Abstract
Rationale
Previous studies have shown that adenosine A2A receptors are colocalized with dopamine D2 receptors on striatal neurons. Activation of these two receptors has antagonistic effects under a number of conditions suggesting that stimulation of adenosine A2A receptors may have behavioral effects resembling those produced by blockade of dopamine D2 receptors, but this possibility has been investigated in a limited number of situations.
Objective
We compared the effects of the adenosine A2A agonist CGS-21680 and the preferential D2 dopamine antagonist haloperidol in a situation in which dopamine blockade produces a distinctive pattern of behavioral effects.
Materials and methods
Six rats were trained to lever press for food reward on a fixed ratio 15 schedule of reinforcement and then tested after being injected with various doses of CGS-21680 (0.064, 0.128, and 0.25 mg/kg) and haloperidol (0.25 and 0.1 mg/kg).
Results
Haloperidol produced a dose-dependent suppression of lever pressing with mean response rates declining across the duration of the test session. CGS-21680 also produced a dose-dependent suppression of responding, but this effect was not temporally graded, and responding was equivalently suppressed across the duration of the session. Additionally, CGS-21680 increased post-reinforcement pause duration to a much greater extent than did haloperidol.
Conclusions
On this task, the behavioral effects of CGS-21680 do not resemble those produced by haloperidol. Several explanations of this discrepancy are possible, the most likely being that the observed behavioral effects of CGS-21680 result from an action at a site other than D2 receptor-expressing striatal neurons.
Keywords: Operant behavior, FR schedule, Post-reinforcement pause, Basal ganglia, Striatum, Indirect-path cells, Neuroleptics, Antipsychotic, Sodium pentobarbital, Adenosine, Operant, Dopamine
Introduction
In recent years, much attention has been focused on the A2A adenosine receptor. This receptor is found at very high levels in the striatum where it is concentrated on striatopallidal neurons which also express D2 dopamine receptors (Augood and Emson 1994; Fink et al. 1992; Schiffmann et al. 1991). Stimulation of dopamine D2 and adenosine A2A receptors has opposite effects on the production of cAMP in these cells (Svenningsson et al. 1998), and A2A receptor activation reduces the affinity of dopamine at D2 receptors through a direct intramembrane interaction (Fuxe et al. 2005). Consistent with these antagonistic interactions at the molecular level, A2A receptor activation can also antagonize many of the neurochemical and behavioral effects produced by stimulation of D2 dopamine receptors. For example, the prototypic A2A agonist CGS-21680 is able to block D2 agonist-induced inhibition of pallidal GABA release (Ferre et al. 1993; Mayfield et al. 1996). CGS-21680 has also been shown to block both the rotation (Morelli et al. 1994, 1995) and the pallidal Fos expression (Morelli et al. 1995) induced by D2 agonists in rats with unilateral 6-OHDA lesions. Furthermore, A2A agonists, like D2 antagonists, inhibit apomorphine stereotypy (Rimondini et al. 1998) and antagonize both the locomotor hyperactivity (Rimondini et al. 1997) and the striatal Fos expression induced by injections of amphetamine (Turgeon et al. 1996).
These results suggest that the effects of adenosine A2A agonists resemble those of selective D2 antagonists, and several authors have suggested that these agents, like D2 antagonists, may be clinically useful as antipsychotic agents (Fuxe et al. 2007; Hauber and Munkle 1997; Rimondini et al. 1997; Weiss et al. 2003; Wardas 2008). It has also been suggested that they may be useful in the control of abnormal or stereotypic movements in Huntington’s disease (Popoli et al. 1994) and autism (Tanimura et al. 2010). In order to evaluate these possibilities and gain greater insight into the function of A2A adenosine receptors, it would clearly be useful to know how close the parallels in the behavioral effects of A2A agonists and dopamine antagonists really are, especially in situations more complex than those involved in measuring locomotor activity and rotation. The available evidence on this point with systemic administration of these drugs is relatively limited. Font et al. (2008) reported that CGS-21680 suppressed food-reinforced lever pressing but, unlike D2 antagonists, did not induce a compensatory increase in the intake of a freely available, but less preferred, food. These authors concluded that the resemblance between the effects of CGS-21680 and D2 antagonists was superficial and that the effects of the adenosine agonist were most likely secondary to sedative effects (Mingote et al. 2008), not shared by D2 antagonists. In the current study, we further explored this issue using a different behavioral paradigm. D2 antagonists exert a highly characteristic temporal pattern of effects on operant behavior in which responding is relatively normal at the start of testing but becomes progressively suppressed across the duration of the test session (Fowler 1990; Hammond et al. 1991; Sanger 1986; Sanger and Perrault 1995; Wirtshafter and Asin 1985; Wise et al. 1978a, b). This pattern of effects resembles that seen in extinction (we have referred to it as “pseudoextinction,” since reward is always present (Wirtshafter and Asin 1985) and has supported the controversial theory that dopamine plays an essential role in reinforcement. The temporal pattern of the effects of CGS-21680 on operant behavior has not previously been examined; if the actions of this drug do indeed resemble those of classical D2 antagonists, one would expect that it would also induce an “extinction-like” decline in responding. In the current study, we tested this possibility by comparing the effects of a range of doses of CGS-21680 to those produced by the preferential D2 dopamine antagonist haloperidol. For comparative purposes, we also examined the sedative–hypnotic agent sodium pentobarbital.
Methods
Animals
Nine adult male Sprague Dawley-derived rats weighing approximately 350 g were obtained from a colony maintained by the Psychology Department of the University of Illinois at Chicago. The animals were housed individually and maintained on a 12:12-h light/dark schedule. All testing took place during the light phase of the cycle. Beginning 1 week prior to the start of magazine training, rats were placed on a restricted feeding schedule until their body weights had declined to 85% of their initial weights. Thereafter, animals were weighed daily, and their food rations adjusted to maintain them at these weights. Unrestricted access to water was available throughout the experiments. Experimental procedures were approved by the U.I.C. Animal Care Committee.
Apparatus
Behavioral testing was conducted in operant conditioning chambers manufactured by Lehigh Valley Electronics. The boxes measured 24 cm wide by 20.5 cm deep by 26 cm high and were each equipped with two levers that were separated by the food hopper. The levers were mounted 2.5 cm above the grid floor and extended 2 cm into the box. Only the right side lever was active during the sessions. Standard 45 mg Noyes food pellets were used as reinforcers and were delivered on depression of the levers. The boxes were installed inside sound-attenuating chambers with an exhaust fan running to provide ventilation. Illumination during the sessions was provided by a house light located at the top of the back wall of the boxes. The operant conditioning schedules were controlled by a computer located in an adjacent room using MED-PC software (Med Associates, St. Albans, VT). The times at which individual lever presses were made and reinforcers delivered were measured to the nearest 0.1 s. Cumulative records were constructed using the SOFTCR program (Med Associates). Post-reinforcement pauses (PRPs) were also measured in animals performing on the fixed ratio 15 (FR15) schedule (see below). In the majority of cases, the inter-response intervals following reinforcer delivery were substantially longer than those occurring during execution of the ratio, and this value was taken as the PRP. In about 20% of cases, however, one or, rarely, two very short (mean=0.18 s) inter-response intervals occurred immediately following reinforcer delivery which were then followed by a much longer pause typical of those usually seen after reinforcement. Since these short inter-response times are not long enough for the animals to move to the hopper, retrieve the food, and press the lever, these trials presumably represent occasions in which the rat emitted one or two extra lever presses before visiting the hopper. In these cases, therefore, the first response exceeding 0.5 s was taken as the PRP (mean value=6.3 s).
Drugs
Haloperidol and CGS-21680 (2-p-(2-carboxyethyl) phenethylamino-5′-N-ethylcarboxamidoadenosine) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Haloperidol was prepared in 30% propylene glycol, which was acidified with the minimum amount of acetic acid necessary to dissolve the compound. CGS-21680 was prepared in 30% dimethylsulfoxide. Sodium pentobarbital (Sigma) was dissolved in a solution of 40% propylene glycol.
Procedure
All subjects received 3 days of 20-min-long magazine training sessions during which individual food pellets were presented on a fixed-time 45-s schedule. Animals were then manually shaped to lever press, after which they were placed on a continuous reinforcement (FR1) schedule for two daily 20-min sessions. Over the next 2 weeks, the schedule requirement was gradually increased from FR1 to FR15. Animals were run 5 days per week for the remainder of the experiments. Rats were run for 7 days under the FR15 schedule after which drug testing was begun. Six animals were used in the main experiment; these subjects were first tested with various doses of CGS-21680. Rats were placed in the operant conditioning chambers for 20-min-long sessions beginning 20 min following intraperitoneal (i.p.) injections of vehicle or of CGS-21680 at doses of 0.064, 0.128, or 0.256 mg/kg. Individual subjects received these four injections in a counterbalanced order, and at least 3 days intervened between successive drug tests, during which time subjects received at least two daily runs in the operant conditioning chambers. Four days following the completion of testing with CGS-21680, analogous methods were used to examine the response to injections of haloperidol; this drug was administered subcutaneously, and rats were run 30 min following s.c. injections of either vehicle or haloperidol at doses of 0.025 or 0.1 mg/kg. Three days following the completion of these studies, rats were tested, using identical methods, following i.p. injections of vehicle or of sodium pentobarbital at doses of either 10 or 15 mg/kg. Three additional animals were trained on the FR15 schedule as described above; as a part of another experiment, these animals were tested for 1 day on extinction and then retrained for 3 days on the FR15 schedule after which they were tested 1 h following injections of the high dose of haloperidol using methods identical to those described above.
Results
CGS-21680
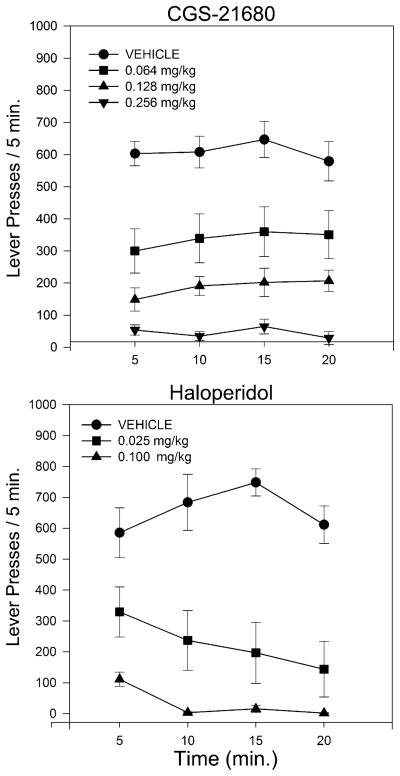
CGS-21680 produced a marked, dose-dependent suppression of lever pressing which was monotonic in all six of the subjects (upper panel of Fig. 1). When responding was examined in 5-min time bins (Fig. 1), it was apparent that mean rates of responding for drug-treated animals tended to be relatively stable across the duration of the session. We examined these data statistically by means of a 4×4 (dose × time bin) repeated measures ANOVA which indicated a significant effect of CGS-21680 dose (F(3,15)=40.56, p< 0.0001) but no effect of time or of the dose × time interaction (p>0.1), indicating that CGS-21680 treatment did not alter the distribution of responding across the session We also examined latencies to initiate lever pressing following placement in the operant conditioning chambers; mean latencies were almost identical at the three lowest doses (39.8±17.8, 45.9±24.4, and 41.1±11.9 s) and tended to increase slightly at the highest dose (77.6±22.6 s), but a one-way repeated measures ANOVA indicated that these differences were not statistically significant (F<1).
Fig. 1.
Upper panel Lever presses in 5-min time bins across the 20-min test session by animals pretreated with vehicle or various doses of CGS-21680. Error bars indicate SEMs. Lower panel: Lever presses in 5-min time bins across the 20-min test session by animals pretreated with vehicle or various doses of haloperidol. Error bars indicate SEMs
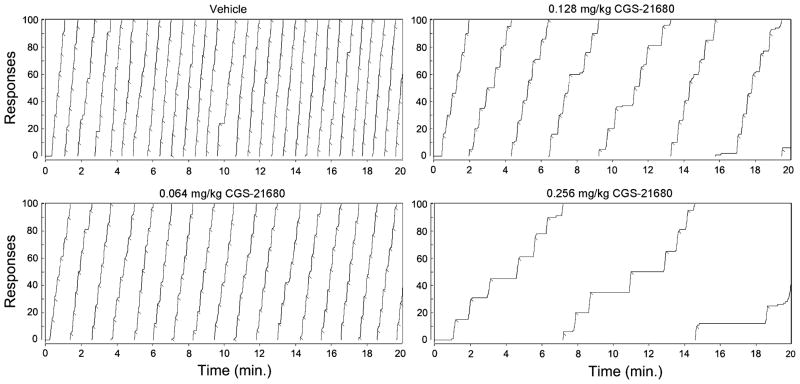
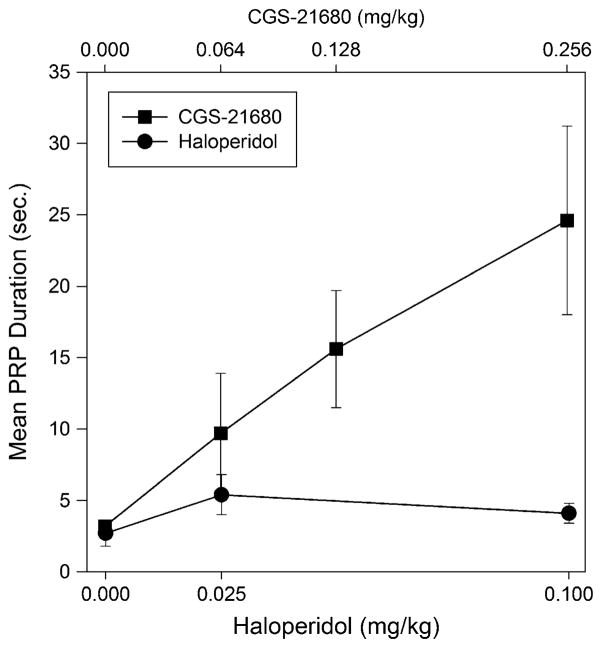
Examination of cumulative records indicated that all subjects displayed a characteristic pattern of responding following treatment with the adenosine agonist. As an example, Fig. 2 displays the cumulative records of a single representative subject following injections of vehicle or of the various doses of CGS-21680. As can be seen in the upper left panel of the figure, this subject, following vehicle injections, pressed at a high and constant rate throughout the session, with only brief pauses apparent after reinforcements. As the dose of CGS-21680 was incremented, the overall rate of responding decreased, and pronounced pauses became apparent following reinforcements. Nonetheless, there were no consistent changes in the rate of responding across the duration of the session. In order to quantitatively explore the effects of CGS-21680 on PRP duration, we examined the mean duration of the first two PRPs across all of the subjects. As can be seen in the upper trace of Fig. 3, mean PRP duration increased almost linearly with CGS-21680 dose. A one-way repeated measures ANOVA conducted on log PRP duration indicated a significant effect of CGS-21260 dose (p<0.01). (We examined just the first two PRPs in order to make these data comparable to those obtained under haloperidol (see below), as one subject under that condition completed only two ratios. Similar results were obtained if PRPs were examined across the entire session; e.g., the mean overall PRP duration after saline was 2.0±0.3 versus 29.8±8.4 s after 0.250 mg/kg CGS-21680.)
Fig. 2.
Cumulative records showing the lever pressing performance of a single animal treated with vehicle (upper left) or with CGS-21680 at doses of 0.064 (lower left), 0.128 (upper right), or 0.256 mg/kg (lower right). The trace resets down to 0 every 100 presses, and the crosshatches indicate delivery of food pellets. This subject was tested first with CGS-21680 at a dose of 0.064 mg/kg, followed sequentially by 0.265 mg/kg, vehicle, and 0.128 mg/kg)
Fig. 3.
Mean duration of the first two PRPs following treatment with haloperidol or its vehicle (filled circles) or CGS-21680 and its vehicle (squares). Error bars indicate SEMs
Haloperidol
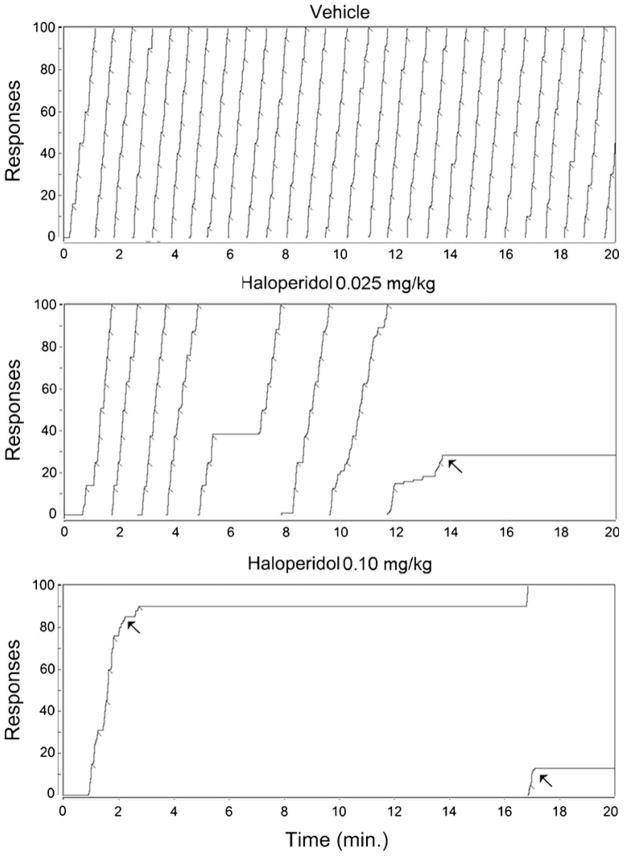
The lower panel of Fig. 1 demonstrates that haloperidol, like CGS-21680, produced a dose-dependent decrease in overall rate of responding which was monotonic in all six subjects. As can be seen in the figure, the rate of responding after haloperidol, but not vehicle, tended to decrease across time, a pattern which was seen in all of the animals at the high dose and five of the six animals at the low dose. A 3×4 (dose × time bin) repeated measures ANOVA indicated significant effects of both dose (F(2,19)=39.6, p<0.0001) and dose × time interaction (F(6,30)=4.35, p<0.003). Post hoc tests of linear trends indicated that response rates declined significantly across time at the 0.1-mg/kg dose of haloperidol (p< 0.006). A similar trend was also seen at the 0.025 dose but was only significant one-tailed (p<0.1). Responding under vehicle was not significantly affected by time (p>0.75). Cumulative records for a representative subject are shown in Fig. 4 where it can be seen that haloperidol treatment resulted in a marked tendency for a within-session decline in the rate of responding. Following treatment with even the highest dose of haloperidol, this subject began pressing at a relatively normal rate before beginning to slow down. Furthermore, the “break and run” pattern, characterized by marked prolongation of post-reinforcement pauses, that was so apparent after CGS-21680 treatment is not evident here. The mean duration of the first two PRPs after treatment with vehicle or haloperidol is shown in Fig. 3 where it can be seen that, in contrast to the effects observed after CGS-21680, there was only a small trend for PRP duration to increase during the first two trials, which was not significant by ANOVA (p>0.2). In many cases, pauses in responding could be seen in the cumulative records of haloperidol-treated rats (c.f., Fig. 4), but these did not seem to be specifically associated with post-reinforcement pauses, as was the case for animals treated with CGS-21680. In order to evaluate this possibility, we determined for each animal, across all of its haloperidol runs, the percentage of the pauses in responding longer than 15 s that were post-reinforcement pauses. This analysis indicated that only 46% of these stoppages occurred following food delivery, and the majority of the pauses therefore interrupted the performance of the ratio.
Fig. 4.
Cumulative records showing the lever-pressing performance of a single animal treated with vehicle (top) or with haloperidol at doses of 0.025 (middle) or 0.10 mg/kg (bottom). Records are from the same animal shown in Fig. 2, and this subject was tested first with the 0.10-mg/kg dose of haloperidol, followed by the 0.025 dose and finally after vehicle. The arrows indicate examples of the rat initiating pauses a substantial number of presses into the ratio
In order to confirm the decremental nature of the haloperidol effect, three additional animals were tested 1 h following injections of haloperidol; all of these subjects showed a decrement in responding across the test session with responses declining from 411±90 (mean ± SEM) in the first 5-min period to 51±51 in the last.
Sodium pentobarbital
Injections of sodium pentobarbital also tended to suppress lever pressing; mean total responses (±SEM) across the test session were 2,658±187, 1,622±322, and 1,408±268 for the saline, 10 and 15 mg/kg doses, respectively. In contrast to the decremental pattern seen with haloperidol, rates of responding actually tended to increase slightly across the duration of the session. Analysis of these results by means of a 3×4 (dose × time bin) ANOVA indicated a significant effect of dose (F(2,19)=7.17, p<0.01), but neither the time nor the dose × time interaction were significant (p>0.2). PRP duration, measured as described above, tended to increase following pentobarbital injections, but this effect was also not significant.
Discussion
In agreement with the results of previous studies (Font et al. 2008; Mingote et al. 2008), the current findings indicate that the prototypical selective adenosine A2A agonist CGS-21680, like the preferential D2 antagonist haloperidol, is able to produce a marked suppression of food-reinforced lever pressing in rats. It should be noted that the doses used here were much lower than those which have been reported to induce catalepsy (Kafka and Corbett 1996; Rimondini et al. 1997) and are similar to those which have been reported to antagonize the locomotor activity induced by amphetamine (1 mg/kg) (Rimondini et al. 1997).
Although the behavioral suppression produced by CGS-21680 in the current study superficially resembled that produced by haloperidol, closer examination revealed marked differences in the effects of the two drugs. In agreement with the results of previous investigators (Fowler 1990; Sanger 1986; Sanger and Perrault 1995; Wirtshafter and Asin 1985), haloperidol produced a temporally graded suppression of fixed ratio responding with performance being relatively high at the start of the session but decaying thereafter. Similar “pseudoextinction” patterns have been reported after treatment with a variety of neuroleptic drugs (Sanger 1986; Sanger and Perrault 1995; Wise et al. 1978b). We observed a similar pattern of responding in three additional animals tested 1 h following haloperidol administration, suggesting that the within-session decline in response rate was not related to the time course of drug absorption or metabolism. It is likely that this pattern of effects results primarily from blockade of dopamine D2-like receptors since the selective D1-like antagonist SCH-23390 does not produce a within-session decline in responding (Sanger 1987). In agreement with the report of Fowler (1990), examination of individual cumulative records suggested that much of the decrement in the performance seen in haloperidol-treated animals was due to relatively sudden terminations of responding before the ends of the sessions. It is extremely unlikely that the within-session decline we observed after haloperidol was somehow the result of the animals’ prior experience with CGS-21680, because other authors have observed similar patterns in drug-naive rats (Wise et al. 1978b).
In contrast, we found that the effects of CGS-21680 did not vary as a function of time; performance in these animals appeared to be as suppressed at the start of the session as it was at the end. We also observed a nondecremental pattern of responding following injections of the sedative agent sodium pentobarbital, a result similar to that reported by Hammond et al. (1991). Similar findings of nondecremental attenuation of responding have been reported following injections of a variety of compounds including dantrolene (Hammond et al. 1991), chlordiazepoxide, clonidine, morphine, and methocarbamol (Sanger 1986). These contrasting temporal patterns of deficits indicate that different drugs are able to suppress operant behavior through diverse behaviorally distinguishable mechanisms; the current findings additionally provide further evidence that the production of within-session declines in response output on FR schedules is an uncommon effect which currently appears to be restricted to D2 dopamine blockers.
In addition to producing different temporal patterns of lever pressing, haloperidol and CGS-21680 also differed in their effects on PRPs. Examination of cumulative records indicated that higher doses CGS-21680 produced large increases in the duration of post-reinforcement pauses that were present from the beginning of the sessions and continued throughout their duration. The basis for this effect is uncertain, as is type of behavior engaged in during the pauses, but a number of other pharmacological and behavioral manipulations (Grove and Thompson 1970; Sidman and Stebbins 1954) have also been shown to produce relatively selective effects on this variable, and its sensitivity must reflect something about the basic nature of fixed ratio schedules. In contrast, haloperidol had a much smaller influence on PRPs and did not significantly alter their duration during the initial ratios. Although individual long PRPs could be seen after haloperidol, they were erratic in their occurrence, and prolonged pauses were not exclusively linked to PRPs; in fact, about half of the long pauses in responding after haloperidol did not follow reinforcement. Similar observations have been made following treatment with reserpine (Dews 1956).
The present results indicate that systemic administration of CGS-21680 produces effects on behavior maintained on a fixed ratio schedule that are clearly distinct from those seen after blockade of dopamine D2 receptors. In fact, the current results demonstrate a double dissociation of the two drugs; thus, haloperidol, but not CGS-21680, produces a within-session decline in response rate, whereas the latter drug produces a more marked effect on PRPs. These results are compatible with those of Font et al. (2008) who found that haloperidol, but not CGS-21680, induced a reallocation of effort in a concurrent operant/free feeding situation. These discrepancies are, perhaps, surprising given the extent of colocalization of A2A and D2 receptors on striatopallidal neurons. Several explanations are possible. First, the behavioral effects of CGS-21680 observed here may not have been mediated through striatopallidal cells. A2A receptors are found at a number of other sites in the brain and periphery (Cunha et al. 1994; Rosin et al. 1998), and, even within the striatum, CGS-21680 has been found to exert presynaptic effects (Quiroz et al. 2009). It is possible that CGS-21680 may produce suppressive effects through an action at these loci at doses lower than those needed to influence striatopallidal neurons. This possibility is supported by the fact that most studies which have observed effects on striatal functioning have used doses substantially higher than those employed here (Boegman and Vincent 1996; Karcz-Kubicha et al. 2006; Morelli et al. 1994, 1995; Pinna et al. 1997). If this hypothesis is correct, it is possible that CGS-21680 might exert effects more similar to those of dopamine antagonists if it were injected directly into the brain. A result of this type has indeed been observed by Font et al. (2008) who found that intra-accumbens injections of CGS-21680 reduced lever pressing but increased food intake in a concurrent operant/free food task. (It should be noted that the pseudoextinction pattern may be more closely related to the dorsal striatum than to the nucleus accumbens (Beninger and Ranaldi 1993).) A second possibility is that while the effects of both CGS-21680 and haloperidol may be exerted through striatopallidal neurons, the two drugs may affect these cells in distinct ways. Fowler (1990), for example, has suggested that the extinction-like decline in performance induced by neuroleptics results from a specific interaction between dopamine receptor blockade and changes in dopamine availability across the duration of the session. If this theory is correct, neuroleptic-like effects would not be expected to result from drugs which influence striatopallidal neurons through nondopaminergic mechanisms. It is interesting that intra-accumbens infusions of CGS-21680 have been found to induce sleep (Satoh et al. 1999), an effect which may be related to the sedation reported after systemic injections (Mingote et al. 2008).
The functional interrelations between D2 and A2A receptors have suggested to a number of authors that adenosine A2A agonists may have utility as antipsychotics (Fuxe et al. 2007; Hauber and Munkle 1997; Rimondini et al. 1997; Weiss et al. 2003; Wardas 2008). The current findings provide no support for this theory insofar as the effects of this agent did not resemble those of a classical antipsychotic agent. It is not clear, however, that the production of within-session declines in operant behavior is correlated with the antipsychotic effects of neuroleptics rather than their extrapyramidal side effects. The latter possibility is suggested by the fact that graded decrements in responding are not prominent after the atypical neuroleptic clozapine (Sanger 1986). On the other hand, it is unlikely that A2A agonists would be useful as anti-psychotics or as treatments for autistic or choreatic movement disorders (Popoli et al. 1994; Tanimura et al. 2010), if they have behavioral side effects at doses lower than those needed to alter the function of striatal indirect-path cells. Further studies will be needed to sort out these possibilities.
Acknowledgments
This publication is based upon work supported by grants 0641943 from the National Science Foundation, R01DK071738 from the National Institute of Diabetes and Digestive and Kidney Diseases, and R03DA020802 from the National Institute for Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation or the National Institutes of Health.
Contributor Information
Chris Jones-Cage, College of the Desert, 43-500 Monterey Avenue, Palm Desert, CA 92260, USA.
Thomas R. Stratford, Laboratory of Integrative Neuroscience and Department of Psychology (M/C 285), University of Illinois at Chicago, 1007 W. Harrison St., Chicago, IL 60607-7137, USA
David Wirtshafter, Email: davew@uic.edu, Laboratory of Integrative Neuroscience and Department of Psychology (M/C 285), University of Illinois at Chicago, 1007 W. Harrison St., Chicago, IL 60607-7137, USA.
References
- Augood SJ, Emson PC. Adenosine A2a receptor mRNA is expressed by enkephalin cells but not by somatostatin cells in rat striatum: a co-expression study. Mol Brain Res. 1994;22:204–210. doi: 10.1016/0169-328x(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Ranaldi R. Microinjections of flupenthixol into the caudate-putamen but not the nucleus accumbens, amygdala or frontal cortex of rats produce intra-session declines in food-rewarded operant responding. Behav Brain Res. 1993;55:203–212. doi: 10.1016/0166-4328(93)90116-8. [DOI] [PubMed] [Google Scholar]
- Boegman RJ, Vincent SR. Involvement of adenosine and glutamate receptors in the induction of c-fos in the striatum by haloperidol. Synapse. 1996;22:70–77. doi: 10.1002/(SICI)1098-2396(199601)22:1<70::AID-SYN8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Johansson B, van der Ploeg I, Sebastiao AM, Ribeiro JA, Fredhlom BB. Evidence for functionally important adenosine A2a receptors in the rat hippocampus. Brain Res. 1994;649:298–216. doi: 10.1016/0006-8993(94)91066-9. [DOI] [PubMed] [Google Scholar]
- Dews PB. Modification by drugs of performance on simple schedules of positive reinforcement. Ann N Y Acad Sci. 1956;65:268–281. doi: 10.1111/j.1749-6632.1956.tb49639.x. [DOI] [PubMed] [Google Scholar]
- Ferre S, O’Connor WTO, Fuxe K, Ungerstedt U. The striopallidal neuron: a main locus for adenosine-dopamine interactions in the brain. J Neurosci. 1993;13:5402–5406. doi: 10.1523/JNEUROSCI.13-12-05402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund R, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Font L, Mingote S, Farrar AM, Percira M, Worden L, Stopper C, Port RG, Salamone JD. Intra-accumbens injections of the adenosine A2A agonist CGS 21680 affect effort-related choice behavior in rats. Psychopharmacol. 2008;199:515–526. doi: 10.1007/s00213-008-1174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SC. Neuroleptics produce within-session response decrements: facts and theories. Drug Dev Res. 1990;20:101–116. [Google Scholar]
- Fuxe K, Ferre S, Canals M, Torvinen M, Terasmaa A, Marcellino D, Goldberg SR, Staines W, Jacobsen KX, Woods AS, Agnati LF, Franco R. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci. 2005;26:209–220. doi: 10.1385/JMN:26:2-3:209. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their releveance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Grove R, Thompson T. The effects of pentobarbital on performance mantained by an interlocking fixed-ratio fixed-interval reinforcement schedule. In: Thompson T, Pickens R, Meisch RA, editors. Readings in behavioral pharmacology. Appleton; New York: 1970. pp. 583–593. [Google Scholar]
- Hammond EO, Torok ML, Ettenberg A. Different patterns of behavior produced by haloperidol, pentobarbital, and dantrolene in tests of unconditioned locomotion and operant responding. Psychopharmacol. 1991;104:150–156. doi: 10.1007/BF02244170. [DOI] [PubMed] [Google Scholar]
- Hauber W, Munkle M. Motor depressant effects mediated by dopamine D2 and adenosine A2a receptors in the nucleus accumbens and caudate putamen. Eur J Pharmacol. 1997;323:127–131. doi: 10.1016/s0014-2999(97)00040-x. [DOI] [PubMed] [Google Scholar]
- Kafka SH, Corbett R. Selective adenosine A2a receptor/dopamine D2 receptor interactions in animal models of schizophrenia. Eur J Pharmacol. 1996;295:147–154. doi: 10.1016/0014-2999(95)00668-0. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Ferre S, Diaz-Ruiz O, Quiroz-Milina C, Goldberg SR, Hope BT, Morales M. Stimulation of adenosine receptors selectively activates gene expression in striatal enkephalinergic neurons. Neuropsychopharmacol. 2006;31:2173–2179. doi: 10.1038/sj.npp.1301035. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Larson G, Orona RA, Zahniser NR. Opposing actions of adenosine A2a and dopamine D2 receptor activation on GABA release in the basal ganglia: evidence for an A2a/D2 receptor interaction in globus pallidus. Synapse. 1996;22:132–238. doi: 10.1002/(SICI)1098-2396(199602)22:2<132::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mingote S, Pereira M, Farrar AM, McLaughlin PJ, Salamone JD. Systemic administration of the adenosine A2A agonist CGS 21680 induced sedation at doses that suppress lever pressing and food intake. Pharmacol Biochem Behav. 2008;89:345–351. doi: 10.1016/j.pbb.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Fenu S, Pinna A, Di Chiara G. Adenosine A2 receptors interact negatively with dopamine D1 and D2 receptors in unilaterally 6-hydroxydopamine-lesioned rats. Eur J Pharmacol. 1994;251:21–25. doi: 10.1016/0014-2999(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Morelli M, Pinna A, Wardas J, Di Chiara G. Adenosine A2 receptors stimulate c-fos expression in striatal neurons of 6-hydroxydopamine lesioned rats. Neurosci. 1995;67:49–55. doi: 10.1016/0306-4522(94)00602-2. [DOI] [PubMed] [Google Scholar]
- Pinna A, Wardas J, Cristalli G, Morelli M. Adenosine A2a receptor agonists increase Fos-like immunoreactivity in mesolimbic areas. Brain Res. 1997;759:41–49. doi: 10.1016/s0006-8993(97)00214-x. [DOI] [PubMed] [Google Scholar]
- Popoli P, Pezzola A, Reggiio R, Caporali MG, De Carolis AS. CGS 21680 antagonizes motor hyperactivity in a rat model of Huntington’s disease. Eur J Pharmacol. 1994;257:R5–R6. doi: 10.1016/0014-2999(94)90715-3. [DOI] [PubMed] [Google Scholar]
- Quiroz C, Lujan R, Uchigashima M, Simoes AP, Lerner TN, Borycz J, Kachroo A, Canas PM, Orru M, Schwarzschild MA, Rosin DL, Kreitzer AC, Cunha RA, Watanabe M, Ferre S. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. Scientific World Journal. 2009;9:1321–13344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Ferre S, Ogren SO, Fuxe K. Adenosine A2a agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacol. 1997;27:82–91. doi: 10.1016/S0893-133X(97)00033-X. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Ferre S, Gimenez-Llort L, Ogren SO, Fuxe K. Differential effects of selective adenosine A1 and A2A receptor agonists on dopamine receptor agonist-induced behavioural responses in rats. Eur J Neurosci. 1998;347:153–158. doi: 10.1016/s0014-2999(98)00107-1. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodward RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2a receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- Sanger DJ. Resonse decrement patterns after neuroleptic and non-neuroleptic drugs. Psychopharmacol. 1986;89:98–104. doi: 10.1007/BF00175198. [DOI] [PubMed] [Google Scholar]
- Sanger DJ. The actions of SCH 23390, a D1 receptor antagonist, on operant and avoidance behavior in rats. Pharmacol Biochem Behav. 1987;26:509–513. doi: 10.1016/0091-3057(87)90157-2. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Perrault G. Effects of typical and atypical antipsychotic drugs on response decrement patterns in rats. J Pharmacol Exp Ther. 1995;272:708–713. [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Kolke N, Tokunaga Y, Maeda T, Hayaishi O. Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur J Neurosci. 1999;11:1587–1597. doi: 10.1046/j.1460-9568.1999.00569.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. The striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not substance P neurons: an in-situ hybridization study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Sidman M, Stebbins WC. Satiation effects under fixed-ratio schedules of reinforcement. J Comp Physiol Psychol. 1954;47:114–116. doi: 10.1037/h0054127. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Rogoni F, Fredholm BB, Greengard P, Fisone G. Activation of adenosine A2a and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARP-32 in distinct populations of striatal projection neurons. Neurosci. 1998;84:223–228. doi: 10.1016/s0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Vaziri S, Lewis MH. Indirect basal ganglia pathway mediation of repetitive behavior: attenuation by adenosine receptor agonists. Behav Bain Res. 2010;210:116–122. doi: 10.1016/j.bbr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon SM, Pollack AE, Schusheim L, Fink JS. Effects of selective adenosine A1 and A2a agonists on amphetamine-induced locomotion and c-Fos in striatum and nucleus accumbens. Brain Res. 1996;707:75–80. doi: 10.1016/0006-8993(95)01223-0. [DOI] [PubMed] [Google Scholar]
- Wardas J. Potential role of adenosine A2A receptors in the treatment of schizophrenia. Front Biosci. 2008;13:4071–4096. doi: 10.2741/2995. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Whawell E, Upton R, Dourish CT. Potential for antipxychotic and psychotomimetic effects of A2A receptor modulation. Neurol. 2003;61(suppl 6):S88–S93. doi: 10.1212/01.wnl.0000095221.30476.8a. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE. Haloperidol and nonreinforcement produce different patterns of response slowing in a food reinforced runway task. Pharmacol Biochem Behav. 1985;22:661–663. doi: 10.1016/0091-3057(85)90509-x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, de Wit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Sci. 1978a;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, Legault L. Major attenuation of food reward with performance-sparing doses of pimozide in the rat. Can J Psychol. 1978b;32:77–85. doi: 10.1037/h0081678. [DOI] [PubMed] [Google Scholar]