Abstract
Introduction:
The epidemiology and pathology of meningioma in Nigeria are still evolving and little has been published about this tumor in Nigeria, especially in the southeast region. The aim of this paper is to compare the characteristics of intracranial meningioma managed in our center with the pattern reported in the literature worldwide.
Materials and Methods:
Retrospective analysis of prospectively recorded data of patients managed for intracranial meningioma between January 2002 and December 2010 at a Private neurosurgery Hospital in Enugu, Nigeria. We excluded patients whose histology results were inconclusive.
Results:
Meningiomas constituted 23.8% of all intracranial tumors seen in the period. The male to female ratio was 1:1.1. The peak age range for males and females were in the fifth and sixth decades, respectively. The most common location is the Olfactory groove in 26.5% of patients followed by convexity in 23.5%. Presentation varied with anatomical location of tumor. Patients with olfactory groove meningioma (OGM) mostly presented late with personality changes and evidence of raised ICP. Tuberculum sellar and sphenoid region tumors presented earlier with visual impairment with or without hormonal abnormalities. Seizures occurred in 30.9% of all patients and in 45% of those with convexity meningiomas. Only 57.4% of the patients were managed surgically and there was no gender difference in this group. WHO grade1 tumors were the most common histological types occurring in 84.6%. One patient had atypical meningioma and two had anaplastic tumors.
Conclusion:
The pattern of meningioma in our area may have geographical differences in location and histology. Childhood meningioma was rare.
Keywords: Epidemiology, geographical differences, intracranial meningioma, tumors
Introduction
Meningiomas are the most common primary brain tumors in adults with presentations ranging from incidentally identified slow growing lesions to highly aggressive tumors. Therapeutic approaches therefore may be quite challenging. Surgical resection provides the best possibility of control. Adjuvant radiotherapy is useful for incomplete resections and/or malignant varieties, but systemic chemotherapy usually with hydroxyureas has been disappointing.[1]
The epidemiology of meningiomas as with other primary brain tumors shows interesting regional variations.[2] Meningiomas are now the most frequently reported primary brain tumors in the USA. The central brain tumor registry report between 1992 and 1994[3] showed that meningiomas account for 26% of primary brain tumors and the recent update between 2002 and 2006 show an apparent increase to 33.8% of all intracranial tumors. This trend over the decades however is not considered to be a true increase in incidence[4,5] but may be due to improvement in practice and diagnostic facilities. Meningiomas are understudied in Nigeria and most African countries. They account for 23% of intracranial tumors in all age groups in Nigeria.[6] This estimate may be low since the studies were carried out in major hospitals rather than within the population. It has been shown that the prevalence of subclinical disease from autopsy studies and incidental MRI findings is up to 2.8% of the population.[7,8]
Meningiomas exhibit a characteristic gender difference with a male to female ratio of 1:2.2.[9,10] There is a documented increase in breast cancer patients[11] and tumor enlargement has been noted during pregnancy.[12,13] Meningiomas occur most frequently in middle-aged and elderly patients, with peak incidence in the eighth and ninth decades.[14] Reports from Africa and Asia indicate earlier presentation in the sixth and seventh decades.[15,16] Radiation-induced meningiomas also appear to have an earlier presentation.[17]
Meningiomas are uncommon in children and in this population it tends to be more frequent in males and more aggressive. This paper aims to compare the characteristics of intracranial meningiomas managed in our center with the pattern reported in the literature worldwide.
Materials and Methods
This paper is a retrospective analysis of prospectively recorded data of patients managed for intracranial meningioma between January 2002 and December 2010 at Memfys Hospital (MHN) Enugu. The Hospital is a privately run, Neurosurgical center and a recognized center for postgraduate training in Neurosurgery and related disciplines. The classification in terms of location was based on pre-operative CT and MRI studies. Demographic data was analyzed for all patients but only patients who had surgery and histological diagnosis were analyzed for outcome. Following acute neurosurgical interventions patients are stepped down to acute or chronic rehabilitation in other hospitals. Follow up was over an average period of 3 months. Post operative imaging was done when possible, usually following clinical deterioration. The hospital's Ethical committee approved the study.
Results
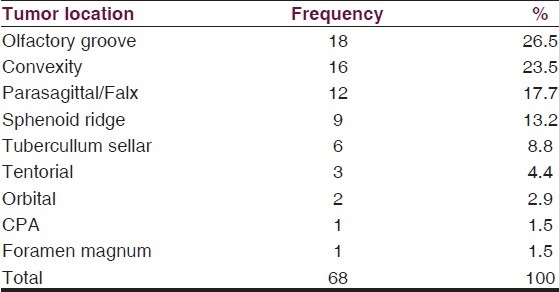
There were 68 cases of intracranial meningioma from a total of 286 intracranial tumors (23.8%). The male to female ratio was 1:1.1. The peak age range for males and females were in the fifth and sixth decades, respectively [Table 1]. The locations were Olfactory groove in 26.5% of patients, convexity in 23.5%, Parasagittal/Falx in 17.7%, sphenoid ridge (13.2%), Tubercullum sellar (8.8%). Others locations include Tentorial, orbital, cerebello-pontine angle, and foramen magnum [Table 2].
Table 1.
Demography
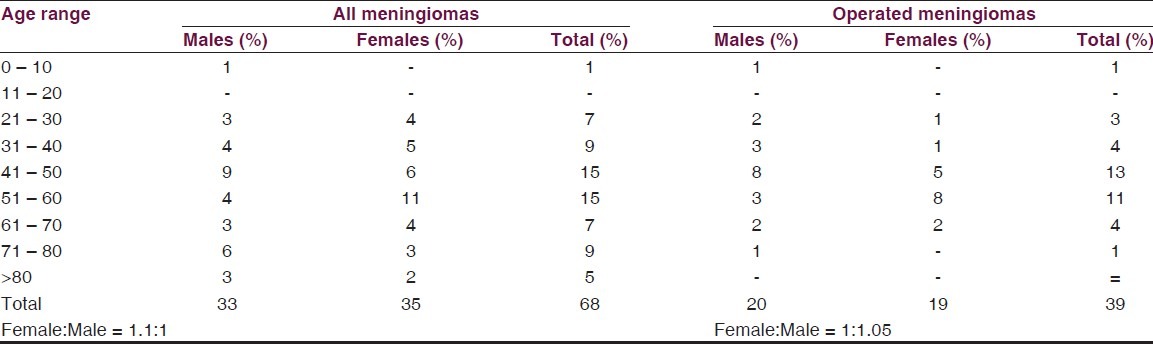
Table 2.
Tumor location
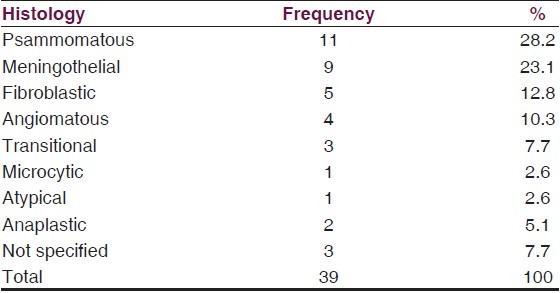
Patients with olfactory groove meningiomas presented mostly with personality changes and evidence of raised ICP. Tuberculum sellar and sphenoid region tumors presented earlier with visual impairment with or without hormonal abnormalities [Table 3]. Seizure occurred in 30.9% of all patients and in 45% of those with convexity meningiomas. Only 39 (57.4%) of the patients were managed surgically. There was a slight male predominance in this group with a male to female ratio of 1.05:1. Histology was predominantly benign. 91.7% of those whose histology were clearly characterized had WHO grade 1 tumors with psammomatous (28.2%) and meningothelial (23.1%) being the main subtypes. There were one atypical and two anaplastic tumors [Table 4].
Table 3.
Clinical presentation
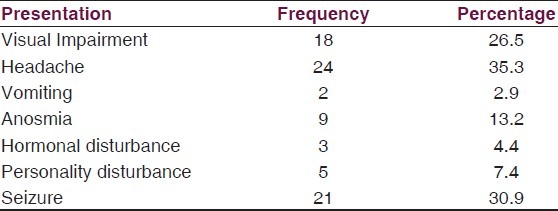
Table 4.
Histology pattern in operated cases
Discussion
Meningiomas are known to have a definite female preponderance. The prevalence of meningioma in the general population presenting for CT and MRI in Enugu, Nigeria, show a slight female predominance (M: F = 1:1.1). Other works from Nigeria also indicate a similar male to female ratio of 1:1.1,[15] which is still lower than the marked female predilection of 2:1 in the USA.[9] Interestingly, more males with meningiomas presented for treatment (M: F = 1.05:1) in this series than is generally reported. This may not reflect a true pattern but evidence of accessibility of private care to the different genders. We note also a gender difference in the peak age incidence with males presenting on the average a decade earlier. This supports the contention that males have more access to care. Meningioma risk increases with age in both male and female[18] but this was not apparent from our data. In this series, the prevalence of meningioma drops sharply following the peak. This we believe, also points to concentration of care resources on the young and more productive.
Most of our cases are diagnosed a decade earlier than reported in more developed countries.[14] This may represent a true geographical peculiarity giving that patients present with more advanced disease. A similar trend is seen from other reports from Africa and Asia.[16,19] There is a similar tendency to earlier presentation in patients with radiation-induced meningioma.[17] Childhood cases of meningiomas are rare worldwide and this is confirmed in this series. There was one case in a pre-adolescent child and this occurred in a male in keeping with the known reversal of the gender incidence in this age group.[20,21]
Olfactory groove is the most common anatomical location of meningioma in this series. Although not significant, this is different from other studies in Africa where the most common is convexity followed by parasagittal.[15,19,22] We did not have any intraventricular or multiple meningiomas. Presentation varied with anatomical location of tumor. Most patients with OGM presented late with personality changes and evidence of raised ICP because of the large size of their tumors. Tuberculum sellar and sphenoid region tumors presented earlier with visual impairment with or without hormonal abnormalities. Seizure occurred in 30.9% of all patients and in 45% of those with convexity meningiomas. We routinely give prophylactic anticonvulsants in our pre-operative cases. Only 39 (57.4%) of the patients were managed surgically.
Meningiomas are believed to arise from arachnoid cap cells and although monoclonal like other cancers, they are predominantly benign in behavior.[18] There is a preponderance of psammomatous meningioma in the histological pattern of our patients. Although not significant, this trend differs from the literature where meningothelial is the most common. Atypical meniningioma is reported to be more common in Blacks than in Whites and Hispanics in the USA.[9] Only 2.6% showed this histological pattern in our series.
Conclusion
In spite of the limitations of one center study and the retrospective nature, this study indicates some peculiar epidemiological differences in patterns of meningioma in our locality. There is a case for multicenter collaborative studies to address these issues. There is also need to explore the reasons for these differences, which may include genetic differences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Newton HB. Hydroxyurea chemotherapy in the treatment of meningiomas. Neurosurg Focus. 2007;23:E11. doi: 10.3171/FOC-07/10/E11. [DOI] [PubMed] [Google Scholar]
- 2.Ohaegbulam SC. Geographical neurosurgery. Neurol Res. 1999;21:161–70. doi: 10.1080/01616412.1999.11740912. [DOI] [PubMed] [Google Scholar]
- 3.2001 CBTRUS Statistical Report: Primary Brain Tumors in the United States, 1992-1997. Available from: http://www.cbtrus.org/2001/2001stats_report.htm .
- 4.Radhakrishnan K, Mokri B, Parisi JE, O’Fallon WM, Sunku J, Kurland LT. The trends in incidence of primary brain tumors in the population of Rochester. Minnesota. Ann Neurol. 1995;37:67–73. doi: 10.1002/ana.410370113. [DOI] [PubMed] [Google Scholar]
- 5.Codera S, Bottacchi E, D’Alessandro G, Machado D, De Gonda F, Corso G. Epidemiology of primary intracranial tumours in NW Italy, a population based study: Stable incidence in the last two decades. J Neurol. 2002;249:281–4. doi: 10.1007/s004150200005. [DOI] [PubMed] [Google Scholar]
- 6.Idowu O, Akang EE, Malomo A. Symptomatic primary intracranial neoplasms in Nigeria, West Africa. J Neurol Sci Turk. 2007;24:212–8. [Google Scholar]
- 7.Krampla W, Newrkla S, Pfisterer W, Jungwirth S, Fischer P, Leitha T, et al. Frequency and risk factors for meningioma in clinically healthy 75-year-old patients: Results of the Transdanube Ageing Study (VITA) Cancer. 2004;100:1208–12. doi: 10.1002/cncr.20088. [DOI] [PubMed] [Google Scholar]
- 8.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–8. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 9.Central Brain Tumor Registry of the United States. 2010 CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. Available from: http://www.cbtrus.org/reports/reports.html .
- 10.Louis DN, Scheithauer BW, Budka H, von Deimling A, Kepes JJ. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2000. Meningiomas. [Google Scholar]
- 11.Custer BS, Koepsell TD, Mueller BA. The Association between Breast Carcinoma and Meningioma in Women. Cancer. 2002;94:1626–35. doi: 10.1002/cncr.10410. [DOI] [PubMed] [Google Scholar]
- 12.Lesch KP, Schott W, Engl HG, Gross S, Thierauf P. Gonadal steroid receptors in meningiomas. J Neurol. 1987;234:328–33. doi: 10.1007/BF00314289. [DOI] [PubMed] [Google Scholar]
- 13.Schrell UM, Fahlbusch R. Hormonal manipulation of cerebral meningiomas. In: Al-Mefty O, editor. Meningiomas. New York: Raven Press; 1991. pp. 273–83. [Google Scholar]
- 14.Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG, et al. Descriptive epidemiology of primary brain and CNS tumors: Results from the Central Brain Tumor Registry of the United States, 1990-1994. Neuro Oncol. 1999;1:14–25. doi: 10.1093/neuonc/1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odebode TO, Akang EE, Shokunbi MT, Malamo AO, Ogunseyinde AO. Factors influencing visual and clinical outcome in Nigerian patients with cranial meningioma. J Clin Neurosci. 2006;13:649–54. doi: 10.1016/j.jocn.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita I, Yang GR, Piao HZ, Sun SC, Fukui M. Comparative study of brain tumours treated at China Medical University, China and Kyushu University, Japan. Fukuoka Igaku Zasshi. 1992;83:386–91. [PubMed] [Google Scholar]
- 17.Sadetzki S, Flint-Richter P, Ben-Tal T, Nass D. Radiation-induced meningioma: A descriptive study of 253 cases. J Neurosurg. 2002;97:1078–82. doi: 10.3171/jns.2002.97.5.1078. [DOI] [PubMed] [Google Scholar]
- 18.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–14. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruberti RF. Surgery of Meningiomas: A review of 215 cases. Afr J Neurol Sci. 1995;14 [Google Scholar]
- 20.Li X, Zhao J. Intracranial meningiomas of childhood and adolescence: Report of 34 cases with follow-up. Childs Nerv Syst. 2009;25:1411–7. doi: 10.1007/s00381-009-0949-9. [DOI] [PubMed] [Google Scholar]
- 21.Menon G, Nair S, Sudhir J, Rao BR, Mathew A, Bahuleyan B. Childhood and adolescent meningiomas: A report of 38 cases and review of literature. Acta Neurochir (Wien) 2009;151:239–44. doi: 10.1007/s00701-009-0206-8. discussion 244. [DOI] [PubMed] [Google Scholar]
- 22.Vivier J, Bardien S, Van der Merwe L, Brusnicky J, Zaharie D, Keyser R, et al. A study of meningiomas in South Africa: Investigating a correlation between clinical presentation, histopathology and genetic markers. Br J Neurosurg. 2009;23:63–70. doi: 10.1080/02688690802593064. [DOI] [PubMed] [Google Scholar]


