Abstract
Renal cysts are a common radiological finding in both adults and children. They occur in a variety of conditions, and the clinical presentation, management, and prognosis varies widely. In this article, we discuss the major causes of renal cysts in children and adults with a particular focus on the most common genetic forms. Many cystoproteins have been localized to the cilia centrosome complex (CCC). We consider the evidence for a universal ‘cilia hypothesis’ for cyst formation and the evidence for non-ciliary proteins in cyst formation.
Keywords: Renal cysts, Primary cilia, Centrosomes, Ciliopathies
Introduction
Renal cystic diseases comprise one of the most common genetic causes of kidney disease in man. As a group, they are, however, remarkably heterogenous and may present as part of rare syndromes. Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited cause of end-stage renal disease (ESRD) in adults and accounts for 10 % of all ESRD. Nephronophthisis, an autosomal recessive condition is the most common inherited cause of ESRD in children. In this article, we summarize the major genetic causes of cystic disease in children and adults. Due to space considerations, non-genetic and syndromic causes are only mentioned in brief but should be borne in mind as part of the differential diagnosis (Table 1).
Table 1.
Diseases commonly associated with a cystic phenotype
| MIM Gene/locus number | Disease | Inheritance, genes | Incidence. Male; Female | Age at presentation | Renal features | Extra-renal features |
|---|---|---|---|---|---|---|
| Genetic disorders | ||||||
| *606702 | Autosomal recessive polycystic kidney disease | A.R. PKHD1 (6p21.1-p12) | 1 in 20,000 (gene frequency 1 in 70) M:F 1:1 | Neonatal, childhood | Abdominal masses, polyuria, polydipsia, UTIs, ESRD | Oligohydramnios if severe, hypertension, ascending cholangitis |
| *601313 | Autosomal dominant polycystic kidney disease | A.D. PKD1 (16p13.3-p13.12), PKD2 (4q21-q23) | 1 in 400-1 in 1,000 M:F 1:1 but renal phenotype may be more severe in males | Usually 20–40 years | Clinical findings in children rare. In adults, abdominal pain, UTIs, ESRD | Clinical findings in children rare. In adults, hypertension, sub-arachnoid hemorrhage |
| *607100 | Nephronophthisis | A.R. 13 causative genes NPHP1-13 | 1 in 50,000 M:F 1:1 | Three forms. Infancy, childhood, adolescence | Polyuria, polydipsia, enuresis, ESRD | Growth retardation, anemia, (visual loss, liver fibrosis, cerebellar ataxia if associated with another syndrome) |
| *243305 | ||||||
| *608002 | ||||||
| *607215 | ||||||
| *609237 | ||||||
| *610142 | ||||||
| *608539 | ||||||
| *610937 | ||||||
| *609799 | ||||||
| *609884 | ||||||
| *613524 | ||||||
| *612014 | ||||||
| *614377 | ||||||
| %174000, *191845 | Medullary cystic kidney disease | A.D. MCKD1, MCKD2/UMOD | Rare. M:F 1:1 | Early adulthood | Clinical findings in children rare. In adults-ESRD | Clinical findings in children rare, may develop gout. In adults, gout |
| *189907 | HNF1β-related diseases | HNF1β (17q12) | ? M:F 1:1 | Any age | Highly variable. Hyperechogenic kidneys, multicystic kidney disease, renal agenesis, renal hypoplasia, cystic dysplasia, or hyperuricemic tubulointerstitial nephropathy not associated with UMOD mutation [88] | Congenital anomalies of the urinary tract, pancreas atrophy, liver abnormalities, maturity-onset diabetes of the young type 5 and genital malformations |
| *608537 | Von Hippel–Lindau disease | A.D. VHL (3p25.3) | 1 in 36,000 M:F 1:1 | Childhood, adolescence or adulthood. Mean age 26 | Renal symptoms rare during childhood. Adults-renal cysts, renal cell carcinoma (RCC) | Clinical findings in children rare. In adults, central nervous system (CNS) hemangioblastomas, retinal hemangioblastomas, pheochromocytoma, pancreatic cysts |
| *605284 | Tuberous sclerosis complex | A.D. High rate of spontaneous mutations. TSC1 (9q34), TSC2 (16p13.3) | 1 in 1,000 M:F 1:1 but female morbidity and mortality rates higher | Childhood | Renal symptoms rare during childhood. Renal angiomyolipomas, renal cysts, ESRD | Numerous systemic findings. Facial angiofibromas, cardiac rhabdomyomas, lymphangioleiomyomatosis, retinal hamartomas |
| *191092 | ||||||
| Renal cysts in malformative syndromes | Varies according to syndrome (including Meckel–Gruber, Bardet-Biedl, Ehlers-Danlos, Trisomy 13, 18 and 21, and Zellweger syndromes) | |||||
| Non-genetic disorders-developmental | ||||||
| Medullary sponge kidney | Mutations in GDNF have been linked. May be part of other syndromes | 1 in 2,000–20,000 M:F 1:1 (but may be more severe in females) | 20–50 years but may present younger | Hematuria, UTI, calculi | ||
| Multicystic renal dysplasia | Usually sporadic but familial disease has occurred (PAX 2). Also associated with many syndromes | 1 in 4,000 M:F 2:1 | Usually detected prenatally or soon after birth | Abdominal mass, flank pain, UTI | Hypertension | |
| Non-genetic disorders-acquired | ||||||
| Acquired renal cystic disease | Acquired | 7–22 % of pre-dialysis patients. >90 % 10 years post-dialysis | Any age depending on age of development of ESRD | Flank pain, bleeding, RCC | ||
| Simple renal cysts | Acquired | Very common. Incidence increases with age. M:F 2:1 | Any age. Usually incidental finding | Clinical findings rare especially in children. In adults, pain, bleeding, infection | Clinical findings rare especially in children | |
| Multilocular renal cysts | Acquired | Rare | Any age but often early childhood | Often asymptomatic. Can present with abdominal mass, abdominal pain, or hematuria | Hypertension | |
| Hypokalemic renal cysts | Acquired | Any age | Usually cysts do not cause symptoms | |||
| Glomerulocystic kidney - Genetic and non-genetic forms | ||||||
| GCK in PKD | ADPKD | M:F 1:1. | Any age | As per primary disease | As per primary disease | |
| Hereditary GCKD | ADGCKD and HNF1β mutations | M:F 1:1 | Any age | Abdominal masses, renal insufficiency, flank pain, hematuria | Hypertension | |
| Syndromic GCK | As per syndrome | As per syndrome | Any age | As per syndromes, e.g., X-linked dominant oral-facial-digital syndrome type 1, tuberous sclerosis | ||
| Obstructive GCK | Any age | Associated with renal dysplasia. Urinary tract infections | ||||
| Sporadic GCK | May be a de novo mutation, ischemic, or drug-induced | Any age | Abdominal masses, renal insufficiency, flank pain, hematuria. Described post-hemolytic uremic syndrome [115] | Hypertension | ||
MIM Mendelian inheritance of man; AD autosomal dominant, AR autosomal recessive, RCC renal cell carcinoma
Autosomal recessive polycystic kidney disease (ARPKD)
Autosomal recessive polycystic kidney disease (ARPKD) is caused by mutations in a single gene, PKHD1. The incidence is 1 in 20,000 live births and the gene frequency is 1 in 70. Over 300 mutations have been identified and genotype-phenotype correlations have been described [1]. PKHD1 is located on chromosome 6p12 and encodes the protein fibrocystin/polyductin [2, 3].
Pathophysiology and histopathology
The exact function of fibrocystin is unknown. It is expressed on primary cilia and the cytoplasmic tail contains a ciliary targeting sequence [4, 5]. ARPKD is one of a number of conditions grouped together as ‘ciliopathies’[6]. Cystogenesis is probably caused by aberrant cell proliferation and apoptosis but it is still unclear how this is regulated. Down regulation of PKHD1 induces cell apoptosis [7]. Fibrocystin binds to and functionally interacts with the PKD2 protein, polycystin 2 [8, 9]. A genetic interaction between Pkd1 and Pkhd1 has been reported though no direct interaction was demonstrated between fibrocystin and the PKD1 protein, polycystin 1 [10].
Patients with ARPKD have diffuse dilatation and elongation of the renal collecting ducts. Cysts are usually small (<3 mm) but as their number increases, the kidneys enlarge. At autopsy, there is poor corticomedullary differentiation due to the extension of the collecting ducts from the medulla to cortex. In fetuses and neonates, there is a correlation between genotype and the degree of collecting duct extension and cortical tubule lesions [11].
All patients with ARPKD have congenital hepatic fibrosis (CHF) or Caroli disease. The histological basis of CHF is a ductal plate malformation which consists of portal fibrosis, bile duct proliferation and hypoplasia of portal vein branches leading to portal hypertension [12]. The pathognomonic feature of Caroli disease is the presence of non-obstructive dilated intrahepatic bile ducts.
Clinical presentation
ARPKD affects males and females equally and can affect all racial groups. There is a wide spectrum in the age of presentation which correlates with disease severity. Those with the most severe phenotype present in the neonatal period with oligohydramnios, pulmonary hypoplasia and Potter facies. Although ARPKD has been classically reported in childhood or adolescence, mild or late onset disease can present in adult life, occasionally as isolated CHF [13]. In a single-center retrospective study, the actuarial renal survival of ARPKD children surviving the first month of life was 86 % at 1 year [14]. Similarly, a second retrospective study showed that overall survival 20 years after diagnosis depended largely on the age at presentation, i.e., 36 % (< 1 year), 80 % (1–20 years) and 88 % (>20 year) [13]. In a study of 164 neonatal survivors with ARPKD, the renal survival rates were 86 % at 5 years, 71 % at 10 years, and 42 % at 20 years [15]. Thus apart from severe neonatal presentation, overall survival is surprisingly good.
Individuals may develop urinary concentrating defects and present with polyuria and polydipsia. Recurrent urinary tract infections (UTIs) may occur and hypertension is common, leading to cardiac hypertrophy and congestive cardiac failure in severe cases. Portal hypertension due to congenital hepatic fibrosis leading to splenomegaly and varices can be the presenting feature. Although hepatocellular function is usually preserved, ascending suppurative cholangitis can lead to hepatic failure [12]. Progression to ESRD is common.
Investigations
The diagnosis of ARPKD relies mainly on imaging. Ultrasound can make the diagnosis prenatally by showing bilaterally enlarged echogenic kidneys. In the neonate, ultrasound shows bilateral smooth enlarged kidneys which are diffusely echogenic with poor corticomedullary differentiation. The liver may also show an increase in parenchymal echogenicity. Studies have suggested that the kidney size stays stable or reduces with increasing age with increased echogenicity in the cortex [16]. There may be macrocysts in the liver and pancreas and splenomegaly. Portal hypertension is associated with reversal of hepatic blood flow detectable by Doppler ultrasound.
Several studies have shown a clear genotype-phenotype correlation for ARPKD. In one study, the presence of a single truncating PKHD1 mutation was associated with death in the first month of life whereas the presence of a missense mutation was associated with survival beyond the first month. All patients inheriting two truncating mutations were uniformly associated with perinatal or neonatal death [17].
Mutation testing is now available with an overall detection rate of 77 % [13, 15]. Most mutations are private and numerous polymorphisms have been reported in PKHD1. In European populations, T36M is the most common mutation reported (15–20 %) and could represent a founder mutation [15]. Linkage analysis could be unreliable since other genes may phenocopy ARPKD.
Treatment
ARPKD is not curable. In the neonatal period, mechanical ventilation may be required for pulmonary hypoplasia or respiratory compromise due to enlarged kidneys. Bilateral nephrectomy may be indicated. In anuric individuals, continuous veno-venous hemofiltration (CVVH) or peritoneal dialysis can be used. Hypertension and urinary tract infections should be treated adequately.
Hepatic complications are common. Bacterial cholangitis is serious and must be treated promptly. Esophageal varices can be treated by sclerotherapy, banding or insertion of a portocaval shunt. A final option is partial liver transplantation, sometimes combined with renal transplantation [18].
The mammalian target of rapamycin (mTOR) pathway is activated in ARPKD kidneys suggesting that mTOR might be a potential target to slow cyst development [19]. In animal models of experimental polycystic kidney disease, mTOR inhibition was shown to reduce cyst growth and slow the loss of renal function [20]. (but see later).
Prognosis
Currently it is estimated that 23–30 % of affected individuals die in the first year of life [14, 15]. For those that survive the first year of life, 10-year survival is estimated to be 82 % [15]. Significant intrafamilial variability has been reported in some families indicating the influence of modifying genes [17].
Autosomal dominant polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) is among the most common inherited diseases in man. Its prevalence is 1 in 400 to 1 in 1,000. Of affected families, 85 % have mutations in PKD1 (located on the short arm of chromosome 16) which encodes for Polycystin 1. The rest have mutations in PKD2 (located on the long arm of chromosome 4), which encodes for Polycystin 2. Some studies have proposed a third gene but this has not been identified [21, 22]. Individuals with PKD2 mutations present later and have a slower rate of progression to ESRD than individuals with PKD1 mutations [23]. Transheterozygotes with mutations in both PKD1 and PKD2 have been reported; their clinical course is more severe than for individuals with mutations in simple heterozygotes [24].
Pathophysiology and histopathology
Polycystin 1 and 2 form a functional receptor-channel complex and a defect in either protein leads to renal cyst formation and an overlapping clinical phenotype [25, 26]. Both proteins have overlapping subcellular distribution including in primary cilia [27, 28]. However, polycystin-2 is mainly resident in the endoplasmic reticulum (ER) where it can function as a Ca2+ release channel [25, 26]. Unlike ARPKD, cysts can arise from any part of the nephron. Cysts arise as outpouchings connected to the tubular lumen which eventually disconnect. Over time, they enlarge, destroying normal parenchyma and result in bilateral kidney enlargement.
Clinical presentation
Most patients present between 20–40 years of age but can present in childhood (early onset, under 15 years of age) or even in utero (very early onset, less than 2 years of age). Common renal symptoms include abdominal pain, polyuria, UTIs, hematuria and hypertension. Extra-renal features are rare in children. They include hepatic, pancreatic, ovarian, splenic and intestinal cysts. There is an increased prevalence of mitral valve prolapse, aortic aneurysms and intracranial aneurysms [29].
Investigations
Age-banded ultrasound criteria have been established for the diagnosis of ADPKD in families with a positive family history [30]. Computed tomography (CT) and magnetic resonance imaging (MRI) are more sensitive than ultrasonography in the detection of cysts but have not been formally evaluated against ultrasonography [31]. Genetic testing for PKD1 and PKD2 by direct DNA sequencing are now readily available for predictive and diagnostic testing [32, 33]. In a research setting, positive detection rates of up to 89 % have been reported [34]. Of these, 60–65 % had definite PKD1 mutations, 4 % had large deletions/duplications while 20–26 % were missense changes; in this latter group, it may be difficult to assign pathogenicity in the absence of a functional test. Evolutionary conservation, predicted effects on secondary structure or splicing, the absence of other pathogenic changes and segregation analysis within the pedigree will inform the likelihood whether a particular change is pathogenic (but see later). PKD1 is also highly polymorphic (average ten neutral variants per patient) and this complicates the interpretation of any changes found [34].
Predictive testing is indicated in potential live related kidney donors from ADPKD families where imaging is negative or equivocal in the under-40-year age group [35]. Diagnostic testing is indicated in families with very early onset disease (under 2 years of age) where a very high recurrence rate of (∼45 %) has been reported [36]. This could facilitate pre-implantation genetic diagnosis where indicated.
Some early onset cases may relate to the co-inheritance of an incompletely penetrant or hypomorphic PKD1 allele from the ‘normal’ parent which in combination with an inactivating allele, leads to the severe early onset phenotype [37, 38]. Since the penetrance of a single hypomorphic allele is low, segregation may appear as an apparent ‘de novo’ mutation or recessive (phenocopying ARPKD) inheritance. The prevalence of hypomorphic alleles as disease modifiers in early onset or typical onset disease is not known.
The co-inheritance of germline mutations in other cystic disease genes (e.g., HNF1β, PKHD1) alongside PKD1 or PKD2 alleles may similarly lead to a more severe renal phenotype [39]. The rate of new mutation for PKD1 has been estimated at 4–5 % of cases though the possibility of mosaicism or non-paternity should be considered in the absence of a positive family history [40]. Other diseases that may phenocopy ADPKD if present in the carrier state (OFD-1 females) or if other systemic features are mild (e.g., HNF1b, TSC) should form part of the differential diagnosis.
The screening of asymptomatic at risk children is presently not recommended. However, it has been reported that the prevalence of hypertension (15 %), borderline hypertension (15 %) and microalbuminuria (36 %) in children with ADPKD could be higher than previously thought [41] and associated with increased left ventricular mass in children with borderline hypertension [42]. A reasonable course of action would therefore be to monitor at risk children for the development of hypertension and proteinuria and institute antihypertensive treatment early. In future, screening could be offered at an earlier age once an effective intervention is identified.
Treatment
The mainstay of treatment is controlling hypertension, reducing proteinuria and treating infections. Two recent trials failed to demonstrate an improvement or slower rate of decline of estimated glomerular filtration rate (eGFR) with mTOR inhibition [43, 44]. Several other interventional studies are in progress [45, 46].
Prognosis
Heart disease is the most common cause of death among patients with ADPKD, followed by infection and neurological events [47, 48]. Renal function does not usually start to decline until the fourth decade [49].
Nephronophthisis
Nephronophthisis (NPHP) is an autosomal recessive condition caused by more than 13 genes [50]. It has been reported worldwide with an incidence of nine per 8.3 million in the USA and 1 in 50,000 live births in Canada [51]. Clinically, three forms of NPHP (infantile, juvenile and adolescent) are recognized based on the age of presentation (Table 2). Extra-renal features are present in 10–20 % of cases of NPHP [52]. A number of syndromes with nephronophthisis and prominent extra-renal features associated with mutations in NPHP genes have been described. These include Joubert’s syndrome (cerebellar ataxia), Senior-Loken syndrome (tapetoretinal degeneration) and Cogan’s syndrome (oculomotor apraxia) [53]. In addition, some NPHP genes (NPHP6, 8, 11) can phenocopy the lethal Meckel–Gruber syndrome (occipital encephalocele, polydactyly, enlarged dysplastic kidneys).
Table 2.
Cystoproteins and their putative functions
| Disease | Gene | Protein | Localization | Function |
|---|---|---|---|---|
| Autosomal recessive polycystic kidney disease | PKHD1 | Fibrocystin/ polyductin | 6p21.1-p12 | Exact function unknown. Expressed in apical membrane, primary cilia, and centrosomes. Interacts with polycystin-2 |
| Autosomal dominant polycystic kidney disease | PKD1 | Polycystin-1 | 16p13.3-p13.12 | Mechanosensitive or ligand-activated receptor; functionally complexed with polycystin-2. Expressed in basolateral plasma membrane, cilia, centrosomes |
| PKD2 | Polycystin-2 | 4q21-q23 | A non-selective Ca2+ channel. Functionally complexed with polycystin-1. Expressed in ER, basolateral plasma membrane, cilia, centrosomes | |
| Nephronophthisis infantile, juvenile/adolescent (I/J/A) | NPHP1/SLSN1 (J) | Nephrocystin 1 | 2q13 | Involved in primary cilia function and the cell cycle. Some localize to cell–cell junctions (1, 2, 4, 10) or focal adhesions (1) |
| NPHP2/INVS (I) | Inversin | 9q31.1 | ||
| NPHP3/SLSN3 (I,J,A) | Nephrocystin 3 | 3q22.1 | ||
| NPHP4/SLSN4 (J) | Nephrocystin 4 | 1p36.31 | ||
| NPHP5/IQCB1/SLSN5 (J) | Nephrocystin 5 | 3q13.33 | ||
| NPHP6/CEP290/LCA10/SLSN6/JBTS5/MKS4 (J) | Nephrocystin 6 | 12q21.32 | ||
| NPHP7/GLIS2 (J) | GLIS 2 protein | 16p13.3 | ||
| NPHP8/RPGRIP1L/JBTS7/MKS5 (I,J) | Nephrocystin 8 | 16q12.2 | ||
| NPHP9/NEK8 (I/J) | Nephrocystin 9 | 17q11.2 | ||
| NPHP10/SLSN7/SDCCAG8 (J) | Centrosome Colon Cancer Autoantigen Protein (CCCAP) | 1q43 | ||
| NPHP11/MKS3/TMEM67 (J) | Meckelin | 8q22.1 | ||
| NPHP12/TTC21B/JBTS11 (I,J) | IFT139 | 2q24.3 | ||
| NPHP13/WDR19 (A) | IFT144 | 4p14 | ||
| Medullary cystic kidney disease | MCKD1 | 1q21 | Not known | |
| MCKD2 (UMOD) | Uromodulin | 16p12 | Possible role in maintaining integrity of thick ascending loop of Henle. Localization to cilia and centrosomes | |
| HNF1β/TCF2 range | HNF1β | HNF1β protein or Transcription Factor 2 (TCF2) | 17q12 | Regulates the transcription of several key cystic genes |
| Von Hippel–Lindau disease | VHL | VHL protein | 3p26-p25 | Tumor suppressor gene acting through HIF and non-HIF-dependent pathways. Localization to cilia and role in cilia length control [116] |
| Tuberous sclerosis complex | TSC1 | Hamartin | 9q34 | Complexes with tuberin to inhibit mammalian target of mTOR [117] |
| TSC2 | Tuberin | 16p13.3 | Complexes with hamartin. Localizes to primary cilia and regulates cilia length [118] |
I infantile, J juvenile, A adolescent, ER endoplasmic reticulum
Pathophysiology and histopathology
On ultrasound, patients with NPHP may have normal or reduced kidney size, corticomedullary cysts or loss of corticomedullary differentiation. On renal biopsy, tubular atrophy, interstitial fibrosis and changes in the tubular basement membranes (TBM) are prominent features. The TBM is irregularly thickened and often there is an abrupt transition between a thick segment and a normal or disintegrated one [54]. In infantile NPHP, there are typically no TBM changes; the features are those of moderate renal enlargement with cortical microcysts [55].
A total of 13 recessive genes (NPHP1-13) have been linked to the NPHP phenotype, some in single families; these account for less than 30 % of all patients indicating that the majority of NPHP genes are yet to be discovered [56]. In a world-wide cohort of 365 NPHP families, NPHP1 mutations were the most frequent and accounted for 64 % of all identified alleles [50]. The identification of these genes has led to the recognition that mutations in some ‘NPHP’ genes (e.g., NPHP 6, 8, 11, 12) can give rise to both mild (NPHP), intermediate (Joubert’s syndrome, JBTS) and severe (Meckel–Gruber syndrome, MKS) phenotypes. The genetic mechanisms determining phenotype are complex with evidence of gene locus heterogeneity, allelic effects (NPHP6) and modifier genes, all of which interact to determine disease severity and the extent of extrarenal involvement [50].
A common finding in NPHP proteins is their localization to primary cilia or centrosomes [6]. Mutations in these genes therefore impair ciliary function both in renal tubular epithelial cells and in other organs [52]. Some NPHP proteins (NPHP1, 2, 4, 10) also localize to cell–cell junctions or focal adhesions (NPHP1) where they regulate junction integrity and polarity [57–59]. In some cells, distinct ciliary sub-compartment localization has been reported for different NPHP proteins [60]. Of interest, NPHP1 has also been reported to bind polycystin-1 and may regulate apoptosis [61].
The NPHP proteins do not share a high degree of sequence homology but rather form interacting protein complexes which function in distinct pathways or subcellular compartments. A recent study has shown that the NPHP proteins co-purify in at least three separate protein complexes with distinct subcellular locations and functions [62]. NPHP1, 4 and 8 are present in the transition zone of primary cilia and regulate apical junction formation, NPHP 5 and 6 are located at the centrosomes and regulate ciliogenesis whereas the MKS proteins, MKS1 and MKS6 regulate hedgehog signaling. NPHP2/INVS and the JBTS3/AHI1 protein, Jouberin act as molecular bridges between the three modules. These findings provide a molecular link between NPHP, JBTS and MKS and an explanation for the overlapping spectrum seen in these diseases. However, the proteins that make up the transition zone could vary between cell types and organisms [60, 63–65].
Clinical presentation
Juvenile nephronophthisis (ESRD ≥ 4 years) is the most common form of NPHP and the usual age at which NPHP presents (except for NPHP2/INVS). The first symptoms usually develop at 4–6 years of age. Reduced urinary concentrating capacity and increased urinary sodium loss leads to polyuria, polydipsia, enuresis and dehydration. Chronic dehydration and eventually renal insufficiency causes growth retardation and failure to thrive [52]. Due to the mild nature of the initial symptoms, there can often be a delay in diagnosis. As there is salt wasting, blood pressure does not tend to be raised until individuals develop renal failure. A normochromic, normocytic anemia is common and may occur prior to renal insufficiency [66]. ESRD occurs at a mean age of 13 years [67].
The adolescent form was described after identification of the NPHP3 gene in a large Venezuelan family. ESRD occurred at a mean age of 19 [68]. However, the histological features are very similar to the juvenile form and often they are regarded as part of the same disease. The age of ESRD in NPHP3 appears more variable than other forms of NPHP, ranging between 3 to 13 years.
Infantile NPHP (ESRD ≤ 4 years) is usually caused by mutations in NPHP2/INVS although patients with other NPHP mutations can present with infantile ESRD [50, 69]. Situs inversus and ventricular septal defects are commonly associated with NPHP2 [52].
Investigations
Urinalysis shows low specific gravity in early morning samples. Careful ophthalmoscopy should be performed and an MRI to look at the cerebellum if neurological symptoms are present [53].
Genetic testing is possible in many cases, negating the need for renal biopsy. Screening for gene mutations can be guided by the age of presentation and the presence or absence of specific extra-renal features [50, 52]. Asymptomatic siblings can be screened for urinary concentrating defects and abnormalities on USS rather than undergoing genetic testing.
Treatment
There is no specific treatment. NPHP does not recur in the transplanted kidney. Live related heterozygous carriers can donate.
Prognosis
The prognosis depends on the age of presentation and whether there are extra-renal features.
Medullary cystic kidney disease
Medullary cystic kidney disease (MCKD) is a rare autosomal dominant condition leading to ESRD. It generally presents in adulthood but can occur in children [70]. It was once thought to be the same disease entity as juvenile NPHP due to the overlapping phenotypes [71]. The condition is caused by mutations in several genes [72, 73]. The MCKD1 locus has been mapped to chromosome 1q21 but the gene has yet to be identified. The MCKD2 locus on chromosome 16p12 has been identified as the UMOD gene [73].
Recently, it was proposed that MCKD be reclassified as one of four types of autosomal dominant interstitial kidney disease (ADIKD) based on the underlying gene mutation: (1) UMOD: mutations in UMOD are the most common and can present as MCKD2, familial juvenile hyperuricemic nephropathy (FJHN), uromodulin-associated kidney disease (UAKD) and glomerulocystic kidney disease (GCKD); (2) REN: mutations in REN, which encodes renin, have been identified in a few ADIKD families [74]; (3) Chromosome 1q21: also known as MCKD1; (4) Unidentified genes: some families without UMOD or REN mutations and unlinked to chromosome 1 have been reported [75]. This classification does not require the presence of cysts for diagnosis and reflects the highly variable cystic phenotype in ADIKD which is most prominently expressed as MCKD.
Pathophysiology and histopathology
MCKD1
Renal biopsy shows tubular atrophy, interstitial fibrosis, lymphocytic infiltration, splitting or lamellation of the thickened and irregular tubular basement membranes [76]. The glomerular basement membranes are usually unaffected though glomerular sclerosis can occur.
MCKD2
The UMOD gene on chromosome 16p12 encodes for uromodulin, the most abundant protein found in normal human urine and which is expressed specifically in the thick ascending loop of Henle. Its functions are not well understood but it is thought to maintain the watertight properties of the thick ascending loop of Henle and/or protect against UTIs [77, 78]. Mutant uromodulin accumulates in the endoplasmic reticulum leading to tubular cell atrophy and death [79]. One study has reported uromodulin expression in primary cilia and centrosomes [80]. UMOD mutations are associated with hyperuricemia and early onset gout: FJHN is allelic to MCKD2. On biopsy, diffuse tubulointerstitial fibrosis is prominent with occasional tubular dilatations or cysts [81].
Clinical presentation
MCKD1
The clinical picture of MCKD1 is that of a slowly progressive chronic kidney disease. There is minimal proteinuria and hematuria. Hypertension becomes more common as the kidney disease progresses, as does hyperuricemia [76]. The course of the disease is variable with ESRD occurring between 25 and 55 years of age [76, 82].
MCKD2
Hyperuricemia is characteristic of MCKD2. In females, this is often asymptomatic but commonly presents as gout in teenage males [83]. As with MCKD1, progressive renal impairment occurs. Hypertension can occur but proteinuria is minimal. Mild concentrating defects can also occur [84].
Investigations
MCKD1
The absence of significant proteinuria in a patient with renal impairment and a strong family history should be enough to consider MCKD. Cysts are sometimes seen on ultrasound but are not essential for the diagnosis [76]. Renal biopsy can be performed but the findings are non-specific [85]. A definitive diagnosis can be made through linkage analysis if there is linkage to chromosome 1q21.
MCKD2
As with MCKD1, diagnosis often relies on clinical suspicion. Patients with MCKD 2 often have hyperuricemia. Medullary cysts may be seen on ultrasound. The biopsy findings are non-specific. Mutational analysis of the UMOD gene can confirm the diagnosis.
Treatment
MCKD1
Treatment is mainly supportive. The disease does not recur in the transplanted kidney but family members need to be screened if they come forward as live donors.
MCKD2
The management of MCKD2 is the same as MCKD1 except for the possible benefits of allopurinol. Allopurinol can be used to prevent gout but there is conflicting evidence as to whether early allopurinol therapy can also prevent progression to ESRD [81, 83, 86].
Prognosis
The age of progression to ESRD varies between 20 and 70 years of age.
Hepatocyte nuclear factor 1-beta (HNF1β) mutations
Mutations of HNF1β were first identified as a rare cause of maturity-onset diabetes of the young type 5 (MODY5) [87]. It is now recognized that HNF1β mutations are responsible for a range of different renal abnormalities including cystic diseases. The HNF1β gene is located on chromosome 17q12.
Pathophysiology and histopathology
HNF1β is a member of the homeodomain-containing superfamily of transcription factors. It encodes the transcription factor 2 (TCF2) and functions as a homodimer or heterodimer with HNF1α [88]. HNF1β appears to be a master regulator controlling the transcription of multiple cystic genes including PKHD1, PKD2, UMOD and Tg737/polaris [89].
The pleiotropic features of HNF1β mutations can be explained by its role in the development of pancreas, kidney, liver, lung and gut and expression in the neural tube and genital tract [90, 91]. In the embryonic kidney, HNF1β is expressed in the ureteric bud, the comma and s-shaped bodies and in the proximal and distal tubules [90] Although it is not expressed in glomeruli, glomerulocystic kidney disease has been associated with HNF1β mutations (see later). It has been suggested that the formation of glomerular cysts could result from transient obstruction of immature nephrons [92].
Several studies have not found a clear genotype-phenotype correlation between the type of mutation and the severity and/or type of renal disease. There is strong intrafamilial variability suggesting that non-allelic or environmental factors must be important [88, 93]. Mutations may involve heterozygous deletion of the whole gene or small mutations including missense, nonsense, frameshift and splice site mutations. In the studies to date, de novo mutations accounted for a third to half of all cases [87, 93].
Clinical phenotypes
Renal cysts are present in most HNF1β carriers, including some with glomerulocystic kidney disease (GCKD). Renal malformations are also common e.g., single and horseshoe kidneys [88, 93]. HNF1β mutations have been identified in all cases of familial hypoplastic glomerulocystic kidney disease examined so far. This condition is inherited in an autosomal dominant fashion [92]. Not all individuals with HNF1β mutations have diabetes but renal cysts and diabetes (RCAD) is a common phenotype [93]. Prenatally, bilateral hyperechogenic kidneys of normal or moderately enlarged size is the most frequent finding. Other prenatal phenotypes include bilateral multicystic kidney disease (MCD), unilateral MCD, unilateral renal agenesis, unilateral renal hypoplasia, renal macrocysts and isolated upper urinary tract dilation. All patients with unilateral MCD developed postnatal abnormalities in the contralateral kidney [88]. The degree of renal impairment varies widely with some cases of ESRD presenting in childhood or prenatally but other adult cases with normal renal function.
Other features of HNF1β mutations include early onset gout and/or hyperuricemia, genital tract malformations, pancreatic atrophy, abnormal liver function tests and hypomagnesaemia [88, 92]. The underlying pathogenesis of hyperuricemia in some patients is unknown though rarely, it may phenocopy FJHN (see above) [94]. In a seminal study, hypomagnesemia was found in 44 % of children with HNF1β mutations [95] and was associated with urinary magnesium wasting and hypocalciuria in a subset of patients. The likely pathogenesis of this abnormality is a defect in HNF1β mediated transcription of FXYD2, which encodes the γ subunit of Na+-K+-ATPase; mutations in FYXD2 are associated with autosomal dominant hypomagnesemia and hypocalciuria [96].
Investigations
HNF1β mutations should be considered in all individuals with an unidentified cause of cystic kidney disease. Since a significant proportion of mutations are de novo, the absence of a family history should not preclude genetic testing.
Treatment
The treatment is dependent on the degree of renal impairment and the presence of other features such as diabetes.
Prognosis
The prognosis varies widely and is difficult to predict. Genetic counseling is also challenging due to the lack of phenotype-genotype correlation.
Glomerulocystic kidney
The term glomerulocystic kidney (GCK) refers to a kidney with more than 5 % cystic glomeruli [97]. Lennerz proposed a useful classification system and suggested that the term glomerulocystic kidney disease (GCKD) is used for the familial subtypes only [98].
-
Type I:
GCK in PKD
Early onset ADPKD sometimes presents as GCK.
-
Type II:
Hereditary GCK (GCKD)
GCKD includes autosomal dominant GCKD (ADGCKD) due to UMOD mutations, familial hypoplastic GCKD due to HNF1β mutations and GCKD due to mutations that have not yet been classified.
-
Type III:
Syndromic GCK
Syndromic GCK is when GCK occurs as part of a known syndrome without dysplasia, the most common being tuberous sclerosis. Other examples include Zellweger cerebrohepatorenal syndrome, von Hippel Lindau disease and X-linked dominant oral-facial-digital syndrome type 1 (OFD-1).
-
Type IV:
Obstructive GCK
Obstructive GCK is GCK associated with renal dysplasia or urinary obstruction without dysplasia and no evidence of a heritable condition. It is a common cause of GCK.
-
Type V:
Sporadic GCK
Included in this category are ischemic GCK and drug-induced GCK. The ischemic causes are often due to systemic sclerosis or hemolytic uremic syndrome. Lithium is a recognized cause of drug-induced GCK.
Pathophysiology and histopathology
Glomerular cysts are defined as Bowman space dilatation of more than 2 to 3 times the normal size [97]. Glomerular tufts help to distinguish glomerular cysts from cysts of tubular origin but may be absent if the tufts degenerate [98]. The pathogenesis of GCK is unknown although a number of theories have been proposed [98]. There is some evidence that primary cilia dysfunction may be involved [99].
Clinical presentation
The presentation of GCK varies. Infants may present with an abdominal mass or renal insufficiency. In later childhood or in adulthood, the presentation may be with hematuria, hypertension or abdominal pain. In cases of syndromic GCK, patients are likely to present with other features. UTIs may precede diagnosis of GCK in obstructive causes.
Investigations
GCK is not easy to diagnose by ultrasound in the fetus or neonate. It is difficult to distinguish GCK from other cystic renal conditions [100]. In adults, glomerular cysts are often missed by ultrasound or CT. MRI is much more reliable [101]. Renal biopsy may be necessary to distinguish GCK from tubular cystic disease. Genetic testing is possible for certain subtypes of GCK.
Treatment
The treatment will depend on the underlying cause of GCK. Most treatments will be symptomatic.
Prognosis
The prognosis is variable and depends on the underlying cause of GCK and the rate of decline in renal function.
The ciliary hypothesis and cystic disease
Primary cilia as sensory organelles
Primary cilia are hair-like organelles expressed by almost every cell and tissue in the body [102]. Unlike motile cilia, primary cilia are generally non-motile though they are sensitive to bending or flow [103]. Sensory cilia involved in vision and smell are modified primary cilia showing that they can sense visual and olfactory stimuli.
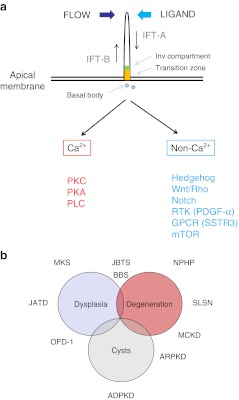
In non-dividing polarized epithelial cells, cilia extend as apical structures consisting of a microtubule core enveloped by a membrane lipid bilayer. Ultrastructurally, all cilia can be classified as ‘9 + 2’ (motile) or ‘9 + 0’ (primary) depending on the presence or absence of a central pair of microtubules. Prior to entering mitosis, cilia are resorbed and both centrosomes form the mitotic spindle poles. As cells become quiescent, cilia reform. More recently, ciliary sub-compartments have been described associated with specific locations of different proteins (Fig. 1a).
Fig. 1.
a Cilia structure and signaling. Primary cilia extend as apical structures with a microtubular core in polarized kidney epithelial cells. They are assembled, disassembled and maintained by a process called intraflagellar transport (IFT). Complex B proteins undergo anterograde transport and complex A undergo retrograde transport. The transition zone (orange) and inversin (Inv, green) ciliary sub-compartments are shown. Cilia signaling can be activated by mechanical bending, flow or ligand binding to a variety of membrane receptors expressed in the ciliary membrane. Cilia signaling can be usefully divided into Ca2+ or non- Ca2+ mediated pathways. Apart from stimulating Ca2+ release from intracellular stores, Ca2+ increases could activate protein kinase C (PKC), protein kinase A (PKA) and phospholipase C (PLC) signaling. Non- Ca2+ signaling could involve multiple receptors including developmental pathways such as Hedgehog, Wnt and Notch, as well as those regulating cell division or cell size such as PDGF-α (platelet-derived growth factor- alpha), SSTR3 (somatostatin receptor 3) or mTOR (mammalian target of rapamycin). b Disease spectrum of the ciliopathies. The phenotypic spectrum of the ciliopathies may overlap and ranges from organ dysplasia, degeneration or fibrosis to cystic change. A number of the common ciliopathies and their typical phenotypes are shown on the diagram
Cilia, flagella and intraflagellar transport
Evolutionarily, cilia are structurally related to (motile) flagella expressed by single-cell organisms such as the green algae, Chlamydomonas reinhardtii. Remarkably, proteins involved in the formation and maintenance of flagella structure, a microtubule-dependent process called intraflagellar transport (IFT) are highly conserved throughout evolution, being also expressed in mammalian cilia [27]. IFT is essential for the assembly, disassembly and maintenance of cilia. In brief, an IFT-B complex (at least 15 proteins) is transported from the cilia base to the cilia tip through the action of the microtubular motor protein kinesin. An IFT-A complex (6 proteins) is transported from the ciliary tip to the base through the action of dynein (Fig. 1a).
Mutations in human IFT proteins have been associated with several skeletal dysplasia syndromes such as Sensenbrenner syndrome (cranioectodermal dysplasia, MIM 218330), Jeune syndrome (asphyxiating thoracic dysplasia, MIM 611263) and short-rib polydactyly (MIM263510). Mutations in two IFT-A proteins (IFT139, 144) syndrome have been reported with in patients with isolated nephronophthisis (NPHP12, 13) indicating a phenotypic overlap between Sensenbrenner syndrome and NPHP.
Cilia, centrosomes and the cell cycle
The link between cilia and cystic disease came from several co-incidental findings [28]. Since these seminal discoveries, many more proteins associated with a cystic kidney phenotype have been localized to primary cilia and their associated structures, the centrosomes. This has led to the formulation of the ‘ciliary hypothesis’ as a unifying pathogenic mechanism for cyst formation [27]. A link to cell-cycle control is also implied by the fact that the mother centriole (or basal body) enucleates the primary cilium (present in non-dividing cells) and that both centrioles also form the mitotic spindle poles (in dividing cells). Some cysto-proteins remain localized at the spindle poles during mitosis. These cilia-associated diseases have been termed ‘ciliopathies’ [6].
Cilia signaling in extrarenal tissues
Abnormalities in cilia structure or function could explain some of the unusual extrarenal features reported in some of these diseases such as situs inversus, retinitis pigmentosa, skeletal or digital abnormalities and central nervous system (CNS) malformations.
Left-right patterning of the body axis is determined by the function of nodal cilia which are motile and transmit an asymmetric Ca2+ signal. The findings of situs inversus in Pkd2 null mice [104] and in several other ciliopathy mutants (e.g., nphp2/invs) indicated that a more general abnormality in cilia function could explain the systemic features of the ciliopathies. In the retina, the connecting cilium represents a modified transition zone which controls the transport of rhodopsin containing vesicles between the inner and outer photoreceptor segments. The finding of retinitis pigmentosa in many ciliopathies correlates with their localization to this structure. Finally, a clear link between hedgehog signaling, a key developmental signaling pathway, and primary cilia signaling has been shown in mouse models [105]. Abnormalities in hedgehog signaling probably underlie the digital and CNS abnormalities seen in some of the ciliopathies. Other signaling pathways that have been linked to primary cilia are the Wingless (Wnt) and Notch pathways, cAMP/PKA, PDGF-α, somatostatin receptor 3 (SSTR3) and mTOR kinase (Fig. 1a). Although these are not associated with discrete phenotypes and may vary between tissues, they do indicate that a variety of ligand-gated receptors can signal through cilia.
Cilia signaling in the kidney
How abnormalities in cilia or centrosome function lead to renal cysts is still unclear. Apart from intercalated cells, it is thought that all renal cells throughout the nephron express primary cilia. Motile (9 + 2) cilia are expressed in the pronephros of lower vertebrates and have been occasionally detected in human kidney [106, 107]. In the latter, they could represent a recapitulation to an embryonic phenotype or a dedifferentiation phenotype to injury.
Planar cell polarity (PCP) and oriented cell division (OCD) are two key processes that are critical for maintaining tubular diameter during tubular elongation in development or repair following injury. Renal epithelial cell cilia are sensitive to flow [103]. Defects in OCD have been reported in several cystic mouse mutants linked to cilia structure (Kif3a) or function (HNF1b) [108, 109]. The non-canonical Wnt pathway is likely to be important in PCP regulation in the kidney [110] and the switch between the two limbs of Wnt signaling mediated through primary cilia flow sensing [111]. Pkd1 mutant tubular cells have defects in cilia-mediated flow-induced Ca2+ signaling [112]. There is conflicting evidence as to whether abnormalities in OCD or PCP are critical to the onset of cyst formation in ADPKD or ARPKD [113, 114].
Non-ciliary mutants and renal cysts
It is helpful to consider that in the vast majority of the ciliopathies, renal cysts are rare unless associated with organ dysplasia (Meckel’s) or of late-onset when associated with degeneration (NPHP). These phenotypes appear distinct from those of more ‘classical’ cystic diseases such as ADPKD and ARPKD, even though these are included in the ciliopathies. This could mean either that the ADPKD or ARPKD proteins serve more distinct ciliary functions (from the other ciliopathy proteins) or that their non-ciliary functions may be more important in preventing cyst formation. In this regard, it is worth noting that polycystin proteins are also prominently expressed at the basolateral membrane in epithelial cells (where they may regulate cell adhesion) and that polycystin-2 can also function as an ER located Ca2+ release channel [25]. We cannot easily differentiate the relative importance of ciliary and non-ciliary functions of the two proteins at present. Similarly, several of the NPHP genes (NPHP1, 2, 4, 10) are clearly expressed at the basolateral epithelial membrane where they could regulate epithelial morphogenesis independent of their cilia function.
Other cystogenic proteins that have exclusive non-ciliary locations are the ER proteins, Sec63 (MIM 608648) and PRKCSH (MIM 177060), which are associated with autosomal dominant polycystic liver disease (ADPLD), the mitochondrial protein XPNPEP3 (MIM 613553) which is associated with a nephronophthisis-like phenotype (NPHP-L1) with occasional cysts, the basement membrane proteins, Laminin α5 (LAMA5, MIM 608648) and collagen IVα1 (COL4A1, MIM 601033) which when mutated, can be associated with renal cysts [6].
These observations suggest two possibilities. First, normal ciliary structure or function is essential in the pathogenesis of all cystic diseases and that even ‘non-ciliary’ cystogenic proteins may somehow interfere with the processing, trafficking or function of key ciliary mediators. An alternative explanation is that there are both ciliary and non-ciliary pathways involved in the pathogenesis of cysts.
Conclusions
Mutations in genes that affect ciliary structure or function are increasingly recognized as an important group of diseases (the ciliopathies). Nonetheless, they are highly heterogenous and it may be helpful to think of a phenotypic spectrum ranging from dysplasia to degeneration or cystic change (Fig. 1b). The occasional phenotypic overlap between NPHP, JBTS and MKS illustrates this well. The mechanistic link between cilia dysfunction and cystic transformation in the kidney remains to be fully elucidated.
Key summary points
Cystic kidney disease can be genetic, developmental or acquired.
Many genetic cystic diseases have been termed ciliopathies due to the colocalization of multiple cystoproteins in primary cilia.
The age at presentation and clinical features are wide-ranging.
Many cystic kidney diseases have extra-renal features or form part of a syndrome.
The mainstay of treatment at present is the management of the complications of renal impairment.
Key research points
Two negative trials of mTOR inhibitors in ADPKD were recently reported.
Other interventional studies are looking at V2 receptor antagonists, somatostatin, renin-angiotensin system blockade and statin use in slowing cyst development in ADPKD.
The function of the primary cilium and its dysfunction in disease remains a focus of intense research investigation.
Questions (answers are provided following the reference list)
- The PKHD1 gene found on chromosome 6p21 encodes for
- Polycystin
- Fibrocystin
- Inversin
- Nephrocystin 1
- Neurofibromin
- Hepatic involvement is associated with
- HNF1β mutations
- ARPKD
- Nephronophthisis
- Medullary sponge kidney
- All of the above
- Individuals with juvenile nephronophthisis
- Generally have enlarged kidneys
- Have glomerular cysts
- Usually have tubular cell hypertrophy on renal biopsy
- Usually have an irregularly thickened tubular basement membrane on biopsy
- Rarely present before 10 years of age
- The following condition is never inherited in an autosomal dominant fashion
- Tuberous sclerosis complex
- Medullary cystic kidney disease
- Von Hippel–Lindau disease
- Nephronophthisis
- Hereditary glomerulocystic kidney disease
- The HNF1β gene is located on
- Chromosome 13
- Chromosome 14
- Chromosome 15
- Chromosome 16
- Chromosome 17
- Multicystic dysplastic kidney
- More commonly affects the left kidney
- Is more common in girls
- Is usually bilateral
- Is rarely detected ante-natally
- Is usually diagnosed by biopsy
- Familial hypoplastic glomerulocystic kidney disease is associated with mutations of
- MCKD2
- HNF1β
- TSC1
- TSC2
- NPHP1
- Early onset gout is a feature of
- ADPKD
- ARPKD
- Nephronophthisis
- Medullary cystic kidney disease
- Acquired cystic kidney disease
- The incidence of autosomal recessive polycystic kidney disease is approximately
- 1 in 5,000 live births
- 1 in 20,000 live births
- 1 in 40,000 live births
- 1 in 80,000 live births
- 1 in 100,000 live births
- Polyuria and polydipsia are often present in
- Autosomal recessive polycystic kidney disease
- Juvenile nephronophthisis
- Medullary sponge kidney
- a, b, and c
- None of the above
Footnotes
Answers
1.b
2.e
3.d
4.d
5.e
6.a
7.b
8.d
9.b
10. d
References
- 1.Bergmann C, Senderek J, Kupper F, Schneider F, Dornia C, Windelen E, Eggermann T, Rudnik-Schoneborn S, Kirfel J, Furu L, Onuchic LF, Rossetti S, Harris PC, Somlo S, Guay-Woodford L, Germino GG, Moser M, Buttner R, Zerres K. PKHD1 mutations in autosomal recessive polycystic kidney disease (ARPKD) Hum Mutat. 2004;23:453–463. doi: 10.1002/humu.20029. [DOI] [PubMed] [Google Scholar]
- 2.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 3.Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 5.Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2010;188:21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrandt F, Benzing T, Katsanis N (2011) Ciliopathies. N Engl J Med 364:1533–1543 [DOI] [PMC free article] [PubMed]
- 7.Sun L, Wang S, Hu C, Zhang X. Down-regulation of PKHD1 induces cell apoptosis through PI3K and NF-kappaB pathways. Ex Cell Res. 2011;317:932–940. doi: 10.1016/j.yexcr.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Kim I, Fu Y, Hui K, Moeckel G, Mai W, Li C, Liang D, Zhao P, Ma J, Chen XZ, George AL, Jr, Coffey RJ, Feng ZP, Wu G. Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol. 2008;19:455–468. doi: 10.1681/ASN.2007070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Gonzalez MA, Menezes LF, Piontek KB, Kaimori J, Huso DL, Watnick T, Onuchic LF, Guay-Woodford LM, Germino GG. Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet. 2007;16:1940–1950. doi: 10.1093/hmg/ddm141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denamur E, Delezoide AL, Alberti C, Bourillon A, Gubler MC, Bouvier R, Pascaud O, Elion J, Grandchamp B, Michel-Calemard L, Missy P, Zaccaria I, Nagard H, Gerard B, Loirat C, Barbet J, Beaufrere AM, Berchel C, Bessieres B, Boudjemaa S, Buenerd A, Carles D, Clemenson A, Dechelotte P, Devisme L, Dijoud F, Esperandieu O, Fallet C, Gonzales M, Hillion Y, Jacob B, Joubert M, Kermanach P, Lallemand A, Laquerriere A, Laurent N, Liprandi A, Loeuillet L, Loget P, Martinovic J, Menez F, Narcy F, Roux JJ, Rouleau-Dubois C, Sinico M, Tantau J, Wann AR. Genotype-phenotype correlations in fetuses and neonates with autosomal recessive polycystic kidney disease. Kidney Int. 2010;77:350–358. doi: 10.1038/ki.2009.440. [DOI] [PubMed] [Google Scholar]
- 12.Gunay-Aygun M. Liver and kidney disease in ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:296–306. doi: 10.1002/ajmg.c.30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adeva M, El-Youssef M, Rossetti S, Kamath PS, Kubly V, Consugar MB, Milliner DM, King BF, Torres VE, Harris PC. Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD) Medicine. 2006;85:1–21. doi: 10.1097/01.md.0000200165.90373.9a. [DOI] [PubMed] [Google Scholar]
- 14.Roy S, Dillon MJ, Trompeter RS, Barratt TM. Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatr Nephrol. 1997;11:302–306. doi: 10.1007/s004670050281. [DOI] [PubMed] [Google Scholar]
- 15.Bergmann C, Senderek J, Windelen E, Kupper F, Middeldorf I, Schneider F, Dornia C, Rudnik-Schoneborn S, Konrad M, Schmitt CP, Seeman T, Neuhaus TJ, Vester U, Kirfel J, Buttner R, Zerres K. Clinical consequences of PKHD1 mutations in 164 patients with autosomal-recessive polycystic kidney disease (ARPKD) Kidney Int. 2005;67:829–848. doi: 10.1111/j.1523-1755.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 16.Blickman JG, Bramson RT, Herrin JT. Autosomal recessive polycystic kidney disease: long-term sonographic findings in patients surviving the neonatal period. Am J Roentgenol. 1995;164:1247–1250. doi: 10.2214/ajr.164.5.7717240. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann C, Senderek J, Sedlacek B, Pegiazoglou I, Puglia P, Eggermann T, Rudnik-Schoneborn S, Furu L, Onuchic LF, Baca M, Germino GG, Guay-Woodford L, Somlo S, Moser M, Buttner R, Zerres K. Spectrum of mutations in the gene for autosomal recessive polycystic kidney disease (ARPKD/PKHD1) J Am Soc Nephrol. 2003;14:76–89. doi: 10.1097/01.ASN.0000039578.55705.6E. [DOI] [PubMed] [Google Scholar]
- 18.Dell KM, Avner ED (2011) GeneReviews. Polycystic kidney disease, autosomal recessive. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, (eds) http://www.ncbi.nlm.nih.gov/books/NBK1326/
- 19.Becker JU, Saez AO, Zerres K, Witzke O, Hoyer PF, Schmid KW, Kribben A, Bergmann C, Nurnberger J. The mTOR pathway is activated in human autosomal-recessive polycystic kidney disease. Kidney Blood Press Res. 2010;33:129–138. doi: 10.1159/000314380. [DOI] [PubMed] [Google Scholar]
- 20.Wuthrich RP, Kistler AD, Serra AL. Impact of mammalian target of rapamycin inhibition on autosomal-dominant polycystic kidney disease. Transplant Proc. 2010;42:S44–S46. doi: 10.1016/j.transproceed.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Pei ADPY. Is there a third gene for autosomal dominant polycystic kidney disease? Kidney Intl. 1998;54:1759–1761. doi: 10.1046/j.1523-1755.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- 22.Turco AE, Clementi M, Rossetti S, Tenconi R, Pignatti PF. An Italian family with autosomal dominant polycystic kidney disease unlinked to either the PKD1 or PKD2 gene. Am J Kidney Dis. 1996;28:759–761. doi: 10.1016/S0272-6386(96)90261-9. [DOI] [PubMed] [Google Scholar]
- 23.Hateboer N, Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353:103–107. doi: 10.1016/S0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 24.Pei Y, Paterson AD, Wang KR, He N, Hefferton D, Watnick T, Germino GG, Parfrey P, Somlo S, St George-Hyslop P. Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet. 2001;68:355–363. doi: 10.1086/318188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giamarchi A, Feng S, Rodat-Despoix L, Xu Y, Bubenshchikova E, Newby LJ, Hao J, Gaudioso C, Crest M, Lupas AN, Honore E, Williamson MP, Obara T, Ong AC, Delmas P. A polycystin-2 (TRPP2) dimerization domain essential for the function of heteromeric polycystin complexes. EMBO J. 2010;29:1176–1191. doi: 10.1038/emboj.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ, Ong AC. Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J Biol Chem. 2002;277:20763–20773. doi: 10.1074/jbc.M107788200. [DOI] [PubMed] [Google Scholar]
- 27.Pazour GJ. Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol. 2004;15:2528–2536. doi: 10.1097/01.ASN.0000141055.57643.E0. [DOI] [PubMed] [Google Scholar]
- 28.Ong AC, Wheatley DN. Polycystic kidney disease–the ciliary connection. Lancet. 2003;361:774–776. doi: 10.1016/S0140-6736(03)12662-1. [DOI] [PubMed] [Google Scholar]
- 29.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 30.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zand MS, Strang J, Dumlao M, Rubens D, Erturk E, Bronsther O. Screening a living kidney donor for polycystic kidney disease using heavily T2-weighted MRI. Am J Kidney Dis. 2001;37:612–619. doi: 10.1053/ajkd.2001.22089. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X, Paterson AD, Zahirieh A, He N, Wang K, Pei Y. Molecular diagnostics in autosomal dominant polycystic kidney disease: utility and limitations. Clin J Am Soc Nephrol : CJASN. 2008;3:146–152. doi: 10.2215/CJN.03430807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pei Y. Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2011;118:c19–c30. doi: 10.1159/000320887. [DOI] [PubMed] [Google Scholar]
- 34.Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, Zhang QJ, Thompson PA, Miller JP, Harris PC. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 35.Huang E, Samaniego-Picota M, McCune T, Melancon JK, Montgomery RA, Ugarte R, Kraus E, Womer K, Rabb H, Watnick T. DNA testing for live kidney donors at risk for autosomal dominant polycystic kidney disease. Transplantation. 2009;87:133–137. doi: 10.1097/TP.0b013e318191e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zerres K, Rudnik-Schoneborn S, Deget F. Childhood onset autosomal dominant polycystic kidney disease in sibs: clinical picture and recurrence risk. German working group on paediatric nephrology (Arbeitsgemeinschaft fur padiatrische Nephrologie) J Med Genet. 1993;30:583–588. doi: 10.1136/jmg.30.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, Hoff WG, Niaudet WP, Torres VE, Harris PC. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009;75:848–855. doi: 10.1038/ki.2008.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peral B, Ong AC, San Millan JL, Gamble V, Rees L, Harris PC. A stable, nonsense mutation associated with a case of infantile onset polycystic kidney disease 1 (PKD1) Hum Mol Genet. 1996;5:539–542. doi: 10.1093/hmg/5.4.539. [DOI] [PubMed] [Google Scholar]
- 39.Bergmann C, Bothmer J, Ortiz Bruchle N, Venghaus A, Frank V, Fehrenbach H, Hampel T, Pape L, Buske A, Jonsson J, Sarioglu N, Santos A, Ferreira JC, Becker JU, Cremer R, Hoefele J, Benz MR, Weber LT, Buettner R, Zerres K. Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol : JASN. 2011;22:2047–2056. doi: 10.1681/ASN.2010101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connor A, Lunt PW, Dolling C, Patel Y, Meredith AL, Gardner A, Hamilton NK, Dudley CR. Mosaicism in autosomal dominant polycystic kidney disease revealed by genetic testing to enable living related renal transplantation. Am J Transplant. 2008;8:232–237. doi: 10.1111/j.1600-6143.2007.02030.x. [DOI] [PubMed] [Google Scholar]
- 41.Mekahli D, Woolf AS, Bockenhauer D. Similar renal outcomes in children with ADPKD diagnosed by screening or presenting with symptoms. Pediatr Nephrol. 2010;25:2275–2282. doi: 10.1007/s00467-010-1617-8. [DOI] [PubMed] [Google Scholar]
- 42.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74:1192–1196. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Buchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 44.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wuthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 45.Chang MY, Ong AC. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin Pract. 2011;120:c25–c35. doi: 10.1159/000334166. [DOI] [PubMed] [Google Scholar]
- 46.Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Invest Drugs. 2010;19:315–328. doi: 10.1517/13543781003588491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001;38:777–784. doi: 10.1053/ajkd.2001.27720. [DOI] [PubMed] [Google Scholar]
- 48.Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5:2048–2056. doi: 10.1681/ASN.V5122048. [DOI] [PubMed] [Google Scholar]
- 49.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaki M, Hoefele J, Allen SJ, Ramaswami G, Janssen S, Bergmann C, Heckenlively JR, Otto EA, Hildebrandt F. Genotype-phenotype correlation in 440 patients with NPHP-related ciliopathies. Kidney Int. 2011;80:1239–1245. doi: 10.1038/ki.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 52.Salomon R, Saunier S, Niaudet P. Nephronophthisis. Pediatr Nephrol. 2009;24:2333–2344. doi: 10.1007/s00467-008-0840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simms RJ, Eley L, Sayer JA. Nephronophthisis. Eur J Hum Genet. 2009;17:406–416. doi: 10.1038/ejhg.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zollinger HU, Mihatsch MJ, Edefonti A, Gaboardi F, Imbasciati E, Lennert T. Nephronophthisis (medullary cystic disease of the kidney). A study using electron microscopy, immunofluorescence, and a review of the morphological findings. Helv Paediatr Acta. 1980;35:509–530. [PubMed] [Google Scholar]
- 55.Gagnadoux MF, Bacri JL, Broyer M, Habib R. Infantile chronic tubulo-interstitial nephritis with cortical microcysts: variant of nephronophthisis or new disease entity? Pediatr Nephrol. 1989;3:50–55. doi: 10.1007/BF00859626. [DOI] [PubMed] [Google Scholar]
- 56.Otto EA, Ramaswami G, Janssen S, Chaki M, Allen SJ, Zhou W, Airik R, Hurd TW, Ghosh AK, Wolf MT, Hoppe B, Neuhaus TJ, Bockenhauer D, Milford DV, Soliman NA, Antignac C, Saunier S, Johnson CA, Hildebrandt F. Mutation analysis of 18 nephronophthisis associated ciliopathy disease genes using a DNA pooling and next generation sequencing strategy. J Med Genet. 2011;48:105–116. doi: 10.1136/jmg.2010.082552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delous M, Hellman NE, Gaude HM, Silbermann F, Bivic A, Salomon R, Antignac C, Saunier S. Nephrocystin-1 and nephrocystin-4 are required for epithelial morphogenesis and associate with PALS1/PATJ and Par6. Hum Mol Genet. 2009;18:4711–4723. doi: 10.1093/hmg/ddp434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mollet G, Silbermann F, Delous M, Salomon R, Antignac C, Saunier S. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet. 2005;14:645–656. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- 59.Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, Reeuwijk J, Letteboer SJ, Sang L, Giles RH, Liu Q, Coene KL, Estrada-Cuzcano A, Collin RW, McLaughlin HM, Held S, Kasanuki JM, Ramaswami G, Conte J, Lopez I, Washburn J, Macdonald J, Hu J, Yamashita Y, Maher ER, Guay-Woodford LM, Neumann HP, Obermuller N, Koenekoop RK, Bergmann C, Bei X, Lewis RA, Katsanis N, Lopes V, Williams DS, Lyons RH, Dang CV, Brito DA, Dias MB, Zhang X, Cavalcoli JD, Nurnberg G, Nurnberg P, Pierce EA, Jackson PK, Antignac C, Saunier S, Roepman R, Dollfus H, Khanna H, Hildebrandt F. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nature Genet. 2010;42:840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shiba D, Manning DK, Koga H, Beier DR, Yokoyama T. Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton (Hoboken) 2010;67:112–119. doi: 10.1002/cm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wodarczyk C, Distefano G, Rowe I, Gaetani M, Bricoli B, Muorah M, Spitaleri A, Mannella V, Ricchiuto P, Pema M, Castelli M, Casanova AE, Mollica L, Banzi M, Boca M, Antignac C, Saunier S, Musco G, Boletta A. Nephrocystin-1 forms a complex with polycystin-1 via a polyproline motif/SH3 domain interaction and regulates the apoptotic response in mammals. PLoS One. 2010;5:e12719. doi: 10.1371/journal.pone.0012719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, Huntzicker EG, Sfakianos MK, Sandoval W, Bazan JF, Kulkarni P, Garcia-Gonzalo FR, Seol AD, O'Toole JF, Held S, Reutter HM, Lane WS, Rafiq MA, Noor A, Ansar M, Devi AR, Sheffield VC, Slusarski DC, Vincent JB, Doherty DA, Hildebrandt F, Reiter JF, Jackson PK. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, Garcia-Verdugo JM, Katsanis N, Hildebrandt F, Reiter JF. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nature Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, Yoder BK, Leroux MR. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN, Greenberg CR, McLeod DR, Bernier FP, Chudley AE, Muller T, Shboul M, Logan CV, Loucks CM, Beaulieu CL, Bowie RV, Bell SM, Adkins J, Zuniga FI, Ross KD, Wang J, Ban MR, Becker C, Nurnberg P, Douglas S, Craft CM, Akimenko MA, Hegele RA, Ober C, Utermann G, Bolz HJ, Bulman DE, Katsanis N, Blacque OE, Doherty D, Parboosingh JS, Leroux MR, Johnson CA, Boycott KM. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet. 2011;89:713–730. doi: 10.1016/j.ajhg.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ala-Mello S, Kivivuori SM, Ronnholm KA, Koskimies O, Siimes MA. Mechanism underlying early anaemia in children with familial juvenile nephronophthisis. Pediatr Nephrol. 1996;10:578–581. doi: 10.1007/s004670050164. [DOI] [PubMed] [Google Scholar]
- 67.Hildebrandt F, Strahm B, Nothwang HG, Gretz N, Schnieders B, Singh-Sawhney I, Kutt R, Vollmer M, Brandis M. Molecular genetic identification of families with juvenile nephronophthisis type 1: rate of progression to renal failure. APN Study Group. Arbeitsgemeinschaft fur padiatrische Nephrologie. Kidney Int. 1997;51:261–269. doi: 10.1038/ki.1997.31. [DOI] [PubMed] [Google Scholar]
- 68.Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, Sudbrak R, Antignac C, Gretz N, Walz G, Schermer B, Benzing T, Hildebrandt F, Omran H. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet. 2003;34:455–459. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- 69.Tory K, Rousset-Rouviere C, Gubler MC, Moriniere V, Pawtowski A, Becker C, Guyot C, Gie S, Frishberg Y, Nivet H, Deschenes G, Cochat P, Gagnadoux MF, Saunier S, Antignac C, Salomon R. Mutations of NPHP2 and NPHP3 in infantile nephronophthisis. Kidney Int. 2009;75:839–847. doi: 10.1038/ki.2008.662. [DOI] [PubMed] [Google Scholar]
- 70.Wolf MT, Beck BB, Zaucke F, Kunze A, Misselwitz J, Ruley J, Ronda T, Fischer A, Eifinger F, Licht C, Otto E, Hoppe B, Hildebrandt F. The Uromodulin C744G mutation causes MCKD2 and FJHN in children and adults and may be due to a possible founder effect. Kidney Int. 2007;71:574–581. doi: 10.1038/sj.ki.5002089. [DOI] [PubMed] [Google Scholar]
- 71.Chamberlin BC, Hagge WW, Stickler GB. Juvenile nephronophthisis and medullary cystic disease. Mayo Clin Proc. 1977;52:485–491. [PubMed] [Google Scholar]
- 72.Wolf MT, Vlem B, Hennies HC, Zalewski I, Karle SM, Puetz M, Panther F, Otto E, Fuchshuber A, Lameire N, Loeys B, Hildebrandt F. Telomeric refinement of the MCKD1 locus on chromosome 1q21. Kidney Int. 2004;66:580–585. doi: 10.1111/j.1523-1755.2004.00799.x. [DOI] [PubMed] [Google Scholar]
- 73.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zivna M, Hulkova H, Matignon M, Hodanova K, Vylet'al P, Kalbacova M, Baresova V, Sikora J, Blazkova H, Zivny J, Ivanek R, Stranecky V, Sovova J, Claes K, Lerut E, Fryns JP, Hart PS, Hart TC, Adams JN, Pawtowski A, Clemessy M, Gasc JM, Gubler MC, Antignac C, Elleder M, Kapp K, Grimbert P, Bleyer AJ, Kmoch S. Dominant renin gene mutations associated with early-onset hyperuricemia, anemia, and chronic kidney failure. Am J Hum Genet. 2009;85:204–213. doi: 10.1016/j.ajhg.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kroiss SHK, Berthold S, Rüschendorf F, Scolari F, Caridi G, Ghiggeri GM, Hildebrandt F, Fuchshuber A. Evidence of further genetic heterogeneity in autosomal dominant medullary cystic kidney disease. Nephro Dial Transplant. 2000;15:818–821. doi: 10.1093/ndt/15.6.818. [DOI] [PubMed] [Google Scholar]
- 76.Stavrou C, Koptides M, Tombazos C, Psara E, Patsias C, Zouvani I, Kyriacou K, Hildebrandt F, Christofides T, Pierides A, Deltas CC. Autosomal-dominant medullary cystic kidney disease type 1: clinical and molecular findings in six large Cypriot families. Kidney Int. 2002;62:1385–1394. doi: 10.1111/j.1523-1755.2002.kid581.x. [DOI] [PubMed] [Google Scholar]
- 77.Hoyer JRSS, Vernier RL. Tamm-Horsfall glycoprotein: ultrastructural immunoperoxidase localization in rat kidney. Lab Invest. 1979;41:168–173. [PubMed] [Google Scholar]
- 78.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis. 2003;42:658–676. doi: 10.1016/S0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 79.Bleyer AJHT, Willingham MC, Iskandar SS, Gorry MC, Trachtman H. Clinico-pathologic findings in medullary cystic kidney disease type 2. Pediatr Nephrol. 2005;20:824–827. doi: 10.1007/s00467-004-1719-2. [DOI] [PubMed] [Google Scholar]
- 80.Zaucke F, Boehnlein JM, Steffens S, Polishchuk RS, Rampoldi L, Fischer A, Pasch A, Boehm CW, Baasner A, Attanasio M, Hoppe B, Hopfer H, Beck BB, Sayer JA, Hildebrandt F, Wolf MT. Uromodulin is expressed in renal primary cilia and UMOD mutations result in decreased ciliary uromodulin expression. Hum Mol Genet. 2010;19:1985–1997. doi: 10.1093/hmg/ddq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puig JG, Miranda ME, Mateos FA, Picazo ML, Jimenez ML, Calvin TS, Gil AA. Hereditary nephropathy associated with hyperuricemia and gout. Arch Intern Med. 1993;153:357–365. doi: 10.1001/archinte.1993.00410030063009. [DOI] [PubMed] [Google Scholar]
- 82.Auranen MA-MS, Turunen JA, JärveläI Further evidence for linkage of autosomal-dominant medullary cystic kidney disease on chromosome 1q21. Kidney Int. 2001;60:1225–1232. doi: 10.1046/j.1523-1755.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- 83.Bleyer AJ, Woodard AS, Shihabi Z, Sandhu J, Zhu H, Satko SG, Weller N, Deterding E, McBride D, Gorry MC, Xu L, Ganier D, Hart TC. Clinical characterization of a family with a mutation in the uromodulin (Tamm-Horsfall glycoprotein) gene. Kidney Intl. 2003;64:36–42. doi: 10.1046/j.1523-1755.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 84.Scolari F, Caridi G, Rampoldi L, Tardanico R, Izzi C, Pirulli D, Amoroso A, Casari G, Ghiggeri GM. Uromodulin storage diseases: clinical aspects and mechanisms. Am J Kidney Dis. 2004;44:987–999. doi: 10.1053/j.ajkd.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 85.Kiser RL, Wolf MT, Martin JL, Zalewski I, Attanasio M, Hildebrandt F, Klemmer P. Medullary cystic kidney disease type 1 in a large Native-American kindred. Am J Kidney Dis. 2004;44:611–617. [PubMed] [Google Scholar]
- 86.Fairbanks LD, Cameron JS, Venkat-Raman G, Rigden SP, Rees L, Van THW, Mansell M, Pattison J, Goldsmith DJ, Simmonds HA. Early treatment with allopurinol in familial juvenile hyerpuricaemic nephropathy (FJHN) ameliorates the long-term progression of renal disease. QJM. 2002;95:597–607. doi: 10.1093/qjmed/95.9.597. [DOI] [PubMed] [Google Scholar]
- 87.Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet. 1997;17:384–385. doi: 10.1038/ng1297-384. [DOI] [PubMed] [Google Scholar]
- 88.Heidet L, Decramer S, Pawtowski A, Moriniere V, Bandin F, Knebelmann B, Lebre AS, Faguer S, Guigonis V, Antignac C, Salomon R. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol : CJASN. 2010;5:1079–1090. doi: 10.2215/CJN.06810909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23:1657–1668. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coffinier C, Barra J, Babinet C, Yaniv M. Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev. 1999;89:211–213. doi: 10.1016/S0925-4773(99)00221-X. [DOI] [PubMed] [Google Scholar]
- 91.Reber M, Cereghini S. Variant hepatocyte nuclear factor 1 expression in the mouse genital tract. Mech Dev. 2001;100:75–78. doi: 10.1016/S0925-4773(00)00493-7. [DOI] [PubMed] [Google Scholar]
- 92.Bingham C, Hattersley AT. Renal cysts and diabetes syndrome resulting from mutations in hepatocyte nuclear factor-1beta. Nephrol Dial Transplant. 2004;19:2703–2708. doi: 10.1093/ndt/gfh348. [DOI] [PubMed] [Google Scholar]
- 93.Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet. 2006;43:84–90. doi: 10.1136/jmg.2005.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bingham C, Ellard S, Hoff WG, Simmonds HA, Marinaki AM, Badman MK, Winocour PH, Stride A, Lockwood CR, Nicholls AJ, Owen KR, Spyer G, Pearson ER, Hattersley AT. Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1beta gene mutation. Kidney Int. 2003;63:1645–1651. doi: 10.1046/j.1523-1755.2003.00903.x. [DOI] [PubMed] [Google Scholar]
- 95.Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, Hennekam RC, Ledermann SE, Rees L, Hoff W, Marks SD, Trompeter RS, Tullus K, Winyard PJ, Cansick J, Mushtaq I, Dhillon HK, Bingham C, Edghill EL, Shroff R, Stanescu H, Ryffel GU, Ellard S, Bockenhauer D. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol : JASN. 2009;20:1123–1131. doi: 10.1681/ASN.2008060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meij IC, Koenderink JB, Bokhoven H, Assink KF, Groenestege WT, Pont JJ, Bindels RJ, Monnens LA, Heuvel LP, Knoers NV. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+), K(+)-ATPase gamma-subunit. Nature Genet. 2000;26:265–266. doi: 10.1038/81543. [DOI] [PubMed] [Google Scholar]
- 97.Bernstein J, Landing BH. Glomerulocystic kidney diseases. Prog Clin Biol Res. 1989;305:27–43. [PubMed] [Google Scholar]
- 98.Lennerz JK, Spence DC, Iskandar SS, Dehner LP, Liapis H. Glomerulocystic kidney: one hundred-year perspective. Arch Pathol Lab Med. 2010;134:583–605. doi: 10.5858/134.4.583. [DOI] [PubMed] [Google Scholar]
- 99.Bissler JJ, Siroky BJ, Yin H. Glomerulocystic kidney disease. Pediatr Nephrol. 2010;25:2049–2056. doi: 10.1007/s00467-009-1416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fitch SJ, Stapleton FB. Ultrasonographic features of glomerulocystic disease in infancy: similarity to infantile polycystic kidney disease. Pediatr Radiol. 1986;16:400–402. doi: 10.1007/BF02386818. [DOI] [PubMed] [Google Scholar]
- 101.Borges Oliva MR, Hsing J, Rybicki FJ, Fennessy F, Mortele KJ, Ros PR. Glomerulocystic kidney disease: MRI findings. Abdom Imaging. 2003;28:889–892. doi: 10.1007/s00261-003-0024-z. [DOI] [PubMed] [Google Scholar]
- 102.Zimmerman KW. Beitrage zur kenntnis einiger Drusen und Epithelien. Arch Mikrosk Anat. 1898;52:552–706. doi: 10.1007/BF02975837. [DOI] [Google Scholar]
- 103.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 104.Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002;12:938–943. doi: 10.1016/S0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- 105.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 106.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- 107.Ong AC, Wagner B. Detection of proximal tubular motile cilia in a patient with renal sarcoidosis associated with hypercalcemia. Am J Kidney Dis. 2005;45:1096–1099. doi: 10.1053/j.ajkd.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 108.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 110.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 113.Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, Somlo S. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol. 2010;21:295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luyten A, Su X, Gondela S, Chen Y, Rompani S, Takakura A, Zhou J. Aberrant regulation of planar cell polarity in polycystic kidney disease. J Am Soc Nephrol. 2010;21:1521–1532. doi: 10.1681/ASN.2010010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amir G, Rosenmann E, Drukker A. Acquired glomerulocystic kidney disease following haemolytic-uraemic syndrome. Pediatr Nephrol. 1995;9:614–616. doi: 10.1007/BF00860954. [DOI] [PubMed] [Google Scholar]
- 116.Mans DA, Voest EE, Giles RH. All along the watchtower: is the cilium a tumor suppressor organelle? Biochim Biophys Acta. 2008;1786:114–125. doi: 10.1016/j.bbcan.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, Watnick T, Henske EP. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet. 2009;18:151–163. doi: 10.1093/hmg/ddn325. [DOI] [PMC free article] [PubMed] [Google Scholar]