly larger transverse diameter of the foramen magnum in 35 children with verified Chiari I malformation, as compared to the control group.[32] However, Furtado et al. found no statistical difference in the area and linear dimensions of the foramen magnum in children with Chiari I malformations and control groups.[33] Early developments of symptoms were observed in shorter AP diameters of foramen magnum.[34] Patients with stenosis of foramen magnum, such as craniometaphyseal dysplasia, Jeune's asphyxiating thoracic dystrophy, and spherophakia-brachymorphism (Marchesani's syndrome),[35–37] Beare-Stevenson syndrome (a craniofacial syndrome characterized by hypertrophy of the bony margins),[38] could develop early and severe symptoms. Tubbs et al. hypothesized that the anatomy of the craniocervical region may be different in CM 1 patients with syringomyelia who develop syringobulbia compared to other patients of CM 1 with and without syringomyelia. Although no single morphometrical difference was found in patients with CM 1 and syringobulbia compared to other patients with CM 1 in their series.[39]
Understanding the bony anatomy of foramen magnum is important for the transcondylar approach. Muthukumar et al. found that the occipital condyle can be safely drilled for a distance of 12 mm from the posterior margin before encountering the hypoglossal canal. In 20% of the skulls, the occipital condyle protrudes significantly into the foramen magnum. Wide and sagittally inclined occipital condyles, medially protuberant occipital condyles along with a foramen magnum index of more than 1.2, will require much more extensive bony resection than otherwise.[40]
Strengths and limitations of study
The strength of our study is large sample size. We did the morphometric analysis in dry skull as well as in CT scan of head injury patients without any bony or soft tissue abnormality. This study does have some limitations. Pediatric age group, which is the predominant age group for anomalies of this region, has not been studied. The head injury group and the dry skull group may not be comparable as we could not determine age and gender in the dry skull
Conclusion
Normal ranges of various dimensions of PF and foramen magnum for Indian population were determined which could serve as future reference. Dry skull dimensions could be different from CT scan measurement due to shrinkage or demineralization.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Bagley CA, Pindrik JA, Bookland MJ, Camara-Quintana JQ, Carson BS. Cervicomedullary decompression for foramen magnum stenosis in achondroplasia. J Neurosurg. 2006;104(3 Suppl):166–72. doi: 10.3171/ped.2006.104.3.166. [DOI] [PubMed] [Google Scholar]
- 2.Dickman C, Spetzler RF, Sonntag VK. Surgery of the craniovertebral junction. 1st ed. New York, NY: Thieme Medical Publishers; 1998. [Google Scholar]
- 3.Wang H, Rosenbaum AE, Reid CS, Zinreich SJ, Pyeritz RE. Pediatric patients with achondroplasia: CT evaluation of the craniocervical junction. Radiology. 1987;164:515–9. doi: 10.1148/radiology.164.2.3602395. [DOI] [PubMed] [Google Scholar]
- 4.Sherekar SK, Yadav YR, Basoor AS, Baghel A, Adam N. Clinical implications of alignment of upper and lower cervical spine. Neurol India. 2006;54:264–7. doi: 10.4103/0028-3886.27149. [DOI] [PubMed] [Google Scholar]
- 5.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 6.Tubbs RS, Griessenauer CJ, Loukas M, Shoja MM, Cohen-Gadol AA. Morphometric analysis of the foramen magnum: An anatomic study. Neurosurgery. 2010;66:385–8. doi: 10.1227/01.NEU.0000363407.78399.BA. [DOI] [PubMed] [Google Scholar]
- 7.Aydin S, Hanimoglu H, Tanriverdi T, Yentur E, Kaynar MY. Chiari type I malformations in adults: A morphometric analysis of the posterior cranial fossa. Surg Neurol. 2005;64:237–41. doi: 10.1016/j.surneu.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Yadav YR, Basoor A, Jain G, Nelson A. Expanding traumatic intracerebral contusion/hematoma. Neurol India. 2006;54:377–81. doi: 10.4103/0028-3886.28109. [DOI] [PubMed] [Google Scholar]
- 9.Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari type I malformations identified on magnetic resonance imaging. J Neurosurg. 2000;92:920–6. doi: 10.3171/jns.2000.92.6.0920. [DOI] [PubMed] [Google Scholar]
- 10.Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, et al. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005–17. doi: 10.1097/00006123-199905000-00042. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa M, Sakamoto H, Hakuba A, Nakanishi N, Inoue Y. Pathogenesis of Chiari malformation: A morphometric study of the posterior cranial fossa. J Neurosurg. 1997;86:40–7. doi: 10.3171/jns.1997.86.1.0040. [DOI] [PubMed] [Google Scholar]
- 12.Schady W, Medcalfe RA, Butler F. The incidence of craniocervical bony anomalies in the adult Chiari malformation. J Neurosurg. 1987;82:193–203. doi: 10.1016/0022-510x(87)90018-9. [DOI] [PubMed] [Google Scholar]
- 13.Stovner LJ, Bergan U, Nilsen G, Sjaastad O. Posterior cranial fossa dimensions in the Chiari I malformation: Relation to pathogenesis and clinical presentation. Neuroradiology. 1993;35:113–8. doi: 10.1007/BF00593966. [DOI] [PubMed] [Google Scholar]
- 14.Vega A, Quintana F, Berciano J. Basichondrocranium anomalies in adult Chiari type I malformation: A morphometric study. J Neurol Sci. 1990;99:137–45. doi: 10.1016/0022-510x(90)90150-l. [DOI] [PubMed] [Google Scholar]
- 15.Tubbs RS, Elton S, Grabb P, Dockery SE, Bartolucci AA, Oakes WJ. Analysis of the posterior fossa in children with the Chiari 0 malformation. Neurosurgery. 2001;48:1050–5. doi: 10.1097/00006123-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Wu YW, Chin CT, Chan KM, Barkovich AJ, Ferriero DM. Pediatric Chiari I malformations: Do clinical and radiological features correlate? Neurology. 1999;53:1271–6. doi: 10.1212/wnl.53.6.1271. [DOI] [PubMed] [Google Scholar]
- 17.Ball MJ, Dayan AD. Pathogenesis of syringomyelia. Lancet. 1972;2:799–801. doi: 10.1016/s0140-6736(72)92152-6. [DOI] [PubMed] [Google Scholar]
- 18.du Boulay G, Shah SH, Currie JC, Logue V. The mechanism of hydromyelia in Chiari type I malformations. Br J Radiol. 1974;47:579–87. doi: 10.1259/0007-1285-47-561-579. [DOI] [PubMed] [Google Scholar]
- 19.Gardner WJ, Goodall RJ. The surgical management of Arnold-Chiari malformation in adults.An explanation of its mechanism and importance of encephalography in diagnosis. J Neurosurg. 1950;7:199–206. doi: 10.3171/jns.1950.7.3.0199. [DOI] [PubMed] [Google Scholar]
- 20.Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellartonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3–15. doi: 10.3171/jns.1994.80.1.0003. [DOI] [PubMed] [Google Scholar]
- 21.Grant RA, Heuer GG, Carrión GM, Adzick NS, Schwartz ES, Stein SC, et al. Morphometric analysis of posterior fossa after in utero myelomeningocele repair. J Neurosurg Pediatr. 2011;7:362–8. doi: 10.3171/2011.1.PEDS10234. [DOI] [PubMed] [Google Scholar]
- 22.Noudel R, Jovenin N, Eap C, Scherpereel B, Pierot L, Rousseaux P. Incidence of basioccipital hypoplasia in Chiari malformation type I: Comparative morphometric study of the posterior cranial fossa. Clinical article. J Neurosurg. 2009;111:1046–52. doi: 10.3171/2009.2.JNS08284. [DOI] [PubMed] [Google Scholar]
- 23.Badie B, Mendoza D, Batzdorf U. Posterior fossa volume and response to suboccipital decompression in patients with Chiari I malformation. Neurosurgery. 1995;37:214–8. doi: 10.1227/00006123-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Sgouros S, Kountouri M, Natarajan K. Posterior fossa volume in children with Chiari malformation Type I.J Neurosurg 2006;105(2 Suppl):101-6. Comment in J Neurosurg. 2006;105(2 Suppl):101–6. doi: 10.3171/ped.2006.105.2.101. Comment in J Neurosurg 2007;106(4 Suppl):329;author reply 329-30. [DOI] [PubMed] [Google Scholar]
- 25.Trigylidas T, Baronia B, Vassilyadi M, Ventureyra EC. Posterior fossa dimension and volume estimates in pediatric patients with Chiari I malformations. Childs Nerv Syst. 2008;24:329–36. doi: 10.1007/s00381-007-0432-4. [DOI] [PubMed] [Google Scholar]
- 26.Tubbs RS, Hill M, Loukas M, Shoja MM, Oakes WJ. Volumetric analysis of the posterior cranial fossa in a family with four generations of the Chiari malformation Type I. J Neurosurg Pediatr. 2008;1:21–4. doi: 10.3171/PED-08/01/021. [DOI] [PubMed] [Google Scholar]
- 27.Goel A, Bhatjiwale M, Desai K. Basilar invagination: A study based on 190 surgically treated patients. J Neurosurg. 1998;88:962–8. doi: 10.3171/jns.1998.88.6.0962. [DOI] [PubMed] [Google Scholar]
- 28.Lang J. Clinical anatomy of the posterior cranial fossa and its foramina. Stuttgart, Germany: Thieme; 1991. [Google Scholar]
- 29.Hecht JT, Nelson FW, Butler IJ, Horton WA, Scott CI, Jr, Wassman ER, et al. Computerized tomography of the foramen magnum: Achondroplastic values compared to normal standards. Am J Med Genet. 1985;20:355–60. doi: 10.1002/ajmg.1320200219. [DOI] [PubMed] [Google Scholar]
- 30.Hunter AG, Bankier A, Rogers JG, Sillence D, Scott CI., Jr Medical complications of achondroplasia: A multicentre patient review. J Med Genet. 1998;35:705–12. doi: 10.1136/jmg.35.9.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pauli RM, Horton VK, Glinski LP, Reiser CA. Prospective assessment of risks for cervicomedullary-junction compression in infants with achondroplasia. Am J Hum Genet. 1995;56:732–44. [PMC free article] [PubMed] [Google Scholar]
- 32.Bliesener JA, Schmidt LR. Normal and pathological growth of the foramen occipitale magnum shown in the plain radiography. Pediatr Radiol. 1980;10:65–9. doi: 10.1007/BF01001741. [DOI] [PubMed] [Google Scholar]
- 33.Furtado SV, Thakre DJ, Venkatesh PK, Reddy K, Hegde AS. Morphometric analysis of foramen magnum dimensions and intracranial volume in pediatric Chiari I malformation. Acta Neurochir (Wien) 2010;152:221–7. doi: 10.1007/s00701-009-0480-5. [DOI] [PubMed] [Google Scholar]
- 34.Menezes AH. Primary craniovertebral anomalies and the hindbrain herniation syndrome (Chiari I): Data base analysis. Pediatr Neurosurg. 1995;23:260–9. doi: 10.1159/000120969. [DOI] [PubMed] [Google Scholar]
- 35.Boltshauser E, Schmitt B, Wichmann W, Valavanis A, Sailer H, Yonekawa Y. Cerebellomedullary compression in recessive craniometaphyseal dysplasia. Neuroradiology. 1996;38(Suppl 1):S193–5. doi: 10.1007/BF02278158. [DOI] [PubMed] [Google Scholar]
- 36.Ferrier S, Nusslé D, Friedli B, Ferrier PE. Marchesani's syndrome (spherophakia- brachymorphism) Helv Paediatr Acta. 1980;35:185–98. [PubMed] [Google Scholar]
- 37.Knisely AS, Steigman CK. Stenosis of the foramen magnum and rostral spinal canal, with spinal cord deformity, in Jeune's asphyxiating thoracic dystrophy. Pediatr Pathol. 1989;9:299–305. doi: 10.3109/15513818909037734. [DOI] [PubMed] [Google Scholar]
- 38.Upmeyer S, Bothwell M, Tobias JD. Perioperative care of a patient with Beare- Stevenson syndrome. Paediatr Anaesth. 2005;15:1131–6. doi: 10.1111/j.1460-9592.2005.01585.x. [DOI] [PubMed] [Google Scholar]
- 39.Tubbs RS, Bailey M, Barrow WC, Loukas M, Shoja MM, Oakes WJ. Morphometricanalysis of the craniocervical juncture in children with Chiari I malformation and concomitant syringobulbia. Childs Nerv Syst. 2009;25:689–92. doi: 10.1007/s00381-009-0810-1. [DOI] [PubMed] [Google Scholar]
- 40.Muthukumar N, Swaminathan R, Venkatesh G, Bhanumathy SP. A morphometric analysis of the foramen magnum region as it relates to the transcondylar approach. Acta Neurochir (Wien) 2005;147:889–95. doi: 10.1007/s00701-005-0555-x. [DOI] [PubMed] [Google Scholar]


