Abstract
Background
Cancer can lead to weight loss, anorexia, and poor nutritional status, which are associated with decreased survival in cancer patients.
Methods
Male cancer patients (n = 136) were followed for a mean time of 4.5 years. Variables were obtained at baseline: cancer stage, albumin, hemoglobin, tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, bioavailable testosterone, appetite questionnaire, and weight change from baseline to 18 months. Primary statistical tests included Kaplan–Meier survival analysis and Cox proportional hazard regression (PHREG).
Results
Univariate PHREG showed that cancer stage, albumin, hemoglobin, TNF-α, IL-6, and weight change were each significantly associated with mortality risk (P < 0.05), but bioavailable testosterone was not. Multivariate PHREG analysis established that weight change and albumin were jointly statistically significant even after adjusting for stage.
Conclusion
In this sample of male oncology patients, cancer stage, serum albumin, and weight loss predicted survival. High levels of inflammatory markers and hemoglobin are associated with increased mortality, but do not significantly improve the ability to predict survival above and beyond cancer stage, albumin, and weight loss.
Electronic supplementary material
The online version of this article (doi:10.1007/s13539-012-0075-5) contains supplementary material, which is available to authorized users.
Keywords: Inflammation, Anorexia, Interleukin-6, TNF-α, Testosterone
Background
Almost 1.5 million new cases of cancer are diagnosed annually in the USA [1]. Weight loss is commonly observed in several types of cancer, and poor nutritional status has been associated with decreased survival [2] and poorer quality of life [3] in the cancer population. Several factors can negatively impact nutritional status and lead to weight loss, including appetite changes, inflammation [4–6], and hypermetabolism [7].
Certain parameters, such as weight change and serum albumin levels, are commonly used as part of nutritional assessments in this setting and have been shown to predict survival [8]. Activation of proinflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor alpha (TNF-α), are also thought to decrease appetite, increase muscle wasting, decrease food intake, and contribute to a hypermetabolic state [9–13] in the setting of cancer. Hence, they also are being proposed as prognostic indicators.
Cancer cachexia is a metabolic syndrome that results in overall weight, fat, and muscle loss. Poor appetite, inflammation, insulin resistance, and protein catabolism are associated with this syndrome [14]. Cachexia is very prevalent in cancer patients occurring in over 80 % of gastric, pancreatic, and esophageal cancer, 70 % of head and neck cancer, and approximately 60 % of lung, colorectal, and prostate cancer [15]. Previously, cachexia was defined as a body mass index (BMI) of <20 and/or an unintentional weight loss of ≥5 % in the previous 6 months [2] in the setting of underlying disease, such as cancer. A more recent definition has been proposed, which would incorporate the ≥5 % weight loss over 12 months or less plus three of the following: decreased muscle strength, BMI of <20, fatigue, anorexia, low fat-free mass index, and abnormal biochemistry (increased inflammatory markers such as CRP or IL-6, anemia (hemoglobin of <12 g/dL), or low serum albumin of <3.2 g/dL) [16]. However, this definition has not been fully validated in a clinical setting.
Low testosterone levels are commonly seen in cancer patients [17] and are associated with decrease muscle mass, fatigue, increased inflammation, and poor quality of life in other settings. However, whether testosterone levels are associated with decreased survival in the setting of cancer is not known.
The specific aim of this research was to investigate the role of traditional nutritional markers, such as appetite, serum albumin, and weight loss, and newer inflammatory markers and hormones in cancer survival. Given the new proposed diagnosis of cachexia, this study additionally aimed to examine these biochemical markers in the new definition of cachexia for their impact on survival. This study tested the hypothesis that survival in male cancer patients is predicted by nutritional status (weight change percent, serum albumin, hemoglobin, and perception of appetite), hormones, and inflammatory markers.
Methods
Subjects
All study protocols and procedures were reviewed and approved by the Baylor College of Medicine Institutional Review Board and Michael E. DeBakey VAMC Research and Development Committee for Protection of Human Subjects. This study was a cross-sectional design at recruitment, with a retrospective follow-up component gathered from the electronic medical records. Upon recruitment, blood draws and other data were gathered. At two follow-up time points, the electronic medical record was used to retrospectively gather additional data on weight and survival. Protocols, laboratory assay procedures, and participants for a subset of this cohort have been described earlier [9]. Participants were included if they were adult males with cancer. Women were not included in this study due to the small percentage of females in this population (i.e., veterans with cancer). All cancer diagnoses other than nonmelanomatous skin cancer and all stages were included. Exclusion criteria were identified due to their potential influences on body weight, nutritional intake, or metabolic rate: physician-recorded diagnosis of formally evaluated dysphagia (by speech pathologists), illicit drug or alcohol abuse, congestive heart failure, abnormal liver function, severe chronic obstructive pulmonary disease, uncontrolled diabetes (fasting glucose levels of >140 mg/dL or random glucose levels of >200 mg/dL), thyroid disease, kidney disease, active infection, history of neuroendocrine tumor, diagnosed eating disorders, or use of orexigenic agents (e.g., glucocorticoids, progesterone, testosterone, and antiandrogens). Subjects were recruited between September 2003 and October 2009 from the MEDVAMC Cancer Center outpatient clinic. Human subjects' approval allowed researchers to use the electronic medical chart to review and gather weight and survival data for an extended period before and after baseline enrollment measures were taken. All survival data were collected until August 2010 or until death, whichever occurred first. Subjects were followed for a maximum of 1,500 days.
Variables
The following variables were measured at baseline: demographic and other data regarding type of cancer and cachexia diagnosis, cancer stage, weight history, serum albumin, hemoglobin, TNF-α, IL-6, bioavailable testosterone, and visual analogue scale (VAS) for appetite questionnaire results. The VAS questions were adapted from the Edmonton Symptom Assessment System to focus more on appetite and food intake (available in Supplemental Material), which has been shown to be reliable in the cancer population [18–20]. The VAS consisted of eight questions about appetite and nausea/vomiting. Nausea and vomiting are common issues affecting food intake for cancer patients. Subjects indicated a number from zero to nine (zero equating a less favorable or “decreased” response to the appetite question) for six of the questions. The scale was reversed (zero equating a more favorable response) for one question that asked: “How much nausea have you had on average over the previous week?” The last question asked patients to categorically choose: “How many times have you vomited over the past week?”
Typically before and after study enrollment, these subjects were seen quarterly as part of their medical or oncology care. Weights were followed for a maximum of 18 months after study enrollment via medical chart review. This follow-up time interval was selected to minimize missing weight data. Weights were recorded every 3 months during 18 months of follow-up. Weight change percent was calculated using patient's body weight at enrollment (baseline) and 18 months postenrollment (follow-up). If the subject died before the 18 months, the last weight recorded was used to calculate the percent of weight change. The Social Security Death Index [21] and medical record were used to enter date of death, current to the day that statistical analyses were performed (approximately 4.5 years after recruitment).
Statistics
All statistical analyses were performed using SPSS software (SPSS 12.0, 2004; Somers, New York). Statistical significance was assigned to each comparison for P < 0.05, and appropriate post hoc tests were used when needed during analyses of variance. It was anticipated that cancer stage may be highly associated with survival. The primary design of this study was to apply the stepwise SPSS Cox Proportional Hazards Regression procedure (PHREG) to determine which variables, in addition to cancer stage, were significant predictors of mortality.
Results
One hundred thirty-six men were included in the analysis. Cancer diagnoses included: 38 lung (11 small cell carcinoma and 27 non-small cell carcinoma), 28 colorectal, 14 prostate, 11 head and neck, seven adenocarcinoma of unknown origin, seven lymphomas, five squamous cell cancers of unknown origin, four leukemias, three renal cancers, three melanomas, three sarcomas, three liver cancers, two bladder and one subject each representative of: thyroid, multiple myeloma, ampulloma, brain, intestinal, appendix, basal cell, fibrosarcoma, chondrosarcoma, and male breast cancers.
Descriptive statistics
Descriptive statistics, categorized by cancer stage, are summarized in Table 1. Twelve stage I patients survived (71 %), along with 17 stage II (65 %), 21 stage III (62 %) and 11 stage IV patients (23 %). There was a significant difference when comparing cancer stages II and III in age (P = 0.02), but no difference between races (Caucasian vs. all others P = 0.059). Mean (SD) concentration of bioavailable testosterone, cytokines and other variables are displayed in Table 2. VAS questions which were not significant are not shown.
Table 1.
Descriptive statistics by cancer stage
| Cancer stage | ||||
|---|---|---|---|---|
| I | II | III | IV | |
| n = 17 | n = 26 | n = 34 | n = 48 | |
| Age (mean, range) | 66 (54–83) | 68 (44–85)a | 62 (50–73)a | 65 (42–92) |
| Raceb | ||||
| Caucasian (%) | 13 (76) | 14 (54) | 29 (85) | 34 (71) |
| African-American | 4 | 9 | 3 | 12 |
| Hispanic | 0 | 2 | 2 | 1 |
| Asian | 0 | 1 | 0 | 1 |
| Baseline weight (kg; mean, range) | 89.1 (56.8–112.3) | 82.7 (60.5–106.4) | 86.4 (49.1–115) | 81.8 (41.8–121.8) |
| Weight loss of ≥5 % in previous 6 months (%)c | 2 (12) | 5 (19) | 4 (12) | 15 (31) |
| Deaths recorded (%) | 5 (29) | 9 (35) | 13 (38) | 37 (77) |
aSignificantly different between cancer stages II and III at α = 0.05 (ANOVA with Tukey's b post hoc test)
bMissing values for cancer stage were noted for one African-American subject and ten white subjects
cEquivalent to the percentage of individuals with cachexia according to the traditional definition of cachexia (>5 % weight loss)
Table 2.
Descriptive statistics for all variables
| Variable | Number | Mean (SD) | Range |
|---|---|---|---|
| Survival (days) | 136 | 986 (561) | 5–1,500 |
| Baseline serum albumin (g/dL) | 130 | 3.50 (0.55) | 1.5–4.7 |
| Baseline hemoglobin (g/dL) | 129 | 12.5 (1.88) | 7.4–16.2 |
| TNF-α (pg/mL) | 127 | 4.58 (6.21) | 0.00–58.5 |
| IL-6 (pg/mL) | 127 | 9.55 (18.6) | 0.18–130.6 |
| Bioavailable testosterone (ng/dL) | 112 | 60.2 (52.0) | 0.29–311 |
| VAS 5 (score)a | 134 | 5.3 (2.9) | 0–10 |
| Weight change (%)b | 134 | −4.56 (11.1) | −29.9 to 21.9 |
aVAS 5 is a question from a visual analogue scale questionnaire for appetite (see Supplemental Materials). The VAS 5 question: “How would you rate/describe your appetite?” is rated on self-report on a scale of 0 “decreased” to 9 “increased”
bWeight change percent was calculated in kilogram changed from baseline up to 18 months postenrollment, divided by baseline weight. The range of weight change was 24 kg lost to 16.5 kg gained. If the subject died before the 18 months, the last weight recorded was used to calculate weight change percent. Weights were recorded every 3 months for 18 months. A survival time of 1,500 represents the subject surviving until the end of the study period (e.g., maximum survival)
Survival analysis and ANOVA
The effect of cancer stage on survival was based on the SPSS Kaplan Meier analysis and revealed that cancer stage IV survival (median survival = 370 days) was significantly different from cancer stages I, II, and III (899, 859 and 739 days respectively, P < 0.0005). Therefore, all subsequent analyses controlled for the effect of cancer stage. Additionally, other analyses determined variables associated with cancer stage, and a one-way ANOVA was performed for each variable by cancer stage. The only significant result (F = 3.49, df = 3, n = 123; P = 0.02) found during the global ANOVA and post hoc t testing was that weight loss percent in the previous 6 months before baseline was significantly different between cancer stages II (mean weight gain of 5.4 %) and IV (mean weight loss of −1.1 %). All other variables (serum albumin, appetite score, hemoglobin, testosterone, or inflammatory markers) did not significantly differ by cancer stage.
Hazards analysis
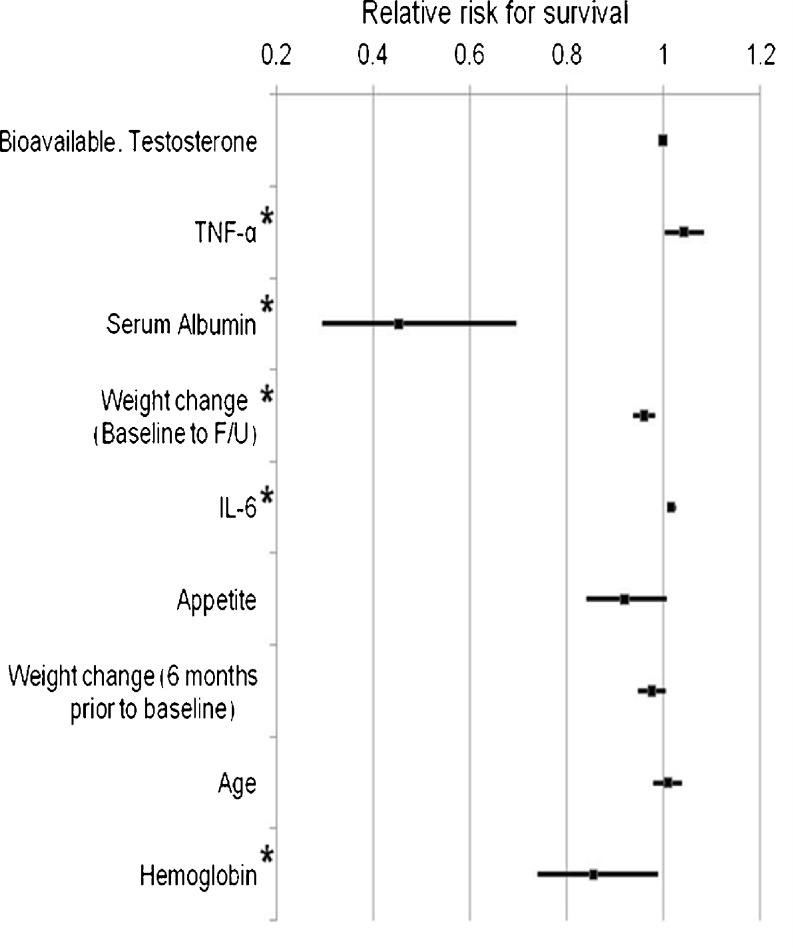
A Cox regression model was subsequently analyzed for each of the variables, where each model would also account for the effect of cancer stage. The results showed that weight change percent, serum albumin, hemoglobin, TNF-α, and IL-6 were significantly associated with mortality (Fig. 1). The hazard of mortality was significantly greater with greater weight loss from baseline to follow-up, low serum albumin, low hemoglobin, and high TNF-α and IL-6. Hazard ratios for bioavailable testosterone, appetite scores, and age were not statistically significant.
Fig. 1.
Cox proportional hazard regression models including the effects of different variables on survival accounting for cancer stage. *P < 0.05. All variables were assessed at baseline. Weight change (“baseline to F/U”) was measured from baseline to 18 months follow-up. Weight change (“6 months prior to baseline”) was measured from 6 months prior to baseline. Appetite is measured using a visual analogue scale question: “How would you rate/describe your appetite?” and is rated on self-report on a scale of 0 to 9 (Supplemental Materials)
To determine which combination of variables predicted survival jointly and independently of cancer stage, the preceding significant results were entered in a stepwise Cox proportional hazard regression analysis. The cancer stage variable was forced into the model at step one to account for its role in mortality. Then, the five additional variables affecting the greatest additional changes (serum albumin, weight change percent, IL-6, TNF-α, and hemoglobin) were entered in a stepwise manner until no further significant improvement could be attained. This stepwise Cox proportional hazard regression showed that the variables having the greatest prediction of survival were weight change percent and serum albumin (Table 3; P = 0.002 and 0.001, respectively). Greater mortality was associated with greater weight loss and lower serum albumin. In this model, hemoglobin, IL-6, and TNF-α did not remain significant predictors of survival when cancer stage, weight loss from baseline to follow-up and serum albumin were also considered. Due to the greater number of stage IV patients, the same Cox proportional hazard regression was performed on this subset of subjects (data not shown). The results did not differ from those described above.
Table 3.
Cox proportional hazards stepwise regression analysis results for independently significant variables
| Variable | Number | df | Parameter estimate | SEE | P value | Hazard ratio | 95 % CI |
|---|---|---|---|---|---|---|---|
| Cancer stagea | 125 | 1 | 0.634 | 0.175 | <0.0005 | 1.89 | 1.338–2.657 |
| Serum albumin (g/dL) | 130 | 1 | −0.588 | 0.293 | 0.045 | 0.556 | 0.313–0.986 |
| Weight change (%) | 134 | 1 | −0.035 | 0.012 | 0.002 | 0.965 | 0.944–0.988 |
| IL-6 (pg/mL) | 127 | 1 | 0.003 | 0.007 | 0.635 | 1.00 | 0.990–1.016 |
| TNF-α (pg/mL) | 127 | 1 | 0.030 | 0.023 | 0.204 | 1.03 | 0.984–1.078 |
| Hemoglobin (g/dL) | 129 | 1 | −0.065 | 0.088 | 0.463 | 0.937 | 0.788–1.115 |
aCancer stage was forced into the stepwise analysis. Subsequent variables' P values represent their significance above and beyond the effect of cancer stage in step one of the Cox proportional hazard regression model. Only three of these variables remained significant after stepwise analysis (cancer stage, P < 0.0005; serum albumin, P = 0.001; and weight change, P = 0.002)
Discussion
Cachexia and poor nutritional status are associated with worsened prognosis in many chronic conditions including cancer [22, 23]. However, they often remain undiagnosed and untreated in spite of the significant burden that they represent to cancer patients. In this population, nutritional risk factors traditionally associated with greater mortality include weight loss, serum albumin, and anorexia [24]. More recently, proinflammatory cytokines [6, 9] and other hormonal factors, including testosterone levels [17, 25], have been postulated to play a role in the development of cachexia, although their prognostic value has not been fully established. This study aimed at establishing the relationship between survival and these collective factors in a group of male cancer patients.
The hypothesis that nutritionally related variables predict survival was supported by the results. As expected, cancer stage was the strongest predictor of survival. However, weight loss from baseline to 18-month follow-up and low serum albumin predicted decreased survival above and beyond the effect of cancer stage. This is in agreement with previous reports of a loss of >8.1 kg of body weight and serum albumin levels of <3.5 mg/dL were associated with shorter survival in terminally ill patients [8], and that lean body mass loss was associated with declining serum albumin, and that low serum albumin predicted mortality [26] in geriatric cancer patients. While loss of lean body mass measured by total body potassium is the ultimate indicator of cachexia progression, this is not a clinically available test (there are only four sites in the USA that have the capability to measure total body potassium, and none of them offers this test for clinical purposes). Based on this, we sought to establish the predictive value and relative contribution of a battery of tests (most of them are inexpensive and already clinically available) on mortality. Simons et al. [27] found that lower levels of serum albumin, testosterone, and IGF-1 were associated with a greater weight loss (≥10 %) in a sample of lung cancer patients. Our data suggest that these markers are associated with poor survival not only in terminally ill subjects, but also in nonterminally ill patients with earlier stages of disease. We are not aware of any other study of this nature performed in nonterminal patients.
The role of inflammatory markers in cancer patients has been investigated before [9, 27, 28]. However, whether these markers significantly add to the more traditional markers of survival discussed before is still controversial. In a study of 39 cachectic and noncachectic subjects with stage IV upper gastrointestinal cancers, Dulger et al. [29] found elevated levels of TNF-α, IL-1β, and IL-6 in cancer patients when compared to healthy controls. Also, elevated IL-6 and lower serum albumin and transthyretin levels predicted mortality in a geriatric population with cancer cachexia [30]. To the contrary, a study of lung cancer patients undergoing chemotherapy [28] found no significant difference in IL-6 between cancer patients and healthy controls. This same study also found no differences between patient/disease characteristics and TNF-α and C-reactive protein, and that these variables were similar in subjects independent from severity of weight loss. In our study, the inflammatory markers, IL-6 and TNF-α, independently predicted the survival beyond the effect of cancer stage. However, when they were considered alongside serum albumin and weight loss, their significance dwindled. Taken together, the data suggest that even though inflammatory markers predict survival in the cancer population, they may not necessarily provide more information to the clinician when other more readily accessible markers, such as cancer stage, serum albumin, and weight loss, are considered.
A new definition of cachexia has been recently proposed that includes not only the traditional markers of weight loss and anorexia, but also increased inflammatory markers and low serum albumin and hemoglobin, among other factors [16]. Our results support the use of serum albumin along with weight loss in predicting survival in the setting of cancer, but suggest that inflammatory markers may not necessarily improve our ability to predict survival in this population when cancer staging, serum albumin, and weight loss history are available.
Other factors of interest evaluated here included appetite and the anabolic hormone testosterone. Cancer patients who develop anorexia have decreased energy intake [31], and a review of 22 studies by Homsi and Luong [14] found that anorexia is a symptom commonly associated with decreased survival. The prevalence of hypogonadism in male patients with cancer ranges from 40 to 90 % [17, 32], and there is a well-developed body of knowledge suggesting that, in hypogonadal men without cancer, testosterone replacement improves lean body mass, muscle strength, libido, mood, sexual dysfunction, and appetite. This has led to the hypothesis that low testosterone levels contribute to the development of these symptoms in the setting of cancer and ultimately lead to poor outcomes. Recently, low testosterone levels were associated with decreased survival in one study [33], but not in another [34]. In this study, neither appetite scores nor bioavailable testosterone were significant predictors of survival (P = 0.06 and 0.08, respectively). Since there are many other factors that could contribute to the development of anorexia or low testosterone levels (i.e., depression, chemotherapy side effects, opioid use, etc.), it may be that a larger sample would establish their significance.
There were several strengths to this study. Although most of the factors examined in this study have been individually investigated as predictors of survival before, most reports do not take into account their interaction. Given that there is an association between these markers in most cases, the fact that our study included all the significant markers in the final predictive model is the strength of the study. Also, most other studies, looking at these variables, have been limited to subjects with a life expectancy of 6 months or less. The fact that this study included nonterminally ill patients indicates that assessing these variables is also useful in the nonterminal cancer population. The homogeneity of the male sample reduces some variability. Also, the extended length of time was given to follow up maximized survival data in the statistical analysis.
There were also limitations to this study. It was not focused to one type or stage of cancer, and it was a relatively small sample size. Multiple cytokines have been associated with inflammation and cachexia; however, this study looked at IL-6 and TNF-α. Other cytokines or markers of inflammation, such as C-reactive protein, could possibly add more information to the results. It was homogenously male, and results may not be relevant in a female sample. Women were excluded from this study because of the small number of females in this population; the population of women in the Veterans Medical Center, where this study was conducted, is approximately 3 %. We also had a relatively large number of stage IV subjects. However, this sample is representative of our VA population where men, smokers, the elderly, and minority populations are overrepresented, which could explain this. The heterogeneity of our sample due to the variable nature of the disease and different diagnoses is a limitation to our study. This issue potentially could decrease the power of the study, and that is the ability to detect a true difference (i.e., that these factors predict survival according to the a priori hypothesis, but we failed to show this). However, the fact that weight loss, serum albumin, cytokines, and hemoglobin did predict survival, in spite of the heterogeneity of the sample, suggests that this study is adequately powered. The variability introduced in the model by the fluctuating course of the disease is partially accounted for by the large follow-up period (4.5 years).
This study stimulates ideas for future hypotheses. Our data suggest that simple and inexpensive testing may be helpful to assess an oncology patient's long-term risk of mortality. Calculations of body weight change and measuring serum albumin may give the clinician a more accurate picture of patient's prognosis without the need for more expensive laboratory testing, such as cytokine or hormone measurements. Further studies in a larger cohort of patients would be needed in order to confirm these findings and to determine if preventing weight loss in this population improves survival. Future research should investigate the impact of nutritional interventions (including counseling by trained dietitians, behavioral therapy, orexigenic agents, and nutritional supplements) along with other promising interventions, such as anabolic agents, resistance training, and anti-inflammatory agents (or a combination of them) on weight loss, serum albumin, survival, quality of life, and treatment tolerance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 22 kb)
Acknowledgments
This material is based upon work supported by the Dept. of Veterans Affairs (MREP, SCVAHNCDA, MERIT awards BX000507 and CX000174). Dr. Garcia receives research support from Abbott, Aeterna Zentaris, and Helsinn Therapeutics. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle [35].
Conflict of interest
The authors declare that they have no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Maltoni M, Nanni O, Pirovano M, Scarpi E, Indelli M, Martini C, et al. Successful validation of the palliative prognostic score in terminally ill cancer patients. Italian multicenter study group on palliative care. J Pain Symptom Manage. 1999;17:240–7. doi: 10.1016/S0885-3924(98)00146-8. [DOI] [PubMed] [Google Scholar]
- 3.Strasser F, Bruera ED. Update on anorexia and cachexia. Hematol Oncol Clin North Am. 2002;16:589–617. doi: 10.1016/S0889-8588(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 4.Sonti G, Ilyin SE, Plata-Salaman CR. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am J Physiol. 1996;270:R1394–402. doi: 10.1152/ajpregu.1996.270.6.R1394. [DOI] [PubMed] [Google Scholar]
- 5.Opara EI, Laviano A, Meguid MM. Correlation between food intake and cerebrospinal fluid interleukin 1 alpha in anorectic tumor-bearing rats. Nutrition. 1995;11:678–9. [PubMed] [Google Scholar]
- 6.Laviano A, Gleason JR, Meguid MM, Yang ZJ, Cangiano C, Rossi Fanelli F. Effects of intra-VMN mianserin and IL-1ra on meal number in anorectic tumor-bearing rats. J Investig Med. 2000;48:40–8. [PubMed] [Google Scholar]
- 7.Jatoi A, Daly BD, Hughes VA, Dallal GE, Kehayias J, Roubenoff R. Do patients with nonmetastatic non-small cell lung cancer demonstrate altered resting energy expenditure? Ann Thorac Surg. 2001;72:348–51. doi: 10.1016/S0003-4975(01)02847-8. [DOI] [PubMed] [Google Scholar]
- 8.Vigano A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez-Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med. 2000;160:861–8. doi: 10.1001/archinte.160.6.861. [DOI] [PubMed] [Google Scholar]
- 9.Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–6. doi: 10.1210/jc.2004-1788. [DOI] [PubMed] [Google Scholar]
- 10.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–71. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 11.Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D, Jr, Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. 2003;97:1211–6. doi: 10.1002/cncr.11178. [DOI] [PubMed] [Google Scholar]
- 12.Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279–92. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- 13.Lyngso D, Simonsen L, Bulow J. Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. J Physiol. 2002;543:379–86. doi: 10.1113/jphysiol.2002.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homsi J, Luong D. Symptoms and survival in patients with advanced disease. J Palliat Med. 2007;10:904–9. doi: 10.1089/jpm.2007.0004. [DOI] [PubMed] [Google Scholar]
- 15.Laviano A, Meguid MM. Nutritional issues in cancer management. Nutrition. 1996;12:358–71. doi: 10.1016/S0899-9007(96)80061-X. [DOI] [PubMed] [Google Scholar]
- 16.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JM, Li H, Mann D, Epner D, Hayes TG, Marcelli M, et al. Hypogonadism in male patients with cancer. Cancer. 2006;106:2583–91. doi: 10.1002/cncr.21889. [DOI] [PubMed] [Google Scholar]
- 18.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 19.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–71. doi: 10.1002/(SICI)1097-0142(20000501)88:9<2164::AID-CNCR24>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Heedman PA, Strang P. Symptom assessment in advanced palliative home care for cancer patients using the ESAS: clinical aspects. Anticancer Res. 2001;21:4077–82. [PubMed] [Google Scholar]
- 21.Genealogy Inc. Death master file [database on the Internet]. 2005. http://www.segenealogy.com/_search/vital_ssdi.htm. Accessed 7 Aug 2010.
- 22.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–7. doi: 10.1016/S0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 23.Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med. 1988;148:1586–91. doi: 10.1001/archinte.1988.00380070082020. [DOI] [PubMed] [Google Scholar]
- 24.Vigano A, Donaldson N, Higginson IJ, Bruera E, Mahmud S, Suarez-Almazor M. Quality of life and survival prediction in terminal cancer patients: a multicenter study. Cancer. 2004;101:1090–8. doi: 10.1002/cncr.20472. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer. 2004;100:851–8. doi: 10.1002/cncr.20028. [DOI] [PubMed] [Google Scholar]
- 26.Fouladiun M, Korner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care—correlations with food intake, metabolism, exercise capacity, and hormones. Cancer. 2005;103:2189–98. doi: 10.1002/cncr.21013. [DOI] [PubMed] [Google Scholar]
- 27.Simons JP, Schols AM, Buurman WA, Wouters EF. Weight loss and low body cell mass in males with lung cancer: relationship with systemic inflammation, acute-phase response, resting energy expenditure, and catabolic and anabolic hormones. Clin Sci (Lond) 1999;97:215–23. doi: 10.1042/CS19990021. [DOI] [PubMed] [Google Scholar]
- 28.Tas F, Duranyildiz D, Argon A, Oguz H, Camlica H, Yasasever V, et al. Serum levels of leptin and proinflammatory cytokines in advanced-stage non-small cell lung cancer. Med Oncol. 2005;22:353–8. doi: 10.1385/MO:22:4:353. [DOI] [PubMed] [Google Scholar]
- 29.Dulger H, Alici S, Sekeroglu MR, Erkog R, Ozbek H, Noyan T, et al. Serum levels of leptin and proinflammatory cytokines in patients with gastrointestinal cancer. Int J Clin Pract. 2004;58:545–9. doi: 10.1111/j.1368-5031.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- 30.Yeh SS, Hafner A, Chang CK, Levine DM, Parker TS, Schuster MW. Risk factors relating blood markers of inflammation and nutritional status to survival in cachectic geriatric patients in a randomized clinical trial. J Am Geriatr Soc. 2004;52:1708–12. doi: 10.1111/j.1532-5415.2004.52465.x. [DOI] [PubMed] [Google Scholar]
- 31.Levine JA, Morgan MY. Preservation of macronutrient preferences in cancer anorexia. Br J Cancer. 1998;78:579–81. doi: 10.1038/bjc.1998.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strasser F, Palmer JL, Schover LR, Yusuf SW, Pisters K, Vassilopoulou-Sellin R, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer. 2006;107:2949–57. doi: 10.1002/cncr.22339. [DOI] [PubMed] [Google Scholar]
- 33.Del Fabbro E, Hui D, Nooruddin ZI, Dalal S, Dev R, Freer G, et al. Associations among hypogonadism, C-reactive protein, symptom burden, and survival in male cancer patients with cachexia: a preliminary report. J Pain Symptom Manage. 2010;39:1016–24. doi: 10.1016/j.jpainsymman.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Taira AV, Merrick GS, Galbreath RW, Butler WM, Wallner KE, Allen ZA, et al. Pretreatment serum testosterone and androgen deprivation: effect on disease recurrence and overall survival in prostate cancer patients treated with brachytherapy. Int J Radiat Oncol Biol Phys. 2009;74:1143–9. doi: 10.1016/j.ijrobp.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 35.von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal ofCachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 22 kb)