Abstract
Although head lice are not a major health hazard, they have been a source of irritation and disgust for thousands of years. Despite the use of over-the-counter (OTC) treatments, it has high prevalence, and epidemics occur regularly. Permethrin 1% is currently recommended as a drug of choice, but many areas have shown resistance to this insecticide. A 0.9% suspension of spinosad, a naturally occurring pest control product, has recently been approved by the USFDA for treatment of pediculosis capitis. It acts by enhancing the action of nicotinic acetylcholine, resulting in paralysis of the parasite. Clinical trials show that spinosad is more effective and safe than current drugs of treatment. Additionally, it does not require nit combing. Spinosad appears as a powerful recruit in the battle against head lice.
Keywords: Head lice, pediculicide, spinosad
Pediculus humanus capitis (head louse) is a bloodsucking, wingless arthropod that spends its entire life cycle on the host. The adult head louse is a six-legged obligate parasite—tan to greyish white in color. The female of the species lives up to 3–4 weeks and lays up to 10 eggs (nits) per day with an average of 125 eggs during her lifetime. Eggs hatch in 7–12 days and passes through three nymph stages to become an adult. This cycle repeats itself every 3 weeks.[1]
Worldwide, it affects several billion people globally with a prevalence range of 0–58.9%.[2] Although recent reports are unavailable, one study estimated the prevalence in India to be around 16.59%, going as high as 59.7% in Shillong.[3]
Wet combing, manual removal, and shaving are traditional methods to remove head lice. It is most common among preschool and elementary school aged children. Caregivers and household members of people infested with head lice are at increased risk as also are children with more siblings, longer hair, and the lower socioeconomic group.
Apart from direct costs of treating lice infestation, absenteeism from work of parents and children along with associated anxiety can be troublesome. Besides, it causes embarrassment, low self-esteem, and carries social stigma too.
Untreated infection can lead to persistent itching, excoriation, which can become super infected with methicillin-resistant Staphylococcus aureus (MRSA) or streptococci.[4] Impetigo and local skin infection can lead to local adenopathy. Head lice can transfer Yersinia pestis during blood sucking.[2]
A perfect ovicidal and pediculicidal agent that acts on the louse nervous system requires two treatments separated by 7 days. On Day 0, all lice and eggs with eyespots get killed. Eggs without eyespots develop eyespots by Day 7 and become susceptible. A purely pediculicidal agent requires three applications separated by 1 week each.[4]
Currently, the drug of choice is 1% permethrin. It is pediculicidal but not ovicidal—hence nit combing is emphasized and retreatment is required. Other options are malathion, pyrethrin/piperonyl butoxide, lindane, carbaryl, essential oils, and herbal medications. Oral agents used off label for lice treatment are ivermectin and cotrimoxazole. It may be noted that permethrin resistance has developed in many areas.[5] Misdiagnosis, lack of adherence (noncompliance), inadequate treatment, reinfestation, and lack of ovicidal property contribute to resistance of lice to pediculicides and failure of treatment.
Recently, FDA has approved the topical suspension of spinosad 0.9% for treatment of head lice infestation in patients four years of age and older. It is both pediculicidal and ovicidal.[6,7]
Spinosad is a natural mixture of pediculicidal tetracyclic macrolides—spinosyn A and spinosyn D in the ratio of 5:1 [Figure 1]. It is derived from species of actinomycetes bacteria—Saccharopolyspora spinosa and is a bacterial waste product produced by fermentation on a nutrient food source. It has since long been used as a pesticide and classified by the US Environment Protection Agency as a reduced risk pesticide product.[8,9]
Figure 1.
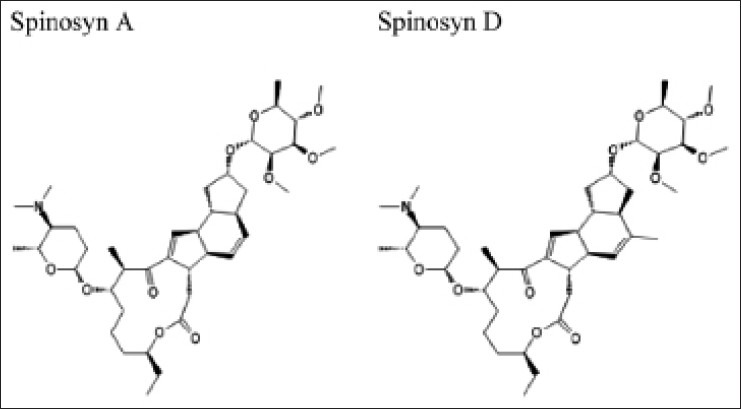
Structure of spinosyn A and spinosyn D
Spinosad overstimulates nerve cells by prolonging electrical impulse across synapses by acting like acetylcholine. Acetylcholinesterase fails to stop such impulse and nerve stimulation. Paralysis is not a primary effect, but results from prolonged hyperexcitation of nervous system leading to neuromuscular fatigue.[10]
Two phase 3, multicenter, randomized investigator/evaluator-blinded studies compared 0.9% spinosad without nit combing to 1% permethrin with combing in 1038 males and females aged ≥6 months. Drugs were administered one to two times during the 21 days home use period on the basis of complete lice eradication after single use or the presence of lice requiring a second treatment. A total of 84.6% (Study 1) and 86.7% (Study 2) of spinosad treated participants were lice-free versus 44.9% and 42.9% permethrin treated participants (P<0.001).[11]
Spinosad is more effective, convenient, and does not require nit combing. Moreover, permethrin-resistant head lice have shown susceptibility to spinosad.[12] Spinosad creme rinse is applied to dry hair, completely covering the scalp first and is then applied outward to the ends of the hair. It is left on scalp and hair for 10 min and then rinsed.
Spinosad is not hazardous by the oral, dermal, ocular, and inhalational route. It is slowly and poorly absorbed by the skin. Eye contact may cause irritation, but corneal injury is unlikely. Prolonged skin contact may cause slight irritation with local redness.[13] In trials, the most common adverse events observed with spinosad versus permethrin were application site redness (3% vs. 7%), redness and irritation to the eyes (2% vs. 3%), or application site irritation (1% vs. 2%). It has shown no neurotoxicity/tumorigenicity/teratogenicity in animals. It is a pregnancy category B medication. It is important not to use in infants as it contains benzyl alcohol as an ingredient, which can cause serious adverse reaction and death in neonates and low birthweight infants. For the same reason, caution is advised in lactating females.
To conclude, Spinosad is a safe and effective agent in our armamentarium against pediculosis capitis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Frankowski BL, Bocchini JA. Head lice. Pediatrics. 2010;126:392–403. doi: 10.1542/peds.2010-1308. [DOI] [PubMed] [Google Scholar]
- 2.Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: A neglected category of poverty associated plagues. Bull World Health Organ. 2009;87:152–9. doi: 10.2471/BLT.07.047308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khokhar A. A study of pediculosis capitis among primary school children in India. Indian J Med Sci. 2002;56:449–52. [PubMed] [Google Scholar]
- 4.Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance and safety considerations. Pediatrics. 2007;119:965–74. doi: 10.1542/peds.2006-3087. [DOI] [PubMed] [Google Scholar]
- 5.Burgess IF, Brown CM, Peock S, Kaufman J. Head lice resistant to pyrethroid insecticides in Britain. BMJ. 1995;311:752. doi: 10.1136/bmj.311.7007.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marina CF, Bond JG, Casas M, Munoz J, Orozco A, Valle J, et al. Spinosad as an effective larvicide for control of Aedes albopictus and Aedes aegypti, vectors of dengue in southern Mexico. Pest Manag Sci. 2011;67:114–21. doi: 10.1002/ps.2043. [DOI] [PubMed] [Google Scholar]
- 7.Kumar AN, Murugan K, Madhiyazhagan P, Prabhu K. Spinosad and neem seed kernel extract as bio-controllinng agents for malarial vector, Anopheles stephensi and non-biting midge, Chironomus circumdatus. Asian Pac J Trop Med. 2011;4:614–8. doi: 10.1016/S1995-7645(11)60158-2. [DOI] [PubMed] [Google Scholar]
- 8.Cole SW, Lundquist LM. Spinosad for treatment of head lice infestation. Ann Pharmacother. 2011;45:954–9. doi: 10.1345/aph.1Q144. [DOI] [PubMed] [Google Scholar]
- 9.Scheinfeld NS. Natroba (spinosad) 0.9% suspension topical suspension for head lice. Skinmed. 2011;9:256. [PubMed] [Google Scholar]
- 10.Salgado VL. Studies on the mode of action of spinosad: Insect symptoms and physiological correlates. Pestic Biochem Physiol. 1998;60:s91–102. [Google Scholar]
- 11.Stough D, Shellabarger S, Quiring J, Gabrielsen AA. Efficacy and safety of spinosad and Permethrin crème rinses for pediculosis capitis (head lice) Pediatrics. 2009;124:e389–95. doi: 10.1542/peds.2008-3762. [DOI] [PubMed] [Google Scholar]
- 12.Mougabure Cueto G, Zerba EN, Picollo MI. Permethrin-resistant head lice (Anoplura: Pediculidae) in Argentina are susceptible to spinosad. J Med Entomol. 2006;43:634–5. doi: 10.1603/0022-2585(2006)43[634:phlapi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349–53. doi: 10.2165/11208070-000000000-00000. [DOI] [PubMed] [Google Scholar]


