Abstract
Human muscle undergoes constant changes. After about age 50, muscle mass decreases at an annual rate of 1–2 %. Muscle strength declines by 1.5 % between ages 50 and 60 and by 3 % thereafter. The reasons for these changes include denervation of motor units and a net conversion of fast type II muscle fibers into slow type I fibers with resulting loss in muscle power necessary for activities of daily living. In addition, lipids are deposited in the muscle, but these changes do not usually lead to a loss in body weight. Once muscle mass in elderly subjects falls below 2 standard deviations of the mean of a young control cohort and the gait speed falls below 0.8 m/s, a clinical diagnosis of sarcopenia can be reached. Assessment of muscle strength using tests such as the short physical performance battery test, the timed get-up-and-go test, or the stair climb power test may also be helpful in establishing the diagnosis. Serum markers may be useful when sarcopenia presence is suspected and may prompt further investigations. Indeed, sarcopenia is one of the four main reasons for loss of muscle mass. On average, it is estimated that 5–13 % of elderly people aged 60–70 years are affected by sarcopenia. The numbers increase to 11–50 % for those aged 80 or above. Sarcopenia may lead to frailty, but not all patients with sarcopenia are frail—sarcopenia is about twice as common as frailty. Several studies have shown that the risk of falls is significantly elevated in subjects with reduced muscle strength. Treatment of sarcopenia remains challenging, but promising results have been obtained using progressive resistance training, testosterone, estrogens, growth hormone, vitamin D, and angiotensin-converting enzyme inhibitors. Interesting nutritional interventions include high-caloric nutritional supplements and essential amino acids that support muscle fiber synthesis.
Keywords: Sarcopenia, Muscle mass, Prevalence, Morbidity
A history of muscle loss
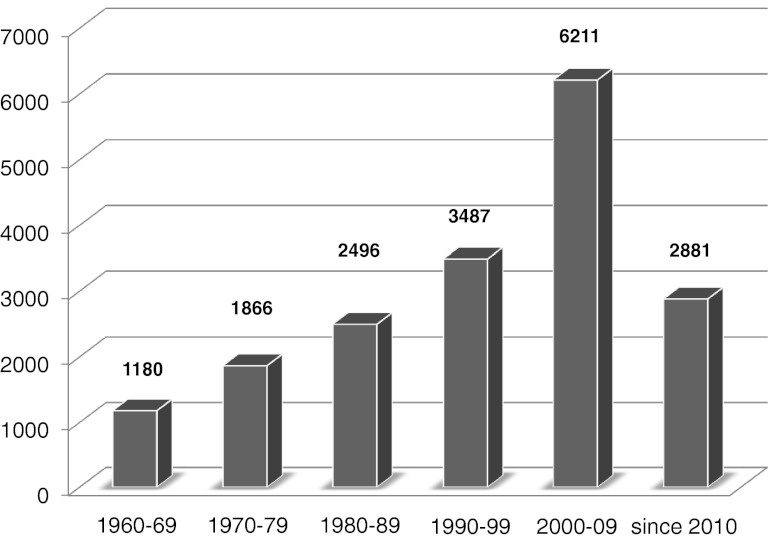
The story of sarcopenia has a rather recent beginning, although a search of the terms muscle wasting and sarcopenia in the online database PubMed buttresses its growing importance over the last years (Fig. 1). Involutional changes of the musculature were described as early as 1931 by Macdonald Critchley, then junior neurologist at King’s College Hospital in London, who wrote that “the entire musculature tends with advancing age to undergo involutional changes, which are manifested as wasting”[1]. He went on saying that “probably the chief cause of this change is to be demonstrated in the general process of senile atrophy, which shows itself in the muscles and elsewhere.” Later on, in the 1970s, Nathan Shock published a series of articles on age-related physiologic functions using data from the first large-scale longitudinal study in this field [2]. Altogether, it evolved that no decline in structure and function is more dramatic than the decline in muscle mass that develops as we age. Irwin Rosenberg realized that if this phenomenon was to be taken seriously, a name was required, and at a meeting in Albuquerque, New Mexico, in 1988, he suggested to use the term sarcopenia [3]. Following the recommendation by Morley, the term took hold over these last 20 years. [4]
Fig. 1.
Number of PubMed entries retrieved after entering the search term “muscle wasting OR sarcopenia”. Assessed on 23 October 2012 from www.pubmed.gov
How to measure muscle mass and muscle strength?
The name sarcopenia is derived from Greek sarx (flesh) and penia (loss), literally meaning poverty of flesh. Sarcopenia is one of the four main reasons for loss of muscle mass, the others being anorexia, dehydration, and cachexia [5, 6]. It is difficult to estimate the prevalence of sarcopenia (Table 1), mostly because of practical difficulties in assessing muscle mass. Many different methodologies have been used over the last 20 years, and new techniques are still being introduced. On average, it is estimated that 5–13 % of elderly people aged 60–70 years are affected by sarcopenia, and the numbers increase to 11–50 % for those aged 80 or above [7]. In line with these data, other sources estimate that 8–40 % of elderly people above the age of 60 years are affected by sarcopenia [8]. Sarcopenia may lead to frailty, but not all patients with sarcopenia are frail. In essence, sarcopenia is about twice as common as frailty [7].
Table 1.
Prevalence of sarcopenia
| Cohort (country) | n (% female) | Age | Sarcopenia definition (assessment method) | Sarcopenia prevalence | Reference |
|---|---|---|---|---|---|
| CHS (USA) | 5,036 (56.4 %) | >65 years | Categories of skeletal mass index, defined as muscle mass normalized for height (BIA) | Moderate sarcopenia, m: 70.7 %, f: 41.9 %; severe sarcopenia, m: 17.1 %, f: 10.7 % | [41] |
| EPIDOS (France) | 1,458 (100 %) | All >70 years; mean 80.3 ± 3.8 years | Appendicular skeletal muscle mass <2 SD below the mean of a young female reference group (DEXA) | 9.5 % | [42] |
| InCHIANTI (Italy) | 1,030 (54.5) | Range: 20–102 | Calf muscle cross-sectional area more than 2 SD below population mean (CT scan) | m: 20 % at 65 years, 70 % at 85 years; f: 5 % at 65 years, 15 % at 85 years | [43] |
| NHANES III (USA) | 14,818 | >18 years; 30 % >60 years | Skeletal mass index was defined as muscle mass/body mass x 100; sarcopenia class I defined as skeletal muscle mass 1–2 SD, sarcopenia class II defined as skeletal muscle mass >2 SD from the mean of young subjects (BIA) | In subjects aged >60 years: sarcopenia class I, m: 45 %, f: 59 %; sarcopenia class II: m: 7 %, f: 10 % | [25] |
| NMEHS (USA) | 808 (47.3 %) | m: 73.6 ± 5.8 years; f: 73.7 ± 6.1 years | Appendicular skeletal muscle mass <2 SD below the mean of a young reference population (substudy of DEXA) | <70 years, m: 13.5–16.9 %, f: 23.1–24.1 %; 70–74 years, m: 18.3–19.8 %, f: 33.3–35.1 %; 75–80 years, m: 26.7–36.4 %, f: 35.3–35.9 %;>80 years, m: 52.6–57.6 %, f: 43.2–60.0 % | [23] |
BIA bioelectrical impedance assessment, CHS Cardiovascular Health Study, CT computed tomography, DEXA dual-energy X-ray absorptiometry, EPIDOS European Patient Information and Documentation Systems, NHANES National Health and Nutrition Examination Survey, NMEHS New Mexico Elder Health Study, SD standard deviation
The broadness in the range of sarcopenia prevalence is partly due to the heterogenecity of study populations, but also due to the different techniques used to assess muscle mass. Dual-energy X-ray absorptiometry (DEXA) is currently considered the gold standard. The name is derived from the fact that two X-ray beams are used with different energy levels of minimal intensity [9]. Other methods used to measure muscle mass include bioelectrical impedance, computed tomography, magnetic resonance imaging, urinary excretion of creatinine, anthropometric assessments, and neutron activation assessments [5]. Depending on the actual technique used in different studies and on the cutoff values chosen, the prevalence of muscle mass may vary considerably (Table 1). Many institutions use handgrip strength as a standard measure for assessing muscle strength. Physical performance can be analyzed using simple and easy-to-do tests such as the short physical performance battery test [10], usual gait speed [11], the timed get-up-and-go test [12], or the stair climb power test [13]. More recently, Scharf and Heineke have argued that “a combination of serum markers, diagnostic imaging, and functional tests of muscle function would constitute an ideal biomarker panel” [14]. A recent consensus statement [15] acknowledges that the list of potential serum markers of sarcopenia is quite long. It embraces markers of inflammation (e.g., C-reactive protein, interleukin-6, and tumor necrosis factor-), clinical parameters, urinary creatinine, hormones (e.g., dehydroepiandrosterone sulfate, testosterone, insulin-like growth factor-1, and vitamin D), products of oxidative damage, or antioxidants [15]. Since all the aforementioned markers are rather indirect measures of muscle loss, novel serum markers directly associated with changes in skeletal muscle mass and function are also promising. The one mentioned in the consensus statement is procollagen type III N-terminal peptide (P3NP) [15]. Another interesting marker in this regard is C-terminal Agrin Fragment, a degeneration product of the neuromuscular junction [16, 17].
Pathophysiological changes in sarcopenia
Using assessments of physical performance it became clear that aging is associated with changes not only in muscle mass but also in muscle composition, contractile, and material properties of muscle as well as in the function of tendons [18]. Therefore, recent consensus definitions include not only changes in muscle mass, but also changes in muscle function like exercise performance. In aging muscle, there is a loss of motor units via denervation. These denervated motor units are recruited by surviving motor units, which puts an increased burden of work on them.
Altogether, there is a net conversion of fast type II muscle fibers into slow type I fibers with resulting loss in muscle power necessary for activities of daily living such as rising from a chair or climbing steps.[18] Other aspects include the deposition of lipids within muscle fibers. These effects — in contrast to cachexia [6] — do not lead to a net loss in body weight, but to a significant reduction in muscle strength. Indeed, in healthy volunteers, the maximal velocity during cycle ergometry decreased by 18 % from the third age decade (20–29 years) to the sixth (50–59 years) [19]. Another 20 % were lost between the seventh (60–69 years) and the ninth age decade (80–89 years) [19]. The loss of maximal oxygen consumption (peak VO2) with increasing age has also been attributed to reduced muscle mass and cardiac output [18]. Our group has recently demonstrated that 19.5 % of a prospectively enrolled cohort of 200 patients with clinically stable chronic heart failure fulfilled the criteria of sarcopenia. Among these patients, muscle wasting remained an independent predictor of reduced peak VO2 after adjusting for age, gender, New York Heart Association class, hemoglobin value, left ventricular ejection fraction, 6-min walk distance, and the number of co-morbidities [20]. Importantly, the risk of falls and consequently of fractures in elderly subjects is closely related to reduced muscle mass as well: Another study found a 2.3-fold increase in the risk of falls after multivariable adjustment among patients in the lower third of handgrip strength as compared to the upper third [21]. Likewise, another large-scale study in more than 2,100 elderly subjects found that a low walking speed is an independent risk factor of falls [22].
Making a diagnosis of sarcopenia
Using the above knowledge, it does not come as a surprise that the likelihood of having a physical disability is higher in elderly subjects who present with height-adjusted appendicular muscle mass 2 standard deviations below the mean of young adult as compared to those with normal muscle mass [23]. Having said that, the difficulty in making a correct diagnosis of sarcopenia is easily understood. Several definitions and diagnostic criteria have been proposed over the last 20 years. Many of them are not easily applicable in daily clinical practice.
A consensus definition formulated by experts from a vast array of different medical fields recently suggested to diagnose sarcopenia when two criteria are fulfilled: (1) a low muscle mass and (2) a low gait speed [24]. Normal muscle mass is defined using data derived from young subjects aged 18–39 years from the Third NHANES population, [25] and the requirement for a diagnosis of sarcopenia is the presence of a muscle mass ≥2 standard deviations below the mean of this reference population. This value can normally be calculated automatically by equipment such as DEXA scanners. A low gait speed is defined as a walking speed below 0.8 m/s in the 4-m walking test [26]. The European Working Group on Sarcopenia in Older People suggested diagnosing sarcopenia when at least two of three criteria apply: (1) low muscle mass, (2) low muscle strength, and/or (3) low physical performance [27]. Cutoff points are defined in a similar manner as by the consensus group mentioned before, namely 2 standard deviations below the mean reference value for muscle mass and muscle strength of a reference population and a gait speed ≤0.8 m/s. A more recent consensus document [28] defines “sarcopenia with limited mobility” as present in a person with muscle loss whose walking speed is equal to or less than 1 m/s or who walks less than 400 m during a 6-min walk, and who has a lean appendicular mass corrected for height squared of 2 standard deviations or more below the mean of healthy persons between 20 and 30 years of age of the same ethnic group. However, these criteria remain cumbersome in daily clinical practice, and easily applicable tests such as handgrip strength testing or one of the biomarkers mentioned above may help to identify patients in need of a more thorough examination.
No consensus has been reached so far as to whether the term sarcopenia should be limited to older persons above 60 years of age or whether it should be used in adults of any age, particularly also in patients with chronic disease [28].
Treatment approaches to sarcopenia
A diagnosis of sarcopenia remains a rare case. But even if the diagnosis is reached, the treatment of sarcopenia remains challenging. Many different approaches have been pursued, but exercise and physical activity are important considerations for both sarcopenia prophylaxis [29, 30] and sarcopenia management [31]. Progressive resistance training, performed two–three times per week by older people, has been shown to improve gait speed, timed get-up-and-go, climbing stairs, and overall muscle strength [32]. Indeed, a recent study in patients with heart failure has shown that daily exercise using a cycle ergometer reduces the expression of ligases from the ubiquitin-proteasome pathway [33]. Nutritional interventions also have an important impact. Current recommendations state that protein should be consumed at a rate of 0.8 g/kg/day, but about 40 % of persons above the age of 70 years do meet this amount [34]. Additional calorie intake of 360 cal per day together with resistance exercise training has been shown to increase leg muscle strength in nursing home residents after 10 weeks. Similar effects were described in cachectic patients [35]. Supplementation of essential amino acids has been shown to improve handgrip strength and 6-min walking distance in elderly subjects after 3 months [36]. Other therapeutic approaches include the use of testosterone, estrogens, growth hormones, vitamin D, and angiotensin-converting enzyme inhibitors [5, 30, 37]. In addition, animal studies have recently reported beneficial effects of soluble activin receptor type IIB (ActRIIB) treatment [38] and myostatin inhibition [39].
The first step in the sarcopenia journey is to create more awareness of this clinical problem, both by the general public and by healthcare professionals. In this respect, the implementation of standardized diagnostic criteria was extremely important. However, sarcopenia is a phenomenon that is present not only in healthy elderly subjects but also in those with chronic illnesses, such as chronic heart or renal failure. More prospective studies are required to understand the prevalence, incidence, phenotype, and the clinical impact of sarcopenia. Such studies are only beginning to emerge. Furthermore, the discussion continues whether sarcopenia, i.e., loss of muscle mass, should be separated from dynopenia, i.e., loss of power. Also, as enthusiasts try to make sarcopenia all encompassing by adding a functional definition, the definition lines between frailty and sarcopenia are becoming blurred. There is a need for a consensus decision regarding the use of these terms.
Acknowledgments
This article is an updated and modified version of an article previously published by the same authors in the Journal of Cachexia, Sarcopenia and Muscle [40]. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, et al. J Cachexia Sarcopenia Muscle 2010; 1:7–8.).
References
- 1.Critchley M. The neurology of old age. Lancet. 1931;217:1331–7. doi: 10.1016/S0140-6736(00)46849-2. [DOI] [Google Scholar]
- 2.Shock NW. Physiologic aspects of aging. J Am Diet Assoc. 1970;56:491–6. [PubMed] [Google Scholar]
- 3.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–1. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE. Aspects of the medical history unique to older persons. JAMA. 1993;269:677–8. doi: 10.1001/jama.269.5.675. [DOI] [PubMed] [Google Scholar]
- 5.Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452–6. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- 6.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morley JE, Kim MJ, Haren MT, Kevorkian R, Banks WA. Frailty and the aging male. Aging Male. 2005;8:135–40. doi: 10.1080/13685530500277232. [DOI] [PubMed] [Google Scholar]
- 8.van Kan A. Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13:708–12. doi: 10.1007/s12603-009-0201-z. [DOI] [PubMed] [Google Scholar]
- 9.Blake GM, Fogelman I. An update on dual-energy x-ray absorptiometry. Semin Nucl Med. 2010;40:62–73. doi: 10.1053/j.semnuclmed.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 11.Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–91. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 12.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 13.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults. Arch Phys Med Rehabil. 2007;88:604–9. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Scharf G, Heineke J. Finding good biomarkers for sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:145–8. doi: 10.1007/s13539-012-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, Van Kan GA, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:181–90. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drey M, Sieber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, et al. C-terminal agrin fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol. 2012. doi:10.1016/j.exger.2012.05.021. [DOI] [PubMed]
- 17.Hettwer S, Dahinden P, Kucsera S, Farina C, Ahmed S, Fariello R, et al. Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol. 2012. doi:10.1016/j.exger.2012.03.002. [DOI] [PubMed]
- 18.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–59. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostka T. Quadriceps maximal power and optimal shortening velocity in 335 men aged 23–88 years. Eur J Appl Physiol. 2005;95:140–5. doi: 10.1007/s00421-005-1390-8. [DOI] [PubMed] [Google Scholar]
- 20.Fülster S, Tacke M, Sandek S, Ebner N, Tschöpe C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J 2012; in press. [DOI] [PubMed]
- 21.Wickham C, Cooper C, Margetts BM, Barker DJ. Muscle strength, activity, housing and the risk of falls in elderly people. Age Ageing. 1989;18:47–51. doi: 10.1093/ageing/18.1.47. [DOI] [PubMed] [Google Scholar]
- 22.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Anderson FH, Cooper C. Falls, sarcopenia, and growth in early life: findings from the Hertfordshire cohort study. Am J Epidemiol. 2006;164:665–71. doi: 10.1093/aje/kwj255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 24.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and precachexia: joint document elaborated by special interest groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–9. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 26.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- 27.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–9. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sipilä S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol. 1995;78:334–40. doi: 10.1152/jappl.1995.78.1.334. [DOI] [PubMed] [Google Scholar]
- 30.Burton LA, Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010;5:217–28. doi: 10.2147/cia.s11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9–21. doi: 10.1007/s13539-010-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009; 3:CD002759. [DOI] [PMC free article] [PubMed]
- 33.Gielen S, Sandri M, Kozarez I, Kratzsch J, Teupser D, Thiery J, et al. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: the randomized Leipzig Exercise Intervention in Chronic Heart Failure and Aging catabolism study. Circulation. 2012;125:2716–27. doi: 10.1161/CIRCULATIONAHA.111.047381. [DOI] [PubMed] [Google Scholar]
- 34.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the health, aging, and body composition (Health ABC) study. Am J Clin Nutr. 2008;87:150–5. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 35.Rozentryt P, von Haehling S, Lainscak M, Nowak JU, Kalantar-Zadeh K, Polonski L, et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle. 2010;1:35–42. doi: 10.1007/s13539-010-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scognamiglio R, Piccolotto R, Negut C, Tiengo A, Avogaro A. Oral amino acids in elderly subjects: effect on myocardial function and walking capacity. Gerontology. 2005;51:302–8. doi: 10.1159/000086366. [DOI] [PubMed] [Google Scholar]
- 37.Fülster S, von Haehling S. A prospective study of the associations between 25-hydroxyvitamin D, sarcopenia progression and physical activity in older adults. Clin Endocrinol. 2011;74:138. doi: 10.1111/j.1365-2265.2010.03888.x. [DOI] [PubMed] [Google Scholar]
- 38.Hagerty L, Lachey JL, Kumar R, Pearsall RS, Sherman M, Seehra J. Age-related lean tissue loss is attenuated by treatment with a form of soluble activin receptor type IIB. J Cachexia Sarcopenia Muscle. 2010;1:65–6. [Google Scholar]
- 39.Murphy KT, Koopman R, Naim T, Léger B, Trieu J, Ibebunjo C, Lynch GS. Antibody-directed myostatin inhibition in 21-mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function. FASEB J. 2010;24:4433–42. doi: 10.1096/fj.10-159608. [DOI] [PubMed] [Google Scholar]
- 40.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–33. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen I. Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 42.Rolland Y, Lauwers-Cances V, Cournot M, Nourhashémi F, Reynish W, Rivière D, et al. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;51:1120–4. doi: 10.1046/j.1532-5415.2003.51362.x. [DOI] [PubMed] [Google Scholar]
- 43.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]