Abstract
Increased expression of protease-activated receptor 1 (PAR1), a G protein-coupled receptor for thrombin, has previously been correlated with breast carcinoma cell invasion. PAR1 is irreversibly proteolytically activated, internalized, and sorted directly to lysosomes, a critical process for the termination of signaling. We determined that activated PAR1 trafficking is severely altered in metastatic breast carcinoma cells but not in nonmetastatic or normal breast epithelial cells. Consequently, the proteolytically activated receptor is not sorted to lysosomes and degraded. Altered trafficking of proteolytically activated PAR1 caused sustained activation of phosphoinositide hydrolysis and extracellular signal-regulated kinase signaling, even after thrombin withdrawal, and enhanced cellular invasion. Thus, our results reveal that a novel alteration in trafficking of activated PAR1 causes persistent signaling and, in addition to other processes and proteins, contributes to breast carcinoma cell invasion.
The genetic changes that initiate tumor cell metastasis and invasion are unclear. The procoagulant activities of tumor cells and surrounding stromal cells that lead to thrombin generation and fibrin deposition appear to provide an important influence on tumor cell invasion and metastasis (3). Although a link between coagulation factors and tumor progression and metastasis has been firmly established, the molecular basis remains poorly understood (7, 26).
Thrombin, the main effector protease of the coagulation cascade, is generated by the actions of tissue factor and other coagulation factors. The first description of thrombin-induced murine tumor cell metastasis was provided by Nierodzik and colleagues (23, 24). Subsequent studies determined that tissue factor, like thrombin, is highly expressed in carcinoma cells and contributes to metastasis (20, 21). In addition, the anticoagulants heparin and warfarin have been shown to significantly decrease experimentally induced pulmonary metastasis in vivo (37). A similar decrease in tumor cell metastasis in vivo was observed with hirudin, a specific inhibitor of thrombin (9). Fibrin, generated by the action of thrombin on fibrinogen, is a significant component of the tumor microenvironment and has been strongly associated with tumor progression and metastasis (7). A recent genetic study revealed that, in fibrinogen-deficient mice, the development of both spontaneous and experimental lung metastases was substantially diminished (27). However, hirudin further diminished the metastatic potential of tumor cells in fibrinogen-deficient mice. Thus, while fibrinogen is one critical component of metastasis, thrombin appears to contribute to tumor cell dissemination through at least one fibrinogen-independent mechanism.
In addition to acting on fibrinogen, thrombin elicits a host of cellular responses through the activation of G protein-coupled protease-activated receptors (PARs): PAR1, -3, and -4 (6). Thrombin activation of PAR1 mediates proliferative signaling and migratory responses in a variety of cell types (19, 29). A role for PAR1 in tumor cell invasion is suggested by increased PAR1 mRNA and protein expression in infiltrating ductal carcinomas of breast tissue specimens and in highly invasive breast carcinoma cell lines; these increases have been correlated with carcinoma cell invasiveness (10, 13). Importantly, the addition of thrombin further increased the invasiveness of certain breast carcinoma cells but had no effect on noninvasive cells. In contrast, PAR1 expression was minimal or absent in premalignant atypical intraductal hyperplasia, normal breast epithelial tissue specimens, and noninvasive cell lines, as well as the B16F10 cell line (10, 13). Additionally, antisense inhibition of PAR1 expression in a highly invasive breast carcinoma cell line caused a reduction in invasive potential (10, 22). Thus, the roles of thrombin and PAR1 and the mechanism by which they contribute to breast carcinoma cell invasion remain poorly understood.
PAR1 is activated by an unusual proteolytic mechanism in which thrombin binds to and cleaves the amino-terminal exodomain of the receptor (6). This cleavage generates a new amino terminus that functions as a tethered ligand by binding intramolecularly to the body of the receptor to cause transmembrane signaling. Because proteolytic activation of PAR1 creates a tethered ligand that cannot diffuse away, the mechanisms that contribute to the termination of receptor signaling are critical in determining the magnitude and duration of thrombin-stimulated signaling in cells.
Signaling by activated PAR1 is terminated at the plasma membrane by phosphorylation and arrestin binding (15, 25). However, we demonstrated previously that downregulation of activated PAR1 by internalization and lysosomal sorting is also critical for the termination of receptor signaling. In fibroblasts, a chimeric PAR1 that internalizes and recycles back to the cell surface showed enhanced and prolonged signaling after activation with thrombin (35, 36). This prolonged signaling was apparently due to recycling and continued signaling by proteolytically activated receptors that returned to the plasma membrane with their tethered ligands intact. In the present study, we determined that activation of PAR1 is necessary and sufficient for thrombin-stimulated breast carcinoma cell invasion and that dysregulated PAR1 trafficking leads to constitutive signaling and promotes cellular invasion.
MATERIALS AND METHODS
Plasmid constructs.
A human PAR1 cDNA sequence was isolated by PCR from a human colon cDNA library (Clontech), sequence verified, and subcloned into the pBabe-puro expression vector. PCR amplification was used to generate a PAR1 cDNA in antisense orientation representing the first exon, as previously described (11), which was subcloned into pBabe-puro. A chimeric PAR1 bearing the substance P receptor carboxyl-terminal tail (P/S C tail) was described previously (35).
Materials and antibodies.
The PAR1 agonist peptide SFLLRN was synthesized at the Chapel Hill Peptide Facility, University of North Carolina. Human α-thrombin (Enzyme Research Laboratories), pertussis toxin (Calbiochem), leech hirudin, α-trypsin treated with tosyl-amido-2-phenylethyl chloromethyl ketone, and trypsin inhibitor (Sigma) were from commercial sources.
A rabbit polyclonal anti-PAR1 antibody was generated as described previously (14). Monoclonal anti-PAR1 antibody (Biodesign International), anti-p42/44 (Santa Cruz Biotechnology), anti-phospho-p42/44 ERK antibodies (Cell Signaling Technology), and EEA1 antibody (Transduction Laboratories) were purchased from commercial sources. Anti-LAMP1 H4A3 monoclonal antibody was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa. The secondary antibodies, goat anti-mouse and anti-rabbit conjugated to horseradish, were from Pierce. Alexa488- and Alexa594-conjugated goat anti-mouse and anti-rabbit antibodies were from Molecular Probes.
Cell culture.
All human breast carcinoma cell lines were obtained from the American Type Culture Collection (ATCC) and were maintained in growth media as recommended by ATCC. HeLa cells stably expressing PAR1 or the P/S C tail were generated and characterized as described previously (33). HEK 293T cells were maintained in growth medium as recommended by ATCC. Human mammary epithelial cells (HMECs) immortalized by ectopic expression of the catalytic subunit of hTERT were kindly provided by Robert Weinberg (Massachusetts Institute of Technology) and were maintained as described previously (8).
Retroviral infection and transfection.
Infectious amphotropic recombinant retrovirus encoding PAR1 or the empty vector was produced in HEK 293T cells using the pVPack retroviral production system (Stratagene) as described previously (28). Cells were infected with retroviral supernatant, and mass cell populations were selected in medium containing 1 μg of puromycin/ml.
Cellular invasion assay.
Cellular invasion was assessed using a procedure modified from that previously described (1). Serum-starved invasive (2,000 to 5,000) or noninvasive (5 × 104 to 2 × 105) breast carcinoma cells were diluted in 500 μl of Dulbecco's minimum essential medium (DMEM) containing 0.1% bovine serum albumin (BSA) with or without agonists and were added to the upper well. The peptide agonist SFLLRRN or α-thrombin (α-Th) was added to the cells in the upper well in order to completely activate PAR1. The assays were performed in this manner to directly assess whether proteolytic activation of PAR1 promotes cellular invasion and to allow interpretation in a consistent and reproducible manner. Hence, α-Th and the peptide agonist were utilized as stimulators of movement, not chemoattractants, in invasion assays (11, 13). Since for the majority of our assays the stimulus was present in the upper and not the lower chamber, some aspect of our assayed invasion might also have been contributed by chemokinesis in response to a reverse gradient. However, since we also observed PAR1-dependent migration in the absence of ligand, we believe that the observed invasion activity was due primarily to PAR1 activation.
In the initial assays that were performed to optimize the assay, it was difficult to interpret in a consistent and reproducible manner whether thrombin stimulation of PAR1 increased breast carcinoma cell invasion, because too many cells invaded the Matrigel when the medium in the lower chamber contained serum or any type of adhesive ligand. Therefore, the Boyden chamber assay was optimized by manipulating the percentage of serum in the bottom well and the density of serum-starved cells plated in the upper well. Thus, serum-free medium containing 0.1% BSA (750 μl) was added to the lower well and cells were incubated for 24 h at 37°C. The filters were fixed and stained with hematoxylin and eosin. The cells on 50% of the membrane area were counted using a 10× objective, and the cells in the bottom well were counted using a hemocytometer.
RT-PCR.
Total RNA was isolated from cells using TRI-reagent (MRC) according to the manufacturer's instructions. PAR1 mRNA was PCR amplified using a sense-strand primer (5′ CAG TTT GGG TCT GAA TTG TGT CG 3′) and an antisense primer (5′ TGC ACG AGC TTA TGC TGC TGA C 3′) as described previously (17). The antisense primer was used in the reverse-transcription (RT) step, and the resulting PCR product contained 591 bases. 18S rRNA was amplified simultaneously using the Alternate 18S Internal Standards primer set (Ambion) and served as an internal loading control. PCR products were resolved by sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis (SDS-8% PAGE), and dried gels were imaged by autoradiography.
Immunoprecipitation and immunoblotting.
To assess PAR1 protein expression, 50 μg of total cell lysate protein was resolved by SDS-PAGE, followed by immunoblot analyses with anti-PAR1 antibody. To assess PAR1 degradation, cells were plated at a density of 106 per 100-mm-diameter plate; grown overnight; serum starved for 30 min in DMEM supplemented with 1 mg of BSA/ml, 10 mM HEPES, and 10 μM cycloheximide; and then stimulated with 50 μM peptide agonist for 120 min. The cells were washed, lysed in 1% Triton X-100 buffer, immunoprecipitated with a mouse monoclonal PAR1 antibody, and immunoblotted as described previously (25). The immunoblots were developed and imaged by autoradiography.
ERK activity was assayed in cells serum starved for 48 to 72 h. During the starvation period, the serum-free medium was replaced every 24 h. The cells were stimulated with 10 nM α-Th for appropriate times, equivalent amounts of lysates were resolved by SDS-PAGE, and ERK activity was assayed by immunoblot analyses with an anti-phospho-p42/44 ERK antibody. The membranes were then reprobed with anti-p42/44 antibody to quantitate total ERK expression.
Internalization and recycling assays.
Internalization and recycling of activated PAR1 were assessed essentially as described previously (36). Briefly, 6 × 105 breast carcinoma cells were aliquoted into 1.7-ml microcentrifuge tubes and incubated with anti-PAR1 antibody for 1 h at 4°C. The cells were agonist treated, washed, and incubated with 200 nM trypsin for 30 min at 4°C to remove receptor-antibody complex that remained on the cell surfaces. The cells were then washed and incubated in medium containing 2 μg of trypsin inhibitor/ml, and the reappearance of receptor-antibody complex on the cell surface was determined after 30 min. The amount of receptor-bound antibody on the cell surface was quantitated at various times by enzyme-linked immunosorbent assay.
Confocal microscopy.
Cells grown on fibronectin-coated glass coverslips (22 by 22 mm) were incubated with or without agonists, fixed, immunostained for PAR1 and either LAMP1 or EEA1, washed, incubated with species-specific fluorophore-conjugated secondary antibodies, and examined by confocal microscopy as previously described (38).
Phosphoinositide (PI) hydrolysis assay.
Breast carcinoma cells (106) plated on 100-mm-diameter dishes were labeled overnight with 1 μCi of [myo-3H]inositol (American Radiochemicals)/ml in inositol-free DMEM containing 1 mg of BSA/ml. The cells were removed, aliquoted (6 × 105) into 1.7-ml microcentrifuge tubes, washed, and agonist treated, and the accumulation of inositol phosphates (IPs) was measured as described previously (36).
RESULTS
Activation of PAR1 enhances invasion of breast carcinoma cells.
Increases in PAR1 mRNA and protein expression have been correlated with invasiveness of certain breast carcinoma tumors and cell lines (10, 13, 24). However, the role of PAR1 activation and the mechanism by which it contributes to cellular invasion are poorly understood. To determine a role for proteolytically activated PAR1 in cellular invasion, we first examined a series of human mammary carcinoma cell lines for PAR1 protein expression and determined the basal invasive potential by examining the ability of cells to migrate through a reconstituted basement membrane. We performed our assays essentially as described previously (11, 13), with thrombin added to the upper well to stimulate PAR1 activation.
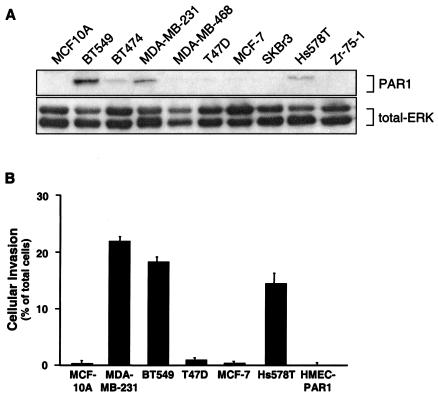
The normal MCF-10A and noninvasive MDA-MB-468, T47D, MCF-7, SKBr3, and Zr-75-1 human breast carcinoma cell lines and the noninvasive hTERT-immortalized normal HMECs did not show significant PAR1 protein expression (data not shown) or exhibit cellular invasion (Fig. 1). In contrast, MDA-MB-231 cells, a highly invasive cell line, expressed an elevated level of PAR1 in comparison to the noninvasive MCF-7 cells (Fig. 1). Similarly, PAR1 protein overexpression also correlated with the invasive capabilities of the BT549 and Hs578T cell lines (Fig. 1). Interestingly, ectopic overexpression and proteolytic activation of PAR1 in HMECs, a normal breast epithelial cell line, did not induce in vitro invasion (Fig. 1B). However, ectopic overexpression and activation of PAR1 in MCF-7 cells, a normally noninvasive breast carcinoma cell line, induced a significant increase in cellular invasion (data not shown). These studies demonstrate that mere overexpression and activation of PAR1 in normal breast epithelial cells is not sufficient to induce invasive potential, suggesting the likelihood that perturbations in other cellular processes and proteins in these breast carcinoma cells likely contribute to cellular invasion. However, these findings do confirm the studies of Nierodzik et al. (22) with B16F10 cells and experimental metastasis and further support the possibility that activation of overexpressed PAR1 in invasive breast carcinoma cell lines contributes to cellular invasion. To investigate this possibility, we used the invasive MDA-MB-231, BT549, and Hs578T cells and the noninvasive hTERT-immortalized normal HMECs to assess a causal relationship between PAR1 overexpression and invasion.
FIG. 1.
PAR1 protein is overexpressed in invasive breast carcinoma cell lines. (A) Lysates prepared from multiple breast carcinoma cell lines were resolved by SDS-15% PAGE (50 μg of total protein), transferred, and immunoblotted with an anti-PAR1 monoclonal antibody. Immunoblotting these same membranes for total ERK with a polyclonal anti-p42/44 ERK antibody served as a control for equal loading. (B) Basal invasion activity of breast carcinoma cell lines was measured using a Matrigel invasion assay. All of the cell lines were serum starved for a minimum of 24 h, and cellular invasion was assessed by the addition of α-Th to the upper well only to stimulate PAR1 activation. The data are expressed as the percentage of cells that invaded compared to the total number of cells seeded in the upper chamber and are reported as the mean percentage plus the standard error of the mean of at least two independent experiments for MCF10A, HMEC-PAR1, and T47D cells; three independent experiments for MCF-7 and SKBR3 cells; and six independent experiments for MDA-MB-231, Hs578T, and BT549. The average numbers of cells invading under basal conditions in a given experiment were between 0 and 100 cells for MCF-10A, T47D, MCF-7, HMEC-PAR1 and SKBr3 and between 200 and 600 cells for MDA-MB-231, BT549, and Hs578T.
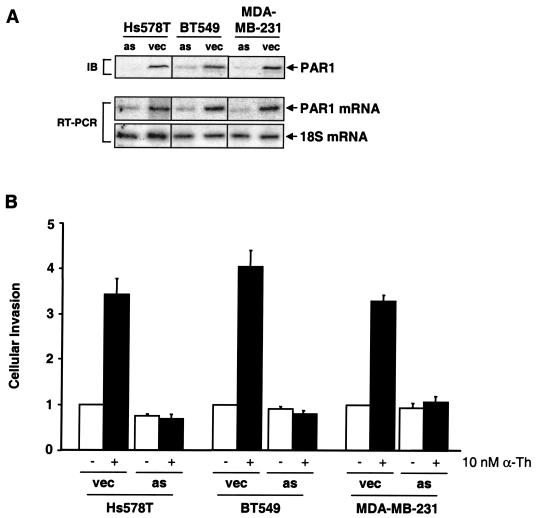
To investigate whether PAR1 overexpression and activation contribute to breast carcinoma cell invasion, we ectopically overexpressed an antisense cDNA construct of PAR1 (10) in Hs578T, BT549, and MDA-MB-231 human breast carcinoma cell lines. RT-PCR revealed a decrease in PAR1 mRNA expression in the antisense-expressing cells compared to vector controls (Fig. 2A). Immunoblot analysis of lysates from antisense-expressing cells confirmed the stable reduction of PAR1 protein expression, whereas expression of the receptor was retained in vector controls. The empty-vector control populations of Hs578T, BT549, and MDA-MB-231 cells retained ligand-dependent stimulation (three- to fourfold) of invasion in response to thrombin (Fig. 2B). However, in cells expressing the PAR1 antisense-cDNA construct, thrombin-mediated activation of PAR1-induced cellular invasion was significantly decreased (Fig. 2B). Taken together, these data strongly suggest that thrombin activation of PAR1 enhances breast carcinoma cell invasion in vitro. However, downregulation of PAR1 expression did not appear to inhibit the basal invasive activity of the invasive breast carcinoma cell lines, which is consistent with the likelihood that the altered functions of other proteins also contribute to invasion by these breast carcinoma cells.
FIG. 2.
Expression and activation of PAR1 enhances thrombin-mediated breast carcinoma cell invasion. (A) Lysates prepared from cells stably expressing PAR1 antisense (as) cDNA or empty vector (vec) were resolved by SDS-PAGE (50 μg of total protein) and immunoblotted using a monoclonal PAR1 antibody. RT-PCR was used to assess PAR1 mRNA levels in antisense and vector control-expressing cells, and 18S mRNA was amplified as a control. (B) MDA-MB-231, BT549, and Hs578T cells expressing empty vector or PAR1 antisense cDNA were serum starved for a minimum of 24 h and then incubated in the absence or presence of 10 nM α-Th, and cellular invasion was assessed. The data are expressed as the increase (n-fold) in cells invaded compared to untreated controls and represent the means plus standard errors of the mean of at least three independent experiments. The average numbers of cells invading after stimulation with 10 nM α-Th in a given experiment were between 0 and 20 for HMEC-PAR1 and between 600 and 1,100 for MDA-MB-231, BT549, and Hs578T.
Activated PAR1 is not degraded in invasive breast carcinoma cells.
To determine if differences in PAR1 degradation may account for altered PAR1 protein levels in invasive breast carcinoma cells, we examined whether PAR1 was efficiently degraded in these cells. In HMECs that ectopically overexpressed PAR1 protein (HMEC-PAR1), we observed that prolonged exposure to SFLLRN agonist peptide caused a significant loss (25% remaining) of PAR1 protein (Fig. 3A), similar to that observed in fibroblasts and HeLa cells (33, 34). In striking contrast, the same treatment of Hs578T, BT549, and MDA-MB-231 cells with SFLLRN showed reduced degradation of endogenous PAR1 and retained 60 to 70% of receptor protein (Fig. 3A). These findings suggest that activated PAR1 is unable to undergo efficient degradation in invasive breast carcinoma cells.
FIG. 3.
Defective degradation of activated PAR1 in invasive breast carcinoma cell lines. Cells plated at a density of 106 were incubated in the absence (0′) or presence (120′) of 50 μM SFLLRN for 120 min at 37°C. The cells were lysed and immunoprecipitated (IP) with monoclonal anti-PAR1 antibody. The immunoprecipitates were resolved by SDS-9% PAGE, transferred, and immunoblotted (IB) with an anti-PAR1 polyclonal antibody.
Aberrant trafficking of activated PAR1 in invasive breast carcinoma cells.
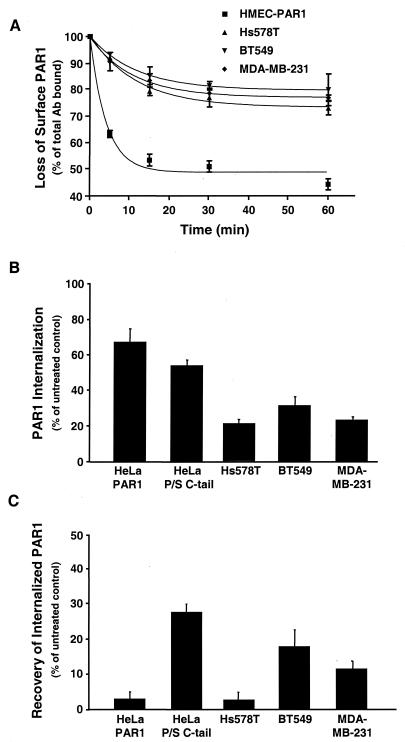
To determine whether the failure of activated PAR1 to be efficiently downregulated was due to an inability of the receptor to be internalized, we examined agonist-induced loss of cell surface PAR1. Thrombin treatment of PAR1-expressing HMECs induced rapid and substantial internalization of PAR1 (Fig. 4A), similar to reported studies using fibroblasts and HeLa cells (33, 34). In striking contrast, the same treatment of Hs578T, BT549, and MDA-MB-231 cells induced a markedly low rate of internalization and only a modest loss (20 to 25%) of endogenous PAR1 from the cell surface following 60 min of agonist exposure. These data suggest that the impaired degradation of PAR1 seen in invasive breast carcinoma cells (Fig. 3A) is due in part to a defect in trafficking of PAR1.
FIG.4.
Agonist-induced internalization and recycling of PAR1 are perturbed in invasive breast carcinoma cells. (A) Cells were incubated in the absence or presence of 10 nM α-Th for the indicated times at 37°C and fixed, and the amount of PAR1 remaining on the cell surface was measured by enzyme-linked immunosorbent assay. The results are expressed as the mean percentage (± standard error of the mean [SEM]) of total antibody bound to untreated controls in two separate experiments in which duplicate determinations were made. (B and C) Cells labeled with anti-PAR1 antibody were incubated in the absence or presence of 50 μM SFLLRN for 60 min. The cells were then trypsin treated, and the amount of receptor-bound antibody reappearing at the cell surface was measured after 30 min. The amount of antibody remaining on the cell surface following trypsin treatment was between 2 and 8% of total binding and was subtracted to obtain the values shown. The results are the amounts of surface-bound antibody detected after agonist incubation at 37°C (B) and after 30 min of recovery (C) and are expressed as the mean percentages (±SEM) of total antibody bound to untreated control cells.
The failure of activated PAR1 to efficiently internalize in invasive cells could be due to slowed internalization or recycling of internalized receptor back to the cell surface. To directly test whether activated PAR1 was recycled, we examined the recovery of previously internalized receptor to the cell surface (36). Exposure of HeLa-PAR1 cells to SFLLRN caused substantial internalization of receptor from the cell surface (Fig. 4B); however, little recovery of receptor (3%) was detected despite the substantial amount that had been internalized previously (Fig. 4C). In contrast, substantial recovery (∼25%) of receptor-bound antibody was seen in agonist-treated HeLa cells expressing a chimeric PAR1 bearing the P/S C tail, which undergoes internalization and recycling rather than lysosomal degradation, as was previously reported (35, 36). The addition of SFLLRN caused a more modest decrease in PAR1 from the cell surface in Hs578T, BT549, and MDA-MB-231 cells (Fig. 4B), consistent with the extent of PAR1 internalization induced by thrombin (Fig. 4A). Interestingly, little recovery of receptor (∼3%) was detected in Hs578T cells. However, BT549 and MDA-MB-231 cells pretreated with agonist showed significant recovery (∼12 to 18%) of previously internalized PAR1 (Fig. 4C). These data are consistent with agonist-dependent internalization and recycling, rather than degradation, of PAR1 in BT549 and MDA-MB-231 cells.
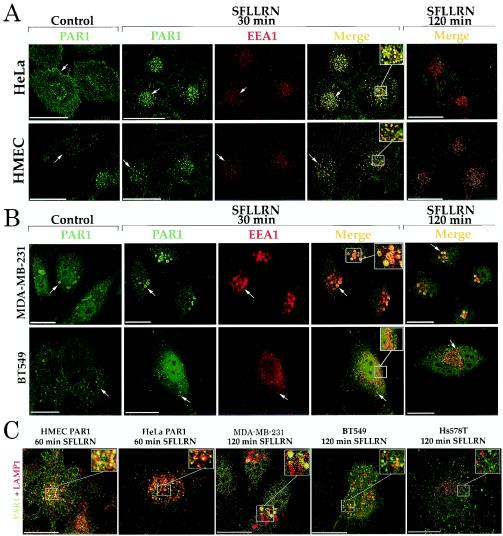
We next determined whether the ability of activated PAR1 to be internalized and sorted to lysosomes was altered in breast carcinoma cells. For these analyses, we examined the trafficking of internalized PAR1 after prolonged agonist treatment by using immunofluorescence confocal microscopy. HMEC-PAR1 cells were used as a control to assess the trafficking of PAR1 in normal breast epithelial cells. PAR1 was located predominantly at the cell surface in unstimulated HeLa cells and HMECs (Fig. 5A). After 30 min of agonist stimulation, PAR1 was robustly internalized to endocytic vesicles and markedly colocalized with EEA1, a specific marker of early endosomes (4). However, after prolonged agonist treatment, PAR1-containing endosomes were no longer apparent, whereas EEA1 localization was not perturbed, suggesting that activated PAR1 is sorted to a degradative pathway in these cells.
FIG. 5.
PAR1 trafficking is dysregulated in invasive breast carcinoma cells. (A) HeLa- and HMEC-PAR1 cells were preincubated with anti-PAR1 antibody at 4°C, washed, and then incubated in the absence (Control) or presence of 50 μM SFLLRN for 30 or 120 min at 37°C. The cells were fixed, permeabilized, and immunostained for PAR1 (green) and EEA1 (red). (B) MDA-MB-231 and BT549 cells were treated as described for panel A. (C) Cells labeled with anti-PAR1 antibody were agonist treated for either 60 or 120 min and then coimmunostained for PAR1 (green) and lysosomal marker protein LAMP1 (red). The images are representative of >100 cells examined in at least three independent experiments. The insets are magnifications of the boxed areas. Scale bar, 19.4 μm. Arrows indicate staining of PAR1-positive intracellular vesicles.
In unstimulated MDA-MB-231 cells, endogenous PAR1 was found on the cell surface and also partially in an intracellular compartment (Fig. 5B). As reported previously (30), this distribution pattern is consistent with tonic cycling of inactivated PAR1 between the cell surface and an intracellular compartment. Upon agonist stimulation, PAR1 was redistributed from the cell surface and was found to be localized predominantly in enlarged early endosomes (Fig. 5B). However, after prolonged agonist exposure, PAR1 remained localized in enlarged early endosomes and failed to sort to a degradative compartment. In BT549 and Hs578T cells, agonist treatment also caused PAR1 to localize to endocytic vesicles and failed to induce the redistribution of receptor to a degradative compartment. Consistent with the lack of receptor degradation in stimulated MDA-MB-231 and BT549 cells, activated PAR1 failed to colocalize with lysosome-associated membrane protein 1 (LAMP1), a marker of late endosomes and lysosomes (Fig. 5C). A similar lack of colocalization of activated PAR1 with LAMP1 was also observed in stimulated Hs578T cells. This distribution in breast carcinoma cells contrasts with that seen in HMECs, in which activated PAR1 showed significant colocalization with LAMP1 after 60 min of agonist exposure.
Persistent signaling by activated PAR1 induces invasion by breast carcinoma cells.
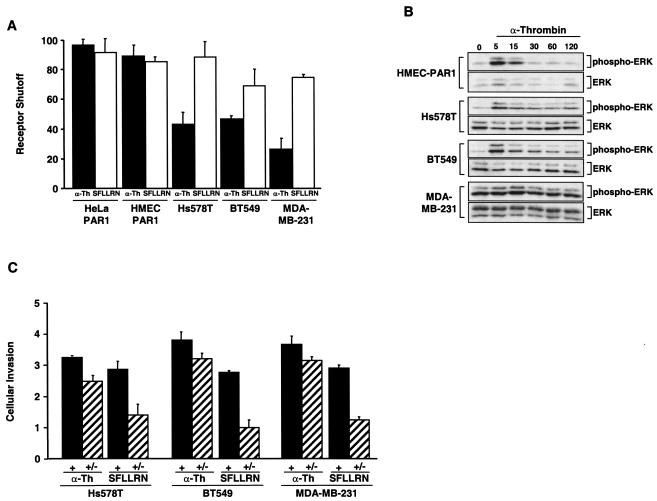
We next determined whether altered trafficking of activated PAR1 resulted in aberrant signaling in invasive cells. It is conceivable that proteolytically activated PAR1 can continue to signal if it remains on the cell surface or if it is internalized and recycled back to the cell surface with its tethered ligand intact. Therefore, we examined whether activation of PAR1 with thrombin or SFLLRN induced persistent signaling in invasive cells by assaying for the accumulation of IPs after agonist removal. In HeLa- and HMEC-PAR1 cells, PAR1 signaling to PI hydrolysis ceased rapidly (>90% shutoff) following the removal of either thrombin or SFLLRN (Fig. 6A), and this rapid shutoff is consistent with the extent of shutoff in PAR1 signaling observed in fibroblasts (36). Interestingly, SFLLRN-stimulated carcinoma cells also showed a substantial (70 to 80%) shutoff in signaling by PAR1 following the removal of this peptide agonist. However, in striking contrast to HeLa cells and HMECs, when activated by thrombin, the breast carcinoma cell lines showed significant defects (20 to 50%) in the ability to shut off signaling in the absence of thrombin. Thus, in contrast to normal breast epithelial cells, proteolytically activated PAR1 signals persistently in invasive breast carcinoma cells after thrombin withdrawal.
FIG. 6.
Persistent signaling by activated PAR1 promotes invasion by breast carcinoma cell lines. (A) Cells labeled with [myo-3H]inositol were incubated with 50 μM SFLLRN or 10 nM α-Th for 60 min at 37°C in the absence of LiCl. Agonists were removed, and the cells were washed with DMEM containing 0.5 U of hirudin/ml and incubated with DMEM (20 mM LiCl and hirudin) at 37°C for an additional 60 min, at which time accumulated 3H-labeled IPs were measured. The results shown are normalized to the amount of IP species generated when agonist was added with LiCl and are expressed as the mean percent (plus standard error of the mean [SEM]) shutoff of at least two separate experiments. (B) Cells were serum starved for 48 to 72 h and incubated in the presence or absence of 10 nM α-Th for the indicated times (in minutes) at 37°C. Cell lysates were prepared, resolved by SDS-PAGE, and immunoblotted with an anti-phospho-p42/44 ERK antibody. The same membranes were immunoblotted with anti-p42/44 antibody to control for total loading. (C) Cells added to the upper chamber were seeded in medium containing 0.1% BSA and either 10 nM α-Th or 50 μM SFLLRN, and the number of invading cells was determined (+) as described in the legends to Fig. 1 and 2. Other cells were pretreated with α-Th or SFLLRN for 60 min at 37°C, agonists were removed, and the cells were washed with DMEM containing 0.5 U of hirudin/ml and then seeded in the upper chamber in medium containing only 0.1% BSA, and the number of invaded cells was determined (+/−). The data are expressed as the increase (n-fold; mean + SEM) in cellular invasion induced by agonists compared to untreated control cells in at least three separate experiments.
Thrombin stimulation also causes PAR1 activation of the p42/p44 extracellular signal-regulated kinase (ERK) pathway (34), an important mitogenic signaling cascade. Therefore, we determined whether PAR1 activation of ERK is altered in breast carcinoma cells. Thrombin treatment of HMECs caused a robust and transient activation of ERK, with peak activation at 15 min followed by a rapid decrease by 30 min (Fig. 6B), a time course seen previously in other cell types (16, 34). In contrast, thrombin treatment of Hs578T and BT549 cells clearly caused a rapid and sustained (60- to 120-min) activation of ERK. However, thrombin stimulation of MDA-MB-231 cells also induced a rapid activation of ERK with a peak at 15 min. As a result of the high basal phosphorylation state of ERK, which is a consequence of the MDA-MB-231 cells haboring an activated K-Ras allele (12), it is not clear if thrombin stimulation induces sustained activation of ERK in MDA-MB-231 cells. Taken together with the PI analyses, these results suggest that proteolytically activated PAR1 signaling is sustained in invasive breast carcinoma cells even in the absence of thrombin.
We next determined whether proteolytically activated PAR1 was able to promote cellular invasion after the removal of thrombin in the invasive breast carcinoma cell lines. To evaluate this possibility, cells were preincubated initially with thrombin or SFLLRN. The stimulus was then removed, and the cells were evaluated for invasive potential. The continuous exposure to thrombin or SFLLRN induced three- to fourfold increases in cellular invasion (Fig. 6C), results consistent with those shown in Fig. 2. As expected, cellular invasion was not induced following incubation and the removal of SFLLRN. In striking contrast, significant increases (2.5- to 3-fold) in invasion were observed in cells after incubation and the removal of thrombin. Taken together, these data suggest that continued signaling by proteolytically activated PAR1 promotes cellular invasion.
DISCUSSION
In this study, we demonstrate that thrombin activation of PAR1 in breast carcinoma cell lines, but not normal breast epithelial cell lines, causes constitutive signaling and contributes in part to breast carcinoma cell invasion. The proteolytic activation of PAR1 by thrombin resulted in continued signaling, even after thrombin withdrawal, and promoted cellular invasion. This persistent signaling by proteolytically activated PAR1 in specific breast carcinoma cell lines appears to be caused by slowed internalization and/or recycling and a lack of lysosomal degradation of activated receptors rather than aberrant translation. These defects were specific for breast carcinoma cells, as PAR1 overexpression in other cells (HMEC-PAR1 and HeLa-PAR1) produced normal receptor trafficking and shutoff of signaling. Previously, the prolonged G protein-coupled receptor (GPCR) signaling observed in human cancers has been attributed to mutational activation of the GPCR or the Gα subunit, increased gene expression or splicing, or upregulation of ligand expression (18, 39). Our study reveals a novel gain-of-function mechanism for the aberrant activation of GPCR signaling in certain human cancer cells.
The failure to efficiently downregulate activated PAR1 by internalization and lysosomal sorting suggests that increased PAR1 protein expression observed in invasive breast carcinoma cells is due partially to defective receptor trafficking. Though gene mutations acquired during tumorigenesis could lead to increased stability of the receptor protein or constitutive activity, we found no missense mutations in the endogenous PAR1 coding sequence. Furthermore, while the duplication and/or amplification of genes is commonly seen in tumor cells, our analysis by RT-PCR of PAR1 mRNA expression showed comparable transcript levels in various invasive breast cell lines, yet we observed considerable differences in PAR1 protein expression (Fig. 1A). Based on receptor degradation studies we performed in the presence of cycloheximide (Fig. 3A), these differences in protein expression appear not to be attributable to alterations in PAR1 translation. Finally, dysregulated lysosomal sorting of activated PAR1 in breast carcinoma cells is unlikely to be due to a general defect in the trafficking machinery, since intracellular trafficking of transferrin and low-density lipoprotein, as well as lysosomal sorting of LAMP1, are unperturbed. Together, these findings suggest that alterations in gene expression are not solely responsible for PAR1 overexpression and that specific defects in receptor trafficking are also a contributing factor.
There are three temporally distinct processes that mediate termination of PAR1 signaling: desensitization, internalization, and downregulation. PAR1 signaling was efficiently shut off when it was reversibly activated by the agonist peptide SFLLRN in invasive breast carcinoma cells. This suggests that PAR1 desensitization, phosphorylation, and rapid uncoupling from signaling are likely intact in invasive carcinoma cells. It is possible that sustained signaling is due in part to dramatically slowed internalization of activated PAR1, which continues to signal from the cell surface. However, we showed that agonist stimulation promoted PAR1 internalization and caused the redistribution of activated PAR1 to early endosomes in invasive cells. An alternative mechanism for constitutive signaling by activated PAR1 is internalization followed by recycling of proteolytically activated receptors back to the cell surface. Consistent with this hypothesis, we observed significant recovery of previously internalized PAR1 to the cell surface following agonist removal in invasive BT549 and MDA-MB-231 cells. Moreover, we found that internalized PAR1 remained in endosomes and failed to colocalize with the lysosomal marker protein LAMP1 even after prolonged agonist exposure. Finally, an additional mechanism for constitutive signaling by activated PAR1 is signaling from activated receptors in endosomes. A growing body of evidence suggests that multiple signaling receptors remain ligand bound in endosomes (31). Consequently, these active, endocytosed receptors are capable of signaling, as many signaling effectors are found associated with the endosomal compartment. Since proteolytic activation of PAR1 by thrombin generates a tethered ligand (6), it is quite conceivable that the tethered ligand remains bound to the internalized receptor and continues to activate the receptor even in an endosomal compartment. This could explain in part the constitutive signaling observed in Hs578T cells that fail to show significant recycling of the proteoytically activated PAR1 but show substantial signaling following activation and the removal of thrombin. In any case, our findings clearly demonstrate a link between altered PAR1 trafficking and sustained signaling.
It has been shown that PAR1 is internalized via a dynamin- and clathrin-dependent pathway and then sorted to lysosomes (33). The sorting of GPCRs to recycling pathways or to lysosomes for degradation is likely determined by specific protein-protein interactions. It was recently reported that sorting nexin 1 is involved in targeting activated PAR1 to lysosomes (38). Whether defects in sorting nexins or other proteins that facilitate GPCR lysosomal sorting occur in invasive cells is not known. It is also possible that activated PAR1 undergoes endocytosis through an alternate pathway that subverts lysosomal sorting and leads to recycling instead. One such clathrin-independent endocytic pathway that can subvert lysosomal sorting is macropinocytosis. Macropinosomes are dynamic structures with membrane components that likely recycle back to the cell surface (32). Mutationally activated ras can induce macropinocytosis, leading to the formation of large, irregular primary endocytic vesicles by closure of lamellipodia generated at the ruffling membrane (2). Interestingly, MDA-MB-231 cells harbor a constitutively active K-ras (G13D) mutant allele (12), and these cells also contain unusually large endocytic vesicles, suggesting the possibility that activated PAR1 is being internalized via macropinocytosis and recycled back to the plasma membrane. Although ras mutations are not associated with the majority of breast cancers, aberrant activation of Ras by overexpression of receptor tyrosine kinases is seen in many breast carcinomas (5) and thus may induce macropinocytosis and perhaps aberrant PAR1 trafficking.
In conclusion, there is increasing evidence for the role of aberrant GPCR signaling in human oncogenesis (18, 39). Previous studies have described activating mutations in GPCRs and Gα subunits. More frequently, upregulation of ligand expression that promotes autocrine or paracrine mechanisms of GPCR activation has been described for a variety of human cancers. In this study, we report that a novel type of gain of function in PAR1 signaling caused by dysregulated receptor trafficking results in constitutive signaling that in turn contributes to and enhances cellular invasion. These studies provide the first example of how aberrant trafficking may cause constitutive GPCR activation to promote tumor cell invasion. Whether dysregulated receptor trafficking is important for the activation of other GPCRs involved in human cancer progression will be important to determine. The challenge now is to elucidate the mechanisms responsible for the dysregulated PAR1 trafficking that leads to constitutive receptor signaling.
Acknowledgments
We thank David Siderovski for critical review of the manuscript. The hTERT-immortalized HMECs were a kind gift from Robert Weinberg.
This work was supported by National Institutes of Health grants CA63071 and CA92240 (to C.J.D.) and HL67697 (to J.T.). M.A.B. was supported by a Leukemia and Lymphoma Society Postdoctoral Fellowship.
REFERENCES
- 1.Albini, A., Y. Iwamoto, H. K. Kleinman, G. R. Martin, S. A. Aaronson, J. M. Kozlowski, and R. N. McEwan. 1987. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 47:3239-3245. [PubMed] [Google Scholar]
- 2.Bar-Sagi, D., and J. R. Feramisco. 1986. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233:1061-1068. [DOI] [PubMed] [Google Scholar]
- 3.Bissell, M. J., and D. Radisky. 2001. Putting tumours in context. Nat. Rev. Cancer 1:46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christoforidis, S., H. M. McBride, R. D. Burgoyne, and M. Zerial. 1999. The Rab5 effector EEA1 is a core component of endosome docking. Nature 397:621-625. [DOI] [PubMed] [Google Scholar]
- 5.Clark, G. J., and C. J. Der. 1995. Aberrant function of the Ras signal transduction pathway in human breast cancer. Breast Cancer Res. Treat. 35:133-144. [DOI] [PubMed] [Google Scholar]
- 6.Coughlin, S. R. 2000. Thrombin signalling and protease-activated receptors. Nature 407:258-264. [DOI] [PubMed] [Google Scholar]
- 7.Dvorak, H. F., J. A. Nagy, B. Berse, L. F. Brown, K. T. Yeo, T. K. Yeo, A. M. Dvorak, L. van de Water, T. M. Sioussat, and D. R. Senger. 1992. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann. N. Y. Acad. Sci. 667:101-111. [DOI] [PubMed] [Google Scholar]
- 8.Elenbaas, B., L. Spirio, F. Koerner, M. D. Fleming, D. B. Zimonjic, J. L. Donaher, N. C. Popescu, W. C. Hahn, and R. A. Weinberg. 2001. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15:50-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esumi, N., D. Fan, and I. J. Fidler. 1991. Inhibition of murine melanoma experimental metastasis by recombinant desulfatohirudin, a highly specific thrombin inhibitor. Cancer Res. 51:4549-4556. [PubMed] [Google Scholar]
- 10.Even-Ram, S., B. Uziely, P. Cohen, S. Grisaru-Granovsky, M. Maoz, Y. Ginzburg, R. Reich, I. Vlodavsky, and R. Bar-Shavit. 1998. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat. Med. 4:909-914. [DOI] [PubMed] [Google Scholar]
- 11.Even-Ram, S. C., M. Maoz, E. Pokroy, R. Reich, B. Z. Katz, P. Gutwein, P. Altevogt, and R. Bar-Shavit. 2001. Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the alpha beta 5 integrin. J. Biol. Chem. 276:10952-10962. [DOI] [PubMed] [Google Scholar]
- 12.Gilhooly, E. M., and D. P. Rose. 1999. The association between a mutated ras gene and cyclooxygenase-2 expression in human breast cancer cell lines. Int. J. Oncol. 15:267-270. [PubMed] [Google Scholar]
- 13.Henrikson, K. P., S. L. Salazar, J. W. Fenton, and B. T. Pentecost. 1999. Role of thrombin receptor in breast cancer invasiveness. Br. J. Cancer 79:401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung, D. T., T. K. Vu, V. I. Wheaton, K. Ishii, and S. R. Coughlin. 1992. Cloned platelet thrombin receptor is necessary for thrombin-induced platelet activation. J. Clin. Investig. 89:1350-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii, K., J. Chen, M. Ishii, W. J. Koch, N. J. Freedman, R. J. Lefkowitz, and S. R. Coughlin. 1994. Inhibition of thrombin receptor signaling by a G-protein coupled receptor kinase. Functional specificity among G-protein coupled receptor kinases. J. Biol. Chem. 269:1125-1130. [PubMed] [Google Scholar]
- 16.Kahan, C., K. Seuwen, S. Meloche, and J. Pouyssegur. 1992. Coordinate, biphasic activation of p44 mitogen-activated protein kinase and S6 kinase by growth factors in hamster fibroblasts. Evidence for thrombin-induced signals different from phosphoinositide turnover and adenylylcyclase inhibition. J. Biol. Chem. 267:13369-13375. [PubMed] [Google Scholar]
- 17.Kahn, M. L., M. Nakanishi-Matsui, M. J. Shapiro, H. Ishihara, and S. R. Coughlin. 1999. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Investig. 103:879-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinissen, M. J., and J. S. Gutkind. 2001. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 22:368-376. [DOI] [PubMed] [Google Scholar]
- 19.Martin, C. B., G. M. Mahon, M. B. Klinger, R. J. Kay, M. Symons, C. J. Der, and I. P. Whitehead. 2001. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene 20:1953-1963. [DOI] [PubMed] [Google Scholar]
- 20.Mueller, B. M., R. A. Reisfeld, T. S. Edgington, and W. Ruf. 1992. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc. Natl. Acad. Sci. USA 89:11832-11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller, B. M., and W. Ruf. 1998. Requirement for binding of catalytically active factor VIIa in tissue factor-dependent experimental metastasis. J. Clin. Investig. 101:1372-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nierodzik, M. L., K. Chen, K. Takeshita, J. J. Li, Y. Q. Huang, X. S. Feng, M. R. D'Andrea, P. Andrade-Gordon, and S. Karpatkin. 1998. Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood 92:3694-3700. [PubMed] [Google Scholar]
- 23.Nierodzik, M. L., F. Kajumo, and S. Karpatkin. 1992. Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res. 52:3267-3272. [PubMed] [Google Scholar]
- 24.Nierodzik, M. L., A. Plotkin, F. Kajumo, and S. Karpatkin. 1991. Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. J. Clin. Investig. 87:229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paing, M. M., A. B. Stutts, T. A. Kohout, R. J. Lefkowitz, and J. Trejo. 2002. Beta-arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J. Biol. Chem. 277:1292-1300. [DOI] [PubMed] [Google Scholar]
- 26.Palumbo, J. S., and J. L. Degen. 2000. Hemostatic factors in tumor biology. J. Pediatr. Hematol. Oncol. 22:281-287. [DOI] [PubMed] [Google Scholar]
- 27.Palumbo, J. S., K. W. Kombrinck, A. F. Drew, T. S. Grimes, J. H. Kiser, J. L. Degen, and T. H. Bugge. 2000. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 96:3302-3309. [PubMed] [Google Scholar]
- 28.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrano, G. R., and S. R. Coughlin. 1999. The carboxyl tail of protease-activated receptor-1 is required for chemotaxis. Correlation of signal termination and directional migration. J. Biol. Chem. 274:20178-20184. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro, M. J., J. Trejo, D. Zeng, and S. R. Coughlin. 1996. Role of the thrombin receptor's cytoplasmic tail in intracellular trafficking. Distinct determinants for agonist-triggered versus tonic internalization and intracellular localization. J. Biol. Chem. 271:32874-32880. [DOI] [PubMed] [Google Scholar]
- 31.Sorkin, A., and M. Von Zastrow. 2002. Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 3:600-614. [DOI] [PubMed] [Google Scholar]
- 32.Swanson, J. A., and C. Watts. 1995. Macropinocytosis. Trends Cell Biol. 5:424-428. [DOI] [PubMed] [Google Scholar]
- 33.Trejo, J., Y. Altschuler, H. W. Fu, K. E. Mostov, and S. R. Coughlin. 2000. Protease-activated receptor-1 down-regulation: a mutant HeLa cell line suggests novel requirements for PAR1 phosphorylation and recruitment to clathrin-coated pits. J. Biol. Chem. 275:31255-31265. [DOI] [PubMed] [Google Scholar]
- 34.Trejo, J., A. J. Connolly, and S. R. Coughlin. 1996. The cloned thrombin receptor is necessary and sufficient for activation of mitogen-activated protein kinase and mitogenesis in mouse lung fibroblasts. Loss of responses in fibroblasts from receptor knockout mice. J. Biol. Chem. 271:21536-21541. [DOI] [PubMed] [Google Scholar]
- 35.Trejo, J., and S. R. Coughlin. 1999. The cytoplasmic tails of protease-activated receptor-1 and substance P receptor specify sorting to lysosomes versus recycling. J. Biol. Chem. 274:2216-2224. [DOI] [PubMed] [Google Scholar]
- 36.Trejo, J., S. R. Hammes, and S. R. Coughlin. 1998. Termination of signaling by protease-activated receptor-1 is linked to lysosomal sorting. Proc. Natl. Acad. Sci. USA 95:13698-13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walz, D. A., and J. W. Fenton. 1994. The role of thrombin in tumor cell metastasis. Invasion Metastasis 14:303-308. [PubMed] [Google Scholar]
- 38.Wang, Y., Y. Zhou, K. Szabo, C. R. Haft, and J. Trejo. 2002. Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol. Biol. Cell 13:1965-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitehead, I. P., I. E. Zohn, and C. J. Der. 2001. Rho GTPase-dependent transformation by G protein-coupled receptors. Oncogene 20:1547-1555. [DOI] [PubMed] [Google Scholar]