Abstract
Background:
Psoriasis may be complicated by contact dermatitis due to an impaired cutaneous barrier. Patch testing helps elucidate sensitizers if any.
Aims:
To determine the prevalence and relevance of secondary contact dermatitis in subjects with psoriasis.
Materials and Methods:
Patch testing with Indian Standard Series was done and readings interpreted after 48 and 96 hours.
Results:
Among 110 subjects 47 (42.7%) showed reactions to at least one antigen. Fifteen (13.6%) reacted to fragrance mix, 10 (9.1%) to nickel sulfate, seven (6.4%) to parthenium, and six (5.5%) to balsam of Peru. Palmoplantar psoriasis was the commonest type of psoriasis patch tested. Fragrance mix was the commonest antigen showing 100% current relevance as an aggravating factor of psoriasis. Cosmetics, beauty preparations, skin and healthcare products followed by topical medications were found to be the most common sources of the patch test positivity.
Conclusions:
Secondary contact dermatitis is common in patients with psoriasis. Patch testing is necessary to determine the triggering or aggravating antigens in these patients to avoid sensitizers and improve quality of life.
Keywords: Allergic contact dermatitis, contact allergy, delayed-type hypersensitivity, fragrance mix, patch testing, psoriasis, relevance
INTRODUCTION
The possibility of delayed-type hypersensitivity to contact allergens in psoriasis has been a topic of debate. While some have suggested that allergic contact dermatitis (ACD) is uncommon in psoriasis, others have reported a frequent association.[1–9] In some psoriasis patients, the site of lesions and resistance to treatment often suggests the involvement of local triggering factors such as ACD.[8]
As lifestyles become more complex, the skin is exposed to an ever-increasing spectrum of chemical and biological allergens. While the skin barrier is relatively impermeable to large molecules, contact allergens, because of their small size, easily penetrate the skin barrier and reach the living layers.[10]
Properly applied and correctly interpreted patch tests are the only scientific proof of ACD.[10] One of the extended applications of patch testing is whenever the physician suspects past or a recent history of superimposed ACD.[11] When the existence of one or more positive reactions is found, relevance must then be determined. The most important question is whether the reaction is a manifestation of the presenting dermatitis or the expression of an ACD that occurred previously.[10]
So far, no Indian studies have been published on contact allergy in psoriasis. This study aims at finding the prevalence and relevance of ACD in psoriasis.
MATERIALS AND METHODS
This prospective, observational study was conducted among 110 patients at the outpatient department of Dermatology, Manipal Hospital, Bangalore, between March 2009 and December 2010. Subjects of any age with any form of psoriasis of more than six months duration who were unresponsive to conventional topical treatments were included. Pregnant women, patients with extensive psoriasis, current or recent dermatitis at patch test site and patients receiving oral prednisolone more than 20 mg/day or recent phototherapy were excluded from the study.
Clinical details were recorded using a standard proforma. KOH mount for fungal identification and skin biopsy were performed in some patients as part of diagnostic work up. All patients were patch tested with the Indian Standard Series (ISS) including parthenium. Written consent was obtained for clinical photographs.
Patch test readings were taken on Day 2 (48 hours) and Day 4 (96 hours) and interpreted according to the International Contact Dermatitis Research Group criteria.[12] Reactions on Day 4 were taken as significant. The relevance of positive reactions was assessed and explained to patients as being related to the present problem or not and for cautioning against future exposure [Figures 1 and 2]. The relevance of positive allergens was recorded as definite, probable, possible, past, or unknown.[13,14] Current relevance was defined as present relevance (definite, probable, or possible). Relevance was considered definite if a use test with the item containing the suspected allergen was positive or a positive patch test to the object/product was observed. For use test, the patient was asked to use the suspected substance in the same way as when the dermatitis developed. For example, if a hand cream is suspected, it is applied over a small marked area (1 × 1 cm) on the hand for 1 week. If an eczematous skin reaction occurs during the test period, the test is considered positive. Relevance was considered probable if the substance identified by patch testing could be verified as present in the unknown skin contactants of the patient. Relevance was considered possible if the patient was exposed to circumstances in which skin contact with materials unknown to contain the allergen would likely occur. The current relevance was tabulated by adding the number of patients with the relevance coded as definite, probable, or possible and converting this to a percentage of patients with a positive test result for the allergen. Source of exposure for each relevant positive allergen was determined based on discussion with the patient. Use test was performed in 10 patients and patch test with patient's own product “as is” was done in 24 patients. Positive ISS antigen reactions in those who had a positive use test or a positive “as is” were grouped as those reactions with a definite relevance. In those patients who had not undergone a use test/“as is” patch test, positive ISS antigen reactions were grouped as those with a possible or probable relevance or past or unknown relevance.
Figure 1.
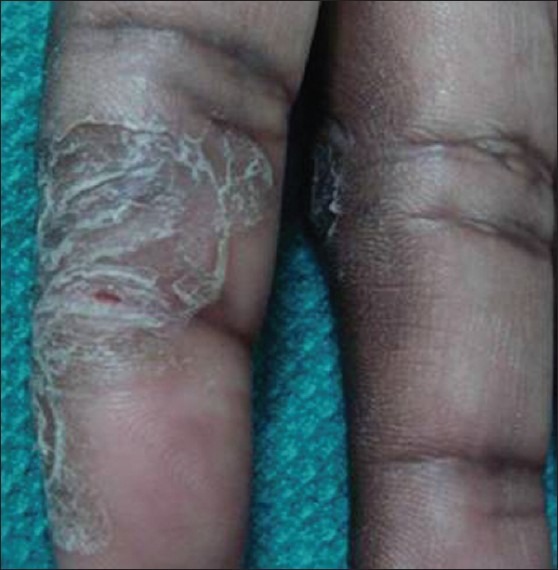
A taxi-driver with chronic, recalcitrant palmar psoriasis was patch tested
Figure 2.
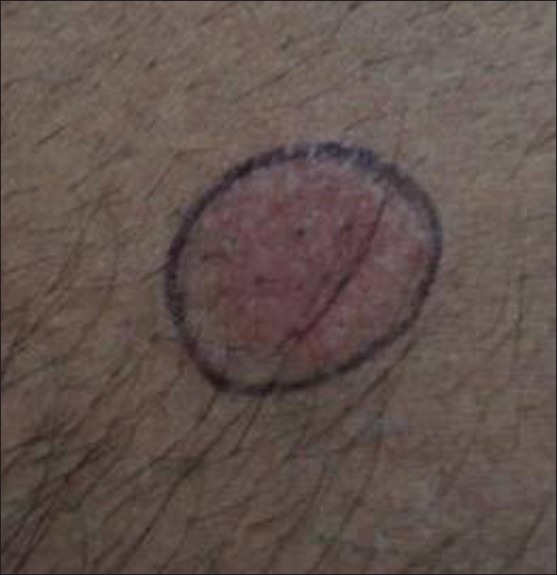
A taxi-driver with chronic, recalcitrant palmar psoriasis was patch tested
The Statistical software namely SPSS 15.0, Stata 8.0, MedCalc 9.0.1, and Systat 11.0 were used for the analysis of the data and Microsoft word and Excel have been used to generate graphs and tables.
RESULTS
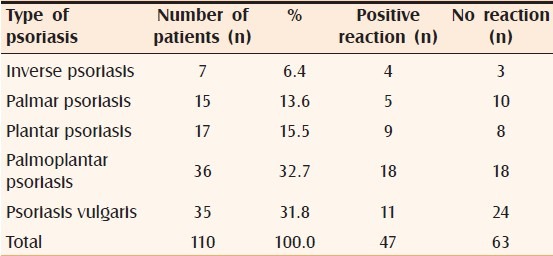
A total of 110 subjects with psoriasis were patch tested. Of them, 55% were male and the rest were female (45%). The age group ranged from 6 to 70 years with an average of 40 years. Most males were in the age group of 21 to 30 years and most females in 51 to 60 years. The duration of illness ranged from two months to 20 years with an average of four years. For most men, the duration of illness was one to two years and in case of women, it was more than two years. 20% of subjects had intense pruritus over legs, palms, and soles; 13.6% had burning sensation over palms and soles; 9.1% had lichenification over the legs; and 7.3% had hyperpigmentation over the legs. Palmoplantar psoriasis (32.7%) was the most commonly patch-tested type of psoriasis [Table 1]. Diagnosis of palmoplantar psoriasis was made clinically by the presence of psoriasiform, scaly plaques with well-demarcated margins; presence of papules and plaques elsewhere in the body that showed a positive Auspitz sign (this was observed in 28 (25.4%) patients); nail changes like pitting and subungual hyperkeratosis. In doubtful circumstances, a skin biopsy was performed (42 patients). Tinea manuum and pedis and atopic dermatitis were ruled out on clinical grounds and by performing 10% KOH mount and serum IgE tests. Personal history of atopy was found in 12 patients (10.9%), family history of atopy in five patients (4.5%) who had a final diagnosis of psoriasis.
Table 1.
Different types of psoriasis that were patch tested
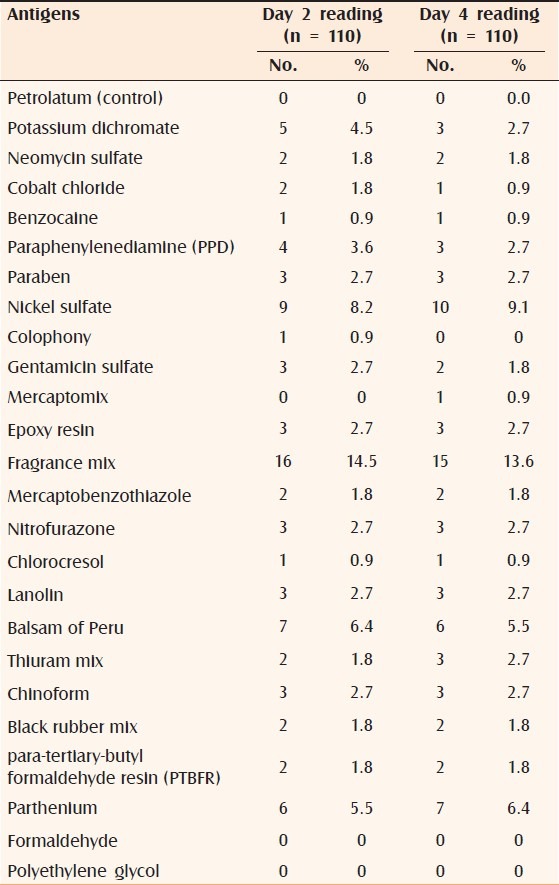
The commonly tested positive allergens were fragrance mix (13.6%), nickel sulphate (9.1%), parthenium (6.4%), and balsam of Peru (5.5%) [Table 2]. 47.3% of subjects showed a positive reaction on Day 2 and 42.7% of the subjects showed positive reaction to one or more allergens on Day 4. 28.2% showed reaction to only one allergen; 8.2% to two allergens; 1.8% to three allergens, and 4.5% to more than three allergens.
Table 2.
Number of patients tested for positive for each antigen
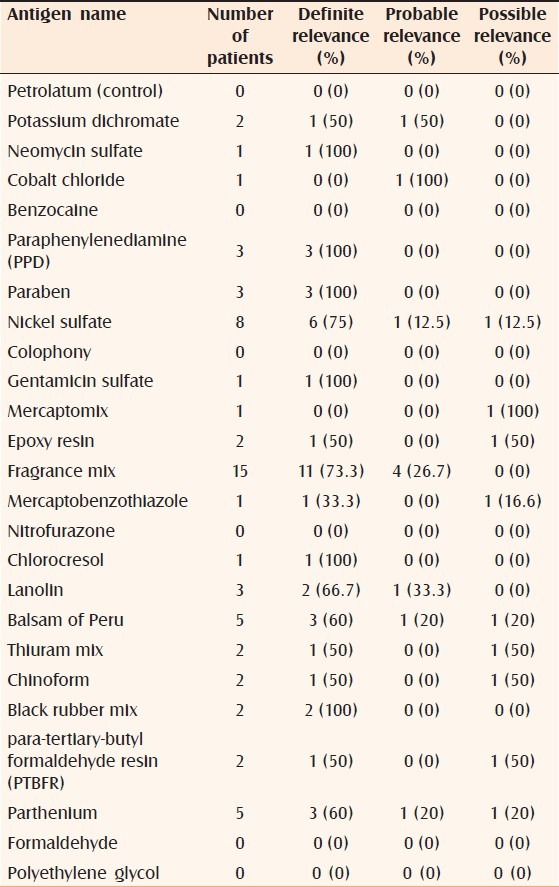
In the current study, fragrance mix, PPD (paraphenylenediamine), paraben mix, lanolin, black rubber mix, para-tertiary-butyl formaldehyde resin, mercapto mix, chlorocresol, and cobalt chloride showed 100% current relevance [Table 3], followed by balsam of Peru (83.3%), nickel sulfate (80%), and parthenium (71.4%). Nitrofurazone had 100% past relevance followed by neomycin sulfate (50%) and gentamicin sulfate (50%). Under current relevance, PPD, paraben mix, chlorocresol, black rubber mix, neomycin sulfate, and gentamicin sulfate had 100% definite relevance [Table 4], followed by nickel sulfate (75%) and fragrance mix (73.3%). Cobalt chloride had a high (100%) probable relevance and mercapto mix had a high (100%) possible relevance.
Table 3.
Overall relevance of positive antigens
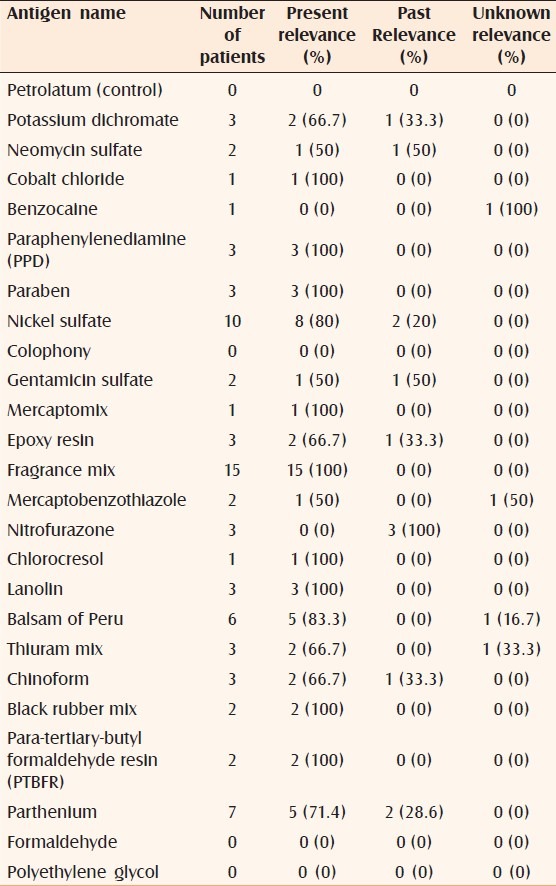
Table 4.
Present relevance of positive antigens
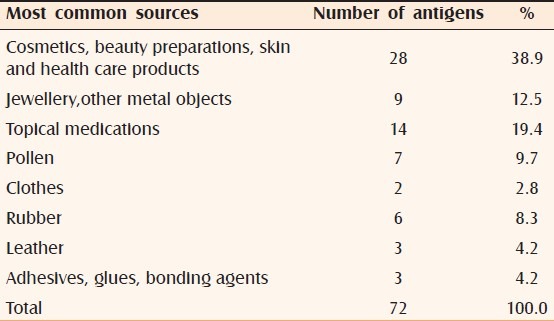
Allergens that were most commonly relevant were those that are found in different topical products like fragrances, topical medications, and preservatives [Table 5]. These allergens are often found in cosmetic products or healthcare products and hence form the major sources of allergens in patients with psoriasis.
Table 5.
Most common sources of the positive relevant antigens
DISCUSSION
Any chronic dermatosis such as psoriasis can be complicated by contact dermatitis due to an impaired cutaneous barrier. Pre-existing or concomitant constitutional and/or irritant contact dermatitis damages the skin, affecting its barrier function and producing increased opportunities for allergen absorption and secondary sensitization. The longer the duration of the disease, greater is the chance of sensitization. The extent to which contact allergens play a role in the etiology of psoriasis has always been contemplated.
It has been suggested that ACD is uncommon in psoriasis.[1–6] Some studies say that ACD has been under-represented in patients with psoriasis.[7] Heule et al.[8] found positive patch tests in 68% of psoriatic patients. Other studies have observed positivity in 20 to 25% of patients with psoriasis.[3,9,15–17] One study[2] showed no difference in the frequency of allergic reactions between atopics, healthy persons, or psoriatics, whereas Henseler and Christophers[7] calculated that ACD was 3 times less frequent in patients with psoriasis compared with a control group. Another study by Stinco et al.[18] revealed that the number of positive patch tests in patients with psoriasis were similar to that in normal population, nickel being the commonest allergen in both groups, which was in agreement with other studies.[3,9,19] Nickel sulfate, coal tar, dithranol, and fragrance mix were the most common allergens in most studies.[3,8,9,15–18,20,21]
This study reveals that 47/110 (42.7%) of the subjects had a positive patch test to one or more allergens. Fragrance mix, nickel sulfate, parthenium, and balsam of Peru were the most common sensitizers. Fragrance mix showed 100% current relevance and nickel sulfate showed 80% current relevance to the existing dermatitis.
Fragrances found in skin-care products are major culprits in ACD. Relevance and avoidance of these allergens can be tricky when one considers the large variety of products each person uses on a daily basis and the choices available. It is possible that careful screening of skin products and avoidance of the selected antigens may alleviate chronic, recalcitrant psoriasis.
Patients confirmed as having positive and relevant contact allergy have been shown to have a significant improvement in both perceived eczema severity and Dermatology Life Quality Index score two months after patch testing.[22] ACD significantly affects Quality of Life (QoL), especially when it affects the hands, face or is occupationally related. Emotional impact is therefore an important measure of QoL in ACD patients. Outcomes in patients with ACD were improved by early diagnosis and subjects enjoyed their best QoL six to twelve months after patch testing.[23] Patch testing and interpretation have been found to bring about greater improvement in the disease severity index and percentage disease activity than diagnosis without patch testing. Patch testing was found to be the most cost-effective in patients with a disease duration of two months to one year.[24,25]
To conclude, secondary contact dermatitis is common in patients with psoriasis and patch testing is necessary to determine the triggering or aggravating factors in these patients, to avoid sensitizers and to improve QoL.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moss C, Friedmann PS, Shuster S. Impaired contact hypersensitivity in untreated psoriasis and the effects of photochemotherapy and dithranol/UVB. Br J Dermatol. 1981;105:503–8. doi: 10.1111/j.1365-2133.1981.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 2.Fedler R, Stromer K. Nickel sensitivity in atopics, psoriasis and healthy subjects. Contact Dermatitis. 1993;29:65–9. doi: 10.1111/j.1600-0536.1993.tb03484.x. [DOI] [PubMed] [Google Scholar]
- 3.Fleming CJ, Burden AD. Contact allergy in psoriasis. Contact Dermatitis. 1997;36:274–6. doi: 10.1111/j.1600-0536.1997.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 4.Epstein WL, Maibach HI. Immunologic competence of patients with psoriasis receiving cytotoxic drug therapy. Arch Dermatol. 1965;91:599–606. doi: 10.1001/archderm.1965.01600120031006. [DOI] [PubMed] [Google Scholar]
- 5.Rinbaud P, Meynadier J, Guilhou JJ, et al. A study of immunity to cellular mediation in psoriasis. Bull Soc Franc Dermatol Syph. 1973;1:477–8. [Google Scholar]
- 6.Silvani S, Spettoli E, Stacul F, Tosti A. Contact dermatitis in psoriasis to propolis. Contact Dermatitis. 1997;37:48–9. doi: 10.1111/j.1600-0536.1997.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 7.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982–6. doi: 10.1016/0190-9622(95)91336-x. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WM8-4CTD2P7-B&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=b112dcffbc180b7695b04eac68d9b351 - m4.cor* [DOI] [PubMed] [Google Scholar]
- 8.Heule F, Tahapary GJ, Bello CR, van Joost T. Delayed-type hypersensitivity to contact allergens in psoriasis. A clinical evaluation. Contact Dermatitis. 1998;38:78–82. doi: 10.1111/j.1600-0536.1998.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 9.Barile M, Cozzani E, Anonide A, Usiglio D, Burroni A, Guarrera M. Is contact allergy rare in psoriatics? Contact Dermatitis. 1996;35:113–4. doi: 10.1111/j.1600-0536.1996.tb02309.x. http://www.sciencedirect.com/science?_ob=MImg&_imagekey= B6WM8-4CTD2P7-B-1&_cdi=6928&_user=10&_orig=search&_coverDate=06%2F30%2F1995&_sk=999679993&view=c&wchp=dGLzVtb-zSkWA&md5=e0e1d080c8920b0dddf3d0395a77fd15&ie=/sdarticle.pdf . [DOI] [PubMed] [Google Scholar]
- 10.Rietschel RL, Fowler JF., Jr . Practical Aspects of Patch Testing. In: Rietschel RL, Fowler JF Jr, editors. Fisher's contact dermatitis. 6th ed. Hamilton: BC Decker Inc; 2008. pp. 11–29. [Google Scholar]
- 11.Lachapelle JM. The spectrum of diseases for which patch testing is recommended. In: Lachepelle JM, Maibach HI, editors. Patch testing prich testing: A practical guide. Springer: Berlin; 2003. pp. 7–26. [Google Scholar]
- 12.Ghosh S. 1st ed. Kolkata: Institute of Allergic and Immunologic Skin Diseases; 2006. Atlas and Synopsis of Contact and Occupational dermatology; pp. 25–61. [Google Scholar]
- 13.Pratt MD, Belsito DV, DeLeo VA, Fowler JF, Jr, Fransway AF, Maibach HI, et al. North American Contact Dermatitis Group patch-test results, 2001-2002 study period. Dermatitis. 2004;15:176–83. [PubMed] [Google Scholar]
- 14.Zug KA, Rietschel RL, Warshaw EM, Belsito DV, Taylor JS, Maibach HI, et al. The value of patch testing patients with a scattered generalized distribution of dermatitis: retrospective cross-sectional analyses of North American Contact Dermatitis Group data, 2001 to 2004. J Am Acad Dermatol. 2008;59:426–31. doi: 10.1016/j.jaad.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra V, Kaur I, Saraswat A, Kumar B. Frequency of patch-test positivity in patients with psoriasis: a prospective controlled study. Acta Derm Venereol. 2002;82:432–5. doi: 10.1080/000155502762064566. [DOI] [PubMed] [Google Scholar]
- 16.Clark AR, Sherertz EF. The incidence of allergic contact dermatitis in patients with psoriasis vulgaris. Am J Contact Dermat. 1998;9:96–9. [PubMed] [Google Scholar]
- 17.Pigatto PD. Atopy and contact sensitization in psoriasis. Acta Derm Venereol Suppl (Stockh) 2000;211(Suppl):19–20. doi: 10.1080/00015550050500077. [DOI] [PubMed] [Google Scholar]
- 18.Stinco G, Frattasio A, De Francesco V, Bragadin G, Patrone P. Frequency of delayed-type hypersensitivity to contact allergens in psoriatic patients. Contact Dermatitis. 1999;40:323–4. doi: 10.1111/j.1600-0536.1999.tb06083.x. [DOI] [PubMed] [Google Scholar]
- 19.Dave VK, Cross D. Non-occupational metal-related contact reactions in women with psoriasis. Contact Dermatitis. 1989;21:194–5. doi: 10.1111/j.1600-0536.1989.tb04735.x. [DOI] [PubMed] [Google Scholar]
- 20.Burden AD, Muston H, Beck MH. Intolerance and contact allergy to tar and dithranol in psoriasis. Contact Dermatitis. 1994;31:185–6. doi: 10.1111/j.1600-0536.1994.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 21.Katugampola RP, Hughes TM, Mills CM, Stone NM. Allergic contact dermatitis complicating pustular psoriasis in two patients. Br J Dermatol. 2007;156:788–90. doi: 10.1111/j.1365-2133.2007.07776.x. [DOI] [PubMed] [Google Scholar]
- 22.Thomson KF, Wilkinson SM, Sommer S, Pollock B. Eczema: quality of life by body site and the effect of patch testing. Br J Dermatol. 2002;146:627–30. doi: 10.1046/j.1365-2133.2002.04692.x. [DOI] [PubMed] [Google Scholar]
- 23.Kadyk DL, McCarter K, Achen F, Belsito DV. Quality of life in patients with allergic contact dermatitis. J Am Acad Dermatol. 2003;49:1037–48. doi: 10.1016/s0190-9622(03)02112-1. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopalan R, Kallal JE, Fowler JF, Jr, Sherertz EF. A retrospective evaluation of patch testing in patients diagnosed with allergic contact dermatitis. Cutis. 1996;57:360–4. [PubMed] [Google Scholar]
- 25.Rajagopalan R, Anderson RT, Sarma S, Retchin C, Jones J. The use of decision-analytical modelling in economic evaluation of patch testing in allergic contact dermatitis. Pharmacoeconomics. 1998;14:79–95. doi: 10.2165/00019053-199814010-00008. [DOI] [PubMed] [Google Scholar]


