Abstract
TNF-α is one of the key proinflammatory cytokines in pathogenesis of rheumatoid arthritis (RA). TNF-α was also found to enhance synthesis of leptin. Leptin is mainly adipocyte-derived hormone controlling appetite and energy expenditure. It acts through inhibition of neuropeptide Y secretion. It is possible that TNF-α-induced leptin secretion contributes to body mass reduction in patients with RA. The study was designed to determine the influence of inactivation of the TNF-α with infliximab on plasma leptin and neuropeptide Y concentrations in patients with RA. Sixteen female patients with RA treated with infliximab and 16 healthy women were investigated. Plasma leptin and neuropeptide Y concentrations were determined before, during and after 1 year management of the patients with infliximab and were compared with body mass index and body fatty and lean mass. There was no difference in plasma leptin concentration between the rheumatoid patients before therapy and the controls (15.6 ± 1.85 and 14.5 ± 2.15 ng/ml, respectively). Neuropeptide Y concentration was higher in the patients than in the controls (54.5 ± 3.96 and 24.8 ± 2.80 pmol/l, respectively). Treatment with infliximab resulted in enhancement in leptin concentration (18.5 ± 2.34 ng/ml) and a slight increase in neuropeptide Y concentration (58.7 ± 4.66 pmol/l). Physiological relationship between leptin and body mass was shown in the patients and was not altered during the treatment. There was no significant correlation between the disease activity and plasma leptin or neuropeptide Y concentrations.
Keywords: Rheumatoid arthritis, Infliximab, Leptin, Neuropeptide Y, Body mass
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease and is associated with reduction of the body mass of some of the patients. Pathomechanism of body mass reduction that occurs in patients with chronic inflammatory diseases remains still unclear.
TNF-α is one of the main proinflammatory cytokines that plays a key role in pathogenesis of rheumatoid arthritis. It has been shown that TNF-α increases secretion of leptin, as known as ob protein, hormone produced mainly by adipocytes, as well as the hormone that decreases appetite and food intake by inhibition of neuropeptide Y (NPY) secretion [1, 2]. Leptin inhibits releasing some other orexigenic (stimulating the appetite) neurotransmitters such as galanin [3], orexin A and B [4] or agouti-related protein [5] and simultaneously increases level of some anorexigenic factors such as corticoliberin [6], glucagon-like peptide-1 [7], melanotropin [8] and cocaine- and amphetamine-regulated transcript. Many studies focus on the role of leptin as a specific lipostat because it inhibits directly accumulation of the intracellular lipids by reducing the synthesis of fatty acids and triglycerides and lowering oxidation of fatty acids [9]. It has been also shown that leptin increases energy expenditure by inhibiting oxidative phosphorylation [10]. Under physiological conditions, plasma leptin concentration correlates with mass of fatty tissue [11] and depends on gender, and a higher leptin concentration was shown in women [12, 13].
There are more and more reports indicating influence of TNF-α on increase in the ob gene expression and leptin synthesis [14]. It has been suggested that cytokine-dependent hyperleptinaemia may be a potential cause of body mass reduction in patients with RA. Chronic long-term administration of TNF-α to mice resulted in lowering of body mass [15–18].
Infliximab, a chimeric monoclonal antibody acting by blocking both soluble and cell membrane-bound forms of TNF-α, is widely used for treatment of patients with RA [19]. The aim of the study was evaluation of the effect of infliximab on plasma leptin and neuropeptide Y concentrations in patients with RA.
Patients and methods
The study group consisted of 16 female patients with RA treated with infliximab (Remicade). All of them were in the postmenopausal period and did not receive hormonal replacement therapy.
Sixteen age—body mass index (BMI)—matched healthy women were investigated as the controls. All patients have active disease and had not received remission after application of at last two disease-modified drugs. Infliximab treatment was administered 7.1 ± 1.0 years after onset of arthritis. Infliximab was administered intravenously in a dose of 3 mg/kg of body mass as 2-h infusion. The infusions were repeated after 2 and 6 weeks after the first infusion, and subsequently every 8 weeks (to total number of infusions–9).
Patients were also given prednisone in a dose of 5.7 ± 1.08 mg/day and methotrexate in a dose of 9.3 ± 0.53 mg/week. All patients received folic acid in the dose of 5 mg/day. The patients were not treated with folic acid during the day they were receiving methotrexate.
At least 4 weeks before the beginning of therapy with infliximab, during the whole period of treatment and 8 weeks after the 9th infusion of infliximab, the doses of additional medication were unchanged.
Only female patients, which on the basis of clinical examination and results of additional tests were possible to exclude potential factors that might have some influence on the body mass and plasma leptin concentration, that is, thyroid disorders, other endocrinopathy, renal insufficiency, heart failure, arterial hypertension, diabetes mellitus, hyperlipidemia, neoplastic disease or mental disease, were included in this study. None of those patients smoked cigarettes.
Plasma leptin and neuropeptide Y concentrations were measured: before treatment, after first infusion, after second infusion (i.e. 2 weeks from first infusion), after fourth infusion (i.e. 14 weeks from beginning of therapy), after sixth infusion (i.e. 30 weeks from beginning of therapy), after ninth dose (i.e. 54 weeks from beginning of therapy) and in follow-up, that is, 62 weeks from the beginning of therapy. Blood samples were taken in the morning (8.00 AM) after overnight fasting. Plasma was stored at −20°C. In patients treated with infliximab, blood samples were drawn in the next day after the drug infusion. Plasma leptin concentration was measured with radioimmunoassay method (Human leptin RIA KIT from Linco Research Inc. USA) and plasma NPY concentration using radioimmunoassay method with Euria-NPY kit (Euro-Diagnostica Sweden).
The following indices were determined: body mass, body mass index (BMI) and waist-to-hip ratio (WHR). Content of fatty tissue and lean mass was determined with densitometric method before and after treatment (DEXA Dual Energy X-ray Absorptiometry, Lunar DPXL). Densitometric measurements (mass of fatty tissue and lean mass) and the determination of WHR took place only in patients treated with infliximab.
Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level and platelet count (PLT) were assayed with routine used methods. DAS 28 was calculated as the disease activity index.
Statistical analysis Results were expressed as mean ± standard error of mean (SEM). Fisher`s test was used to analyse normal distribution of data, and Student`s t-test and U Mann–Whitney test were used appropriately. Correlation analysis was performed using Pearson`s coefficient or Kendall tau coefficient calculation.
Results
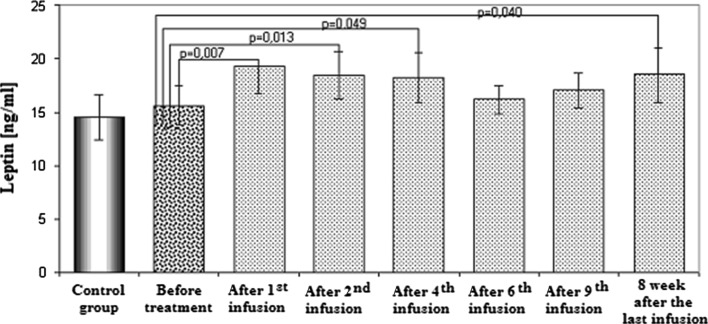
Mean plasma leptin concentration is shown in Fig. 1. There was no difference between rheumatoid patients before infliximab treatment and the controls. An increase in plasma leptin concentration was found after the 1st, 2nd and 4th infusion of infliximab. It was followed by some decrease in leptin concentration; thus, plasma leptin (after the 6th and 9th infusion) and enhanced plasma leptin were shown 8 weeks after the last infusion.
Fig. 1.
The plasma leptin concentrations in control and study groups before treatment, during the treatment and after the treatment with infliximab (mean values ± SEM)
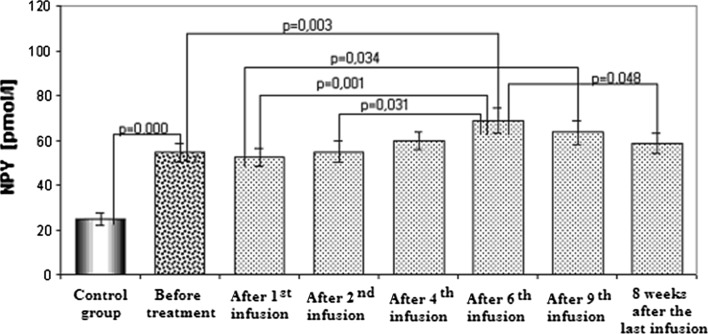
Plasma NPY concentration in rheumatoid patients before the medication with infliximab was almost twice higher than in healthy controls (Fig. 2). NPY concentration was shown to be high during the treatment with infliximab, and additional increase in NPY concentration was found after 6th and 9th infusion. Eight weeks after the last infusion, NPY concentration was still enhanced but slightly reduced as compared with NPY concentration after the 6th infusion.
Fig. 2.
The plasma neuropeptide Y concentrations in the control group and in the study group before the treatment, during the therapy and after the treatment with infliximab (mean values ± SEM)
CRP, ESR and PLT were higher in the rheumatoid patients. During the medication with infliximab, a significant reduction in CRP, ESR and PLT was found; also, the results shown after the 9th infusion, that is, 8 weeks after the last infusion, were still higher than the normal values (data not shown).
BMI of the RA patients was 26.0 ± 1.29 kg/m2 before the treatment with infliximab. There was no difference in BMI after the medication although some increasing tendency was shown (BMI, 8 weeks after the last infusion 26.6 ± 1.23 kg/m2). Similar tendency was observed in body mass (69.2 ± 3.64 kg before the treatment, 70.8 ± 3.44 kg 8 weeks after the last infusion) and waist-to-hip ratio (0.81 ± 0.01 versus 0.83 ± 0.01).
Leptin/BMI ratio was found to shown increasing tendency during the treatment. In the patients before infliximab medication, the ratio was 0.57 ± 0.04, while after the treatment was 0.66 ± 0.07. The leptin/BMI ratio in the controls was 0.53 ± 0.06.
Determination of fatty tissue and lean mass was carried out in RA patients before and after infliximab therapy. Results are summarised in Table 1. An increase in total body mass of fatty tissue as well as fatty tissue of the upper and lower limbs was found. The increase was accompanied by a slight decrease in lean mass of the trunk and upper limbs (Table 1).
Table 1.
The mass of fatty tissue and lean mass in rheumatoid patients determined before and after the therapy with infliximab (mean ± SEM)
| Time of study | Total body mass of fatty tissue (g) | Total lean mass (g) | Mass of fatty tissue of the trunk (g) | Lean mass of the trunk (g) | Mass of fatty tissue of the upper limbs (g) | Lean mass of the upper limbs (g) | Mass of fatty tissue of the lower limbs (g) | Lean mass of the lower limbs (g) |
|---|---|---|---|---|---|---|---|---|
| Before therapy | 26681.2 ± 2916.9 | 40249.6 ± 1466.3 | 13285.2 ± 1631.6 | 20702.0 ± 934.7 | 2416.8 ± 288.8 | 3925.5 ± 154.5 | 9507.2 ± 965.9 | 13214.0 ± 502.2 |
| After therapy | 28075.8 ± 2611.5a | 40530.9 ± 1078.3 | 1323.1 ± 1335.8 | 19816.1 ± 658.8b | 3266.7 ± 435.8c | 4656.2 ± 204.1d | 9922.1 ± 846.2e | 13554.8 ± 364.8 |
Statistical significance of the difference: a P = 0.002; b P = 0.034; c P = 0.004; d P = 0.002; e P = 0.006
Plasma leptin concentration was found to be positively correlated with BMI both in controls and in patients before and during medication with infliximab (Table 2). Leptin was also found to correlate with body mass and total body fatty mass tissue, lean mass and waist-to-hip ratio (Table 3). There was no correlation between either plasma NPY and BMI or leptin and NPY. There was no correlation between plasma NPY and body mass and fatty tissue mass, except positive correlation between NPY and fatty mass of the trunk (τ = 0.478, P < 0.012), fatty mass of the lower limbs (τ = 0.382, P < 0.046) and waist-to-hip ratio (τ = 0.495, P < 0.007) all after treatment. There was also no correlation between plasma leptin concentration and inflammatory indices (CRP, ESR and PLT) as well as diseases activity (DAS 28), but 8 weeks after the last infusion, a positive correlation was found (leptin versus DAS28, τ = 0.433, P < 0.041).
Table 2.
Correlation between plasma leptin concentration and body mass index in control group and in study group before the treatment and after sequential infusions of infliximab
| Correlation | τ | P |
|---|---|---|
| Leptin/BMI CG | 0.545 | 0.003 |
| Leptin/BMI RAbefore 1st infusion | 0.533 | 0.004 |
| Leptin/BMI RA1 | 0.516 | 0.005 |
| Leptin/BMI RA2 | 0.483 | 0.009 |
| Leptin/BMI RA4 | 0.437 | 0.018 |
| Leptin/BMI RA6 | 0.393 | 0.033 |
| Leptin/BMI RA9 | 0.483 | 0.009 |
| Leptin/BMI RAafter treatment | 0.383 | 0.038 |
CG control group, RA before 1st RA group before 1st infusion of infliximab, RA 1,2,4,6,9 RA group after sequential infusions,RA after tr RA group after the treatment
Table 3.
The correlation between plasma leptin concentration and total body weight, total body fatty tissue mass, lean mass and waist-to-hip ratio before and after therapy with infliximab and between plasma leptin concentration and body weight after sequential infusions of infliximab
| τ | P | τ | P | |
|---|---|---|---|---|
| Leptin/body weight | 0.633 | 0.000 | 0.493 | 0.007 |
| Leptin/total body fatty tissue | 0.717 | 0.000 | 0.523 | 0.006 |
| Leptin/total body lean mass | 0.461 | 0.028 | 0.142 | NS |
| Leptin/fatty tissue mass of the trunk | 0.692 | 0.001 | 0.542 | 0.004 |
| Leptin/lean mass of the trunk | 0.512 | 0.014 | 0.314 | NS |
| Leptin/fatty tissue mass of upper limbs | 0.615 | 0.003 | 0.581 | 0.002 |
| Leptin/lean mass of upper limbs | 0.512 | 0.014 | 0.200 | NS |
| Leptin/fatty tissue mass of lower limbs | 0.589 | 0.005 | 0.333 | NS |
| Leptin/lean mass of lower limbs | 0.307 | NS | −0.047 | NS |
| Leptin/WHR | 0.229 | NS | 0.366 | 0.048 |
| Leptin/body weight after 1st infusion | Leptin/body weight after 2nd infusion | Leptin/body weight after 4th infusion | Leptin/body weight after 6th infusion | Leptin/body weight after 9th infusion | |||||
|---|---|---|---|---|---|---|---|---|---|
| τ = 0.583 | P = 0.001 | τ = 0.533 | P = 0.004 | τ = 0.531 | P = 0.004 | τ = 0.393 | P = 0.033 | τ = 0.477 | P = 0.010 |
NS not statistically significant
A positive correlation between plasma NPY concentration and inflammatory indices was shown only before medication (NPY versus CRP, τ = 0.506, P < 0.006; NPY versus DAS 28, τ = 0.393, P < 0.033). There was no correlation between inflammatory indices and body indices (body mass, BMI, WHR, total fatty tissue and total lean mass). Multivariate regression analysis performed with dependent variable—plasma leptin concentration—and independent variables—BMI, WHR, total body fatty tissue mass, total body lean mass, CRP, ESR, PLT, DAS 28 score and plasma NPY concentration—in patients with RA treated with infliximab revealed significant positive correlation between plasma leptin concentration and total body fatty tissue mass and significant negative correlation between plasma leptin concentration and total body lean mass (Table 4).
Table 4.
Multivariate regression analysis with dependent variable—plasma leptin concentration—and independent variables—body mass index (BMI), waist-to-hip ratio (WHR), total body fatty tissue mass, total body lean mass, CRP, ESR, platelet count (PLT), DAS28 score and plasma neuropeptide Y concentration in patients with rheumatoid arthritis (R2 = 0.724); only variables that have statistically significant influence on plasma leptin concentration, after elimination of insignificant variables in sequential parts of regression analysis, are shown
| Study parameter | Study group | |
|---|---|---|
| Total body fatty tissue mass | β | 0.001 |
| P | 0.000 | |
| Total body lean mass | β | −0.000 |
| P | 0.013 | |
Discussion
The obtained results indicate for lack of difference in plasma leptin concentration between RA patients and healthy individuals. This finding is concomitant with reports of Nishiya et al. [20], Popa et al. [21, 22] and Anders et al. [23]. The last investigators additionally reported lack of changes in plasma leptin concentration in RA patients before and after treatment with infliximab. On the contrary, Tokarczyk-Knapik et al. [24] reported decreased plasma leptin in patients with RA. A possible source of this discrepancy may be BMI of the RA patients in the study of Tokarczyk-Knapik et al. [24]. Their BMI was lower than that of the control group. Otero et al. [25] reported that patients with RA showed higher plasma leptin concentration than healthy controls.
Gonzalez-Gay et al. [26] showed that anti-TNF-α therapy does not modulate leptin concentration in patients with severe rheumatoid arthritis. In the present study, we reported an increase in plasma leptin concentration in RA patients after management with infliximab. An increase was shown even after the first infusion of the drug and lasted after medication. The enhanced plasma leptin concentration after the first infusions cannot be associated with an increase in BMI of the patients. Some increase in BMI was shown at least after the 4th infusion of infliximab. The observation is similar to report of van Gossum et al. [27] who reported plasma leptin increase after 1 and 4 weeks of infliximab treatment of patients with Crohn disease. It is possible that changes in TNF-α level in RA patients during medication with infliximab are very rapid but transient after early infusions or TNF-α release in inflammatory states has pulsatory nature. It has been shown in vitro that TNF-α has diphasic effect on leptin secretion by adipocytes. Some studies revealed that TNF-α causes only transitory elevated plasma leptin concentration followed by decreased releasing of this hormone. It was shown in in vitro experiments, and this phenomenon is suggested to be explained as the chronic influence of cachectin on disturbance in adipocytes differentiation leading to impairment in leptin production [28]. Granowitz [29] suggested that TNF-α enhances leptin secretion only in cooperation with transforming growth factor-β and TNF-α alone is less potent stimulatory or even exerts inhibitory properties. It is, however, very difficult to use data from in vitro investigations for explanation of phenomena observed in the RA patients. TNF-α was not determined in our study, but clinical efficacy of medication strongly suggests that active TNF-α level at least in the joints was diminished.
Plasma NPY concentration was found to be higher in patients with RA. Treatment with infliximab was associated with some further enhancement of plasma NPY. An increase in plasma NPY concentration in RA can be explained by higher sympathetic activity in RA as it was shown by Dekkers et al. [30].
As expected, some increase in BMI in the RA patients was found after the treatment. This finding is concomitant with other reports [27]. The correlation of plasma leptin concentration with BMI was shown to be unchanged in the RA patients before and during the infliximab medication. Plasma leptin concentration was shown to correlate with fat mass and unexpectedly with lean mass after infliximab treatment. There was no significant correlation between plasma leptin concentration, inflammatory indices and disease activity. Similar results were reported by other authors [20, 23, 24]. Only Targońska-Stępniak et al. [31] showed positive correlation between plasma leptin concentration and DAS28. Popa et al. [21] found inversed correlation between plasma leptin and serum C-reactive protein and interleukin-6 concentration in RA patients. There was no significant correlation between plasma leptin and NPY concentrations.
In summary, it can be concluded that plasma leptin concentration is not significantly altered in RA patients. Infusion of infliximab, anti-TNF-α antibody, produces an increase in plasma leptin that probably is resulted from transient rapid reduction of serum TNF-α. After longer medication, plasma leptin changes are related to BMI in similar pattern as under physiological conditions in healthy individuals.
Further investigations including studies on RA patients with significant body mass deficiency are needed to elucidate leptin–inflammation relationship in RA.
Conclusion
Plasma leptin concentration in patients with rheumatoid arthritis does not differ from healthy individuals. Plasma NPY concentration in patients with rheumatoid arthritis is higher than in healthy individuals. During the therapy with infliximab, a TNF-α antagonist, plasma leptin concentration increases, and there were no changes in plasma NPY concentration during therapy.
In patients with rheumatoid arthritis, physiological relation between plasma leptin concentration and body mass (BMI, fatty tissue mass) is unaltered. The treatment with infliximab does not disturb this correlation.
In patients with rheumatoid arthritis treated with infliximab, the disease activity does not correlate with plasma leptin concentration. There is no significant correlation between the disease activity and plasma NPY concentration in those patients revealed.
It does not seem that leptin plays a crucial role in the reduction of the body mass in patients with rheumatoid arthritis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- BMI
Body mass index
- CRP
C-reactive protein
- DAS28
Diseases activity score 28
- DEXA
Dual-energy X-ray Absorptiometry
- ESR
Erythrocyte sedimentation rate
- NPY
Neuropeptide Y
- PLT
Platelet count
- RA
Rheumatoid arthritis
- TNF-α
Tumour necrosis factor α
- WHR
Waist-to-hip ratio
References
- 1.Jeanrenaud B, Rohner-Jeanrenaud F. Effects of neuropeptides and leptin on nutrient partitioning: dysregulation in obesity. Annu Rev Med. 2001;52:339–351. doi: 10.1146/annurev.med.52.1.339. [DOI] [PubMed] [Google Scholar]
- 2.Lago R, Gómez R, Lago F, Gómez-Reino J, Gualillo O. Leptin beyond body weight regulation-current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252:139–145. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Huang Q, Viale A, Picard F, Nahon J, Richard D. Effects of leptin on melanin concentrating hormone expression in the brain of lean ad obese Lep(ob)/lep(ob) mice. Neuroendocrinology. 1999;69:145–153. doi: 10.1159/000054413. [DOI] [PubMed] [Google Scholar]
- 4.Nowak KW, Maćkowiak P, Świtońska MM, Fabis M, Malendowicz LK. Acute orexin effects on insulin secretion in the rat: in vivo and in vitro studies. Life Sci. 2000;66:449–454. doi: 10.1016/S0024-3205(99)00611-6. [DOI] [PubMed] [Google Scholar]
- 5.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 6.Richard D, Rivest R, Naimi N, Timofeeva E, Rivest S. Expression of corticotropin-releasing factor and its receptors in the brain of lean and obese Zucker rats. Endocrinology. 1996;137:4786–4795. doi: 10.1210/en.137.11.4786. [DOI] [PubMed] [Google Scholar]
- 7.Goldstone AP, Mercer JG, Gunn I, Moar KM, Edwards CMB, Rossi M, Howard JK, Rasheed S, Turton MD, Small C, Heoth MM, O’Shea D, Steere J, Meeran K, Ghatei MA, Hoggard N, Bloom SR. Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents. FEBS Lett. 1997;415:134–138. doi: 10.1016/S0014-5793(97)01103-4. [DOI] [PubMed] [Google Scholar]
- 8.Ebihara K, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Satoh N, Tamaki M, Yoshioka T, Hayse M, Matsuoka N, Aizova-Abe M. Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes. 1999;48:2028–2033. doi: 10.2337/diabetes.48.10.2028. [DOI] [PubMed] [Google Scholar]
- 9.Shimabukuro M, Koyama K, Chen G, Wang MY, Trien F, Lee Y, Newgard CB, Unger RH. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reidy H, Furst P. Decreased white fat cell thermogenesis in obese individuals. Int J Obes. 1997;21:439. doi: 10.1038/sj.ijo.0800425. [DOI] [PubMed] [Google Scholar]
- 11.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephans TW, Nyce MR, Ohannesian JP, Marco CC, Mc Kee LJ, Bauer TL. Serum immunoreactive leptin concentrations in normal weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 12.Ongphiphadhanakaul B, Rajatanavin R, Chanpraserthyothin S, Piaseu N, Chailurkit L. Serum leptin concentration in relation to body fat, gender, sex hormones and metabolic covariates in Thai. J Med Assoc Thai. 1999;82:862–867. [PubMed] [Google Scholar]
- 13.Ambrosius WT, Compton JA, Bowsher RR, Pratt H. Relation of race, age and hormone differences to serum leptin concentrations in children and adolescents. Horm Res. 1998;49:240–246. doi: 10.1159/000023178. [DOI] [PubMed] [Google Scholar]
- 14.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Fridman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraker DL, Stovroff MC, Merino MJ, Norton JA. Tolerance to tumor necrosis factor in rats and the relationship to endotoxin tolerance and toxicity. J Exp Med. 1988;168:95–105. doi: 10.1084/jem.168.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tracey KJ, Wei H, Manogue KR, Fong Y, Hesse DG, Nguyen HT, Kuo GC, Beuhler B, Cotran RS, Cerami A, Lowry SF. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988;167:1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelin J, Moldawer LL, Lonnorth C, Sherry B, Chizzonite R, Lundholm K. Role of endogenous tumor necrosis factor α and interleukin 1 for experimental tumor growth and the development of cancer cachexia. Cancer Res. 1991;51:415–421. [PubMed] [Google Scholar]
- 18.Sherry BA, Gelin J, Fong Y, Marano M, Wei H, Cerami A, Lowry SF, Lundholm KG, Moldawer LL. Anticachectin/tumor necrosis factor-α antibodies attenuate development of cachexia in tumor models. FASEB J. 1989;3:1956–1962. doi: 10.1096/fasebj.3.8.2721856. [DOI] [PubMed] [Google Scholar]
- 19.Knight DM, Trinh H, Le J, Siegel J, Shealy D, Mc Donough M, Scallan B, Moore MA, Vilcek J, Daddona P. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30:1443–1453. doi: 10.1016/0161-5890(93)90106-L. [DOI] [PubMed] [Google Scholar]
- 20.Nishiya K, Nishiyama M, Chang A, Shinto A, Hashimoto K. Serum leptin level in patients with rheumatoid arthritis are correlated with body mass index. Rinsho-Byori. 2002;50:524–527. [PubMed] [Google Scholar]
- 21.Popa C, Netea MG, Barrera P, Van Riel PL, Van der Meer JWM. Chronic inflammation lowers plasma leptin concentrations in patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63(Suppl 1):136. [Google Scholar]
- 22.Popa C, Netea MG, de Graff J, et al. Circulating leptin and adiponectin concentrations during tumor necrosis factor blockade in patients with active rheumatoid arthritis. J Rheumatol. 2009;36:724–730. doi: 10.3899/jrheum.080626. [DOI] [PubMed] [Google Scholar]
- 23.Anders HJ, Rihl M, Heufelder A, Loch O, Schattenkirchner M. Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism. 1999;48:745–748. doi: 10.1016/S0026-0495(99)90174-9. [DOI] [PubMed] [Google Scholar]
- 24.Tokarczyk-Knapik A, Nowicki M, Wyroślak J. The relation between plasma leptin concentration and body fat mass in patients with rheumatoid arthritis. Pol Arch Med Wew. 2002;108:761–767. [PubMed] [Google Scholar]
- 25.Otero M, Lago R, Gomez R, Lago F, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Gay MA, Garcia-Unzueta MT, Berja A, et al. Anti-TNF-α therapy does not modulate leptin in patients with severe rheumatoid arthritis. Clin Exp Rheumatol. 2009;27:222–228. [PubMed] [Google Scholar]
- 27.van Gossum A, Roland S, Vermeire S, Quertinmont E, Gustot T, Gervy C, Deviere J, Franchimont D (2003) Impact of infliximab on weight regulation and lipid metabolism: immuno-neutralization of TNF-α induced leptinemia in Crohn’s disease. Clin Nutr 22(S1): S51
- 28.Zhang HH, Kumar S, Barnett AH, Eggo MC. Tumor necrosis factor: α exerts dual effects on human adipose leptin synthesis and release. Mol Cell Endocrinol. 2000;159:79–88. doi: 10.1016/S0303-7207(99)00194-X. [DOI] [PubMed] [Google Scholar]
- 29.Granowitz EV. Transforming growth factor-beta enhances and proinflammatory cytokines inhibit ob gene expression in 3T3-L adipocytes. Biochem Biophys Res Commun. 1997;240:382–385. doi: 10.1006/bbrc.1997.7663. [DOI] [PubMed] [Google Scholar]
- 30.Dekkers JC, Geenen R, Godaert GL, Bijlsama JW, van Doornen LJ. Elevated sympathetic nervous system activity in patients with recently diagnosed rheumatoid arthritis with active disease. Clin Exp Rheumatol. 2004;22:63–70. [PubMed] [Google Scholar]
- 31.Targońska-Stępniak B, Majdan M, Dryglewska M. Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol Int. 2007;28:585–591. doi: 10.1007/s00296-007-0480-9. [DOI] [PubMed] [Google Scholar]