Abstract
Study Design
Animal study
Objective
Development of an animal model for the study of biochemical changes that occur in the epidural space after intervertebral disc herniation.
Summary of Background Data
Although strong evidence for an inflammatory component exists, the biochemical processes underlying pain following disc herniation remain unknown.
Methods
Epidural lavage was performed in 48 rats after L5 dorsal root ganglion (DRG) exposure at baseline and 3, 6, or 24 hours after placement of autologous nucleus pulposus (NP) (N = 15), saline (N = 15), or NP + an interferon-gamma antibody (anti-IFNγ; N = 18) directly onto the DRG. Multiplex assays quantifying interleukin (IL-)-1-α, IL-1β, IL-2, IL-4, IL-6, IL-10, TNFα, IFNγ and GM-CSF were performed. NP (N = 7) was also analyzed for these cytokines by placing NP into saline and measuring the relative concentration.
Results
Cytokines measured low at baseline (0–100pg/ml) in all groups. Compared to saline, NP application caused IL-6 elevation, peaking at T=3hr, that was prevented by anti-IFNγ. NP induced elevation of TNFα, peaking at T=24hr and was prevented by anti-IFNγ. IFNγ was elevated after NP at T=3hr and T=24hr. IL-1α was similar after saline versus NP. The concentrations of IL-1β and IL-10 were elevated at T=3hr, 6hr and 24hr in all groups without between-groups difference. The level of IL-4 peaked at T=3hr in the NP group and was different than saline and NP +anti-IFNγ groups but the time effect was insignificant. There was no change for GM-CSF. The concentration of cytokines measured in normal NP was < 2pg/ml for all cytokines except TNFα.
Conclusion
In this model of acute non-compressive disc herniation, NP caused the elevation of epidural IL-6, TNFα and IFNγ; all attenuated by IFNγ blockade. IL-1β and IL-10 were both significantly elevated by saline alone and their response was not prevented by IFNγ blockade. This model may prove useful for the study of the biochemical processes by which NP induces inflammation-induced nerve root irritation and radiculopathic pain.
Keywords: Cytokine, Disc herniation, Inflammation, Epidural, Rat, IL-6, TNF-alpha, Interferon-gamma
Introduction
An association between low back and radiculopathic pain and herniation of nucleus pulposus (HNP) from the intervertebral disc has been recognized for decades,1,2 but the specific mechanisms remain unknown. Traditional thought and utilization of compressive models focused on a mechanism by which mechanical pressure is placed on the dorsal root ganglion (DRG) or nerve roots. However, evidence increasingly supports a mechanism by which neural irritation may result from a combination of compressive and non-compressive etiologies or even in the absence of compression.1,3–9 The observation in human studies that mechanical nerve root stimulation is painful only after prior exposure to nucleus pulposus (NP)10,11 supports both this concept and the possibility that the inflammatory milieu resulting from disc herniation without compression may be sufficient to induce symptoms.1,12 Furthermore, it has been shown that application of NP to the nerve roots or DRG can rapidly sensitize spinal dorsal horn neuronal responses4,5,13,14 or primary afferent neurons.15
The mediators of neuronal sensitization are under investigation, and may include the recruitment of cytokines to the area of inflammation via immunomodulatory cells such as satellite glia or Schwann cells, and resident macrophages.16,17 As NP has been sequestered from circulation since embryologic formation18 it is immunologically naïve, thereby inducing an autoimmune-mediated inflammatory response once herniated.1,12,19,20 It is likely that multiple modulators are involved in a single or in multiple inflammatory cascades. Evidence of this is provided by the clinical efficacy of broad immunosuppressive agents such as steroids and experimental findings that doxycycline, which can modulate expression of several different cytokines, is more effective at alleviating pain behavior than is blockade of a specific pro-inflammatory cytokine such as tumor necrosis factor-alpha (TNFα).21
Few studies have evaluated cytokines in the epidural space in humans with neural root irritation thought to result from disc herniation, partially because of difficulty in accessing this potential space in clinical practice.22,23 Furthermore, clinical variability, including time to presentation, makes this problem difficult to address in clinical studies. An animal model may therefore prove beneficial for further investigation of the mediators involved in NP-induced inflammation and neural sensitization. Cytokine expression was therefore quantified in the epidural fluid around the fifth lumbar dorsal root ganglion (L5 DRG) using lavage at baseline and at various time-points following the application of autologous NP to the L5 DRG. In addition, because interferon-gamma (IFNγ) is a pro-inflammatory cytokine produced by multiple cell types24–27 and can cause the upregulation of various cytokines via the activation of microglia and other cells,26–28 the co-application of a soluble anti-IFNγ antibody with autologous NP was also investigated.
Methods and Methods
Animals
Forty-eight adult male Sprague-Dawley rats (300–400g, Charles River Laboratories) were used in this study. The animals were housed in a temperature-controlled room (22 ± 1°C) in a 12 hour light/dark cycle with food and water provided ad libitum. The experimental procedures were approved by the Stanford University Institutional Animal Care and Use Committee.
L5 Dorsal Root Ganglion Exposure
Rats were anesthetized with isoflurane 3–4% in a chamber, then moved to mask anesthesia with 1.5–2.5% maintenance isoflurane during surgery. The isoflurane concentration was adjusted so that a strong paw pinch failed to evoke a withdrawal response prior to the incision. An incision over the L5-L6 spinous processes was made and the paraspinous muscles were dissected free from the left L5-L6 lamina. Using a dissection microscope and micro-rongeurs, the left L5 inferior articular facet was resected, but the superior articular process of L6 was left intact to minimize DRG trauma and bleeding. This procedure allowed visualization of most of the dorsal side of the left L5 DRG and has been previously described.4,5 The ligamentum flavum was removed between L5 and L6 on the left side, but the dura was left intact.
Epidural lavage of dorsal root ganglion
Baseline lavage was performed following exposure of the left L5 DRG using a micro-pipettor to apply 50 μl of phosphate-buffered saline (pH = 7.4) to the DRG. The solution was allowed to sit for 5 seconds prior to aspiration back into the same pipette. The aspirate was placed into a microcentrifuge tube containing a mix of protease inhibitors (Complete, Roche Diagnostics, Indianapolis, IN; 1 tablet per 1.5ml PBS then aliquoted into 30μl per tube) and kept at −80°C prior to analysis. Multiple time points were analyzed; for 3, 6, and 24 hr post-surgery, animals were randomly assigned to one of three groups based on the solution applied to the DRG: saline only (n = 15), nucleus pulposus (NP) only (n = 15) or NP + co-application of a soluble anti-IFNγ (n = 18) (50 μl of 0.1 mg/ml; R&D Systems, MN, USA).
Coccygeal disc exposure
For animals in the NP or NP + anti-IFNγ groups, a coccygeal disc at the base of the tail was exposed through a skin incision approximately 10 mm in length and 7 mm in width on the dorsal surface of the tail.5 Connective tissue overlying the disc was removed until the outermost surface of the annulus fibrosus was visible. The annulus was incised and the nucleus pulposus was excised with the tip of a #11 scalpel blade and placed onto the exposed L5 DRG immediately. The incision was not taken laterally past the flat surface of the tail, thus avoiding the vasculature, resulting in minimal bleeding. For the animals in the 24-hour experimental group, the spine skin incision was closed with wound clips and the tail skin incision was closed with a 6-0 prolene suture.
Measurement of cytokines in normal nucleus pulposus
In a separate experiment, autologous NP was extracted from a single coccygeal tail disc of multiple rats (n=7) using the same procedure as that described above and placed immediately into a microcentrifuge tube with protease inhibitor and frozen for later analysis. The entire NP was always extracted and placed into a constant volume of solution so that the relative concentration measured between samples was of consistent dilution.
Cytokine analysis
The concentrations of 9 cytokines and chemokines (IFNγ, IL-2, IL-4, IL-6, IL-10, GM-CSF, TNFα, IL-1β and IL-1α) were quantified in samples using a rat 9-plex inflammatory cytokine panel and the BioPlex 200 System (Bio-Rad, Hercules, CA), following the manufacturer’s protocol in a 96-well plate format. This assay utilizes antibodies linked to polystyrene beads containing different levels of fluorophores and has been validated against standard ELISAs.29
Statistical analysis
Analysis of variance (ANOVA) with post-hoc Bonferroni correction for multiple comparisons was used to compare mean cytokine concentrations across the 3 time points compared to baseline and to make comparisons between treatment groups. This analysis was performed using SPSS 9.0 software (Chicago, USA). A p-value < 0.05 was taken as statistically significant.
Results
Data are reported as mean ± standard error of the mean (SEM).
Normal nucleus pulposus
The concentration of TNFα and IL-1α were measured at 64 ± 13 pg/mL and 2 ± 1 pg/ml, respectively. All other cytokines measured were 0 ± 0 pg/ml in the autologous NP (data not shown).
Epidural dorsal root ganglion lavage
IL-6 expression after NP application to the L5 DRG demonstrated a rapid increase over baseline, peaking at 3hr (p < 0.001) and falling back to baseline by 24hr (p = 1.00), respectively, and was significantly greater compared to after saline application (Figure 1; p < 0.005). Soluble anti-IFNγ co-application prevented the enhancement of IL-6 when compared to saline; therefore, the saline and NP+anti-IFNγ mean results were not statistically different (p = 1.0).
Figure 1.
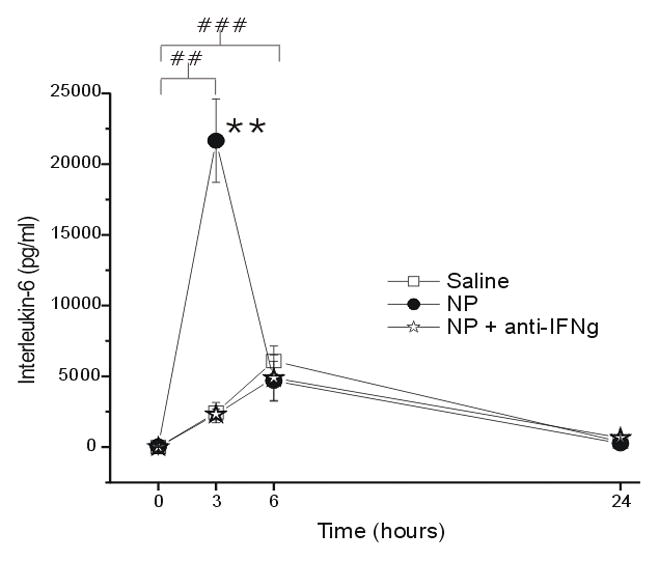
The concentration of interleukin-6 (IL-6; y-axis, picograms/milliliter) measured in the epidural space overlying the L5 DRG at baseline (Time=0hr) and 3, 6 and 24 hr after the application of saline (N = 15), autologous nucleus pulposus (NP=15) or NP + a neutralizing antibody to interferon-gamma (anti-IFNγ; N=18). ## p<0.005 at T=3hr and T=6hr compared to T=0hr; ** p<0.001 between-groups difference between NP and saline groups.
IL-1β expression after saline, NP, and NP+anti-IFNγ application increased over baseline with a peak at 6hr (Figure 2). Although IL-1β levels were statistically significantly elevated at all time points compared to baseline (p < 0.005), there was no difference between NP (p = 1.0) or NP+anti-IFNγ (p = 0.66) treatment groups compared to saline.
Figure 2.
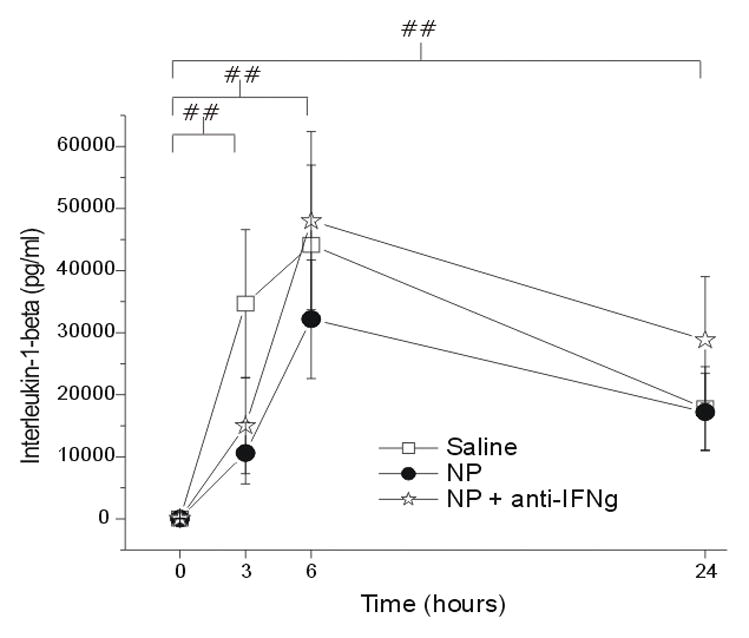
The concentration of interleukin-1-beta (IL-1β; y-axis, picograms/milliliter) measured in the epidural space overlying the L5 DRG at baseline (Time=0hr) and 3, 6 and 24 hr after the application of saline (N = 15), autologous nucleus pulposus (NP=15) or NP + a neutralizing antibody to interferon-gamma (anti-IFNγ; N=18). ## p<0.005 at T=3, 6, 24hr compared to T=0hr.
IL-10 expression peaked at 6hr post saline application and the level of IL-10 was significantly elevated at all time points when compared to baseline in all groups (Figure 3; p < 0.005). However, there was no significant difference between the saline and either NP (p = 0.93) or NP+anti-IFNγ (p = 0.68) groups at any time point.
Figure 3.
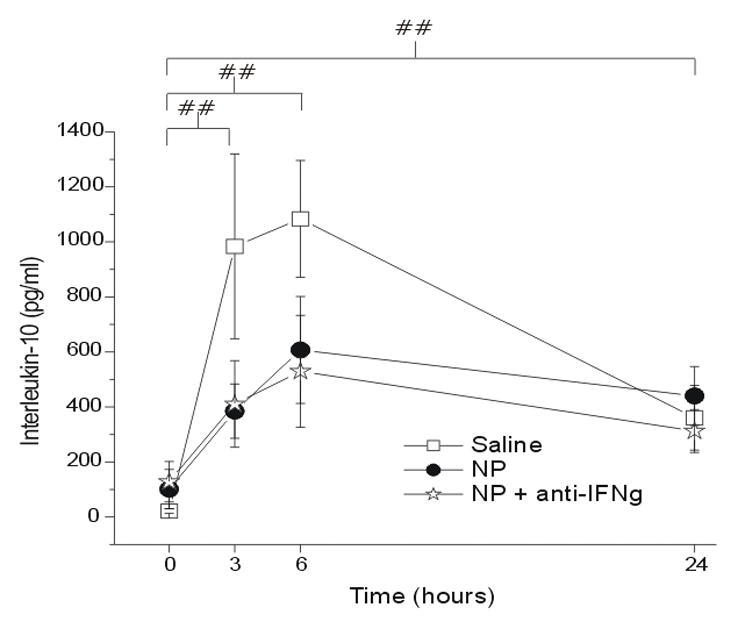
The concentration of interleukin-10 (IL-10; y-axis, picograms/milliliter) measured in the epidural space overlying the L5 DRG at baseline (Time=0hr) and 3, 6 and 24 hr after the application of saline (N = 15), autologous nucleus pulposus (NP=15) or NP + a neutralizing antibody to interferon-gamma (anti-IFNγ; N=18). ## p<0.005 at T=3, 6, 24hr compared to T=0hr.
TNFα levels peaked at 24hr after NP application (Figure 4). Significant differences in expression over time were seen between baseline and 3hr (p < 0.05), 6hr (p < 0.05) and 24hr (p < 0.001). NP application caused a statistically significant increase in expression compared to the saline control group (p < 0.05). The co-application of anti-IFNγ prevented NP-induced elevation of TNFα levels, resulting in no statistically significant difference between the NP+anti-IFNγ and saline groups (p = 0.32).
Figure 4.
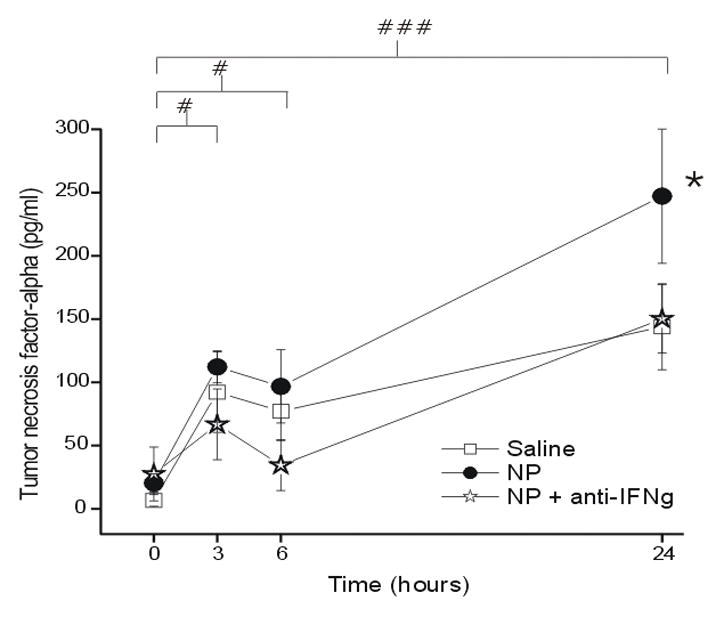
The concentration of tumor necrosis factor-alpha (TNFα; y-axis, picograms/milliliter) measured in the epidural space overlying the L5 DRG at baseline (Time=0hr) and 3, 6 and 24 hr after the application of saline (N = 15), autologous nucleus pulposus (NP=15) or NP + a neutralizing antibody to interferon-gamma (anti-IFNγ; N=18). # p<0.05 at T=3hr and T=6 compared to baseline; ###p<0.001 at T=24hr compared to baseline. * p<0.05 between-groups difference between NP and saline groups.
The measured concentration of IFNγ was also significantly greater in the NP group compared to the saline group, peaking at 3hr (p < 0.05; Figure 5). Time differences of IFNγ concentration were seen between baseline, 3hr and 24hr (p < 0.005). There was also a significantly lower concentration of IFNγ after NP+anti-IFNγ compared to NP alone at 24hr; therefore, there was no difference between the saline and NP+anti-IFNγ groups at 24hr (p = 0.63).
Figure 5.
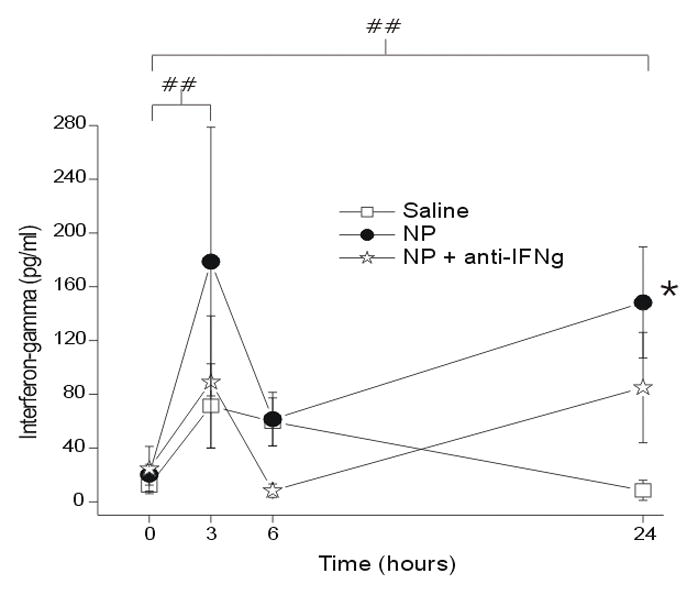
The concentration of interferon gamma (IFNγ; y-axis, picograms/milliliter) measured in the epidural space overlying the L5 DRG at baseline (Time=0hr) and 3, 6 and 24 hr after the application of saline (N = 15), autologous nucleus pulposus (NP=15) or NP + a neutralizing antibody to interferon-gamma (anti-IFNγ; N=18). ## p<0.005 at T=3, 24hr compared to T=0hr; * p<0.05 between-groups difference between NP and saline groups.
The concentration of IL-1α was similar after both saline and NP treatment (p = 1.00) but was significantly elevated at 24hr (p < 0.001) in the NP+anti-IFNγ compared to the saline group (p < 0.05; Figure 6). The measured concentrations were not significantly elevated compared to baseline at 3hr (p = 1.0) and 6hr (p = 0.11).
Figure 6.
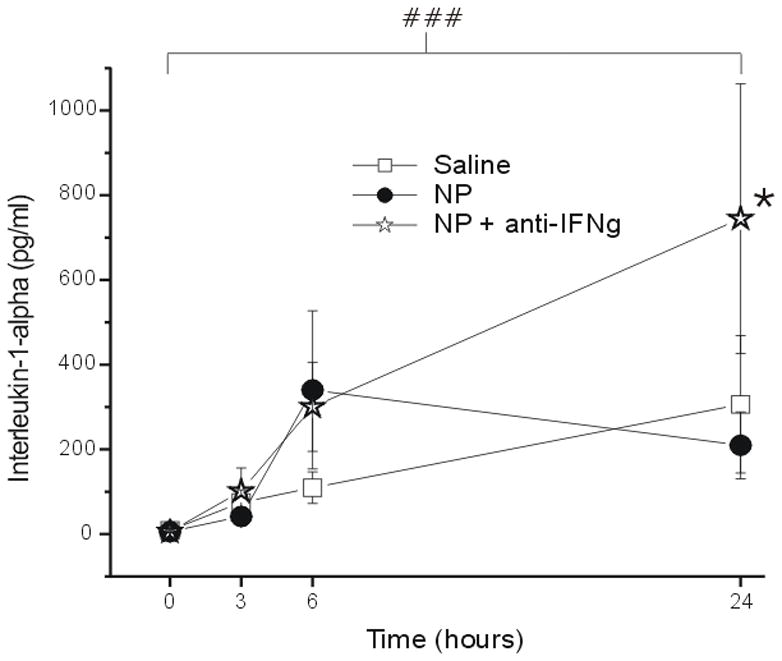
The concentration of interleukin-1-alpha (IL-1α; y-axis, picograms/milliliter) measured in the epidural space overlying the L5 DRG at baseline (Time=0hr) and 3, 6 and 24 hr after the application of saline (N = 15), autologous nucleus pulposus (NP=15) or NP + a neutralizing antibody to interferon-gamma (anti-IFNγ; N=18). ### p<0.001 at T=24hr compared to T=0hr; * p<0.05 between-groups difference between NP+anti-IFNγ and saline group.
Although the concentrations of IL-2 (Figure 7) and IL-4 (Figure 8) increased over baseline (Figure 7) there was no statistically significant difference between groups over time. There was no significant change over time (p = 1.0) and no group difference (p > 0.05) for the concentration of GM-CSF (data not shown).
Figure 7.
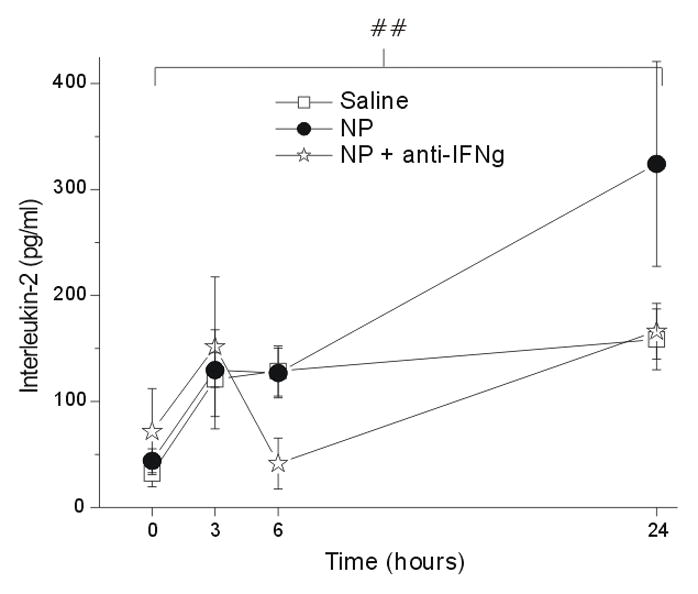
The concentration of interleukin-2 (IL-2; y-axis, picograms/milliliter) measured in the epidural space overlying the L5 DRG at baseline (Time=0hr) and 3, 6 and 24 hr after the application of saline (N = 15), autologous nucleus pulposus (NP=15) or NP + a neutralizing antibody to interferon-gamma (anti-IFNγ; N=18). ## p<0.001 at T=24hr compared to T=0hr.
Figure 8.
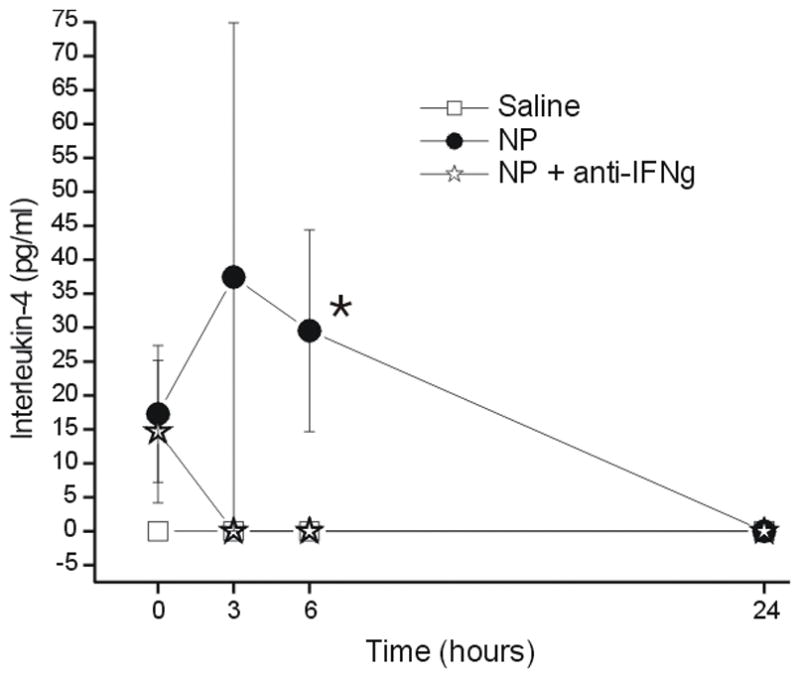
The concentration of interleukin-4 (IL-4; y-axis, picograms/milliliter) measured in the epidural space overlying the L5 DRG at baseline (Time=0hr) and 3, 6 and 24 hr after the application of saline (N = 15), autologous nucleus pulposus (NP=15) or NP + a neutralizing antibody to interferon-gamma (anti-IFNγ; N=18). * p<0.05 between-groups difference between NP and saline groups.
Discussion
In the present study the concentrations of several pro-inflammatory (TNFα, IL-1a, IL-1β, IL-6, GM-CSF), anti-inflammatory (IL-10), T-helper-1 (IFNγ, IL-2) and Th-2 (IL-4) cell-derived cytokines were measured over the course of 24 hours. This study provides an animal model to investigate the biochemical mediators by which exposure of NP results in neural root irritation. Prior animal behavioral data demonstrate pro-inflammatory cytokines can initiate or increase allodynia and hyperalgesia,16,22,30–32 while anti-inflammatory cytokines can act as negative feedback regulators of these cytokines.16,33 Thus regulation of the balance of pro- and anti-inflammatory mediators could potentially play a role in pain from neural root irritation following disc herniation.5,14,34
Nucleus pulposus-induced cytokine expression of IL-6, TNFα, IFNγ, IL-4
The concentrations of IL-6, TNFα, IFNγ and IL-4 were significantly elevated over time after application of NP compared to saline alone. Although there was a marked and significant elevation of IL-1β, IL-10 and IL-2 over time from baseline levels, there was not a significant difference between the saline and NP application groups, indicating that these cytokines are involved in the inflammatory response to surgery but their expression is not further enhanced by the presence of NP. This could represent either a physiologic ceiling effect of the expression of these cytokines or a divergent inflammatory cascade that varies from that which is mediated by NP.
Interleukin-6 is a pro-inflammatory cytokine produced by several cell types, including of the mast- and glial-cell lineages,35–38 and has been shown to sensitize joint nociceptors39 and induce hyperalgesia, possibly via the cyclooxygenase-prostaglandin-E2 pathway.40,41 In the present study the concentration of IL-6 around the L5 DRG was significantly elevated by the surgery but to a significantly greater extent following NP application. This NP-induced enhancement of IL-6 was prevented by co-application of a neutralizing soluble IFNγ antibody, thus providing evidence of an inflammatory pathway that involves IL-6 in an IFNγ-dependent cascade induced by NP.
An NP-induced upregulation of TNFα was also observed in the present study. TNFα is a pro-inflammatory cytokine that plays a critical role in the inflammatory response initiation.42,43 It is produced by various cell types including Schwann cells after nerve injury,42,44,45 neutrophils,46 and infiltrating and resident macrophages during inflammation.8,47 TNFα may play a role in nociceptor sensitization, allodynia, and hyperalgesia43,48,49 and a positive correlation between its tissue concentration and neuropathic pain has been observed.50,51 In addition, there is prior evidence that NP-induced enhancement of spinal cord nociceptive responses may involve TNFα.5 There was a small measurable concentration of TNFα in the autologous NP. Therefore, the TNFα measured in the epidural lavage may be the result of both an inherent supply from within the NP as well as from the production by inflammatory cells locally or recruited to the region in response to NP.
It has been shown that IL-6 and its receptor may be modulated by both TNFα and IFNγ52 and there is evidence that IL-6 upregulation is a function of the concentrations of TNFα and IL-1β.53,54 TNFα has been shown to induce the production of IL-1β, IL-6, and IL-8,7 and there is evidence that IL-1β and IL-6 may act synergistically.53 These findings may be in agreement with the results of the present study observation that IFNγ neutralization prevented the NP-induced enhancement of both IL-6 and TNFα. Although the present study did not observe a significant elevation of IL-1β after NP compared to saline, there was a very marked elevation in IL-1β levels over time following the surgery, an observation also consistent with these previous studies demonstrating complex interactions between TNFα, IFNγ, IL-6 and IL-1β.
The concentration of IFNγ after NP application was significantly greater than after saline. IFNγ was undetectable in the autologous NP lavage. Therefore, IFNγ production may be a mediator of an inflammatory response induced by NP exposure. It has been shown that IFNγ is produced by multiple cell types including natural killer cells, macrophages, activated type 1 helper T-cells, astrocytes and damaged neurons,24–27 and is a key pro-inflammatory cytokine that can sensitize spinal cord dorsal horn neurons,55–57 possibly via the activation of microglia.26–28, 58
Interleukin-4 has been shown to inhibit the expression of pro-inflammatory cytokines IL-1β, TNFα, and IL-659,60 as well as the activation of macrophages and microglia – accounting for its antinociceptive properties.59,61 The concentration of IL-4, an anti-inflammatory cytokine, was significantly greater in the NP group compared to the saline group by ANOVA. However, because the post-hoc test did not find a significant time effect it cannot be concluded with confidence that NP induces the elevation of IL-4 over time compared to saline.
Study limitations
The epidural or intradiscal concentration of cytokines reported in the present study should not be taken as absolute values since there was some dilution upon lavage and placement of the sample into the microfuge tube containing protease inhibitor. However, since the volumes of lavage and protease inhibitor were known and were consistent across animals, this should not affect relative concentration comparisons. Theoretically, the dilution could have reduced the levels of the cytokines (except TNFα) to undetectable levels in the normal NP samples. This dilution was unavoidable, however, as any quantitative protein concentration assay requires some volume of fluid.
The amount of neutralizing soluble IFNγ antibody used in this study (50 μl of 0.1 mg/ml) was based upon results of in vitro neutralization studies provided by the manufacturer whereby the neutralization dose (ND50) is typically 0.08–0.2 μg/ml of antibody in the presence of 2.5 ng/ml of recombinant rat IFNγ. Because no similar prior in vivo study had been performed, it was unknown how much neutralizing antibody would be required to prevent in vivo IFNγ activity after saline or NP application.
The study time points of 3, 6 and 24hrs following HNP may appear to be on a much shorter time span compared to the usual clinical presentation of patients with radiculopathy. However, with the use of standard allometric scaling of physiologic parameters one can estimate that 3, 6 and 24 hr time points in the 0.35kg rat are equivalent to as many as 7, 14 and 56 days, respectively, in the 75kg human.62,63 The time points used in this study may therefore be clinically relevant to the time course of clinically significant HNP-induced radiculopathy.
Conclusions and future studies
The present study has provided an in vivo animal model by which to investigate the biochemical changes that occur in the epidural space contiguous with the dorsal root ganglia and nerve root following exposure of nucleus pulposus by disc herniation. Exposure of nucleus pulposus induced a significant elevation of IL-6, TNFα and IFNγ in a manner that is prevented by neutralization of soluble IFNγ. The surgery alone induced a significant elevation of expression of IL-1β, IL-10, IL-1α and IL-2 and NP did not cause further enhanced levels. The level of GM-CSF was unchanged over time following surgery. The animal model presented may be useful for further studies investigating not only the epidural but also the neuronal changes within the nerve root and dorsal root ganglion that occur following disc herniation. Finally, this model may enable the evaluation of various inflammatory modulators that may be developed for potential therapeutics. It will be important to determine the correlation of these findings with pain behavior in future studies.
Key Points.
The present study describes a new animal model of non-compressive disc herniation
Nucleus pulposus application to the L5 DRG resulted in rapid and marked elevation of epidural IL-6, TNFα, IFNγ and IL-4
IFNγ blockade prevented NP-induced elevation of IL-6, TNFα and IFNγ
There was a marked elevation of IL-1β and IL-10 in response to surgery that was not further enhanced by NP
IL-6, TNFα and IFNγ may interact in an important way in the development of neural root irritation caused by exposure of nucleus pulposus from a disc herniation
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s). Grant funds were received in support of this work. One or more of the author(s) has/have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this manuscript: e.g., honoraria, gifts, consultancies, royalties, stocks, stock options, decision making position.
References
- 1.McCarron RF, Wimpee MW, Hudkins PG, et al. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine (Phila Pa 1976) 1987;12:760–4. doi: 10.1097/00007632-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl O. Hyperalgesia of the lumbar nerve roots in sciatica. Acta Orthop Scand. 1966;37:367–74. doi: 10.3109/17453676608989427. [DOI] [PubMed] [Google Scholar]
- 3.Brisby H, Olmarker K, Larsson K, et al. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11:62–6. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuellar JM, Montesano PX, Antognini JF, et al. Application of nucleus pulposus to L5 dorsal root ganglion in rats enhances nociceptive dorsal horn neuronal windup. J Neurophysiol. 2005;94:35–48. doi: 10.1152/jn.00762.2004. [DOI] [PubMed] [Google Scholar]
- 5.Cuellar JM, Montesano PX, Carstens E. Role of TNF-alpha in sensitization of nociceptive dorsal horn neurons induced by application of nucleus pulposus to L5 dorsal root ganglion in rats. Pain. 2004;110:578–87. doi: 10.1016/j.pain.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Olmarker K. Combination of two cytokine inhibitors reduces nucleus pulposus-induced nerve injury more than using each inhibitor separately. Open Orthop J. 2011;5:151–3. doi: 10.2174/1874325001105010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–7. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Rothman SM, Huang Z, Lee KE, et al. Cytokine mRNA expression in painful radiculopathy. J Pain. 2009;10:90–9. doi: 10.1016/j.jpain.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernstrom V. Disk rupture with surgical observations. Acta Chir Scand Suppl. 1960:258. [Google Scholar]
- 10.Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181–7. [PubMed] [Google Scholar]
- 11.Smyth MJ, Wright V. Sciatica and the intervertebral disc; an experimental study. J Bone Joint Surg Am. 1958;40-A:1401–18. [PubMed] [Google Scholar]
- 12.Marshall LL, Trethewie ER, Curtain CC. Chemical radiculitis. A clinical, physiological and immunological study. Clin Orthop Relat Res. 1977:61–7. [PubMed] [Google Scholar]
- 13.Anzai H, Hamba M, Onda A, et al. Epidural application of nucleus pulposus enhances nociresponses of rat dorsal horn neurons. Spine (Phila Pa 1976) 2002;27:E50–5. doi: 10.1097/00007632-200202010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Onda A, Yabuki S, Kikuchi S. Effects of neutralizing antibodies to tumor necrosis factor-alpha on nucleus pulposus-induced abnormal nociresponses in rat dorsal horn neurons. Spine (Phila Pa 1976) 2003;28:967–72. doi: 10.1097/01.BRS.0000061984.08703.0C. [DOI] [PubMed] [Google Scholar]
- 15.Takebayashi T, Cavanaugh JM, Cuneyt Ozaktay A, et al. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine (Phila Pa 1976) 2001;26:940–5. doi: 10.1097/00007632-200104150-00018. [DOI] [PubMed] [Google Scholar]
- 16.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Watkins LR, Hutchinson MR, Milligan ED, et al. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. 2007;56:148–69. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch C, Schajowicz F. Studies on structural changes in the lumbar annulus fibrosus. Acta Orthop Scand. 1953;22:184–231. doi: 10.3109/17453675208989006. [DOI] [PubMed] [Google Scholar]
- 19.Gertzbein SD, Tile M, Gross A, et al. Autoimmunity in degenerative disc disease of the lumbar spine. Orthop Clin North Am. 1975;6:67–73. [PubMed] [Google Scholar]
- 20.Habtemariam A, Gronblad M, Virri J, et al. Immunocytochemical localization of immunoglobulins in disc herniations. Spine (Phila Pa 1976) 1996;21:1864–9. doi: 10.1097/00007632-199608150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine (Phila Pa 1976) 1998;23:2538–44. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 22.Scuderi GJ, Cuellar JM, Cuellar VG, et al. Epidural interferon gamma-immunoreactivity: a biomarker for lumbar nerve root irritation. Spine (Phila Pa 1976) 2009;34:2311–7. doi: 10.1097/BRS.0b013e3181af06b6. [DOI] [PubMed] [Google Scholar]
- 23.Golish SR, Hanna LS, Bowser RP, et al. Outcome of lumbar epidural steroid injection is predicted by assay of a complex of fibronectin and aggrecan from epidural lavage. Spine (Phila Pa 1976) 2011;36:1464–9. doi: 10.1097/BRS.0b013e3181f40e88. [DOI] [PubMed] [Google Scholar]
- 24.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 25.Chan SH, Kobayashi M, Santoli D, et al. Mechanisms of IFN-gamma induction by natural killer cell stimulatory factor (NKSF/IL-12). Role of transcription and mRNA stability in the synergistic interaction between NKSF and IL-2. J Immunol. 1992;148:92–8. [PubMed] [Google Scholar]
- 26.Racz I, Nadal X, Alferink J, et al. Interferon-gamma is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain. J Neurosci. 2008;28:12136–45. doi: 10.1523/JNEUROSCI.3402-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costigan M, Moss A, Latremoliere A, et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29:14415–22. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuda M, Masuda T, Kitano J, et al. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:8032–7. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jager W, te Velthuis H, Prakken BJ, et al. Simultaenous Detection of 15 Human Cytokines in a Single Sample of Stimulated Peripheral Blood Mononuclear Cells. Clinical and Diagnostic Laboratory Immunology. 2003;10:133–9. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata Y, Olmarker K, Larsson K, et al. Production of tumor necrosis factor-alpha from porcine nucleus pulposus cells at various time points in cell culture under conditions of nutritional deficiency. Cytokine. 2006;34:206–11. doi: 10.1016/j.cyto.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Obreja O, Rathee PK, Lips KS, et al. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16:1497–503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- 32.Sung CS, Wen ZH, Chang WK, et al. Intrathecal interleukin-1beta administration induces thermal hyperalgesia by activating inducible nitric oxide synthase expression in the rat spinal cord. Brain Res. 2004;1015:145–53. doi: 10.1016/j.brainres.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 33.Strle K, Zhou JH, Shen WH, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–49. [PubMed] [Google Scholar]
- 34.Homma Y, Brull SJ, Zhang JM. A comparison of chronic pain behavior following local application of tumor necrosis factor alpha to the normal and mechanically compressed lumbar ganglia in the rat. Pain. 2002;95:239–46. doi: 10.1016/S0304-3959(01)00404-3. [DOI] [PubMed] [Google Scholar]
- 35.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–64. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Bolin LM, Verity AN, Silver JE, et al. Interleukin-6 production by Schwann cells and induction in sciatic nerve injury. J Neurochem. 1995;64:850–8. doi: 10.1046/j.1471-4159.1995.64020850.x. [DOI] [PubMed] [Google Scholar]
- 37.Ito Y, Yamamoto M, Li M, et al. Differential temporal expression of mRNAs for ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), interleukin-6 (IL-6), and their receptors (CNTFR alpha, LIFR beta, IL-6R alpha and gp130) in injured peripheral nerves. Brain Res. 1998;793:321–7. doi: 10.1016/s0006-8993(98)00242-x. [DOI] [PubMed] [Google Scholar]
- 38.Kurek JB, Austin L, Cheema SS, et al. Up-regulation of leukaemia inhibitory factor and interleukin-6 in transected sciatic nerve and muscle following denervation. Neuromuscul Disord. 1996;6:105–14. doi: 10.1016/0960-8966(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 39.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56:351–9. doi: 10.1002/art.22282. [DOI] [PubMed] [Google Scholar]
- 40.Anderson GD, Hauser SD, McGarity KL, et al. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–9. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samad TA, Moore KA, Sapirstein A, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–5. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 42.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–60. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunha FQ, Poole S, Lorenzetti BB, et al. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–4. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murwani R, Hodgkinson S, Armati P. Tumor necrosis factor alpha and interleukin-6 mRNA expression in neonatal Lewis rat Schwann cells and a neonatal rat Schwann cell line following interferon gamma stimulation. J Neuroimmunol. 1996;71:65–71. doi: 10.1016/s0165-5728(96)00131-2. [DOI] [PubMed] [Google Scholar]
- 45.Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996;7:2897–901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- 46.Witko-Sarsat V, Rieu P, Descamps-Latscha B, et al. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–53. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 47.Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998;151:138–42. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- 48.Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85:145–51. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- 49.Perkins MN, Kelly D, Davis AJ. Bradykinin B1 and B2 receptor mechanisms and cytokine-induced hyperalgesia in the rat. Can J Physiol Pharmacol. 1995;73:832–6. doi: 10.1139/y95-113. [DOI] [PubMed] [Google Scholar]
- 50.Lindenlaub T, Sommer C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol. 2003;105:593–602. doi: 10.1007/s00401-003-0689-y. [DOI] [PubMed] [Google Scholar]
- 51.Shafer DM, Assael L, White LB, et al. Tumor necrosis factor-alpha as a biochemical marker of pain and outcome in temporomandibular joints with internal derangements. J Oral Maxillofac Surg. 1994;52:786–91. doi: 10.1016/0278-2391(94)90217-8. discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 52.Sanceau J, Wijdenes J, Revel M, et al. IL-6 and IL-6 receptor modulation by IFN-gamma and tumor necrosis factor-alpha in human monocytic cell line (THP-1). Priming effect of IFN-gamma. J Immunol. 1991;147:2630–7. [PubMed] [Google Scholar]
- 53.Streit WJ, Semple-Rowland SL, Hurley SD, et al. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- 54.Watkins LR, Hansen MK, Nguyen KT, et al. Dynamic regulation of the proinflammatory cytokine, interleukin-1beta: molecular biology for non-molecular biologists. Life Sci. 1999;65:449–81. doi: 10.1016/s0024-3205(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 55.Vikman KS, Hill RH, Backstrom E, et al. Interferon-gamma induces characteristics of central sensitization in spinal dorsal horn neurons in vitro. Pain. 2003;106:241–51. doi: 10.1016/S0304-3959(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 56.Vikman KS, Siddall PJ, Duggan AW. Increased responsiveness of rat dorsal horn neurons in vivo following prolonged intrathecal exposure to interferon-gamma. Neuroscience. 2005;135:969–77. doi: 10.1016/j.neuroscience.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 57.Vikman KS, Duggan AW, Siddall PJ. Interferon-gamma induced disruption of GABAergic inhibition in the spinal dorsal horn in vivo. Pain. 2007;133:18–28. doi: 10.1016/j.pain.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Piehl F, Lidman O. Neuroinflammation in the rat--CNS cells and their role in the regulation of immune reactions. Immunol Rev. 2001;184:212–25. doi: 10.1034/j.1600-065x.2001.1840119.x. [DOI] [PubMed] [Google Scholar]
- 59.Cunha FQ, Poole S, Lorenzetti BB, et al. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-4. Br J Pharmacol. 1999;126:45–50. doi: 10.1038/sj.bjp.0702266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lord CJ, Lamb JR. TH2 cells in allergic inflammation: a target of immunotherapy. Clin Exp Allergy. 1996;26:756–65. [PubMed] [Google Scholar]
- 61.Vale ML, Marques JB, Moreira CA, et al. Antinociceptive effects of interleukin-4, -10, and -13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther. 2003;304:102–8. doi: 10.1124/jpet.102.038703. [DOI] [PubMed] [Google Scholar]
- 62.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–6. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 63.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:351–3. [Google Scholar]


