Abstract
Inactivation of the ARF-p53 tumor suppressor pathway leads to immortalization of murine fibroblasts. The role of this pathway in immortalization of human epithelial cells is not clear. We analyzed the functionality of the p14ARF-p53 pathway in human mammary epithelial cells (MEC) immortalized by human papillomavirus 16 (HPV16) E6, the p53 degradation-defective E6 mutant Y54D, or hTERT. E6-MEC or E6Y54D-MEC maintains high-level expression of p14ARF. Late-passage hTERT-immortalized MEC express p53 but down-regulate p14ARF. Enforced expression of p14ARF induces p53-dependent senescence in hTERT-MEC, while both E6-MEC and E6Y54D-MEC are resistant. We show that E6Y54D inhibits p14ARF-induced activation of p53 without inactivation of the p53-dependent DNA damage response. Hence, p53 degradation and inhibition of p14ARF signaling to p53 are independent functions of HPV16 E6. Our observations imply that long-term proliferation of MEC requires inactivation of the p14ARF-p53 pathway.
After extensive growth in cell culture, primary cells cease proliferating and develop the phenotype called replicative senescence. Replicative senescence is associated with telomere shortening and involves p53- and p16ink4a-dependent mechanisms (reviewed in reference 31). Expression of the catalytic component of telomerase, hTERT, activates telomerase, restores telomeres, and immortalizes some but not all human cells (7, 26, 39, 47).
When placed in culture, primary human mammary epithelial cells (MEC) senesce in two stages. The first is characterized by growth arrest at about 10 population doublings (PDs) and is termed selection or M0 (4, 16). Selection appears to be controlled by the p16ink4a-pRb pathway, as cells can be rescued from M0 by expression of human papillomavirus type 16 (HPV16) E7 (16). Some MEC spontaneously lose p16ink4a expression (8, 17, 22) and give rise to postselection cells. Postselection MEC can be propagated further to about 40 PD until they reach replicative senescence or M1, which can be overcome by expression of HPV16 E6 (5, 42). Postselection, but not preselection, MEC can be also immortalized by hTERT (26), indicating the critical role of telomerase in overcoming replicative senescence. It has been proposed that HPV16 E6 and E7 induce human epithelial cell immortalization by stimulating hTERT expression and blocking the p16ink4a-pRb pathway, respectively (26). MEC grown on feeder layers do not manifest M0 and can be immortalized by hTERT without inactivation of p16ink4a (21).
The contribution of p53 inactivation to epithelial cell immortalization is complex. Two dominant-negative (dn) p53 mutations immortalize MEC, while the majority of mutations do not (9, 20). dn p53 together with E7 immortalizes human foreskin keratinocytes (39, 40). This suggests that the role of E6 is to degrade and inactivate p53, while E7 blocks the p16ink4a-Rb pathway. Apart from targeting p53 for degradation, E6 induces telomerase (27) and also has other biological activities affecting cell growth (reviewed in reference 38). Induction of telomerase by E6 is associated with transcriptional activation of the hTERT gene (48). Several E6 mutants defective in p53 degradation (8S9A10T, F2V, and Y54H) can activate telomerase and immortalize postselection MEC (18, 26, 30). Cells immortalized by these E6 mutants have an intact DNA damage response to actinomycin D (AcD); therefore, elimination of p53 protein or the DNA damage checkpoint is not required for immortalization (26, 30). p14ARF and its murine counterpart p19ARF inhibit Mdm2-mediated proteolysis of p53 and thereby increase p53 protein levels (6, 46, 50). Inactivation of p19ARF elicits immortalization in murine embryonic fibroblasts (MEF) (24, 41); however, the role of p14ARF in immortalization of human cells is not clear. Pre-M1 MEC transduced with hTERT, wild-type E6, or E6 mutant 8S9A10T maintain expression of p14ARF, suggesting that elimination of p14ARF is not required for immortalization (26).
In this study, we analyzed induction of hTERT by E6 and expression levels of p14ARF and p16ink4a genes in early and late passages of MEC transduced with hTERT, E6, or E6 mutant Y54D. Like Y54H, this E6 mutant does not induce p53 degradation (30). We found that, in comparison to primary MEC, early passages of E6-MEC, E6Y54D-MEC, and hTERT-MEC did not alter expression of p14ARF and p16ink4a. In contrast, late passages of MEC immortalized by hTERT down-regulated p14ARF and p16ink4a, indicating negative selection against cells expressing these genes. MEC immortalized by E6 or E6Y54D, while down-regulating p16ink4a to different extents, maintained high levels of p14ARF expression, implying that these cells are resistant to p14ARF inhibition. Based on these observations, we sought to further explore the role of p14ARF in MEC immortalization and the activities of HPV16 E6 in this process.
MATERIALS AND METHODS
Retroviral vectors and infections.
Constructs of HPV16 E6 and various E6 mutants in pLXSN were described before (30); pBabe-puro-hTERT was from R. Weinberg; pWZL-hygro and pWZL-Hygro-H-Ras(V12) were from S. Lowe; and pBabe-puro-p14ARF was from G. Peters. The p14ARF cDNA sequence from pBabe-puro-p14ARF was recloned as a 0.4-kb BamHI-EcoRI fragment into pWZL-hygro. The R248W dn p53 mutant from pBabe-puro-p53R248W was transferred as a BamHI fragment into pLXSN. The LinX-A amphotropic packaging cells were kindly provided by G. Hannon. For production of infectious virus, LinX-A cells were plated in DFCI-1 media (4) and were transfected with FuGene6 (Roche). Retrovirus-containing media was harvested 3 days posttransfection of LinX-A cells cultured at 31°C.
Mammary cell immortalization.
Normal human MEC strain 76N, generously supplied by V. Band, and immortal cells derived from it were grown in DFCI-1 media (D media) as described earlier (4). 76N MEC were transduced by infection with retrovirus supernatant containing pLXSN-16E6, pLXSN-16E6Y54D, or pBabe-puro-hTERT. MEC were infected for 12 h at 31°C and were transferred to 37°C. Cells were selected with the appropriate antibiotic 48 h postinfection (G418, 75 μg/ml, 14 days; puromycin, 0.5 μg/ml, 6 days; and hygromycin, 50 μg/ml, 3 days). MEC were expanded as two populations. One was always maintained in D media, and the other was propagated for 18 PD in D2 media, which is a defined medium lacking serum and bovine pituitary extract and is selective for immortal MEC (5). The PDs calculated for immortal cells correspond to their outgrowth after antibiotic selection.
Induction of p53 response by AcD.
Late-passage immortal MEC were plated at 106 cells per 10-cm dish 24 h before treatment with AcD (0.5 nM). The cells were harvested for protein and RNA analysis 24 h posttreatment.
RNA purification.
Cells were grown to 70% confluence, were washed twice with ice-cold phosphate-buffered saline, and were lysed with 4 M guanidinium thiocyanate, and total RNA was extracted by acid phenol-chloroform (10). The integrity of the RNA was verified by 1.2% agarose gel electrophoresis with 7% formaldehyde. Poly(A) RNA was purified from total RNA with an Oligotex mRNA Kit (Qiagen).
cDNA synthesis and RT-PCR.
Single-stranded cDNA was made from 0.3 μg of poly(A) RNA or 1 μg of total RNA with Superscript II reverse transcriptase (RT) (Gibco) with random primer dN6. cDNA was amplified with Taq polymerase (Gibco). The following primer pairs were used: glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-G3S (dACCACAGTCCATGCCATCAC) and G3A (dTCCACCACCCTGTTGCTGTA); p14ARF-p14S1 (dGTTCTTGGTGACCCTCCGGATT) and p14A2 (dATCAGCACGAGGGCCACAG); p16ink4a-p16S1 (dGCCCAACGCACCGAATAGTT) and p16A1 (dGGGCAGTTGTGGCCCTGTAG); and hTERT-TERT1784S (dCGGAAGAGTGTCTGGAGCAA) and TERT1928A (dGGATGAAGCGGAGTCTGGA).
Quantitative RT-PCR.
cDNA was amplified in the real-time PCR cycler (SmartCycler; Cepheid) by using PCR buffer supplemented with 1.5 mM MgCl2, 5% dimethyl sulfoxide, 0.1× SYBR Green (S-7563; Molecular Probes), 1× additive reagent (Cepheid), 0.5 μM primers for p14ARF (p14S1 and p16A1) or GAPDH (G3S and G3A), and Taq polymerase (0.025 U/μl; Gibco). p14ARF and GAPDH sequences in each cDNA were quantitated by comparison to amplification of external standards of p14ARF or GAPDH DNA, and the ratio of p14ARF to GAPDH was calculated. Experiments were repeated twice for each cDNA sample, and the average ratio of p14ARF/GAPDH was determined.
Northern blots.
Poly(A) RNA was resolved in 1.5% agarose-7% formaldehyde gel, transferred to GeneScreen (NEN Life Science Products), and hybridized with 32P-labeled DNA probe at 42°C overnight in solution containing 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 50% formamide, 2× Denhardt's solution, 100 μg of salmon sperm DNA/ml, and 1% sodium dodecyl sulfate (SDS). The membrane was washed in 1× SSC with 0.1% SDS at 55°C. Images were analyzed on the PhosphorImager Storm 820 (Molecular Dynamics). p16ink4a exon 1β-specific cDNA was generated by PCR with primers p14S1 and p14A2 (dATCAGCACGAGGGCCACAG). The DNA was 32P labeled in asymmetric PCR with p14A2 primer and [α-32P]dATP. The GAPDH cDNA probe was generated by PCR with primers G3S and G3A and 32P-labeled with the Prime-A-Gene Kit (Promega).
Western blots.
Cells were washed twice with ice-cold phosphate-buffered saline and were lysed on plates by scraping in TSG buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, and 10% glycerol). Protein concentrations were determined with a bicinchoninic acid protein assay (Pierce). Proteins were transferred to Immobilon-P membranes (Millipore) and were processed with the following antibodies: anti-p53 DO-1 (Santa Cruz [1:1,000]); anti-p21 polyclonal (sc-397; Santa Cruz [1:1,000]); anti-p21 monoclonal (OP64; Oncogene Research [1:250]); anti-MDM2 (sc-965; Santa Cruz [1:500]); antitubulin (SC-5286; Santa Cruz [1:4,000]); goat-anti-mouse-horseradish peroxidase (Jackson [1:8,000]); goat-anti-rabbit-horseradish peroxidase (Jackson [1:8,000]). p14ARF was detected only with Ab3 (2 μg/ml, clone 14P03; NeoMarkers) but not with FL-132 (Santa Cruz), Ab-1 (Oncogene Sciences), Ab2 (clone 14P02; NeoMarkers), or NB200-11 (Novus). Processed blots were developed with SuperSignal West Dura extended-duration substrate (Pierce). Images were detected and quantitated with the LAS-1000+ luminescent image analyzer (Fuji).
RESULTS
Induction of hTERT by HPV16 E6 in MEC.
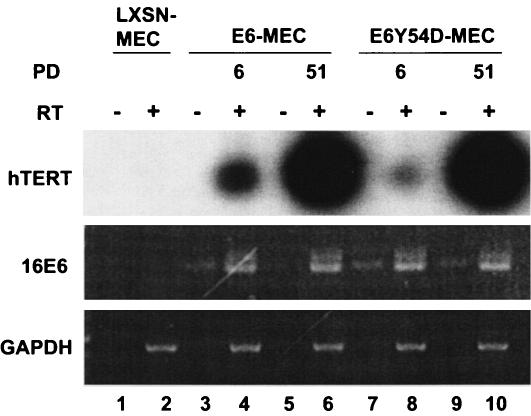
HPV16 E6 and immortalization-competent E6 mutants induce telomerase in MEC (18, 27). Activation of telomerase by E6 correlates with increased transcription of hTERT (35, 48). However, the temporal expression of hTERT by E6 mutants defective in p53 degradation was not tested. Figure 1 shows RT-PCR of hTERT and E6 in early and late passages of MEC transduced with E6 or the E6 mutant Y54D. In primary 76N MEC infected with the control LXSN retrovirus, hTERT transcripts were below detection under these RT-PCR conditions (Fig. 1, lane 2). In MEC transduced with E6 or E6Y54D, induction of the hTERT gene was detected by PD 6 (Fig. 1, lanes 4 and 8). There was a further dramatic increase in the amount of hTERT transcription in late-passage cells (Fig. 1, lanes 6 and 10). Of note, levels of E6 RNA did not change (compare lanes 4, 6, 8, and 10). This suggests that, apart from E6, other secondary events modulate the level of hTERT in MEC. Similar findings were recently reported in primary cervical epithelial cells transduced with E6/E7 oncogenes (2) and in human foreskin keratinocytes transfected with the HPV16 genome (45). Based on these observations, we investigated whether MEC expressing E6 or constitutive hTERT exhibits other adaptations in key pathways during passage beyond senescence checkpoints.
FIG. 1.
Induction of hTERT in MEC by HPV16 E6 and E6 mutant Y54D. Poly(A) RNA was extracted from MEC transduced with recombinant retroviruses and was analyzed by RT-PCR for hTERT, E6, or GAPDH. hTERT RT-PCR products were transferred to GeneScreen membranes and were hybridized with a 32P-labeled hTERT DNA probe generated by asymmetric PCR with primer TERT1928A and [α-32P]dATP. The blot is overexposed to demonstrate a lack of detectable hTERT transcripts in primary 76N MEC infected with LXSN retrovirus. “PD” represents PD after selection with G418.
Expression of p53, p14ARF, and p16ink4a in early and late passages of MEC transduced with hTERT, E6, or E6 mutant Y54D.
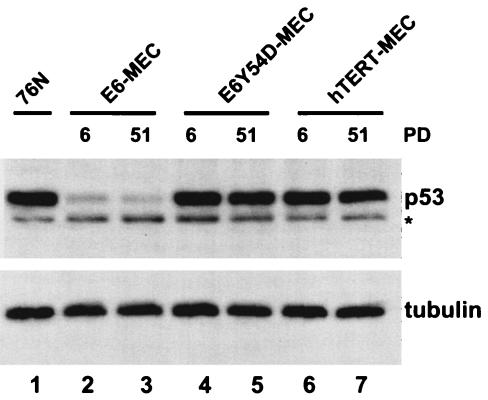
Pre-M1 MEC transduced with hTERT, E6 or E6 mutant 8S9A10T, which does not degrade p53, have high levels of expression of p14ARF while down-regulating p16ink4a (26). However, later passages of immortal MEC were not tested in this report. We analyzed levels of p53, p14ARF, and p16ink4a in early (PD 6) and late (PD 51) passages of MEC infected with retroviruses expressing wild-type E6, E6 mutant Y54D, or hTERT. To avoid clonal variations, we tested pooled populations of cells. As expected, MEC expressing E6 had low levels of p53 protein (Fig. 2, lanes 2 and 3), while both E6Y54D-MEC (lanes 4 and 5) and hTERT-MEC (lanes 6 and 7) had levels of p53 similar to those of primary 76N cells. This confirms that E6-mediated p53 degradation is not required for MEC immortalization (26, 30). In each cell population, the level of p53 protein remained consistent at early or late passage, suggesting that there is no negative selection against expression of p53 during propagation of these MEC.
FIG. 2.
Levels of p53 protein in early and late passages of MEC transduced with wild-type HPV16 E6, E6 mutant Y54D, or hTERT. Total cell lysate equal to 20 μg of protein was loaded per lane and was analyzed by Western blotting. p53 protein was detected with DO-1 antibody, and tubulin was used as loading control. PD, PD after retrovirus infection and antibiotic selection; *, a nonspecific band.
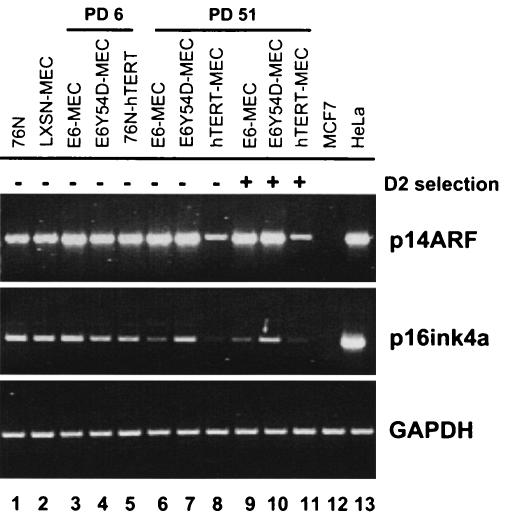
We examined the expression of the p14ARF and p16ink4a genes by RT-PCR (Fig. 3). The primary 76N strains used in this study are post-M0 MEC and express detectable p16ink4a mRNA. A uniform population of preselection 76N cells does not exist for comparison to determine whether p16ink4a was down-regulated during their selection. Immortal MEC are selected by culture in D2 media, which lacks serum and bovine pituitary extract (5). Cell growth in serum-free media may select a subpopulation of cells. Therefore, in addition to the MEC selected in D2, we tested late-passage cells that were propagated in parallel cultures in complete D media. Comparison of 76N MEC (Fig. 3, lane 1) or cells infected with pLXSN empty vector (lane 2) to early-passage cells revealed a slight increase in the levels of p14ARF transcript, with p16ink4a levels only marginally decreased in E6Y54D-MEC and hTERT-MEC (lanes 3, 4, and 5). A different picture emerged from analysis of late-passage cells (lanes 6 to 11). Both E6-MEC and hTERT-MEC down-regulated p16ink4a (lanes 9 and 11), while E6Y54D-MEC exhibited elevated levels of p16ink4a (lane 10). Interestingly, E6-MEC (lane 9) and E6Y54D-MEC (lane 10) both maintained high levels of p14ARF, while hTERT-MEC (lane 11) down-regulated p14ARF. This pattern of p16ink4a and p14ARF expression was not dependent on D2 media, as cells always grown in D media (lanes 6, 7, and 8) had similar levels of expression (compare p14ARF in lanes 6 and 9; lanes 7 and 10; and lanes 8 and 11).
FIG. 3.
RT-PCR of p14ARF and p16ink4a in early and late passages of immortal MEC. Cells transduced with specified retroviruses were grown in D or D2 media and were harvested at different PDs. RNA was analyzed by RT-PCR: p14ARF- 30 cycles, primers p14S1 and p16A1; p16ink4a, 34 cycles, primers p16S1 and p16A1; and GAPDH, 20 cycles, primers G3S and G3A. MCF7 cells do not express p14ARF and p16ink4a due to deletion of the INK4a locus. HeLa cells are from an HPV18-positive cervical cancer cell line and express p14ARF and p16ink4a.
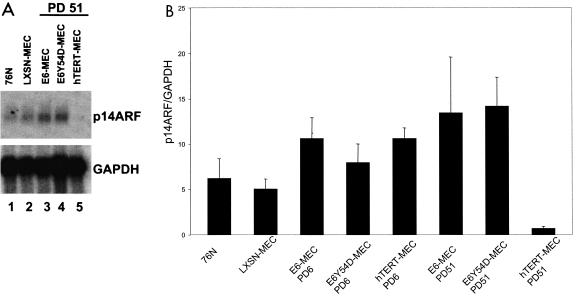
Another report for which Northern blotting was used did not detect down-regulation of p14ARF in early-passage (pre-M1) MEC transduced with hTERT, E6, or the p53 degradation-defective E6 mutant 8S9A10T (26). Therefore, we tested levels of p14ARF expression in MEC by Northern blotting (Fig. 4A) and quantitative real-time RT-PCR (Fig. 4B). Both methods confirmed that expression of p14ARF was down-regulated in late-passage hTERT-MEC while being maintained in E6-MEC and E6Y54D-MEC (Fig. 4A, compare lanes 3 to 5, and B). Real-time RT-PCR revealed a 15-fold reduction of p14ARF transcript from early to late passage in hTERT-MEC. We also confirmed that levels of p14ARF protein in 76N and early- and late-passage hTERT-immortalized MEC (Fig. 5B, lanes 3, 4, and 7) were barely detectable with one of five commercially available antibodies and with use of enhanced high- sensitivity chemiluminescence, indicating that levels of endogenous proteins are very low. p16ink4a protein was not detected by immunoblot in lysates from these cells (data not shown).
FIG. 4.
Expression of p14ARF is down-regulated in late passages of MEC immortalized by hTERT but is maintained in MEC immortalized by E6 or E6 mutant Y54D. (A) Northern blot. Poly(A) RNA from specified cells was hybridized with 32P-labeled probes specific for p14ARF or GAPDH. (B) Quantitative RT-PCR. cDNA was amplified in the real-time PCR cycler with SYBR Green detection of PCR products. p14ARF and GAPDH sequences in each cDNA were quantitated with external standards, and the ratio of p14ARF to GAPDH was calculated. Experiments were repeated twice for each cDNA sample, and the average p14ARF/GAPDH ratio is shown.
FIG. 5.
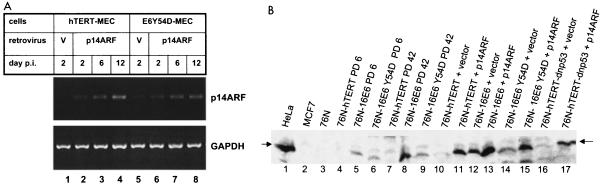
Detection of p14ARF RNA and protein in MEC infected with p14ARF retrovirus. (A) RT-PCR detection of p14ARF. Cells were infected with control pWZL-hygro or pWZL-hygro-p14ARF retrovirus. The day 2 time point is immediately before hygromycin selection was started. p14ARF sequences were amplified in 30 cycles of RT-PCR with primers p14S1 and p14A2. (B) Western blot of p14ARF. Total cell lysate equal to 50 μg of protein was loaded per lane and was analyzed by Western blot. p14ARF protein was detected with the Neomarkers antibody. Lanes 1 and 2 are HeLa (positive) and MCF7 (negative) controls. Early-passage primary MEC strain 76N (lane 3) and hTERT immortal MEC at early (lane 4) and late (lane 7) passage do not express detectable p14ARF protein, which was detectable in wild-type HPV 16 E6 (lanes 5 and 8) and mutant Y54D (lanes 6 and 9) at both early and late passages. Lanes 10 to 17 used lysates from these cells after infection with control (pWZL-hygro) or pWZL-hygro-p14ARF retrovirus.
Enforced expression of p14ARF in hTERT-MEC induces p53-dependent senescence.
Enforced expression of p19ARF in MEF or p14ARF in human tumor cell lines induces p53-dependent growth arrest (24, 46). Diploid fibroblasts expressing exogenous p14ARF develop the enlarged flat-cell morphology and stain positive for senescence-associated β-galactosidase (SA-β-gal), similar to that seen in cells in replicative senescence (13, 46, 49). It appears that p14ARF-induced growth arrest requires intact p53-p21cip1 signaling, because p53−/− as well as p21cip1−/− diploid human fibroblasts are resistant to p14ARF inhibition (49). Accordingly, E6 rescues human diploid fibroblasts from p14ARF inhibition (13, 46). However, expression of hTERT in diploid fibroblasts does not rescue these cells from p14ARF, indicating that p14ARF-induced senescence is a pathway independent from telomerase (49).
Our RT-PCR data suggest that E6- and E6Y54D-immortalized MEC can tolerate high levels of p14ARF, while hTERT-immortalized cells cannot. To test this, we expressed exogenous p14ARF in immortal MEC by means of retrovirus-mediated gene transfer. Cells were infected with pWZL-hygro-p14ARF or pWZL-hygro control retrovirus. After selection with hygromycin, cells were harvested at specified days for cell counts and protein or RNA studies. Figure 5A shows RT-PCR results for MEC infected with the p14ARF retrovirus. Increased p14ARF RNA levels are seen after hygromycin selection in hTERT and E6Y54D-MEC after infection with the p14ARF virus. Lysates from all cells show increased p14ARF protein analyzed by Western blotting with the Ab3 antibody after infection with pWZL-hygro-p14ARF retrovirus but not with the control pWZL-hygro virus (Fig. 5B, lanes 10 to 17).
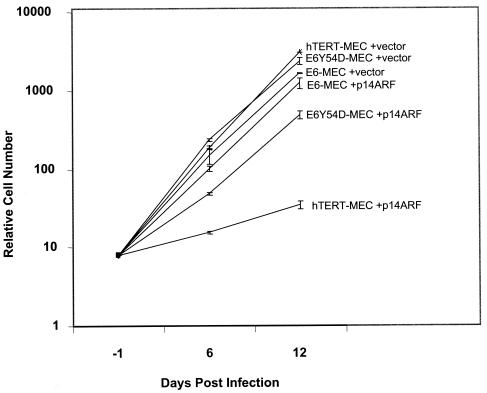
Proliferation of hTERT-MEC was strongly inhibited by p14ARF (Fig. 6). By day 6 postinfection (about three PDs), these cells developed the enlarged flat-cell phenotype with vacuoles characteristic of senescent MEC. By day 12 postinfection, most of the cells had this appearance. In contrast to induced senescence in human fibroblasts (13, 49), we did not detect increased SA-β-gal staining in immortal MEC infected with p14ARFor activated H-RasV12 (data not shown). Lack of SA-β-Gal staining in senescent human epithelial cells was also noted in other studies (V. Band, personal communication). To test whether p14ARF-induced growth arrest in hTERT-MEC is dependent on functional p53, we introduced the dn p53 mutant R248W into hTERT-MEC by retrovirus-mediated gene transfer. The p53R248W inactivates p53-mediated transactivation and extends life span but does not immortalize 76N MEC (9). When infected with p14ARF retrovirus, the hTERT-p53R248W-MEC (T53-MEC) cells displayed no growth arrest and no phenotypic change in comparison to infection with empty vector (data not shown). This proves that p14ARF-induced growth arrest in hTERT-MEC is p53 dependent.
FIG. 6.
Growth of immortal MEC infected with p14ARF retrovirus. Immortal MEC (PD75) were infected with pWZL-hygro-p14ARF retrovirus or pWZL-hygro empty vector. After selection with hygromycin, cells were harvested and counted at the specified days postinfection. Day −1 is when cells were plated for infection. Cells were infected on the next day, designated as day 0. The growth curve is the average of two independent experiments. Note that this is a semilogarithmic graph.
We also tested the effect of exogenous p14ARF on growth of MEC immortalized by E6 or E6Y54D. Based on high-level expression of endogenous p14ARF in late passages of these cells, we predicted that both E6-MEC and E6Y54D-MEC would be resistant to exogenous p14ARF. As expected, growth of E6-MEC was not inhibited by p14ARF (Fig. 6) and there was no difference in phenotype. Interestingly, proliferation of E6Y54D-MEC was transiently inhibited by p14ARF. By day 6 postinfection with p14ARF, the E6Y54D-MEC were a mixed population of small cells and large flat cells mostly without vacuoles. By day 12 postinfection the population was composed mostly of small cells with a doubling rate identical to that of wild-type E6. Therefore, enforced expression of p14ARF in E6Y54D-MEC induces transient growth arrest followed by rapid outgrowth of cells resistant to p14ARF despite the presence of normal levels of wild-type p53.
Activation of p53 by p14ARF is inhibited in MEC immortalized by E6 mutant Y54D.
p14ARF-induced growth arrest is associated with the p53 stabilization and accumulation of the products of p53-responsive genes such as MDM2 and p21cip1 (23, 46). The fact that E6Y54D-MEC rapidly overcome p14ARF-induced growth inhibition suggests that E6Y54D can inactivate p14ARF-induced activation of p53.
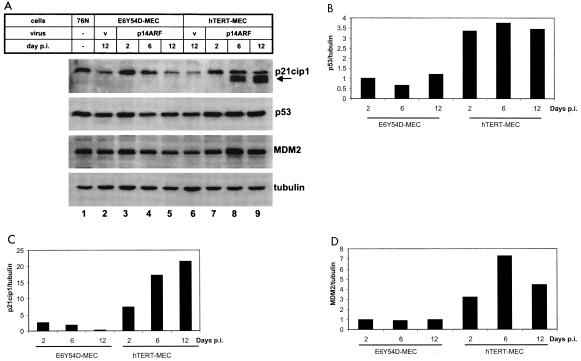
Figure 7A shows levels of p53, p21cip1, and MDM2 proteins in cells infected with p14ARF or control retrovirus. Figure 7B, C, and D depict normalized levels of proteins in comparison to cells infected with pWZL-hygro virus. In hTERT-MEC there was a 3.5-fold increase in p53 protein at day 2 postinfection (Fig. 7A, compare lanes 6 and 7, and B), before the cells' morphology changed. This level of p53 was maintained through day 12 postinfection (Fig. 7B). Accumulation of MDM2 (Fig. 7A, compare lanes 6 and 7, and D) was detected at day 2 postinfection and increased sevenfold by day 6, when the cells developed the large flat-cell senescent phenotype. Accumulation of p21cip1 (Fig. 7C) was also detected at day 2 postinfection (compare lanes 6 and 7 in Fig. 7A). Interestingly, at day 6 postinfection, we reproducibly detected accumulation of smaller peptides cross-reacting with the p21cip1 antibody (Fig. 7A, lane 8). The relative abundance of these faster-migrating p21cip1-specific peptides increased further by day 12 postinfection (Fig. 7A, lane 9). These proteins were also detected by a second p21cip1 monoclonal antibody (data not shown), implying that they are p21cip1 encoded. A p21cip1 antiserum showed only a single band at the predicted position (data not shown). Total levels of p21cip1-specific peptides in hTERT-MEC increased more than 20-fold by day 12 postinfection (Fig. 7C). In E6-MEC and T53-MEC infected with p14ARF retrovirus, there was no accumulation of p21cip1 protein (data not shown).
FIG. 7.
Accumulation of p53, p21cip1, and MDM2 proteins in p14ARF-infected cells. (A) Cells were infected with p14ARF retrovirus or empty vector as described in the Fig. 6 legend, and total cell lysates corresponding to 20 μg of protein were analyzed by Western blot. The arrow indicates smaller p21cip1-specific peptides detected with OP64 antibody (Oncogene Research). Virus “V,” empty vector pWZL-hygro. (B to D) Levels of p53, p21cip1, and MDM2 were quantitated with the LAS-1000+ luminescent image analyzer (Fuji) and were normalized to tubulin. Protein levels in cells infected with the empty vector were set at 1.
In contrast to hTERT-MEC, stabilization of p53 or accumulation of MDM2 was not detected in E6Y54D-MEC infected with p14ARF (Fig. 7A, lanes 3 to 5, and B and D). There was a 2.7-fold increase in levels of p21cip1 in E6Y54D-MEC by day 2 postinfection (Fig. 7C). The abundance of p21cip1 gradually returned to basal noninduced levels by day 12 postinfection (Fig. 7A, compare lanes 2 to 5, and C).
AcD-induced DNA damage response in immortal MEC.
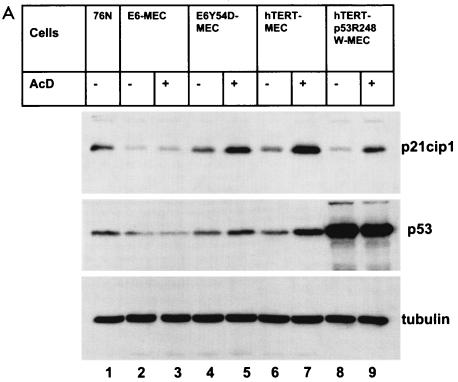
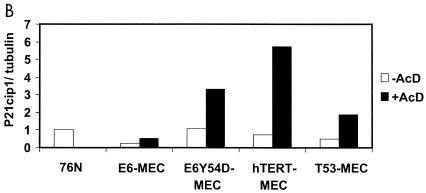
Analysis of levels of p53, p21cip1, and MDM2 proteins in cells infected with p14ARF retrovirus implies that activation of p53 is blocked in E6Y54D-MEC. p53 is activated in response to diverse stress signals (reviewed in reference 3). MEC immortalized by the E6 mutant 8S9A10T, F2V, or Y54H, which does not degrade p53, have an intact DNA damage response to AcD (26, 30). Therefore, we tested activation of p53 by AcD in immortal MEC containing E6, E6Y54D, hTERT, or hTERT+p53R248W (Fig. 8). As expected, there was no accumulation of p53 or p21cip1 in E6-MEC (compare lanes 2 and 3). Both E6Y54D-MEC (lanes 4 and 5) and hTERT-MEC (lanes 6 and 7) accumulated p53 and p21cip1 proteins, indicating activation of p53 and induction of p21cip1. However, E6Y54D-MEC accumulated less p53 and p21cip1 than did hTERT-MEC, indicating that activation of p53 by AcD may be partially compromised by E6Y54D. In contrast to the response induced by p14ARF, there was no accumulation of small p21cip1-specific peptides in hTERT-MEC treated with AcD. Evaluation of p53 stabilization in T53-MEC was not possible due to the large amounts of p53 protein in cells not treated with AcD. Interestingly, a small degree of p21cip1 induction was still present in T53-MEC expressing dn p53 mutant R248W (compare lanes 8 and 9), indicating residual p53 function or p53-independent activation of p21cip1 by AcD.
FIG. 8.
Accumulation of p53 and p21cip1 proteins in AcD-treated cells. (A) Late-passage immortal MEC were treated with AcD, and total cell lysates (20 μg) were analyzed by Western blot as described in the Fig. 7 legend. (B) Levels of p21cip1 were quantitated and normalized to tubulin as described in the Fig. 7 legend. p21cip1 levels in 76N cells were set at 1.
DISCUSSION
In this study we address the role of the p14ARF-p53 and p16ink4a-pRb pathways in MEC immortalization. It has been proposed that replicative senescence is dependent only on telomere shortening, while the p16ink4a-dependent mechanism of replicative senescence is an artifact created by inappropriate growth conditions (36). However, even when MEC are grown on feeder layers, p16ink4a protein levels are down-regulated in late passages of MEC (21). Collectively, our data and other studies (26, 39) indicate negative selection against cells expressing p16ink4a during immortalization and/or propagation of MEC and keratinocytes—either grown on feeder layers or not. Activation of the p16ink4a-pRb pathway represses telomerase activity in cancer cell lines (11, 12). The inverse correlation between expression of p16ink4a and hTERT in MEC raises the question whether down-regulation of p16ink4a can account for increased levels of hTERT in late passage MEC immortalized by 16E6 or E6-Y54D.
Studies on the role of ARF in cell immortalization are complicated by the partial overlapping of ARF and p16ink4a genes. Mutations or deletions in the INK4a locus frequently inactivate both genes, and disruption of ARF might be due to bystander effect of selection against p16ink4a. In murine cells, inactivation of p19ARF leads to immortalization of MEF and pre-B cells (24, 33). Rare selective inactivation of p14ARF with retention of p16ink4a has been reported in human tumors (19, 34, 44), melanoma cell lines (29), a melanoma-neural system tumor syndrome family (37), and a postcrisis clone of human adenoid epithelial cells immortalized by hTERT (15). These data suggest a role for p14ARF in human cancer and cell transformation, independent from p16ink4a. The observation that E6-MEC with inactivated p53 function maintain expression of p14ARF while down-regulating p16ink4a indicates that inactivation of p14ARF in hTERT-MEC is not a consequence of selection against p16ink4a and implies an independent role for p14ARF in MEC immortalization.
Pre-M1 MEC transduced with hTERT, E6 or E6 mutant 8S9A10T maintain high-level p14ARF mRNA (26), suggesting that elimination of p14ARF is not required for MEC proliferation. However, advanced passages of those cells were not tested. In addition, 76N MEC immortalized by Bmi-1 do not down-regulate p14ARF protein, as shown by Western blot analysis (14). In our experiments, the level of endogenous p14ARF protein in primary and hTERT immortal 76N MEC was below the detection limit of Western blotting with several commercially available polyclonal and monoclonal antibodies. MEC immortalized by HPV 16 E6, E6Y54D, and hTERT plus dn p53 had low but detectable p14ARF protein, consistent with the RT-PCR data. In this study down-regulation of p14ARF RNA is shown in two independent populations of late-passage hTERT-MEC. Analysis of the p14ARF expression in other epithelial cells (and other MEC strains) immortalized by hTERT will clarify if inactivation of p14ARF is a common event in such cells.
Enforced expression of p14ARF induces p53-dependent growth arrest and senescence in hTERT-MEC. This is accompanied by accumulation of p53, p21cip1, and MDM2, which is in agreement with p14ARF-induced senescence in fibroblasts (23, 46, 49). Interestingly, in addition to full-size p21cip1, we found accumulation of smaller p21cip1-specific peptides. Such peptides are not detected in hTERT-MEC treated by AcD. The truncated p21cip1 peptides represent amino-terminal fragments of p21cip1, since the carboxy-terminal p21cip1 polyclonal antiserum detects only one full-size p21cip1 in the p14ARF-infected hTERT-MEC (data not shown). The N terminus of p21cip1 has cdk inhibitory activity, while the C terminus binds and inhibits PCNA (32). Other studies have not shown the induction of p21cip1 fragments in cells expressing exogenous p14ARF but used different cell strains and antibodies (23, 46, 49). The origin and significance of the p21cip1-derived peptides are a subject for further investigation.
Previous studies showed that E6 mutants 8S9A10T, F2V, and Y54H do not degrade p53 yet could immortalize MEC (26, 30), identical to the phenotype that we show here for E6Y54D. Interestingly, these mutant E6 proteins all retained ability to bind the ubiquitin ligase E6AP, which is necessary for E6-mediated p53 degradation. Pre-M1 MEC expressing these mutants, as well as hTERT-MEC, express normal levels of p53 and p14ARF and preserve the p53-dependent DNA damage checkpoint. However, here we show that, while cells immortalized by E6Y54D express p53, p14ARF signaling to p53 is inactivated and p14ARF-induced senescence is blocked. Nonetheless, cells harboring E6Y54D remain susceptible to p53 activation by DNA damage. p14ARF is not strictly required for p53-dependent DNA damage response but may modulate it (25). The mechanism for how E6Y54D restricts p14ARF-induced activation of p53 requires further investigation. Different stress signals activate p53 through multiple pathways (3). In tumor cells, the p53-dependent response to hypoxia-like stress may be inactivated while response to AcD is intact (1). The present model is that ARF stabilizes p53 by targeting MDM2 to the nucleolus and blocking p53 ubiquitination by MDM2 (reviewed in reference 43). E6Y54D may block p53 activation by interaction with the p53 protein or by inhibiting another protein(s) required for p53 activation. Interestingly, a recent study shows that immortalization-competent E6 mutants defective in p53 degradation, including E6Y54H, bind and degrade the p53 transcriptional coactivator hADA3 (28). We are currently examining the relationship between E6 binding to cellular targets and inhibition of the p14ARF-p53 pathway.
The mechanism of E6 stimulation of hTERT expression is of active interest. We show a dramatic increase in expression of hTERT in late-passage MEC immortalized by wild-type E6 or E6Y54D. This was not accompanied by an increase in the level of E6. Similar findings were reported in human keratinocytes transduced with HPV16 E6/E7 (2). Our observations in MEC suggest initial E6-dependent induction of low levels of hTERT followed by E6-independent induction of high levels of hTERT expression during propagation of cells. Alternatively, different cells transduced with E6 initially express variable levels of hTERT, and only those with high levels of expression become immortal and are selected for during propagation.
Acknowledgments
We thank Scott Lowe, Robert Weinberg, Gordon Peters for providing constructs, Greg Hannon for retroviral packaging cells, Vimla Band for 76N cells and stimulating discussion, Joanna Parish, Ting Yu and Caroline Lai for assistance with computer graphics and review of the manuscript, and other members of the lab for useful comments. Judith Alamares assisted with the real-time PCR.
This work was supported by NIH grant R01 CA73558.
REFERENCES
- 1.Ashcroft, M., Y. Taya, and K. H. Vousden. 2000. Stress signals utilize multiple pathways to stabilize p53. Mol. Cell. Biol. 20:3224-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baege, A. C., A. Berger, R. Schlegel, and T. Veldman. 2002. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am. J. Pathol. 160:1251-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balint, E. E., and K. H. Vousden. 2001. Activation and activities of the p53 tumour suppressor protein. Br. J. Cancer 85:1813-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Band, V., and R. Sager. 1989. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc. Natl. Acad. Sci. USA 86:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Band, V., D. Zajchowski, V. Kulesa, and R. Sager. 1990. Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc. Natl. Acad. Sci. USA 87:463-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates, S., A. C. Phillips, P. A. Clark, F. Stott, G. Peters, R. L. Ludwig, and K. H. Vousden. 1998. p14ARF links the tumour suppressors RB and p53. Nature 395:124-125. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 8.Brenner, A. J., M. R. Stampfer, and C. M. Aldaz. 1998. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 17:199-205. [DOI] [PubMed] [Google Scholar]
- 9.Cao, Y., Q. Gao, D. E. Wazer, and V. Band. 1997. Abrogation of wild-type p53-mediated transactivation is insufficient for mutant p53-induced immortalization of normal human mammary epithelial cells. Cancer Res. 57:5584-5589. [PubMed] [Google Scholar]
- 10.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 11.Crowe, D. L., and D. C. Nguyen. 2001. Rb and E2F-1 regulate telomerase activity in human cancer cells. Biochim. Biophys. Acta 1518:1-6. [DOI] [PubMed] [Google Scholar]
- 12.DeFilippis, R. A., E. C. Goodwin, L. Wu, and D. DiMaio. 2003. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J. Virol. 77:1551-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimri, G. P., K. Itahana, M. Acosta, and J. Campisi. 2000. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14ARF tumor suppressor. Mol. Cell. Biol. 20:273-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimri, G. P., J. L. Martinez, J. J. Jacobs, P. Keblusek, K. Itahana, M. Van Lohuizen, J. Campisi, D. E. Wazer, and V. Band. 2002. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 62:4736-4745. [PubMed] [Google Scholar]
- 15.Farwell, D. G., K. A. Shera, J. I. Koop, G. A. Bonnet, C. P. Matthews, G. W. Reuther, M. D. Coltrera, J. K. McDougall, and A. J. Klingelhutz. 2000. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 156:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster, S. A., and D. A. Galloway. 1996. Human papillomavirus type 16 E7 alleviates a proliferation block in early passage human mammary epithelial cells. Oncogene 12:1773-1779. [PubMed] [Google Scholar]
- 17.Foster, S. A., D. J. Wong, M. T. Barrett, and D. A. Galloway. 1998. Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol. Cell. Biol. 18:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, Q., L. Singh, A. Kumar, S. Srinivasan, D. E. Wazer, and V. Band. 2001. Human papillomavirus type 16 E6-induced degradation of E6TP1 correlates with its ability to immortalize human mammary epithelial cells. J. Virol. 75:4459-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardie, B., J. M. Cayuela, S. Martini, and F. Sigaux. 1998. Genomic alterations of the p19ARF encoding exons in T-cell acute lymphoblastic leukemia. Blood 91:1016-1020. [PubMed] [Google Scholar]
- 20.Gollahon, L. S., and J. W. Shay. 1996. Immortalization of human mammary epithelial cells transfected with mutant p53 (273his). Oncogene 12:715-725. [PubMed] [Google Scholar]
- 21.Herbert, B. S., W. E. Wright, and J. W. Shay. 2002. p16(INK4a) inactivation is not required to immortalize human mammary epithelial cells. Oncogene 21:7897-7900. [DOI] [PubMed] [Google Scholar]
- 22.Huschtscha, L. I., J. R. Noble, A. A. Neumann, E. L. Moy, P. Barry, J. R. Melki, S. J. Clark, and R. R. Reddel. 1998. Loss of p16INK4 expression by methylation is associated with lifespan extension of human mammary epithelial cells. Cancer Res. 58:3508-3512. [PubMed] [Google Scholar]
- 23.Kamijo, T., J. D. Weber, G. Zambetti, F. Zindy, M. F. Roussel, and C. J. Sherr. 1998. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc. Natl. Acad. Sci. USA 95:8292-8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 25.Khan, S. H., J. Moritsugu, and G. M. Wahl. 2000. Differential requirement for p19ARF in the p53-dependent arrest induced by DNA damage, microtubule disruption, and ribonucleotide depletion. Proc. Natl. Acad. Sci. USA 97:3266-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 27.Klingelhutz, A. J., S. A. Foster, and J. K. McDougall. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79-82. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, A., Y. Zhao, G. Meng, M. Zeng, S. Srinivasan, L. M. Delmolino, Q. Gao, G. Dimri, G. F. Weber, D. E. Wazer, H. Band, and V. Band. 2002. Human papillomavirus oncoprotein E6 inactivates the transcriptional coactivator human ADA3. Mol. Cell. Biol. 22:5801-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar, R., I. Sauroja, K. Punnonen, C. Jansen, and K. Hemminki. 1998. Selective deletion of exon 1 beta of the p19ARF gene in metastatic melanoma cell lines. Genes Chromosomes Cancer 23:273-277. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y., J. J. Chen, Q. S. Gao, S. Dalal, Y. H. Hong, C. P. Mansur, V. Band, and E. J. Androphy. 1999. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J. Virol. 73:7297-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundberg, A. S., W. C. Hahn, P. Gupta, and R. A. Weinberg. 2000. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 12:705-709. [DOI] [PubMed] [Google Scholar]
- 32.Luo, Y., J. Hurwitz, and J. Massague. 1995. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature 375:159-161. [DOI] [PubMed] [Google Scholar]
- 33.Munoz, N., F. X. Bosch, S. de Sanjose, R. Herrero, X. Castellsague, K. V. Shah, P. J. Snijders, and C. J. Meijer. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518-527. [DOI] [PubMed] [Google Scholar]
- 34.Newcomb, E. W., M. Alonso, T. Sung, and D. C. Miller. 2000. Incidence of p14ARF gene deletion in high-grade adult and pediatric astrocytomas. Hum. Pathol. 31:115-119. [DOI] [PubMed] [Google Scholar]
- 35.Oh, S. T., S. Kyo, and L. A. Laimins. 2001. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J. Virol. 75:5559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez, R. D., C. P. Morales, B. S. Herbert, J. M. Rohde, C. Passons, J. W. Shay, and W. E. Wright. 2001. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randerson-Moor, J. A., M. Harland, S. Williams, D. Cuthbert-Heavens, E. Sheridan, J. Aveyard, K. Sibley, L. Whitaker, M. Knowles, J. N. Bishop, and D. T. Bishop. 2001. A germline deletion of p14(ARF) but not CDKN2A in a melanoma-neural system tumour syndrome family. Hum. Mol. Genet. 10:55-62. [DOI] [PubMed] [Google Scholar]
- 38.Rapp, L., and J. J. Chen. 1998. The papillomavirus E6 proteins. Biochim. Biophys. Acta Rev. Cancer 1378:F1-F19. [DOI] [PubMed] [Google Scholar]
- 39.Rheinwald, J. G., W. C. Hahn, M. R. Ramsey, J. Y. Wu, Z. Guo, H. Tsao, M. De Luca, C. Catricala, and K. M. O'Toole. 2002. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell. Biol. 22:5157-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedman, S. A., N. L. Hubbert, W. C. Vass, D. R. Lowy, and J. T. Schiller. 1992. Mutant p53 can substitute for human papillomavirus type-16 E6 in immortalization of human keratinocytes but does not have E6-associated trans-activation or transforming activity. J. Virol. 66:4201-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serrano, M., H. Lee, L. Chin, C. Cordon-Cardo, D. Beach, and R. A. DePinho. 1996. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85:27-37. [DOI] [PubMed] [Google Scholar]
- 42.Shay, J. W., W. E. Wright, D. Brasiskyte, and B. A. Van Der Haegen. 1993. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene 8:1407-1413. [PubMed] [Google Scholar]
- 43.Sherr, C. J. 2001. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2:731-737. [DOI] [PubMed] [Google Scholar]
- 44.Smeds, J., P. Berggren, X. Ma, Z. Xu, K. Hemminki, and R. Kumar. 2002. Genetic status of cell cycle regulators in squamous cell carcinoma of the oesophagus: the CDKN2A (p16(INK4a) and p14(ARF)) and p53 genes are major targets for inactivation. Carcinogenesis 23:645-655. [DOI] [PubMed] [Google Scholar]
- 45.Sprague, D., S. Phillips, C. Mitchell, K. Berger, M. Lace, L. Turek, and A. Klingelhutz. 2002. Telomerase activation in cervical keratinocytes containing stably replicating human papillomavirus type 16 episomes. Virology 301:247. [DOI] [PubMed] [Google Scholar]
- 46.Stott, F. J., S. Bates, M. C. James, B. B. McConnell, M. Starborg, S. Brookes, I. Palmero, K. Ryan, E. Hara, K. H. Vousden, and G. Peters. 1998. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 17:5001-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 48.Veldman, T., I. Horikawa, J. C. Barrett, and R. Schlegel. 2001. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 75:4467-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei, W., R. M. Hemmer, and J. M. Sedivy. 2001. Role of p14ARF in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 21:6748-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, Y., Y. Xiong, and W. G. Yarbrough. 1998. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92:725-734. [DOI] [PubMed] [Google Scholar]