Abstract
Wnt signaling is critical to many aspects of development, and aberrant activation of the Wnt signaling pathway can cause cancer. Dishevelled (Dvl) protein plays a central role in this pathway by transducing the signal from the Wnt receptor complex to the β-catenin destruction complex. Dvl also plays a pivotal role in the planar cell polarity pathway that involves the c-Jun N-terminal kinase (JNK). How functions of Dvl are regulated in these two distinct pathways is not clear. We show that deleting the C-terminal two-thirds of Dvl, which includes the PDZ and DEP domains and is essential for Dvl-induced JNK activation, rendered the molecule a much more potent activator of the β-catenin pathway. We also found that casein kinase Iɛ (CKIɛ), a previously identified positive regulator of Wnt signaling, stimulated Dvl activity in the Wnt pathway, but dramatically inhibited Dvl activity in the JNK pathway. Consistent with this, overexpression of CKIɛ in Drosophila melanogaster stimulated Wnt signaling and disrupted planar cell polarity. We also observed a correlation between the localization and the signaling activity of Dvl in the β-catenin pathway and the JNK pathway. Furthermore, by using RNA interference, we demonstrate that the Drosophila CKIɛ homologue Double time positively regulates the β-catenin pathway through Dvl and negatively regulates the Dvl-induced JNK pathway. We suggest that CKIɛ functions as a molecular switch to direct Dvl from the JNK pathway to the β-catenin pathway, possibly by altering the conformation of the C terminus of Dvl.
The Wnt family of glycoproteins regulate cell growth and cell fate determination in a wide range of metazoan organisms, including Drosophila melanogaster and mammals. Wnts play pivotal roles in diverse developmental processes, such as segmentation in D. melanogaster, control of asymmetric division in Caenorhabditis elegans, and axis formation and patterning of the central nervous system in vertebrates (10, 56).
Genetic experiments in D. melanogaster and biochemical studies in Xenopus and mammalian cells have established a framework for the Wnt signaling pathway (15, 39). In the absence of a Wnt signal, cytoplasmic β-catenin is bound to Axin and constitutively phosphorylated at several N-terminal Ser and Thr residues by glycogen synthase kinase 3 (GSK3); phosphorylated β-catenin is then recognized by β-TrCP and degraded by the ubiquitin-proteasome system. Wnt signaling is initiated by the binding of secreted Wnt protein to its receptors, Frizzled and LRP5/6, activating Dishevelled (Dvl) by an unknown mechanism. Dvl induces the dissociation of the Axin-GSK3-β-catenin complex and the stabilization of β-catenin, presumably through the inactivation of GSK3 and failure to phosphorylate β-catenin. Accumulated β-catenin enters the nucleus, binds to members of LEF/TCF family of transcription factors, and alters the transcription of Wnt target genes.
Deregulated Wnt signaling contributes to tumorigenesis. Wnt-1, the founding member of the Wnt family, was first identified as a gene activated by insertion of mouse mammary tumor provirus, leading to the formation of mouse mammary tumors (36). Activating mutations of β-catenin and inactivating mutations of adenomatous polyposis coli and Axin, whose products are negative regulators of the Wnt signaling pathway, have been associated with various types of human cancers (41).
Some components of the Wnt signaling pathway, including Frizzled and Dvl, are also involved in a β-catenin-independent pathway that determines planar cell polarity (32, 49). The planar cell polarity pathway regulates cytoskeletal organization through small GTPases and the c-Jun N-terminal kinase (JNK). In D. melanogaster, planar cell polarity signaling is essential for proper orientation of hairs and bristles on the thorax, abdomen, wing, and leg as well as correct polarity of ommatidia in the eye. In vertebrates, planar cell polarity is required for cell polarity and movement during gastrulation (52, 55). Analysis of Wingless (Wg) signaling-deficient arm, pan/TCF and zw3/sgg clonal tissue in the wing (6) and eye (9) revealed no polarity-related phenotype, indicating that D. melanogaster Dishevelled (Dsh) is the branching point of the planar cell polarity pathway and the β-catenin pathway.
Three conserved domains have been identified in the Dvl protein, including an amino-terminal DIX domain, a central PDZ domain, and the carboxyl-terminal DEP domain (Fig. 1A). A simplified view is that the DIX and the PDZ domains are required for the β-catenin pathway while the PDZ and the DEP domains are required for planar cell polarity/JNK signaling (6, 9, 33, 60). It is possible that Dvl adopts different conformations and forms different protein complexes in these divergent signaling pathways. The involvement of Dvl in one signaling pathway might exclude its participation in the other pathway. However, it is still not clear how Dvl signaling activities are regulated in these two pathways.
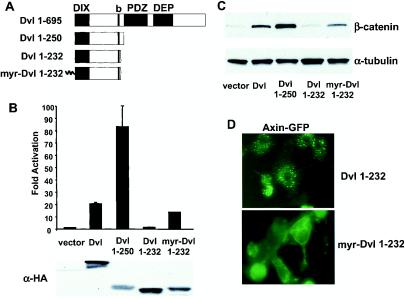
FIG. 1.
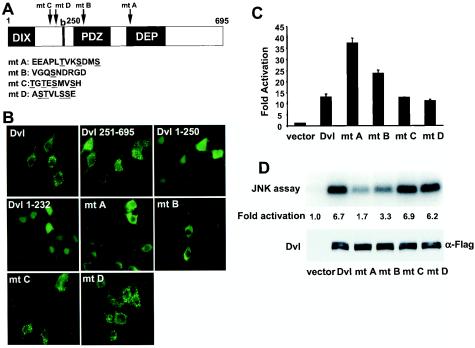
Identification of a hyperactive Dvl mutant that stimulates the β-catenin signaling pathway. (A) Schematic representations of mouse Dvl 1 and its mutants. Domains that are conserved among Dvl molecules in different species are highlighted. The basic region (b) is a region N-terminal to the PDZ domain that contains multiple basic amino acid residues. (B) Effects of Dvl mutants on LEF-1-mediated transcription. 293 cells were plated in 24-well plates and transfected with 0.01 μg of LEF-1 expression plasmid, 0.01 μg of cytomegalovirus-Renilla luciferase expression plasmid, 0.1 μg of LEF-1 luciferase reporter plasmid, and 0.1 μg of Dvl expression plasmid. Empty vector DNA was added to equalize the total amount of DNA (0.5 μg/transfection). The luciferase activities (top panel) were normalized to the Renilla luciferase activities. Each experiment was carried out in triplicate, and error bars represent standard deviations. Dvl proteins were tagged with the HA epitope at their carboxyl termini, and protein expression was examined by immunoblot analysis with anti-HA antibodies (bottom panel). Note that expression of Dvl 1-250 was significantly lower than that of wild-type Dvl. (C) Effects of Dvl mutants on β-catenin stabilization. 293 cells were transfected with the indicated plasmids. Forty-eight hours after transfection, cells were subjected to subcellular fractionation. Equal amounts of cytosolic extracts were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with anti-β-catenin antibodies (top panel). α-Tubulin levels were determined by immunoblotting with anti-α-tubulin antibodies as an internal loading control (bottom panel). (D) Recruitment of Axin-GFP to the plasma membrane by myristoylated Dvl 1-232. Axin-GFP and Dvl 1-232 or myristoylated Dvl 1-232 were coexpressed. The subcellular distributions of Axin-GFP were examined by fluorescence microscopy.
Casein kinase Iɛ (CKIɛ) has been identified as a positive regulator of the Wnt signaling pathway (40, 45). Overexpression of CKIɛ in Xenopus embryos induces axis duplication and rescues UV-treated embryos. Epistasis experiments in Xenopus embryos suggest that CKIɛ functions between Dvl and GSK3 (40). Associations of CKIɛ, Dvl and Axin have been demonstrated by coimmunoprecipitation (45). Using yeast two-hybrid experiments, CKIɛ was shown to bind directly to the PDZ domain of Dvl (40) and the C-terminal region of Axin (30, 44). However, it was also shown that CKIɛ binds directly to the DEP domain of Dvl and indirectly to Axin (22). Recently, it has been suggested that CKIɛ is recruited to the Axin-GSK3-β-catenin complex by the ankyrin repeat protein Diversin (46).
The CKI gene family consists of seven different genes in mammals, CKIα, β, γ1, γ2, γ3, δ, and ɛ (16). The ɛ and δ isoforms are closely related and share a C-terminal domain that is not present in other CKI isoforms. The shared C-terminal region is responsible for the interaction between Dvl and CKIɛ; accordingly, ectopic overexpression of CKIɛ and CKIδ, but not CKIα, induces axis duplication in Xenopus embryos (45). Recent studies suggest that the α isoform of CKI is, in contrast, a negative regulator of Wnt signaling by functioning as a priming kinase for β-catenin and GSK3 (28, 59). In spite of intensive study, the mechanism by which CKIɛ stimulates the Wnt pathway remains elusive. Overexpression of CKIɛ increases the phosphorylation of multiple Wnt pathway components, including Dvl (17, 22, 40), adenomatous polyposis coli (17, 22, 44), Axin (17), TCF (27), and β-catenin (17), but the physiological significance of these modifications is uncertain.
Most of the major components in the Wnt pathway are conserved from Drosophila to mammals. Double time (Dbt) is the D. melanogaster homologue of CKIɛ/δ, but it is not clear what role, if any, Dbt plays in Wnt signaling. For example, Dbt-dsRNA induces the stabilization of Armadillo, the D. melanogaster β-catenin homologue, raising the possibility that Dbt functions differently than its vertebrate homologue (59).
Here, we demonstrate that CKIɛ functions as a molecular switch to direct Dvl from the planar cell polarity/JNK pathway to the β-catenin pathway. In addition, we observe a correlation between the subcellular localizations of Dvl and its signaling activities in the β-catenin and the JNK pathways. Our work also indicates that the D. melanogaster CKIɛ homologue Dbt has a dual role in Wnt signaling, and it positively regulates Wnt signaling through Dishevelled.
MATERIALS AND METHODS
Expression constructs.
Mouse Dvl1 and its mutants were tagged with hemagglutinin (HA) or Flag epitope fused at the carboxyl termini and cloned into mammalian expression vectors under the control of the cytomegalovirus promoter. Dvl-green fluorescent protein (GFP) fusion constructs were generated by cloning mouse Dvl1 into pEGFP-N2 (BioSignal Packard). Site-directed mutagenesis was performed with two-step PCR. All constructs were verified by DNA sequencing. The axin-GFP fusion construct was generated by cloning mouse axin into pEGFP-C1 (Clontech). The LEF-1 reporter constructs were kindly provided by Rudolf Grosschedl. HA-tagged mouse CKIɛ and its K38F mutant expression constructs were provided by Lewis Williams (45). Wild-type CKIɛ and its K38F mutant were also subcloned into the mammalian expression construct pMT21 with the Myc epitope fused to the carboxyl termini.
Wingless, DFz2, Dsh, Armadillo ΔN (lacking amino acid residues 1 to 77), mouse CKIɛ, Dbt (24), Drosophila CKIα, LEF-1-VP16 (3), LEF-1, and Renilla luciferase were cloned into the D. melanogaster expression construct pPac-PL under the control of the D. melanogaster actin promoter. Dsh, mouse CKIɛ, Dbt, and D. melanogaster CKIα were fused with the Myc epitope at the carboxyl termini.
Mammalian cell culture, transfection, and luciferase assays.
Human kidney epithelium 293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (HyClone), 1% glutamine, and 1% penicillin/streptomycin (Gibco) at 37°C in 5% CO2. 293 cells were transfected with Fugene 6 (Roche) according to the manufacturer's instructions. Luciferase assays were performed with the dual luciferase assay kit (Promega) according to the manufacturer's instructions.
Cell fractionation assay.
Cells were washed and scraped on ice into TBS (10 mM Tris-HCl [pH 7.5], 140 mM NaCl, 2 mM dithiothreitol, protease inhibitors). Cells were homogenized with 30 strokes in a Dounce homogenizer, and the nuclei were removed by low-speed centrifugation. The postnuclear supernatants were spun at 100,000 x g for 90 min at 4°C to generate a supernatant or cytosolic fraction. Samples normalized for protein content were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
D. melanogaster S2 cell transfection and dsRNA experiments.
D. melanogaster S2 cells were grown at room temperature in Schneider's Drosophila medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 1% glutamine, and 1% penicillin/streptomycin (Gibco). For transfection, S2 cells were plated into 12-well plates at a density of 0.8 × 106 cells/well 2 h before transfection. Cells were transfected with CellFectin (Invitrogen) according to the protocol provided by the manufacturer. Each well received 0.1 μg of pPac-LEF-1, 0.1 μg of pPac-Renilla, and 0.1 μg of LEF-1-luciferase reporter plasmid and 0.5 of μg effector plasmid. Thirty-six hours after transfection, cells were lysed, and cell lysates were used for the luciferase assay and immunoblot analysis.
Double-stranded RNA (dsRNA) experiments were performed as described (13). PCR was performed with primers containing T7 polymerase binding sites. Complementary single-stranded RNA was generated with a Megascript T7 transcription kit (Ambion), and annealed to form double-stranded RNA. Double-stranded RNA was added to S2 cells at 15 μg/well in 0.5 ml of Drosophila serum-free medium (D-SFM) (Gibco). After a 1-h incubation, 0.5 ml of D-SFM containing 20% fetal calf serum was added. Cells were transfected with the indicated plasmids 24 h after dsRNA treatment. The following genes were targeted with dsRNA: Drosophila CKIα, DDBJ/EMBL/GenBank accession number U55848; Dbt, accession number AF055583; Dsh, accession number L26974; and Drosophila stimulatory G protein α subunit Gαs, accession number M23233. Gαs-dsRNA was used as a control in the RNA interference [RNAi] experiments.
Immunoblot analysis.
Cells were lysed with radioimmunoprecipitation assay (RIPA) buffer (10 mM sodium phosphate [pH 7.4], 100 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM NaF, 10 mM β-glycerophosphate, and protease inhibitors). Equal amounts of protein were subjected to SDS-PAGE, and proteins were transferred onto a nitrocellulose filter. After blocking with 5% dry milk in TBS plus 0.1% Tween-20 for 1 h, the membrane was incubated with the primary antibodies overnight at 4°C and with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Signals were detected by the enhanced chemiluminescence kit (Amersham).
In vitro kinase assay.
To measure JNK activation, an HA-tagged JNK expression construct was cotransfected with Dvl expression constructs into 293 cells. Forty-eight hours after transfection, cells were solubilized with lysis buffer (20 mM Tris [pH 7.5], 10% glycerol, 137 mM NaCl, 1% Triton X-100, 25 mM β-glycerophosphate, 2 mM EDTA, 0.5 mM dithiothreitol, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml), and cell lysates were microcentrifuged at 13,000 rpm for 20 min at 4°C. Cell lysates were incubated with anti-HA antibody for 1 h at 4°C, and the immunocomplex was collected by incubation with 20 μl of protein G-agarose beads for 2 h at 4°C. The beads were washed three times with lysis buffer and twice with kinase buffer (25 mM HEPES [pH 7.4], 25 mM β-glycerophosphate, 25 mM MgCl2, 2 mM dithiothreitol, 0.1 mM sodium orthovanadate). The kinase assays were initiated by the addition of 2 μg of GST-Jun(1-109) substrate protein, and 10 μCi of [γ-32P]ATP in a volume of 25 μl. The reactions were terminated by the addition of Laemmli sample buffer. The phosphorylated proteins were fractionated by SDS-PAGE and examined by autoradiography and PhosphorImager analysis.
Fluorescence microscopy.
293 or COS7 cells were transfected with Dvl-GFP or Axin-GFP expression constructs. Thirty-six hours after transfection, cells were washed once with phosphate-buffered saline and fixed with 2% paraformaldehyde for 20 min at room temperature. Cells were then washed several times with phosphate-buffered saline, mounted with Vetashield mounting medium (Vector), and examined by fluorescence microscopy (Leica).
D. melanogaster wing mounting.
UAS-mCKIɛ transgenic lines were crossed to C765-Gal4 lines. Adult wings from the cross, together with the wild-type and dsh1 homozygous adult wings, were stored in a 1:2 glycerol-ethanol solution. Then they were washed twice in 100% ethanol before being mounted in 5:6 ethanol-lactic acid solution. Coverslips were then sealed and examined under a microscope.
RESULTS
Identifying a hyperactive Dvl mutant that stimulates the β-catenin pathway.
Dvl is a modular protein involved in divergent signaling pathways. It is believed that the DIX and the PDZ domains are required for β-catenin signaling while the PDZ and the DEP domains are essential for planar cell polarity/JNK signaling (8). To begin to ask whether Dvl contains a binding site for a positive regulator of the β-catenin pathway that is recruited by Dvl to the Axin-GSK3-β-catenin complex, we made a series of deletion mutants, hoping to define a minimal fragment of Dvl that stimulates the β-catenin pathway. During this analysis, we discovered that a truncation mutant (Dvl 1-250), which lacks the C-terminal two-thirds of the molecule, functions as a hyperactive protein with respect to the β-catenin pathway.
The ability of Dvl mutants to activate the β-catenin pathway was assessed in cotransfection assays in 293 cells with a LEF-1-luciferase reporter. The Dvl 1-250 mutant (Fig. 1A) activated the reporter to a much higher level than wild-type Dvl, although the protein expression level of Dvl 1-250 is significantly lower than that of wild-type Dvl (Fig. 1B). We have noticed that the levels of wild-type Dvl correlate with its activities on the β-catenin pathway in a wide range of dosages (data not shown), and the largest amount of Dvl used in this experiment did not induce the maximal response. Serial deletion mutants that remove additional C-terminal residues from Dvl 1-250 were also tested for their ability to activate the LEF reporter (Fig. 1 and data not shown). In this analysis, we found that Dvl 1-232 largely lacked activity on the LEF-1 responsive reporter (Fig. 1B). To our knowledge, Dvl 1-250 is the smallest, and possibly the most potent, Dvl mutant for activating the β-catenin pathway. To confirm the signaling activities of various Dvl mutants, we expressed Dvl mutants in 293 cells and tested for their activities to induce β-catenin stabilization (Fig. 1C). The results from the β-catenin stabilization assay were consistent with those from the LEF reporter assay.
It has been reported that the Wnt signal recruits Axin to the plasma membrane and destabilizes it (29). Intriguingly, Dvl also relocates to the plasma membrane upon Wnt stimulation (60), raising the possibility that Dvl facilitates translocation of Axin to the plasma membrane upon Wnt signaling. Indeed, it has recently been shown that Wnt signaling induces membrane-translocation of Axin in a Dvl-dependent manner (14). We asked whether a membrane-tethered form of an inactive form of Dvl could activate the β-catenin pathway by relocating Axin to the plasma membrane. To do this, we added a myristoylation signal to the amino terminus of Dvl 1-232 to anchor it to the plasma membrane. Notably, myristoylation of Dvl 1-232 markedly increased its ability to stimulate LEF-1-mediated transcription (Fig. 1B). This finding was confirmed by the β-catenin stabilization assay (Fig. 1C).
The ability of myristoylated Dvl 1-232 to recruit cotransfected Axin-GFP to the plasma membrane was confirmed (Fig. 1D), implying that myristoylated Dvl 1-232 can activate Wnt signaling by recruiting Axin to the plasma membrane. Although adding a myristoylation signal to Dvl 1-232 increased its activity to stimulate LEF-1-mediated transcription, adding a myristoylation signal to Dvl and Dvl 1-250 decreased their activities (data not shown). Furthermore, ectopic expression of Dvl and Dvl 1-250 did not induce obvious membrane-translocation of Axin-GFP (data not shown). We hypothesize that Dvl 1-250 and myristoylated Dvl 1-232 might activate the β-catenin pathway through different mechanisms: Dvl 1-250 might recruit a positive regulator of the Wnt pathway into the Axin-GSK3-β-catenin complex, while myristoylated Dvl 1-232 might relocate Axin to the plasma membrane and destabilize Axin. It is noteworthy that although myristoylated Dvl 1-232 is more active than Dvl 1-250 in recruiting Axin-GFP to the plasma membrane, its effect on LEF-1-mediated transcription was significantly less than that of Dvl 1-250. Therefore, Dvl seems to have multiple ways to influence Wnt signaling.
We attempted to determine whether the functions of Dvl 1-250 and myristoylated Dvl 1-232 on LEF-1-mediated transcription required the DIX domain. However, deleting the DIX domain from these two proteins rendered them unstable (data not shown). In agreement with the notion that the DIX domain is required for Dvl-induced β-catenin activation, neither DvlΔDIX nor DvlΔ1-250 showed any stimulatory activity on the β-catenin pathway, although these two mutants appeared to be stable (data not shown). Therefore, we assume that the Dvl 1-250 and myristoylated Dvl 1-232 mutants also require the DIX domain for their functions.
Although the region of amino acids 232 to 250 is important for the signaling activity of Dvl 1-250, it is dispensable for the signaling activity of the full-length Dvl (data not shown). Presumably, this region is important for the proper folding of Dvl 1-250, but it is compensated for by the other regions of Dvl in the context of the full-length Dvl.
CKIɛ synergizes with Dvl to activate the β-catenin pathway.
Dvl can be activated by Wnt, possibly through Frizzled, and is responsible for transducing signals from Wnt receptors to the β-catenin destruction complex; however, it is not known how Dvl is instructed to transmit a signal that leads to stabilization of β-catenin. Since CKIɛ positively regulates Wnt signaling through an unknown mechanism, we tested the possibility that CKIɛ stimulates Dvl activity in the β-catenin pathway. Various amounts of CKIɛ and Dvl expression plasmids were transfected into 293 cells together with an LEF-1-expressing plasmid and the LEF luciferase reporter plasmid. At a low dose (50 ng of each), CKI ɛ or Dvl alone caused a modest increase of LEF-1-mediated transcription activity, while coexpression of CKIɛ and Dvl dramatically stimulated LEF-1-mediated transcription (Fig. 2, left panel). At an even lower dose (5 ng of each), CKIɛ or Dvl alone had no discernible effect on LEF-1-mediated transcription, while CKIɛ and Dvl together had an obvious synergistic effect (Fig. 2, right panel). These data are in agreement with a previous report (22) and suggest that CKIɛ and Dvl can synergistically activate the β-catenin pathway.
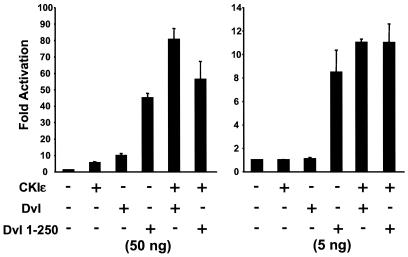
FIG. 2.
Synergy between CKIɛ and Dvl but not CKIɛ and Dvl 1-250 on LEF-1-mediated transcription. 293 cells were transfected with an LEF-1 expression plasmid and an LEF-1 luciferase reporter plasmid as well as the indicated expression constructs. The cytomegalovirus-Renilla luciferase expression plasmid was used to control the transfection efficiency. In the left panel, 50 ng each of CKIɛ or Dvl expression plasmid DNA was used, while in the right panel 5 ng was used.
We also tested whether CKIɛ could also synergize with Dvl 1-250. Intriguingly, no synergistic effect was observed on LEF-1-mediated transcription between CKIɛ and Dvl 1-250 at two 10-fold different dosages (Fig. 2). Since Dvl 1-250 is a much more potent activator of LEF-1-mediated transcription than wild-type Dvl, we propose that the C-terminal two-thirds of Dvl structurally blocks the function of the N-terminal region of the molecule in the β-catenin pathway and that this inhibition can be relieved by either deletion of the C-terminal region of Dvl or CKIɛ-induced phosphorylation of Dvl. This could explain the lack of synergy between CKIɛ and Dvl 1-250.
Overexpression of CKIɛ inhibits Dvl-induced activation of JNK.
Dvl plays a critical role in the planar cell polarity signaling pathway that involves the activation of the small GTPases, Rac and Rho, and the JNK cascade. The DEP domain, but not the DIX domain, has been reported to be essential for the function of Dvl in planar cell polarity/JNK signaling. For example, a Drosophila dsh1 allele, which contains a K417 M mutation in the DEP domain of Dsh, exhibits a planar cell polarity phenotype but not a wg phenotype (6, 9). In tissue culture, overexpression of Dvl activated the JNK pathway, while Dvl mutants with the DEP domain deleted or bearing the corresponding K417 M mutation failed to activate the JNK pathway (9, 33). Although the role of JNK signaling in the planar cell polarity pathway is not yet entirely clear, we used activation of JNK as a surrogate marker for activation of the planar cell polarity pathway in this study.
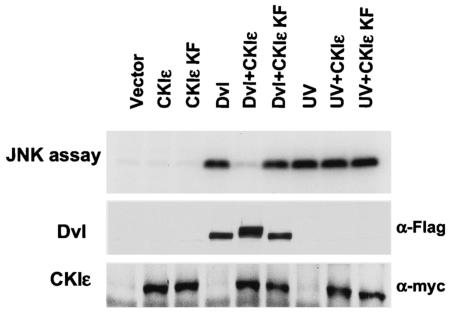
To test the effect of CKIɛ on Dvl-induced JNK activation, we coexpressed CKIɛ and Dvl together with HA-tagged JNK in 293 cells and measured JNK activity by an in vitro kinase assay (Fig. 3). Overexpression of wild-type CKIɛ or kinase-deficient CKIɛ (K38F) had no effect on JNK activation, while overexpression of Dvl strongly activated JNK. Significantly, coexpression of wild-type CKIɛ and Dvl greatly inhibited Dvl-induced JNK activation, while coexpression of the kinase-deficient CKIɛ and Dvl had no effect on the ability of Dvl to activate JNK. In addition, CKIɛ had no effect on UV-induced JNK activation, suggesting that the effect of CKIɛ was specific to the signaling pathway mediated by Dvl. Furthermore, coexpression of CKIɛ and Dvl also induced hyperphosphorylation of Dvl, as indicated by the slower migration of Dvl (Fig. 3, middle panel). Since the kinase activity is required for the inhibitory effect of CKIɛ on Dvl-induced JNK activation, it is not likely that overexpression of CKIɛ simply blocks the interaction between Dvl and its downstream target. Instead, CKIɛ-induced phosphorylation might reduce the affinity between Dvl and its downstream target in the planar cell polarity/JNK pathway.
FIG. 3.
Inhibition of Dvl-induced JNK activation by CKIɛ. 293 cells were cotransfected with the HA-tagged JNK expression construct with Flag-tagged Dvl expression constructs and Myc-tagged CKIɛ expression constructs. CKIɛ KF mutant contains a K38F mutation in the kinase domain and lacks kinase activity. As a control for activation of JNK by an alternative mechanism, cells were treated with UV at 40 J/m2 before cell harvesting. HA-tagged JNK was immunoprecipitated by anti-HA antibodies, and the abilities of JNK to phosphorylate GST-c-Jun were determined. The results were visualized with a PhosphorImager (top panel). The expression of Dvl and CKIɛ was confirmed by immunoblotting with anti-Flag and anti-Myc antibodies (bottom panels).
Overexpression of CKIɛ in D. melanogaster activates Wnt signaling and perturbs planar cell polarity signaling.
Dsh is a key component not only in transducing Wg signaling but also in the planar cell polarity pathway to mediate DFz1 activity (23, 25, 35, 50, 53). To ask if CKIɛ regulates Dsh in D. melanogaster to affect both the Wg signaling and the planar cell polarity pathways, we generated UAS-mouse CKIɛ transgenic flies. Eight individual transgenic lines were obtained. These animals were then used to investigate whether CKIɛ can perturb the normal Wg signaling events or the planar cell polarity pathway.
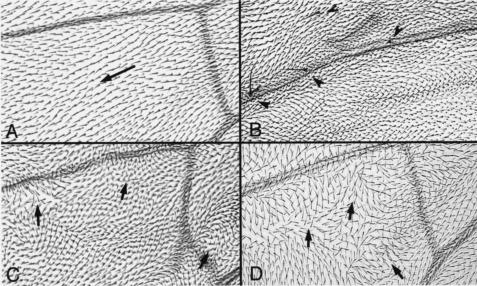
It was reported that activation of Wg signaling in the developing wing can lead to ectopic wing margin bristles in the interior of the wing (5, 7, 62) and that loss of DFz1 function or of DFz1 overexpression disrupts wing hair polarity (1, 21). Here, we examined the effects of CKIɛ on adult wings. With a C765-Gal4 driver, which is expressed ubiquitously in wing (47), we observed ectopic bristles in the interior of the wing, resembling the effect of Wg signal activation (Fig. 4B). Overexpressing CKIɛ also led to a polarity defect (Fig. 4C), although the defect appeared to be weaker than that observed with a dsh1 mutant (Fig. 4D).
FIG. 4.
Effects of overexpressing CKIɛ in adult wings. (A) Wild-type wing pattern in the region distal to the posterior cross vein. The arrow indicates hair orientation. (B) Ectopic bristles were observed in wings with CKIɛ overexpression (indicated by arrowheads), similar to ectopic Wg signal activation in the interior of the wing. (C and D) Further polarity defects are shown here in wings overexpressing CKIɛ (C), similar to the phenotypes observed in dsh1 (D). Arrows indicate irregular orientations of the hairs.
In sum, CKIɛ overexpression in D. melanogaster not only activates Wg signaling, but also perturbs planar cell polarity signaling. However, it is still not clear whether endogenous CKIɛ is required for establish proper planar cell polarity in D. melanogaster wings. Dbt is the Drosophila ortholog of CKIɛ/δ, and it regulates the period of circadian rhythms (24). Dbt is also known as disk overgrowth (Dco), and mutants show strong effects on cell survival and growth control in imaginal disks (63). Therefore, it will be difficult to examine the role of Dbt in the planar cell polarity pathway by loss-of-function assays.
CKIɛ causes a dramatic relocalization of Dvl in cells, and the subcellular localizations of Dvl correlate with its signaling activities.
In multiple experimental systems, antibody staining for exogenous Dvl produces a punctate pattern. The punctate appearance of Dvl does not seem to result solely from overexpression, since endogenous Dvl produces a similar pattern in mesenchymal and epithelial cells in embryonic mouse kidneys (54). Furthermore, endogenous Xenopus Dvl has been found associated with small vesicle-like organelles in fertilized Xenopus eggs (31). It has been suggested that translocation of Xenopus Dvl by small vesicles along microtubules to the prospective dorsal side of the embryo contributes to the local activation of a maternal Wnt pathway and establishment of dorsal cell fate in Xenopus laevis (31).
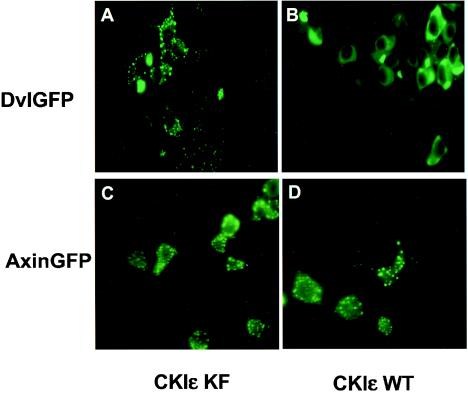
We tested the effect of CKIɛ on the location of Dvl by coexpressing CKIɛ and a full-length Dvl-GFP in 293 cells (Fig. 5). When expressed alone (data not shown) or coexpressed with a kinase-deficient mutant of CKIɛ (Fig. 5A), Dvl-GFP was found in a punctate pattern as previously reported. Coexpressing wild-type CKIɛ caused a dramatic change: the punctate pattern disappeared and Dvl-GFP became evenly distributed throughout the cytoplasm (Fig. 5B). Similar findings were observed when Dvl-GFP and CKIɛ were coexpressed in NIH 3T3 cells (data not shown). Since CKIɛ has been reported to phosphorylate Axin (17), we tested the effect of CKIɛ on the localization of Axin and found that coexpression of CKIɛ had no discernible effect on the punctate localization of Axin-GFP (Fig. 5C, D).
FIG. 5.
Effects of CKIɛ on the subcellular localizations of Dvl. Dvl-GFP (A and B) or Axin-GFP (C and D) was coexpressed with kinase-deficient (A and C) or wild-type (B and D) CKIɛ in 293 cells. The localizations of Dvl-GFP and Axin-GFP proteins were determined by fluorescence microscopy.
To determine which part of Dvl is required for the punctate pattern, deletion mutants of Dvl-GFP were generated, and their subcellular locations were examined by fluoresence microscopy. Deletions of the DIX domain, the PDZ domain, or the DEP domain alone did not dramatically alter the punctate pattern (data not shown). Notably, the C-terminal two-thirds of the Dvl protein (amino acid residues 251 to 695), which includes the PDZ and the DEP domains, produced a punctate pattern very similar to that of wild-type protein (Fig. 6B), suggesting that the punctate pattern was mainly determined by the C-terminal region of Dvl. Although the DIX domain is reportedly required for the vesicular-like pattern of Dvl (6, 11), wild-type Dvl and Dvl ΔDIX have similar localization patterns in our experiments in cultured cells, even at the lowest expression level that allowed detection of Dvl-GFP (data not shown). This discrepancy could be due to the different systems used.
FIG. 6.
Subcellular localizations and signaling activities of Dvl Ser/Thr mutants. (A) Schematic representations of mouse Dvl and its mutants. Twenty-two Dvl mutants were generated. Each mutant had one to four neighboring Ser or Thr residues mutated to Asp. The relative positions of the mutations in the four Dvl mutants (mutants A to D) are indicated by arrows. The wild-type amino acid sequences corresponding to these mutants are listed, with mutated Ser and Thr residues underlined. Mutant A contained T410D, S413D, and S416D mutations. Mutant B contained an S270D mutation. Mutant C contained T135D, T137D, S139D, and S142D mutations. Mutant D contained S179D, T180D, S183D, and S184D mutations. (B) The subcellular localizations of Dvl mutants. 293 cells were transfected with GFP-tagged Dvl expression constructs. The localizations of various Dvl mutants were determined by fluorescence microscopy. Note that mutant A formed some punctate aggregates in the top two cells but was localized diffusely in the remaining cells. To a lesser extent, mutant B was also localized more diffusely. (C) The effects of Dvl Ser/Thr mutants on the β-catenin pathway. 293 cells were cotransfected with a LEF-1 luciferase reporter, a LEF-1 expression construct, and various Dvl mutant expression constructs. (D) The effects of Dvl Ser/Thr mutants on the JNK pathway. 293 cells were cotransfected with HA-tagged JNK and Flag-tagged Dvl expression constructs. JNK activity was measured by immunoprecipitation and in vitro kinase assays. Results were visualized and quantitated by a PhosphorImager (top panel). The expressions of Dvl mutants were confirmed by immunoblotting with anti-Flag antibodies (bottom panel).
Since overexpression of CKIɛ in 293 cells increased the phosphorylation of Dvl and was associated with the dramatic redistribution of Dvl inside cells, we tested whether phosphorylation of Dvl is required for its redistribution. Coexpression of CKIɛ and Dvl caused a significant shift of Dvl in SDS-PAGE gels (Fig. 3), suggesting that many Ser and Thr residues were phosphorylated. Because there are a total of 40 Ser residues and 11 Thr residues on mDvl1, it is difficult to determine which residues are phosphorylated. However, we have shown that a GST fusion protein containing the PDZ domain (residues 221 to 360) and, to a lesser extent, a GST fusion protein containing the DEP domain of Dvl (residues 371 to 500) can be phosphorylated by CKI in vitro (data not shown).
In the absence of definitive mapping of phosphorylation sites, we used an algorithm to predict the potential of each Ser and Thr residue to be phosphorylated (26), systematically mutated Ser and Thr residues on Dvl-GFP to Asp, an acidic amino acid residue that mimics phosphorylated residues, and examined the locations of the resulting mutant proteins as Dvl-GFP fusions in 293 cells (Fig. 6B and data not shown).
Twenty-two such mutants were generated, and each mutant had one to four Ser or Thr residues changed to the acidic residue Asp to mimic phosphorylation. Most of the Ser/Thr residues in Dvl were included in these changes, and all the Ser/Thr residues with high potential of being phosphorylated were changed. Most of these mutants had the same punctate pattern as wild-type protein. Two of them, mutant C (T135D, T137D, S139D, and S142D) and mutant D (S179D, T180D, S183D, and S184D), which affect a Ser and Thr-rich region between the DIX domain and the basic region, had punctate locations similar to those of wild-type Dvl (Fig. 6B). Nevertheless, we found two mutants that were more diffusely localized than wild-type Dvl. One mutant (mutant A) contained mutations in the N terminus of the DEP domain (T410D, S413D, and S416D) and exhibited a diffuse distribution in the cytosol (Fig. 6B), similar to that of wild-type Dvl coexpressed with CKIɛ. Mutant B (S270D), which contains a mutation in the PDZ domain, also appeared to be more diffusely distributed than wild-type Dvl, but to a lesser extent than mutant A (Fig. 6B).
Next, we tested the abilities of these Dvl mutants to activate the β-catenin and JNK pathways. Mutant A and, to a lesser extent, mutant B had higher activity than wild-type Dvl in the LEF-1-mediated transcription assay (Fig. 6C). Significantly, mutant A and, again to a lesser extent, mutant B did not activate JNK as well as did wild-type Dvl. (Fig. 6D). By contrast, mutant C and mutant D were indistinguishable from wild-type Dvl in these two assays. Therefore, the abilities of mutant A and mutant B to activate the β-catenin pathway seem to be inversely correlated with their abilities to activate the JNK pathway, suggesting that the altered amino acid residues may be the natural targets of CKIɛ.
Of the two mutants, mutant A (T410D, S413D, and S416D) had the stronger effects on Dvl localization and activities. The mutated residues are found within the N-terminal region of the DEP domain. It has been postulated that Lys412 is involved in the formation of a membrane targeting surface (57). Introducing negative charges in this region might destabilize the interaction between the DEP domain and membranes, thereby compromising the function of the DEP domain. A mutant with the four potential phosphorylation sites in mutant A and mutant B mutated to the neutral amino acid Ala (S270A, T410A, S413A, and S416A) exhibited a punctate pattern similar to that of wild-type Dvl (data not shown), supporting the idea that negative charges introduced into mutant A and mutant B are responsible for its diffuse pattern of localization. However, this mutant was able to redistribute to a diffuse pattern when coexpressed with CKIɛ (data not shown), suggesting that other sites phosphorylated by CKIɛ must also contribute to the regulation of subcellular localizations of Dvl.
As described earlier, Dvl 1-250 is much more active than wild-type Dvl in its ability to stimulate the β-catenin pathway. This mutant does not contain the PDZ domain or the DEP domain. Consistent with the notion that the DEP domain is essential for Dvl-induced activation of JNK (9, 33), we found that Dvl 1-250 did not activate the JNK pathway (data not shown). We also examined the subcellular localization of Dvl 1-250 in 293 cells (Fig. 6B). Dvl 1-250-GFP was evenly distributed throughout the cells, like other Dvl proteins that are hyperactive in the β-catenin pathway.
Thus, we have observed a correlation between the localization of Dvl and its signaling activities. Dvl with a punctate appearance seems to have less ability to stimulate the β-catenin pathway and more ability to stimulate the JNK pathway. Conversely, Dvl with a diffuse location seems to have a greater ability to stimulate the β-catenin pathway and less ability to stimulate the JNK pathway.
Dbt, the Drosophila CKIɛ homologue, positively regulates Wnt signaling through Dsh and negatively regulates Dsh-dependent JNK/planar cell polarity signaling.
Dbt is the Drosophila ortholog of CKIɛ/δ. Although the function of CKIɛ as a positive regulator of Wnt signaling in vertebrates has been intensively studied, it is not known whether Dbt also has a similar role in D. melanogaster. In fact, a recent study showed that Dbt-dsRNA, like CKIα-dsRNA, induced accumulation of Armadillo in Drosophila S2 cells (59), suggesting that Dbt might function differently from its vertebrate counterpart.
We used a LEF-1 luciferase reporter-based assay to study Wnt signaling in Drosophila S2 cells (48). A positive control—coexpression of D. melanogaster Frizzled 2 (DFz2) and Wg—dramatically stimulated LEF-1-mediated transcription (Fig. 7C), and this increase could be totally blocked by Armadillo-dsRNA (data not shown). We then examined the effect of overexpression of CKIɛ and Dbt on the β-catenin pathway. Overexpression of CKIɛ in Drosophila S2 cells stimulated LEF-1-mediated transcription (Fig. 7C), consistent with the Wg phenotype observed in the Drosophila wing after ectopic overexpression of CKIɛ (Fig. 4). Overexpression of Dbt in S2 cells also greatly increased LEF-1-mediated transcriptional activity (Fig. 7A), and this increase could be totally blocked by Armadillo-dsRNA (data not shown). The stimulatory effect of Dbt on the β-catenin pathway requires the kinase activity of Dbt; a Dbt K38D mutant with a mutation in the crucial lysine in the kinase domain lacked any LEF-1-stimulating activity, although it was expressed at a similar level as wild-type Dbt (Fig. 7A).
FIG. 7.
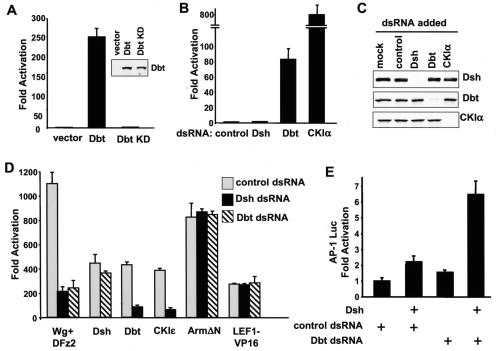
Function of Dbt and Dsh during β-catenin and JNK/planar cell polarity signaling in Drosophila S2 cells. (A) Overexpression of Dbt activates Wnt signaling in S2 cells; 0.2 μg of empty vector or Myc-tagged Dbt cDNA in a D. melanogaster expression plasmid was cotransfected with 0.1 μg each of Drosophila expression construct with the coding sequence for LEF-1, Renilla luciferase, and LEF-1 luciferase reporter. The experiments were done in triplicate, and the luciferase activities were normalized to Renilla luciferase activity. The Dbt KD mutant contains a K38D mutation in the kinase domain and lacks kinase activity. Expression of Myc-tagged Dbt and Dbt KD mutant in S2 cells was confirmed by immunoblotting with anti-Myc antibodies (inset). (B) The effects of Dbt-dsRNA and CKIα-dsRNA on basal LEF-1 transcription. S2 cells were plated into 12-well plates and treated with the indicated dsRNAs at a concentration of 15 μg/well. Twenty-four hours after dsRNA treatment, cells were transfected with the LEF-1 reporter plasmids. Cells were lysed 36 h after transfection, and luciferase activities were measured. (C) Effectiveness and specificity of Dsh-, Dbt-, and CKIα-dsRNA. S2 cells were treated with the indicated dsRNA and transfected with Myc-tagged Dsh, Dbt, or CKIα expression constructs. Thirty-six hours after transfection, cells were lysed and subjected to Western blotting with anti-Myc antibodies. (D) The effects of Dbt-dsRNA and Dsh-dsRNA on activated LEF-1-mediated transcription. S2 cells were plated into 12-well plates and treated with the indicated dsRNAs at a concentration of 15 μg/well. Twenty-four hours after dsRNA treatment, cells were transfected with the indicated effector plasmid together with LEF-1 reporter plasmids. Cells were lysed 36 h after transfection, and luciferase activities were measured. (E) The effects of Dbt-dsRNA on Dsh-dependent JNK signaling. S2 cells were treated with control or Dbt-dsRNA and then transfected with a Dsh expression plasmid together with an AP-1 luciferase reporter plasmid and a cytomegalovirus-Renilla luciferase reporter plasmid. Cells were lysed 48 h after transfection, and luciferase activities were measured.
These results suggest that Dbt is a positive regulator of Wnt signaling. Results from overexpression experiments should be treated cautiously, because overexpression of a component of a multimeric protein complex can sometimes function as a dominant-negative inhibitor. To avoid these potential problems, we also performed loss-of-function experiments with dsRNA (Fig. 7B). First, we tested the effects of various dsRNA on basal LEF-1-mediated transcription activity in S2 cells. Although control dsRNA and Dsh-dsRNA had no effect, Drosophila CKIα-dsRNA dramatically increased LEF-1-mediated transcription activity (about 800-fold). This result is consistent with the recent findings that CKIα phosphorylates Ser 45 of β-catenin and serves as a priming kinase for GSK3 (28, 59).
In agreement with a recent report (59), Dbt-dsRNA also modestly activated LEF-1-mediated transcription (about 80-fold), to a much lower extent than CKIα-dsRNA, but well above background (Fig. 7B). This result suggests that Dbt might help to keep Armadillo activity in check in the resting cells. To test the effectiveness and specificity of dsRNA, S2 cells were treated with various dsRNAs and transfected with Myc-tagged Dsh, Dbt, or CKIα expression constructs. Dsh-, Dbt-, and CKIα-dsRNAs caused the selective disappearance of the corresponding proteins (Fig. 7C), indicating that dsRNA treatment was effective and specific.
Next, we tested the effect of various dsRNAs on Wg- and DFz2-induced LEF-1 activation (Fig. 7D). In agreement with the notion that Dsh functions between Frizzled and Armadillo, Dsh-dsRNA significantly attenuated Wg- and DFz2-induced activation of LEF-1. More interestingly, Dbt-dsRNA also significantly decreased activation of LEF-1 promoter by Wg and DFz2. This result, together with the overexpression data, firmly establishes Dbt as a positive regulator of Wnt signaling in Drosophila cells.
It has been suggested that CKIɛ functions downstream of Dvl and upstream of GSK3 on the basis of experiments in Xenopus embryos with a dominant negative Dvl mutant Xdd1 and a casein kinase inhibitor CKI-7 (40). As also shown in Fig. 7D, we attempted to examine the relationship between Dbt and Dsh with dsRNA. Dbt-dsRNA only slightly decreased Dsh-induced LEF-1-mediated transcription; in contrast, Dsh-dsRNA significantly blocked CKIɛ and Dbt-induced LEF-1-mediated transcription. These results suggest that CKIɛ and Dbt function upstream of Dsh. As a control, neither Dsh-dsRNA nor Dbt-dsRNA had any effect on the ability of stabilized Armadillo (Arm ΔN) or LEF-1-VP16 to activate expression of the luciferase reporter, showing that the effects of dsRNA were specific.
Overexpression of CKIɛ strongly inhibits Dvl-induced JNK activation in mammalian cells (Fig. 3). To test whether endogenous CKIɛ plays a role in modulating the JNK/planar cell polarity pathway in S2 cells, we used a reporter construct containing multiple AP-1 elements to monitor JNK activation and asked whether depleting Dbt with dsRNA would affect Dsh-dependent activation of JNK. As shown in Fig. 7E, Dbt-dsRNA significantly increased the stimulatory effect of Dsh on the AP-1 luciferase reporter. This experiment suggests that endogenous Dbt inhibits Dsh-dependent planar cell polarity/JNK signaling. It should be noted that the AP-1 reporter is only a surrogate maker for planar cell polarity/JNK signaling. Future experiments will be necessary to directly examine the role of Dbt in planar cell polarity signaling in vivo.
Taken together, our data suggest that Dbt positively regulates Wnt signaling through Dsh and negatively modulates Dsh-induced JNK/planar cell polarity signaling.
DISCUSSION
Dvl plays a pivotal role in both the Wnt/β-catenin and the planar cell polarity/JNK signaling pathways. Our study has identified CKIɛ as a key regulator of Dvl in both pathways. We observed a correlation between the subcellular localizations of Dvl and the activities of Dvl in these pathways. Furthermore, we showed that the Drosophila CKIɛ homologue Dbt positively regulates the β-catenin pathway through Dvl and negatively regulates the Dvl-dependent planar cell polarity/JNK pathway.
Dvl is a phosphoprotein that contains many potential phosphorylation sites, and its phosphorylation can be increased by Wnt stimulation. It is possible that Wnt-induced hyperphosphorylation activates Dvl in the β-catenin pathway, although a correlation between Dvl hyperphosphorylation and activation of the planar cell polarity pathway has also been shown (4, 42). Phosphorylation of Dvl is likely to be a dynamic process, and phosphorylation of Dvl at different sites in divergent biological processes might have different effects on Dvl signaling activities.
Dvl is implicated in regulation of both the β-catenin and the planar cell polarity pathways, and it may form different complexes within the two pathways. For example, activation of one pathway might sequester Dvl into one type of complex and render it unavailable to the other pathway. It is possible that cells can transmit only β-catenin signals or only JNK/planar cell polarity signals, depending on the status of certain factors, such as Dvl, in these cells. This could explain why ectopic expression of Wg impairs the planar cell polarity phenotype induced by excess DFz1, while overexpression of DFz1 produces embryos that develop lawns of denticles reminiscent of wg mutants (6). However, it seems more likely that both β-catenin and planar cell polarity signaling can occur in the same cells (19), through different pools of Dvl present in different complexes. Since both β-catenin and planar cell polarity signals are transmitted through Dvl, regulating Dvl conformation by CKIɛ-induced phosphorylation may not only influence which signal is transmitted, but may also determine the strength of each signal that passes through Dvl.
Recently, several Dvl-binding proteins have been identified that affect the β-catenin and JNK/planar cell polarity pathways. For instance, naked cuticle (Nkd) and Dapper are negative regulators of Wnt signaling; they interact with Dsh and interfere with its function in both the β-catenin and JNK/planar cell polarity pathways (12, 43, 58, 61). Frodo, another Dvl binding protein, synergizes with Dvl in the β-catenin pathway (18). Stbm (Strabismus/Van Gogh), a membrane protein involved in planar cell polarity, interacts with Dvl, antagonizing β-catenin signaling while activating the JNK signaling pathway (38). Par-1, a protein kinase that interacts with Dvl, increases Dvl activity in β-catenin signaling, while inhibiting its activity in JNK signaling through an unknown mechanism (51).
CKIɛ also interacts with Dvl and affects both β-catenin signaling and JNK/planar cell polarity signaling. Consistent with results from other labs (17, 22, 40, 45), we find that CKIɛ associates with Dvl in vivo and phosphorylates Dvl in vitro (data not shown). Potentially, CKIɛ could modulate signaling by phosphorylating molecules other than Dvl, such as Diversin (46). However, since Dvl is involved in both β-catenin-mediated and JNK/planar cell polarity signaling and since Dvl is a documented substrate of CKIɛ in vitro, we focused our attention on Dvl. The effects of CKIɛ on Dvl signaling activities and subcellular locations can be mimicked by mutating certain potential phosphorylation sites (Fig. 6). Furthermore, the stimulatory effect of CKIɛ on the β-catenin pathway requires Dvl (Fig. 7). Therefore, it is likely that the effects of CKIɛ on the β-catenin and JNK/planar cell polarity pathways are mediated by phosphorylation of Dvl. However, the effect of CKIɛ on Dvl could also be indirect. For example, it is possible that the C-terminal region of Dvl forms a complex with an unknown protein and CKIɛ regulates the formation of the complex by phosphorylating this unknown protein. It is also possible that the effect of CKIɛ on Dvl is mediated by another kinase.
Our results demonstrate that CKIɛ can function as a switch for Dvl: CKIɛ promotes Dvl signaling through β-catenin, and it directs Dvl away from the planar cell polarity/JNK pathway. We also observed a correlation between the subcellular locations of Dvl and the signaling activities of Dvl in the β-catenin and the JNK pathways. First, overexpression of CKIɛ causes a dramatic redistribution of Dvl, from a punctate pattern in the cytoplasm to a diffuse pattern. This change is associated with a significant increase in the activity of Dvl on the β-catenin pathway and a dramatic decrease in activity in the JNK pathway. Second, two Dvl mutants that we generated, especially mutant A, exhibited diffuse patterns of distribution and accordingly had higher activities in the β-catenin pathway and lower activities in the JNK pathway. Third, the Dvl 1-250 mutant adopted a diffuse distribution pattern and was much more potent than wild-type Dvl as an activator of LEF-1-mediated transcription, while it failed to activate the JNK pathway.
Together with work by others (6, 9, 33, 60), our data suggest that the activity of Dvl on the β-catenin pathway is mainly contributed by the N-terminal one-third of the molecule, while the activity of Dvl on JNK signaling is mainly contributed by the C-terminal region. At least when Dvl is overexpressed, the C-terminal two-thirds of the protein seems to have an inhibitory effect on the function of the N terminus. This inhibitory effect can be alleviated either by deletion of the C-terminal domains or by coexpression of CKIɛ. It is possible that the C-terminal part of Dvl enables the protein to adopt a certain conformation and bind to the structures that form the observed punctate pattern. In such a model, this conformation would be incompatible with Dvl activity on the β-catenin pathway, but would be required for Dvl-induced activation of JNK. We speculate that CKIɛ might induce a conformational change of Dvl that releases Dvl from the punctate structures and enhances the interaction between Dvl and its partners in the β-catenin pathway. Indeed, it has been shown that overexpression of CKIɛ enhances the interaction between Dvl and GBP/Frat (20, 27). However, we acknowledge that, although the C-terminal region of Dvl apparently negatively regulates activity via the β-catenin pathway when Dvl is exogenously overexpressed, it might somehow be required for activating the β-catenin pathway through endogenous Dvl present at lower levels.
Despite intensive studies, a full description of the mechanisms by which CKIɛ regulates β-catenin signaling remains elusive. Both positive and negative roles of CKIɛ in the β-catenin pathway have been suggested (2, 40, 45, 46, 59). To further complicate matters, CKIɛ seems to have multiple substrates in the pathway, including Dvl, adenomatous polyposis coli, Axin, β-catenin, and TCF, but the contribution of each is unclear. To further explore this situation, we have taken advantage of the high efficiency of dsRNA in Drosophila S2 cells and the lower gene redundancy in the Drosophila genome. Our studies firmly establish Dbt, the Drosophila CKIɛ homologue, as a positive regulator of β-catenin signaling. More importantly, our dsRNA experiments also demonstrate that the positive effect of CKIɛ on the β-catenin pathway is at least partially mediated by Dvl. Therefore, although CKIɛ can phosphorylate multiple components of the β-catenin pathway and can possibly impinge on the pathway at multiple points, Dvl is one major point of the pathway that CKIɛ acts on.
Our study also suggests that Dbt might have a dual role in Wnt signaling; while Dbt is required for Wnt-induced activation of β-catenin, it also seems to be required for repressing basal β-catenin activity in resting cells (Fig. 7). Notably, knocking down CKIɛ expression in mammalian cells with RNA interference did not induce β-catenin accumulation (28), although potential compensation of CKIɛ function by CKIδ, a CKI isoform related to CKIɛ, could not be ruled out. Further experiments are necessary to understand the potential negative role of CKIɛ in Wnt signaling and to determine whether CKIɛ, like CKIα, negatively regulates Wnt signaling by serving as a priming kinase for GSK3 (2, 59).
Although our study suggests that Dbt is a regulator of the Wnt/β-catenin pathway, it is also known as disk overgrowth (Dco) and is required for cell survival, proliferation, and growth arrest in Drosophila imaginal disks (63). Clonal analysis in the wing demonstrates that dco clones have a cell-autonomous defect in cell proliferation and survival in the wing cells. Although growth failure could be due to the failure of multiple pathways, it is noteworthy that wg- and arm-deficient cells also exhibit cell proliferation defects (34, 37). Further experiments will be necessary to determine whether the growth defect of Dco-deficient clones is partially due to defective Wnt signaling. In addition, since ablating the function of Dbt leads to modest stabilization of β-catenin in S2 cells, it could be interesting to test whether a small rise in basal β-catenin activities might lead to the overgrowth phenotypes in certain fly tissues.
Acknowledgments
We thank Lewis Williams, Dianqing Wu, Shin-ichi Yanagawa, Rudolf Grosschedl, Peter Vogt, Michael Forte, Richard Young, Po Chen, Myriam Zecca, Virginia Cox, and Mary Baylies for providing reagents critical to this work. We also thank William Pao and Suzanne Ortiz for reading the manuscript.
F.C. is supported by a postdoctoral fellowship from the Susan G. Komen Breast Cancer Foundation.
REFERENCES
- 1.Adler, P. N., R. E. Krasnow, and J. Liu. 1997. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr. Biol. 7:940-949. [DOI] [PubMed] [Google Scholar]
- 2.Amit, S., A. Hatzubai, Y. Birman, J. S. Andersen, E. Ben-Shushan, M. Mann, Y. Ben-Neriah, and I. Alkalay. 2002. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16:1066-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki, M., A. Hecht, U. Kruse, R. Kemler, and P. K. Vogt. 1999. Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc. Natl. Acad. Sci. USA 96:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod, J. D. 2001. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axelrod, J. D., K. Matsuno, S. Artavanis-Tsakonas, and N. Perrimon. 1996. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science 271:1826-1832. [DOI] [PubMed] [Google Scholar]
- 6.Axelrod, J. D., J. R. Miller, J. M. Shulman, R. T. Moon, and N. Perrimon. 1998. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 12:2610-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair, S. S. 1994. A role for the segment polarity gene shaggy-zeste white 3 in the specification of regional identity in the developing wing of Drosophila. Dev Biol. 162:229-244. [DOI] [PubMed] [Google Scholar]
- 8.Boutros, M., and M. Mlodzik. 1999. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 83:27-37. [DOI] [PubMed] [Google Scholar]
- 9.Boutros, M., N. Paricio, D. I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109-118. [DOI] [PubMed] [Google Scholar]
- 10.Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. [DOI] [PubMed] [Google Scholar]
- 11.Capelluto, D. G., T. G. Kutateladze, R. Habas, C. V. Finkielstein, X. He, and M. Overduin. 2002. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature 419:726-729. [DOI] [PubMed] [Google Scholar]
- 12.Cheyette, B. N., J. S. Waxman, J. R. Miller, K. Takemaru, L. C. Sheldahl, N. Khlebtsova, E. P. Fox, T. Earnest, and R. T. Moon. 2002. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev. Cell 2:449-461. [DOI] [PubMed] [Google Scholar]
- 13.Clemens, J. C., C. A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B. A. Hemmings, and J. E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 97:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cliffe, A., F. Hamada, and M. Bienz. 2003. A role of Dishevelled in relocating axin to the plasma membrane during Wingless signaling. Curr. Biol. 13:960-966. [DOI] [PubMed] [Google Scholar]
- 15.Dierick, H., and A. Bejsovec. 1999. Cellular mechanisms of wingless/Wnt signal transduction. Curr. Top. Dev. Biol. 43:153-190. [DOI] [PubMed] [Google Scholar]
- 16.Fish, K. J., A. Cegielska, M. E. Getman, G. M. Landes, and D. M. Virshup. 1995. Isolation and characterization of human casein kinase I epsilon (CKI), a novel member of the CKI gene family. J. Biol. Chem. 270:14875-14883. [DOI] [PubMed] [Google Scholar]
- 17.Gao, Z. H., J. M. Seeling, V. Hill, A. Yochum, and D. M. Virshup. 2002. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc. Natl. Acad. Sci. USA 99:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gloy, J., H. Hikasa, and S. Y. Sokol. 2002. Frodo interacts with Dishevelled to transduce Wnt signals. Nat. Cell Biol. 4:351-357. [DOI] [PubMed] [Google Scholar]
- 19.Habas, R., Y. Kato, and X. He. 2001. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107:843-854. [DOI] [PubMed] [Google Scholar]
- 20.Hino, S., T. Michiue, M. Asashima, and A. Kikuchi. 2003. Casein kinase I epsilon enhances the binding of Dvl-1 to Frat-1 and is essential for Wnt-3a-induced accumulation of beta-catenin. J. Biol. Chem. 278:14066-14073. [DOI] [PubMed] [Google Scholar]
- 21.Jones, K. H., J. Liu, and P. N. Adler. 1996. Molecular analysis of EMS-induced frizzled mutations in Drosophila melanogaster. Genetics 142:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishida, M., S. Hino, T. Michiue, H. Yamamoto, S. Kishida, A. Fukui, M. Asashima, and A. Kikuchi. 2001. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase I epsilon. J. Biol. Chem. 276:33147-33155. [DOI] [PubMed] [Google Scholar]
- 23.Klingensmith, J., R. Nusse, and N. Perrimon. 1994. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 8:118-130. [DOI] [PubMed] [Google Scholar]
- 24.Kloss, B., J. L. Price, L. Saez, J. Blau, A. Rothenfluh, C. S. Wesley, and M. W. Young. 1998. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase I epsilon. Cell 94:97-107. [DOI] [PubMed] [Google Scholar]
- 25.Krasnow, R. E., L. L. Wong, and P. N. Adler. 1995. Dishevelled is a component of the frizzled signaling pathway in Drosophila. Development 121:4095-4102. [DOI] [PubMed] [Google Scholar]
- 26.Kreegipuu, A., N. Blom, and S. Brunak. 1999. PhosphoBase, a database of phosphorylation sites: release 2.0. Nucleic Acids Res. 27:237-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, E., A. Salic, and M. W. Kirschner. 2001. Physiological regulation of β-catenin stability by Tcf3 and CK1epsilon. J. Cell Biol. 154:983-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, C., Y. Li, M. Semenov, C. Han, G. H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 29.Mao, J., J. Wang, B. Liu, W. Pan, G. H. Farr 3rd, C. Flynn, H. Yuan, S. Takada, D. Kimelman, L. Li, and D. Wu. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7:801-809. [DOI] [PubMed] [Google Scholar]
- 30.McKay, R. M., J. M. Peters, and J. M. Graff. 2001. The casein kinase I family in Wnt signaling. Dev. Biol. 235:388-396. [DOI] [PubMed] [Google Scholar]
- 31.Miller, J. R., B. A. Rowning, C. A. Larabell, J. A. Yang-Snyder, R. L. Bates, and R. T. Moon. 1999. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J. Cell Biol. 146:427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mlodzik, M. 1999. Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J. 18:6873-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriguchi, T., K. Kawachi, S. Kamakura, N. Masuyama, H. Yamanaka, K. Matsumoto, A. Kikuchi, and E. Nishida. 1999. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem. 274:30957-30962. [DOI] [PubMed] [Google Scholar]
- 34.Neumann, C. J., and S. M. Cohen. 1996. Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development 122:1781-1789. [DOI] [PubMed] [Google Scholar]
- 35.Noordermeer, J., J. Klingensmith, N. Perrimon, and R. Nusse. 1994. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature 367:80-83. [DOI] [PubMed] [Google Scholar]
- 36.Nusse, R., and H. E. Varmus. 1982. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31:99-109. [DOI] [PubMed] [Google Scholar]
- 37.Orsulic, S., and M. Peifer. 1996. An in vivo structure-function study of armadillo, the beta-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signaling. J. Cell Biol. 134:1283-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park, M., and R. T. Moon. 2002. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol. 4:20-25. [DOI] [PubMed] [Google Scholar]
- 39.Peifer, M., and P. Polakis. 2000. Wnt signaling in oncogenesis and embryogenesis-a look outside the nucleus. Science 287:1606-1609. [DOI] [PubMed] [Google Scholar]
- 40.Peters, J. M., R. M. McKay, J. P. McKay, and J. M. Graff. 1999. Casein kinase I transduces Wnt signals. Nature 401:345-350. [DOI] [PubMed] [Google Scholar]
- 41.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 42.Rothbacher, U., M. N. Laurent, M. A. Deardorff, P. S. Klein, K. W. Cho, and S. E. Fraser. 2000. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 19:1010-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rousset, R., J. A. Mack, K. A. Wharton, Jr., J. D. Axelrod, K. M. Cadigan, M. P. Fish, R. Nusse, and M. P. Scott. 2001. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 15:658-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubinfeld, B., D. A. Tice, and P. Polakis. 2001. Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1epsilon. J. Biol. Chem. 276:39037-39045. [DOI] [PubMed] [Google Scholar]
- 45.Sakanaka, C., P. Leong, L. Xu, S. D. Harrison, and L. T. Williams. 1999. Casein kinase Iepsilon in the Wnt pathway: regulation of beta-catenin function. Proc. Natl. Acad. Sci. USA 96:12548-12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarz-Romond, T., C. Asbrand, J. Bakkers, M. Kuhl, H. J. Schaeffer, J. Huelsken, J. Behrens, M. Hammerschmidt, and W. Birchmeier. 2002. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 16:2073-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schweizer, L., and K. Basler. 1998. Drosophila ciD encodes a hybrid Pangolin/Cubitus interruptus protein that diverts the Wingless into the Hedgehog signaling pathway. Mech. Dev. 78:141-151. [DOI] [PubMed] [Google Scholar]
- 48.Schweizer, L., and H. Varmus. 2003. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shulman, J. M., N. Perrimon, and J. D. Axelrod. 1998. Frizzled signaling and the developmental control of cell polarity. Trends Genet. 14:452-458. [DOI] [PubMed] [Google Scholar]
- 50.Siegfried, E., E. L. Wilder, and N. Perrimon. 1994. Components of wingless signalling in Drosophila. Nature 367:76-80. [DOI] [PubMed] [Google Scholar]
- 51.Sun, T. Q., B. Lu, J. J. Feng, C. Reinhard, Y. N. Jan, W. J. Fantl, and L. T. Williams. 2001. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat. Cell Biol. 3:628-636. [DOI] [PubMed] [Google Scholar]
- 52.Tada, M., and J. C. Smith. 2000. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127:2227-2238. [DOI] [PubMed] [Google Scholar]
- 53.Theisen, H., J. Purcell, M. Bennett, D. Kansagara, A. Syed, and J. L. Marsh. 1994. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development 120:347-360. [DOI] [PubMed] [Google Scholar]
- 54.Torres, M. A., and W. J. Nelson. 2000. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J. Cell Biol. 149:1433-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallingford, J. B., B. A. Rowning, K. M. Vogeli, U. Rothbacher, S. E. Fraser, and R. M. Harland. 2000. Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405:81-85. [DOI] [PubMed] [Google Scholar]
- 56.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14:59-88. [DOI] [PubMed] [Google Scholar]
- 57.Wong, H. C., J. Mao, J. T. Nguyen, S. Srinivas, W. Zhang, B. Liu, L. Li, D. Wu, and J. Zheng. 2000. Structural basis of the recognition of the dishevelled DEP domain in the Wnt signaling pathway. Nat. Struct. Biol. 7:1178-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan, D., J. B. Wallingford, T. Q. Sun, A. M. Nelson, C. Sakanaka, C. Reinhard, R. M. Harland, W. J. Fantl, and L. T. Williams. 2001. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc. Natl. Acad. Sci. USA 98:3802-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yanagawa, S., Y. Matsuda, J. S. Lee, H. Matsubayashi, S. Sese, T. Kadowaki, and A. Ishimoto. 2002. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 21:1733-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yanagawa, S., F. van Leeuwen, A. Wodarz, J. Klingensmith, and R. Nusse. 1995. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 9:1087-1097. [DOI] [PubMed] [Google Scholar]
- 61.Zeng, W., K. A. Wharton, Jr., J. A. Mack, K. Wang, M. Gadbaw, K. Suyama, P. S. Klein, and M. P. Scott. 2000. naked cuticle encodes an inducible antagonist of Wnt signalling. Nature 403:789-795. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, J., and R. W. Carthew. 1998. Interactions between Wingless and DFz2 during Drosophila wing development. Development 125:3075-3085. [DOI] [PubMed] [Google Scholar]
- 63.Zilian, O., E. Frei, R. Burke, D. Brentrup, T. Gutjahr, P. J. Bryant, and M. Noll. 1999. double-time is identical to discs overgrown, which is required for cell survival, proliferation and growth arrest in Drosophila imaginal discs. Development 126:5409-5420. [DOI] [PubMed] [Google Scholar]