Abstract
We have previously published results from a screen of 1,040 FDA-approved drugs and bioactives (NINDS Custom Collection) for drugs that protect against neomycin-induced hair cell death (Ou et al., J Assoc Res Otolaryngol 10:191–203, 2009). Further evaluation of this drug library identified eight protective drugs that shared a common quinoline scaffold. These drugs were tested further in terms of their protection against other aminoglycosides, as well as their effect on aminoglycoside uptake. All of the eight quinolines that protected against neomycin were found to protect against short- and long-term gentamicin damage protocols. We then tested the structurally related compounds quinoline, isoquinoline, naphthalene, and indole for protective effects. Of these compounds, indole demonstrated a small but significant amount of protection against neomycin, while quinoline and isoquinoline partially protected against long-term gentamicin damage. We examined whether the protective activity of this group of compounds was related to known targets of the quinoline derivatives. The protective effects did not seem linked to either the cholinergic or histaminergic pathways that are regulated by some members of the quinoline family. However, all eight protective drugs were found to reduce the uptake of aminoglycosides into hair cells. Subsequent experiments suggest that reduction of uptake is the primary mechanism of protection among the quinoline drugs.
Keywords: quinoline ring, hair cell protection, zebrafish
Introduction
The zebrafish is rapidly gaining acceptance as a useful model system for studying hair cell protection and death. Chemical screens using the zebrafish lateral line have led to rapid identification of a number of novel candidate protective drugs (Owens et al. 2008; Ou et al. 2009). Identification of new classes of protective drugs can potentially lead to the identification of new protective pathways on a molecular level. We previously screened a library of 1,040 FDA-approved drugs and bioactives (NINDS Custom Collection II) for drugs that protected against neomycin-induced hair cell death. From that screen, seven protective drugs were initially identified that effectively prevented neomycin-induced hair cell death in the zebrafish lateral line.
The need for additional protective drugs in the inner ear is critical. While many drugs have been demonstrated to successfully protect hair cells against damage (e.g., antioxidants, caspase inhibitors, Stat-1, etc.; Matsui et al. 2002; Wang et al. 2003, 2004; Sha et al. 2006; Campbell et al. 2007; Schmitt et al. 2009), there is currently no FDA-approved drug used for the prevention of hearing loss. Aspirin was demonstrated to protect against aminoglycoside-induced hair cell death (Sha et al. 2006), but required high daily doses (3 g/day). With evidence now that inhibition of one cell death pathway can lead to upregulation of other death pathways, the hypothesis that hair cells will “find a way to die” seems likely (Lin et al. 1999; Yu et al. 2004; Vandenabeele et al. 2006). Identification of multiple protective drugs may allow design of protective drug cocktails to inhibit multiple death pathways.
In general, our analysis of the results of a zebrafish protection screen focus on (1) dose–response function analysis of hits, (2) evaluation of candidate protective drugs against other toxicants, (3) evaluation of known biological targets of hits, (4) evaluation of drugs with similar targets or similar structures, and (5) evaluation of effect on aminoglycoside uptake. These steps serve to better characterize each protectant and help determine whether the mechanism of protection is due to known targets of a drug, or due to off-target effects.
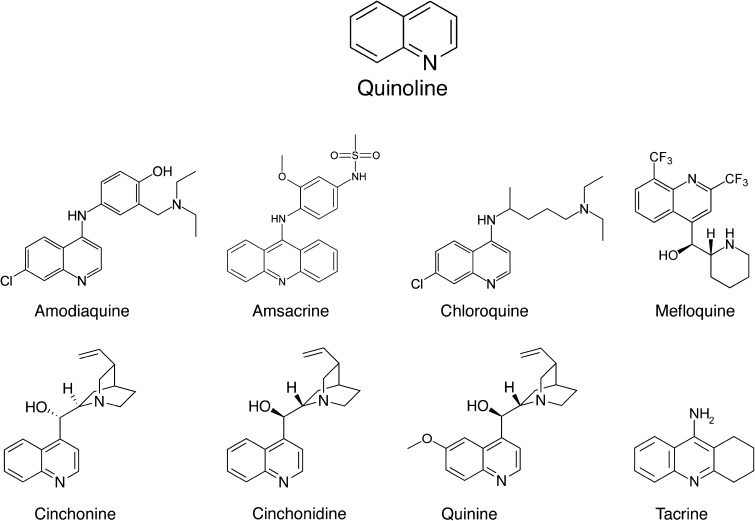
Our original FDA compound screen (Ou et al. 2009) focused on “hits” that demonstrated the greatest protection during the initial screen at a specific dose (100 μM). Further examination of other hits with less robust protection in the screen identified eight additional protective drugs, all sharing a common quinolone core or scaffold (Fig. 1). Six of these drugs are antimalarial drugs—other known functions include antineoplastics and anticholinesterases. In addition, some members of the quinoline ring family are known to inhibit histamine N-methyltransferase (Harle and Baldo 1988; Cumming et al. 1990; Horton et al. 2005). In this study, we examined and characterized the protective profile of this group of protective drugs.
FIG. 1.
Chemical structures of the eight quinoline derivatives found to have protective effects in the zebrafish lateral line. Note that cinchonine and cinchonidine are diastereomers and are both structurally closely related to quinine.
Methods
All zebrafish procedures described have been approved by the University of Washington Animal Care and Use Committee.
Animals
Zebrafish (Danio rerio) embryos of the AB wild-type strain were produced by paired matings of adult fish maintained at 28.5 °C in the University of Washington zebrafish facility. Embryos were maintained at a density of 50 embryos per 100 mm2 petri dish in embryo media (1 mM MgSO4, 120 μM KH2PO4, 74 μM Na2HPO4, 1 mM CaCl2, 500 μM KCl, 15 μM NaCl, and 500 μM NaHCO3 in dH2O). Zebrafish larvae were fed live Paramecia at four days post-fertilization (dpf).
Zebrafish drug screen
The original drug screen of the NINDS Custom Collection II library, which yielded the drugs of interest to this study, is described in Ou et al. (2009). The original study identified seven drugs (including tacrine) with robust protection at the screened concentration of 100 μM. However, additional drugs were found to provide moderate amounts of protection at the screened concentrations and, with further quantitative testing, were demonstrated to provide robust protection. The quinoline derivatives described here were identified in this secondary analysis of the initial screen results from Ou et al. (2009).
Hair cell counts/immunohistochemistry
For all hair cell counts, quantification was done using immunohistochemistry. Zebrafish larvae fixed in 4 % paraformaldehyde overnight at 4 °C were rinsed in phosphate-buffered saline (PBS) three times and then placed in blocking solution (1 % Triton-X, 5 % normal goat serum (NGS) in PBS) for 1–2 h at room temperature. Zebrafish were then incubated with anti-parvalbumin antibody (monoclonal, 1:400 in 1 % Triton-X, 1 % NGS, in PBS) at 4 °C overnight. Negative controls were prepared in the absence of anti-parvalbumin antibody. Zebrafish were rinsed in 1 % Triton-X in PBS (PBS-T) three times and then incubated in Alexa 488 goat anti-mouse fluorescent antibody (1:500, in 1 % Triton-X, 1 % NGS, in PBS) for 4 h. Following secondary antibody labeling, zebrafish were rinsed in PBS-T followed by PBS and mounted between two coverslips in Fluoromount-G (Southern Biotech, Birmingham, AL, USA) for imaging. Mounted specimens were examined using a Zeiss Axioplan II microscope using a FITC filter set at a final magnification of ×200. Hair cells from the SO1, SO2, O1, and OC1 neuromasts (Raible and Kruse 2000) were counted. Eight to 12 fish per dose were counted. Results were calculated as the mean hair cell survival as a percentage of the control (no drug).
Neomycin protection
Five dpf zebrafish larvae were pretreated with each quinoline drug at 0, 10, 50, 100, and 200 μM doses for 1 h, followed by treatment with neomycin 200 μM for 1 h. Additional experiments were performed using 2-h exposure to neomycin to determine whether protection was maintained.
Note that the quinoline drug was still present during neomycin treatment. Zebrafish were then anesthetized with MS222 and fixed in 4 % paraformaldehyde overnight at 4 °C and then processed for immunocytochemistry and hair cell counts.
Variable neomycin dose
We sought to determine whether protection was maintained against a range of neomycin doses. Once the optimal protective dose of quinoline (lowest dose with maximal protection) was determined, five dpf larvae were then pretreated with each quinoline at the optimal dose (drug dependent) followed by treatment with neomycin doses of 0, 100, 200, and 400 μM for 1 h (quinoline still present). Zebrafish were then fixed and processed for hair cell counts.
Gentamicin protection
In order to determine whether the quinoline drugs protected against other aminoglycosides, we tested the drugs against gentamicin. In the zebrafish lateral line, gentamicin is known to cause damage after both short and long damage protocols with seemingly different pathways (Owens et al. 2009). Each quinoline drug was tested at its previously determined optimal protective dose for 1 h, followed by treatment with gentamicin for a short (400 μM × 1 h) or long period (50 μM × 6 h). Zebrafish were then fixed and processed for hair cell counts.
Uptake of Texas red-conjugated gentamicin (GTTR)
Since quinine is a known blocker of the mechanotransduction channel (Farris et al. 2004) and thus a blocker of aminoglycoside uptake, we sought to determine whether other protective quinoline drugs affected the uptake of aminoglycosides. GTTR was prepared as described by Steyger et al. (2003). Five dpf zebrafish larvae were pretreated with protective drugs (100 μM × 15 min), then treated with 50 μM GTTR for 3 and 18 min with the protective drug still present. This dose protocol was chosen because it causes rapid uptake of GTTR without oversaturation or toxicity to hair cells. Larvae were then thoroughly rinsed and placed in MS222 for in vivo imaging. The SO2 neuromast was then examined with fluorescence microscopy and images in the z-plane were acquired at 1 μm sections to quantify the uptake. Fluorescence was quantified using Slidebook software to subtract background fluorescence and calculate mean fluorescence. Mean fluorescence for zebrafish treated with quinoline was compared to untreated controls after exposure to both 3 and 18 min of GTTR.
Inhibition of cholinergic pathways
In addition to evaluating blockade of uptake as a possible mechanism for protection, we also evaluated known activities of the quinoline drugs. Tacrine is known to be an inhibitor of acetylcholinesterase (Drukarch et al. 1987; Pearce and Potter 1988). To determine whether this was the mechanism of protection, we blocked nicotinic and muscarinic receptors of acetylcholine using dihydrobetaerythroidine (DBE) and atropine, respectively. Five dpf larval zebrafish were first exposed to DBE, atropine, or both at 1, 10, or 100 μM concentration for 1 h. Fish were then treated with tacrine 100 μM for 1 h with the cholinergic blocker still present. Zebrafish were then treated with neomycin 200 μM for 1 h, with both the cholinergic blocker and tacrine still present. If cholinergic pathways were involved, one would expect an inhibition of tacrine’s protective effects.
Inhibition of histaminergic pathways
Mefloquine, amodiaquine, and tacrine are known inhibitors of histamine N-methyltransferase (HNMT), which metabolizes and breaks down histamine (Harle and Baldo 1988; Cumming et al. 1990; Horton et al. 2005). Inhibition of HNMT and enhancement of the effects of histamine thus were hypothesized as a possible mechanism of protection. To evaluate this possibility, we blocked H1 and H2 receptors of histamine using diphenhydramine and ranitidine, respectively. Five dpf larval zebrafish were first exposed to diphenhydramine, ranitidine, or both blockers at up to a 100-μM concentration for 1 h (above 100 μM concentration, there was lethality to the fish). Fish were then treated with mefloquine 10 μM for 1 h with the antihistamine still present. Zebrafish were then treated with neomycin 200 μM for 1 h, with both the antihistamine and mefloquine still present. If histamine-related pathways were involved, one would expect an inhibition of mefloquine-related protection.
Structural analogue testing
To potentially define the critical structural components of our quinoline ring derivatives that were involved in hair cell protection, we evaluated for protection among the commercially available structural analogues quinoline, isoquinoline, indole, and naphthalene. Note, in contrast to the eight quinoline derivatives identified by the screen, these four drugs were not identified by the initial zebrafish screen and were chosen due to their structural similarity without any knowledge of their protective capacity. Five dpf zebrafish were pretreated with the structural analogue for 1 h at 0, 10, 50, 100, and 200 μm concentrations, followed by treatment with neomycin (200 μM × 1 h), short-term (400 μM × 1 h) or long-term (50 μM × 6 h) gentamicin. Zebrafish were then fixed for immunohistochemistry, followed by quantification with hair cell counts.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration testing
All candidate drugs were tested at 200 μM concentration to determine whether they interfered with the known bactericidal activity of neomycin. Escherichia coli ATCC 25922 was used to inoculate the antibiotic dilutions. The MIC was tested in accordance with the National Clinical and Laboratory Standards Institute (Wikler 2006, 2007).
Statistics
All values were calculated and presented as the mean value ± 1 SD. Statistical analyses were performed using one- and two-way ANOVA (VassarStats: faculty.Vassar.edu/lowry/VassarStats.html). Results were considered statistically significant if p < 0.05.
Results
Quinoline derivatives protect against neomycin-induced hair cell death
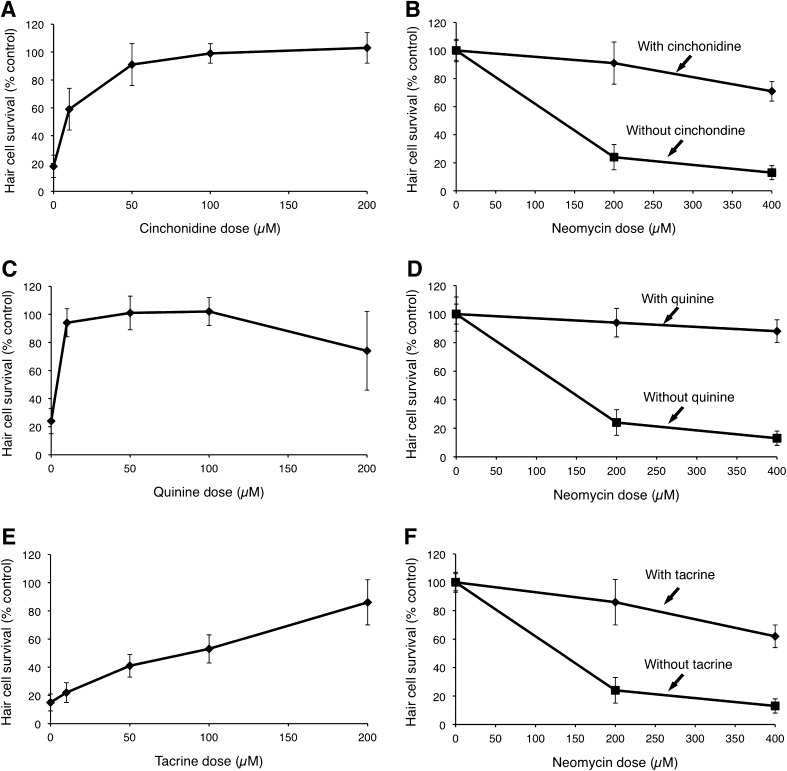
Each of the eight quinoline ring derivatives demonstrated dose-dependent protection against neomycin (p < 0.0001, one-way ANOVA; Fig. 2; Table 1). Amsacrine, quinine, and mefloquine were particularly potent protectants, with ED50 values of less than 10 μM based on linear regression estimates. Tacrine was the least potent protectant with an ED50 of 92 μM. Quinine and mefloquine demonstrated some hair cell toxicity at higher doses, resulting in a decline in hair cell survival at 200 μM doses. We then tested the optimal protective doses of each quinoline against a higher dose of neomycin (400 μM) and found that significant protection was maintained for all eight drugs (p < 0.0001, two-way ANOVA; Fig. 2, Table 2).
FIG. 2.
Dose–response curves for selected quinoline derivatives. A Five dpf zebrafish pretreated with cinchonidine at variable doses for 1 h, followed by treatment with 200 μM neomycin demonstrate significant dose-dependent protection (F(4, 45) = 91.46, p < 0.0001, one-way ANOVA). B Cinchonidine-induced hair cell protection is maintained at higher doses of neomycin (200 and 400 μM). C Zebrafish pretreated with variable doses of quinine for 1 h, followed by treatment with 200 μM neomycin demonstrate significant dose-dependent protection (F(4, 45) = 41.34, p < 0.0001, one-way ANOVA). D Quinine-induced hair cell protection is maintained at higher doses of neomycin (200 and 400 μM). E Zebrafish pretreated with tacrine for 1 h, followed by treatment with 200 μM neomycin demonstrate significant dose-dependent protection (F(4, 45) = 74.83, p < 0.0001, one-way ANOVA). F Tacrine-induced hair cell protection is maintained at higher doses of neomycin (200 and 400 μM). For all graphs, data points represent the mean hair cell survival of eight to ten fish. Error bars = ±1 SD from the mean. Data for these three drugs as well as the remaining five quinoline derivatives are presented in Tables 1 and 2.
TABLE 1.
Hair cell survival (percent of control) and ED50 of protection after pretreatment with increasing doses of quinoline derivative followed by 200 μM neomycin treatment
| Drug | Protective drug concentration | ED50 (μM) | Treatment effect; p value (ANOVA) | |||||
|---|---|---|---|---|---|---|---|---|
| 0 μM | 10 μM | 50 μM | 100 μM | 200 μM | Drug alone (no neo) | |||
| Amsacrine | 16 ± 6 | 103 ± 12** | 108 ± 10** | 102 ± 19** | 102 ± 8** | 107 ± 10 | 4 | F(4, 43) = 105.51, p < 0.0001 |
| Quinine | 24 ± 9 | 94 ± 10** | 101 ± 12** | 102 ± 10** | 74 ± 28** | 64 ± 24 | 4 | F(4, 45) = 41.34, p < 0.0001 |
| Mefloquine | 20 ± 6 | 88 ± 8** | 95 ± 13** | 103 ± 17** | 85 ± 14** | 82 ± 11 | 6 | F(4, 40) = 73.48, p < 0.0001 |
| Cinchonidine | 18 ± 8 | 59 ± 15** | 91 ± 15** | 99 ± 7** | 103 ± 11** | 101 ± 8 | 160 | F(4, 45) = 91.46, p < 0.0001 |
| Amodiaquine | 13 ± 8 | 49 ± 7** | 64 ± 18** | 85 ± 15** | 101 ± 8** | 94 ± 9 | 40 | F(4, 44) = 51.19, p < 0.0001 |
| Chloroquine | 33 ± 13 | 30 ± 20 | 64 ± 16** | 65 ± 20** | 95 ± 10** | 104 ± 11 | 50 | F(4, 41) = 25.15, p < 0.0001 |
| Cinchonine | 17 ± 11 | 52 ± 13** | 50 ± 9** | 61 ± 10** | 78 ± 12** | 104 ± 18 | 64 | F(4, 43) = 37.15, p < 0.0001 |
| Tacrine | 15 ± 6 | 22 ± 7 | 41 ± 8** | 53 ± 10** | 86 ± 16** | 106 ± 12 | 92 | F(4, 45) = 74.83, p < 0.0001 |
Drug alone column indicates hair cell survival in the presence of 200 μM quinoline derivative without neomycin
**p < 0.01, Tukey HSD test
TABLE 2.
Hair cell survival (percent of control) after pretreatment with protective drug (optimal concentration) followed by variable doses of neomycin
| Drug | Neomycin dose (μM) | p value (two-way ANOVA) | |
|---|---|---|---|
| 200 | 400 | ||
| No pretreatment | 24 ± 9 | 13 ± 5 | N/A |
| Amsacrine | 103 ± 12** | 67 ± 4** | F(1, 36) = 165.24, p < 0.0001 |
| Quinine | 94 ± 10** | 88 ± 8** | F(1, 36) = 757.14, p < 0.0001 |
| Mefloquine | 95 ± 13** | 83 ± 10** | F(1, 32) = 603.14, p < 0.0001 |
| Cinchonidine | 91 ± 15** | 71 ± 7** | F(1, 36) = 442.42, p < 0.0001 |
| Amodiaquine | 85 ± 15** | 85 ± 12** | F(1, 36) = 400.68, p < 0.0001 |
| Chloroquine | 95 ± 10** | 73 ± 11** | F(1, 36) = 479.25, p < 0.0001 |
| Cinchonine | 78 ± 12** | 73 ± 15** | F(1, 33) = 257.98, p < 0.0001 |
| Tacrine | 86 ± 16** | 62 ± 8** | F(1, 35) = 293.36, p < 0.0001 |
Zebrafish were pretreated with optimal protective dose from Table 1 (10 μM amsacrine, 10 μM quinine, 10 μM mefloquine, 50 μM cinchonidine, 100 μM amodiaquine, 200 μM chloroquine, 200 μM cinchonine, 200 μM tacrine)
**p < 0.01, two-way ANOVA
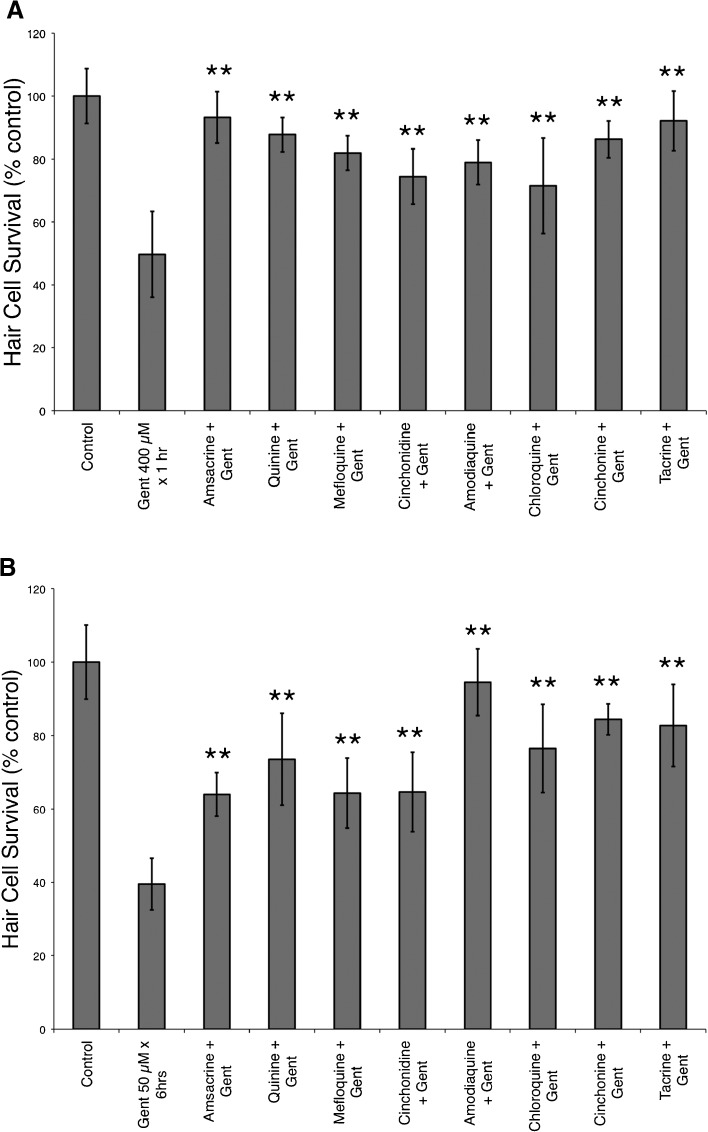
Quinoline derivatives protect against gentamicin-induced hair cell death
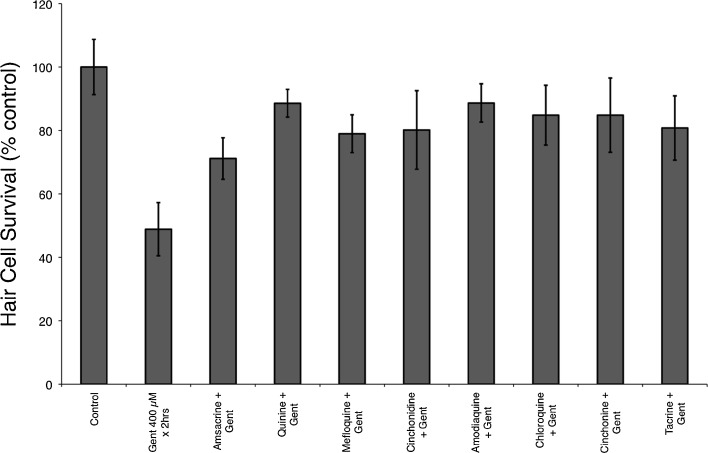
Previously, we have demonstrated that gentamicin causes damage after short- and long-term exposures and that this may occur through different pathways (Owens et al. 2009). When tested against short-term gentamicin (400 μM for 1 h), we found that all eight quinoline derivatives provided significant protection (F(8, 88) = 21.88, p < 0.0001, one-way ANOVA; Fig. 3). We found similar protection from each of the quinoline derivatives against long-term gentamicin damage (50 μM for 6 h) with significant protection (F(8, 75) = 25.47, p < 0.0001, one-way ANOVA; Fig. 3).
FIG. 3.
Quinoline derivatives protect against short-term (400 μM × 1 h) and long-term (50 μM × 6 h) gentamicin damage protocols. Previous studies (Owens et al. 2009) suggest that different death pathways are involved in short- and long-term gentamicin damage in the zebrafish lateral line. A Pretreatment with each of the eight quinoline derivatives provided significant protection against short-term gentamicin-induced hair cell death (**F(8, 88) = 21.88, p < 0.0001, one-way ANOVA). Similarly, in B, each of the eight quinoline derivatives protected against long-term gentamicin-induced hair cell death (**F(8, 75) = 25.47, p < 0.0001, one-way ANOVA). Data bars represent the mean hair cell survival of eight to ten fish. Error bars = ±1 SD from the mean.
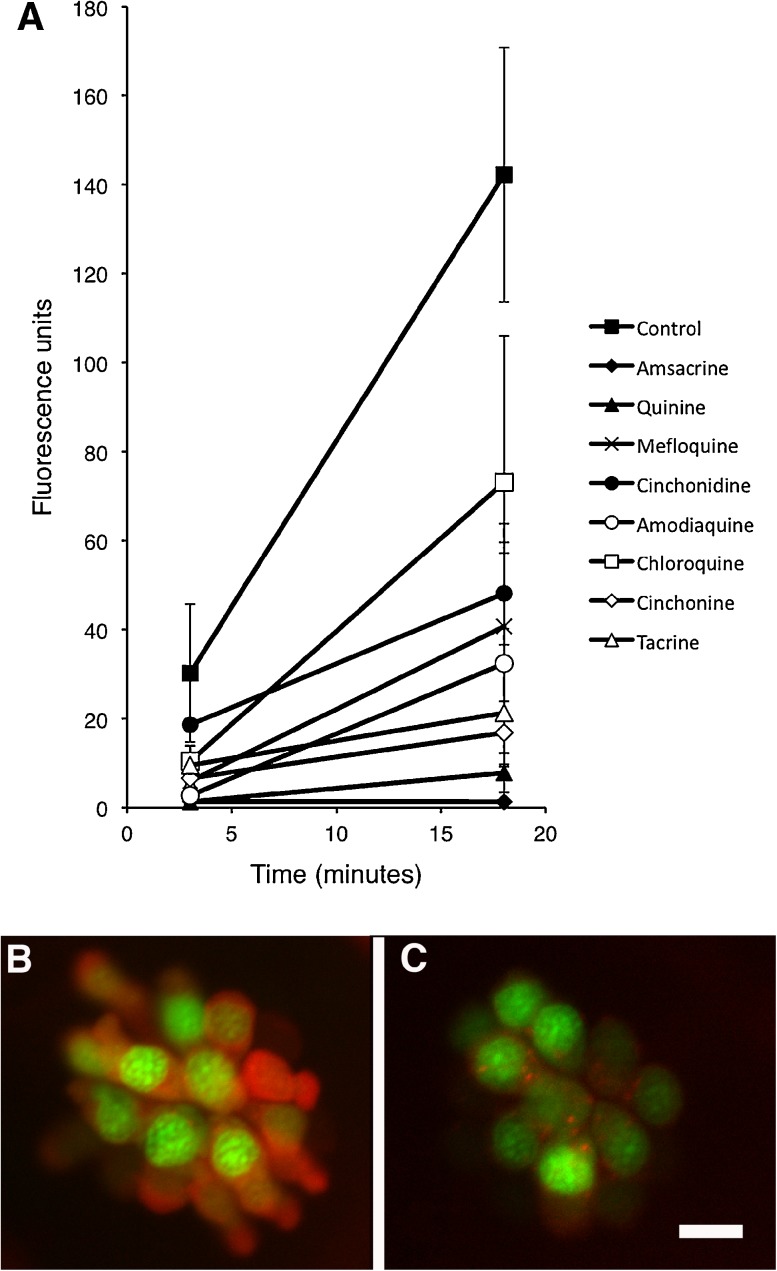
All protective quinoline derivatives reduce uptake of Texas-red conjugated gentamicin
Uptake of GTTR was quantified at 3 and 18 min after administration of GTTR. At 3 min, uptake of GTTR was significantly reduced in all quinoline derivatives except cinchonidine when compared to controls (F(8, 21) = 6.20, p < 0.05, one-way ANOVA; Fig. 4). After 18 min, GTTR uptake was significantly reduced in all quinoline derivatives except chloroquine (F(8, 26) = 10.96, p < 0.05, one-way ANOVA; Fig. 4). The slope of the increase in fluorescence between 3 and 18 min was significantly decreased in all eight of the quinoline derivatives (Fig. 4). For four drugs in particular (amsacrine, quinine, tacrine, and cinchonine), there was essentially no increase in fluorescence from 3 to 18 min.
FIG. 4.
Quinoline derivatives reduce the uptake of Texas red-conjugated gentamicin (GTTR). Five dpf zebrafish were pretreated with quinoline derivatives, then exposed to 50 μM GTTR. SO2 neuromast from each fish was then imaged after 3 and 18 min of exposure to GTTR. Red fluorescence was quantified in z-series through the entire neuromast, after subtraction of background fluorescence. A Fluorescence is reduced after treatment with all eight quinolines relative to control at 3 and 18 min. B Control neuromast after 18 min of exposure to GTTR. Red fluorescence is GTTR, green is YO-PRO1 labeled nuclei. C Cinchonine-treated neuromast after 18 min of exposure to GTTR. GTTR uptake is markedly reduced. Data points in A represent mean fluorescence of five to seven fish (one SO2 neuromast measured per fish). Error bars represent SD. Scale bar in C = 10 μm and applies to B and C.
Longer exposure to gentamicin does not overcome protective effects
We hypothesized that reduced gentamicin uptake might simply be delaying hair cell death by increasing the duration required to reach a lethal gentamicin level. To determine whether this was the case, we tested whether the quinoline derivatives prevented hair cell death from longer exposures to gentamicin (doubling the duration of exposure in the short-term gentamicin damage protocol). We found no significant increase in hair cell death when doubling the duration of gentamicin exposure (Fig. 3A compared to Fig. 5).
FIG. 5.
Increasing the duration of short-term gentamicin exposure from 1 to 2 h did not lead to an increase in hair cell death. GTTR uptake studies demonstrated that all quinoline derivatives reduced the rate of GTTR uptake. One could hypothesize that reducing the rate of uptake would only delay hair cell death; however, doubling the exposure duration from 1 (Fig. 3A) to 2 h (shown here) did not lead to a significant reduction in hair cell survival. Data bars represent the mean hair cell survival of eight to ten fish. Error bars = ±1 SD from the mean.
Inhibition of cholinergic or histaminergic pathways did not affect quinoline-induced hair cell protection
While uptake of aminoglycoside was reduced by the quinoline derivatives, we also examined whether other mechanisms might be involved in protection. Tacrine is known as an acetylcholinesterase inhibitor, which thus increases levels of available acetylcholine. Blockade of acetylcholine activity at nicotinic receptors (with atropine) and muscarinic receptors (with dihydrobetaerythroidine) both failed to affect tacrine-induced hair cell protection, suggesting alternate mechanisms for protection (data not shown). It should be noted that in the original FDA library screen, the cholinesterase inhibitors physostigmine, edrophonium, and huperzine were all tested and not found to be protective (Ou et al. 2009).
In addition, mefloquine is a known inhibitor of histamine-N-methyl transferase. However, as with blockade of acetylcholine, blockade of histaminergic pathways at H1 receptors (with diphenhydramine) and H2 receptors (with ranitidine) also failed to affect mefloquine-induced hair cell protection (data not shown).
Some structural analogues demonstrated partial protection against neomycin and gentamicin
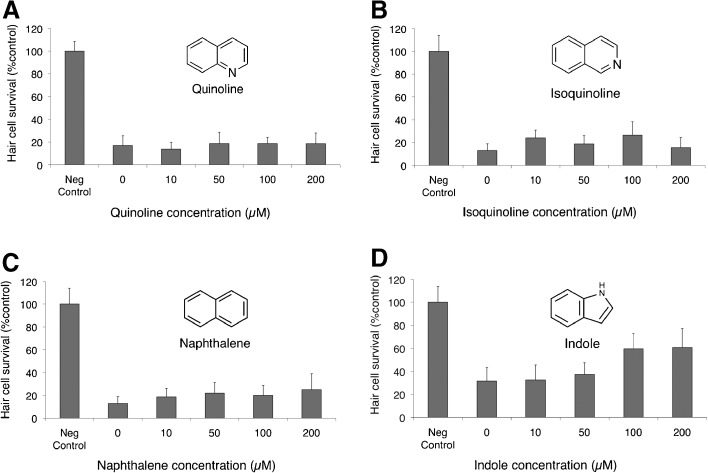
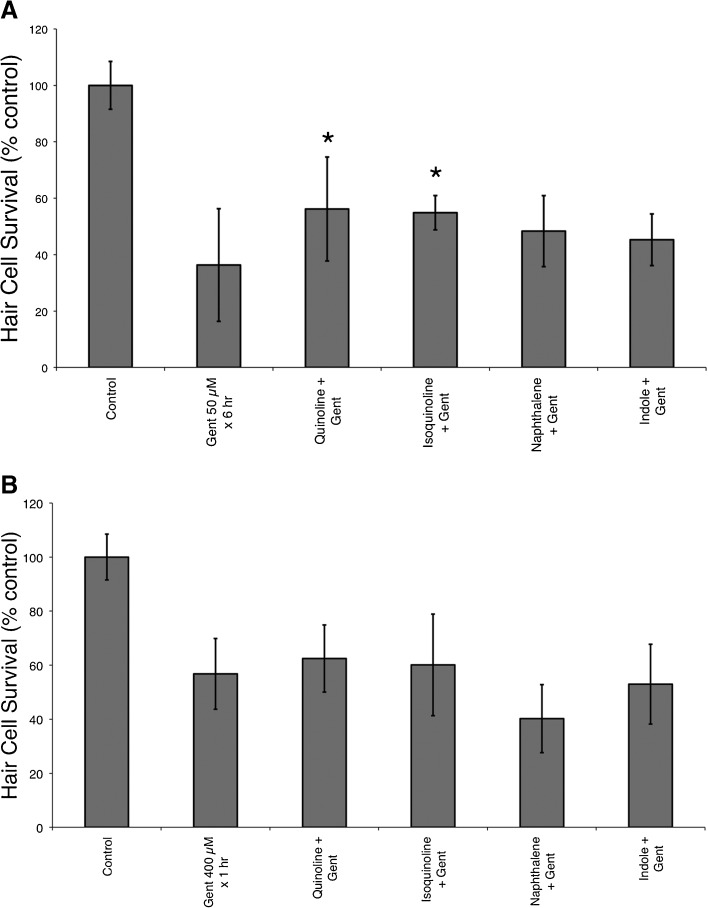
Four structurally related compounds with no known protective effects were tested for protection against neomycin-induced hair cell death (Fig. 6). Of these four compounds, only indole demonstrated a small but significant protection (F(4, 42) =11.72, p < 0.0001, one-way ANOVA) against neomycin-induced hair cell death (Fig. 6) with an increase of hair cell survival from 32 ± 12 to 60 ± 13 % after pretreatment with 100 μM indole. The remaining three compounds, including quinoline itself, failed to provide any protection against neomycin. We then tested whether any of these four structural analogues prevented short- and long-term gentamicin damage. None of these compounds protected against short-term gentamicin; however, quinoline and isoquinoline demonstrated modest but significant protection against long-term gentamicin damage (F(5, 57) = 30.71, p < 0.05, one-way ANOVA; Fig. 7).
FIG. 6.
Structural analogues provide minimal protection against neomycin-induced hair cell death. Five dpf zebrafish were pretreated for 1 h with variable doses of A quinoline, B isoquinoline, C naphthalene, and D indole prior to treatment with 200 μM neomycin for 1 h. Of the four compounds, only indole demonstrated significant protection (F(4, 42) = 11.72, p < 0.0001, one-way ANOVA). Structure of each analogue is depicted alongside each graph. Data bars represent the mean hair cell survival of eight to ten fish. Error bars = ±1 SD from the mean.
FIG. 7.
Structural analogues (quinoline and isoquinoline) demonstrate partial protection against long-term gentamicin damage but do not protect against short-term gentamicin. A Quinoline and isoquinoline partially protected against long-term gentamicin (50 μM × 6 h) damage (*F(5, 57) = 30.71, p < 0.05, one-way ANOVA). B In contrast, none of the structural analogues protected against short-term (400 μM × 1 h) gentamicin-induced hair cell death. Data bars represent the mean hair cell survival of ten fish. Error bars = ±1 SD from the mean.
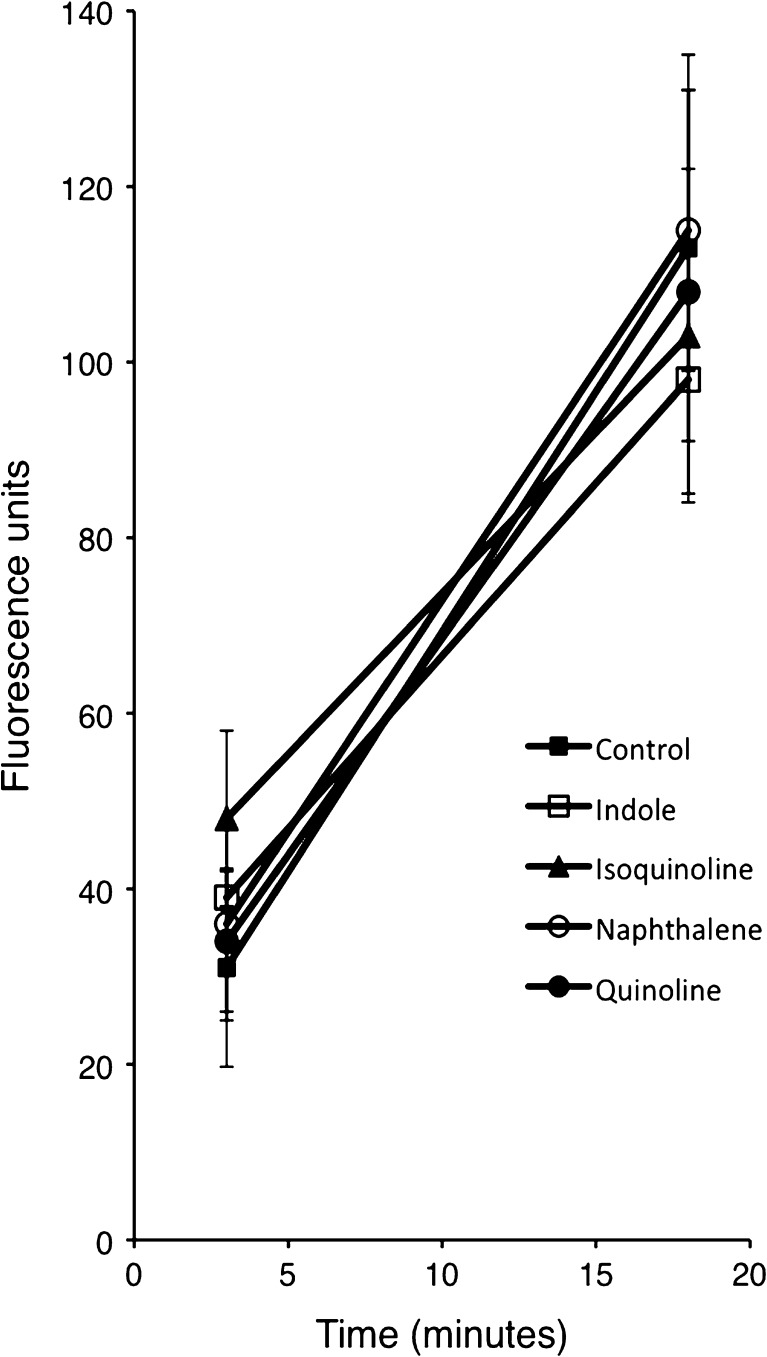
We then tested whether these four structural analogues inhibited the uptake of GTTR. We found that none of the four compounds significantly inhibited the uptake of GTTR at 3 or 18 min (Fig. 8).
FIG. 8.
Structural analogues do not reduce the uptake of Texas red-conjugated gentamicin (GTTR). Five dpf zebrafish were pretreated with structural analogues then exposed to 50 μM GTTR for 3 and 18 min. Red fluorescence was quantified in z-series through the entire neuromast, after subtraction of background fluorescence. There was no significant difference for any analogue at 3 or 18 min when compared to control. Data points represent mean fluorescence of five fish (one SO2 neuromast measured per fish). Error bars = ±1 SD from the mean.
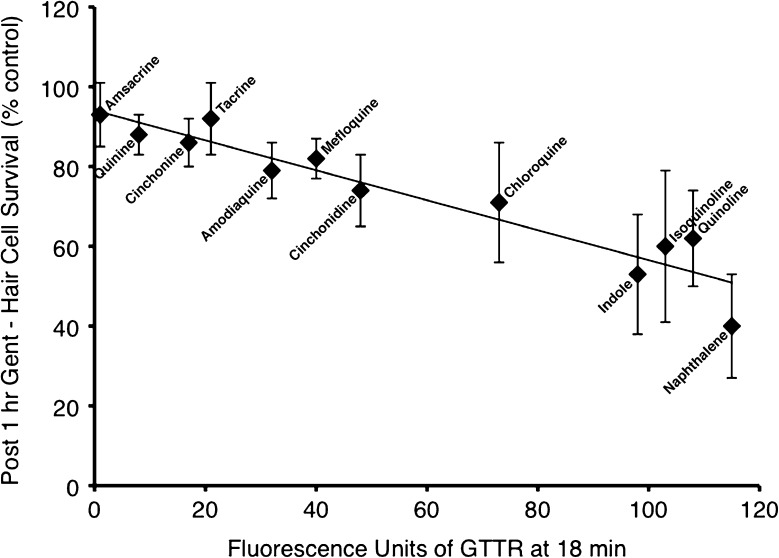
Protection against short-term gentamicin correlated with reduction in GTTR uptake
We examined whether GTTR uptake at 18 min correlated with protection against neomycin or gentamicin. We found a statistically significant correlation between protection against short-term gentamicin and a reduction in GTTR uptake (r2 = 0.896, F(1, 10) = 86.19, p < 0.0001; Fig. 9). In contrast, we found no correlation between GTTR uptake and protection against neomycin (r2 = 0.14; regression data not shown), or protection against long-term gentamicin damage (r2 = 0.503; regression data not shown).
FIG. 9.
Protection against short-term gentamicin correlated with reduction in GTTR uptake at 18 min in quinoline derivatives and structural analogues. Ordinate represents mean hair cell survival (% control; n = 10) after pretreatment with quinoline derivative and exposure to gentamicin 400 μM × 1 h. Abscissa represents mean fluorescence after quinoline pretreatment and 18 min of exposure to GTTR (n = 5). Solid line represents result of linear regression (r 2 = 0.896, F(1, 10) = 86.19, p < 0.0001). Error bars = ±1 SD from the mean.
Inhibition of bactericidal activity
None of the eight protective quinoline derivatives or four structural analogues significantly increased the minimum inhibitory concentration or the minimum bactericidal concentration of neomycin. There was no greater than a twofold change in the minimum inhibitory concentration (4.0 μg/ml) and minimum bactericidal concentration (4.0 μg/ml) of neomycin (up to a twofold increase is considered within the normal variation of the test; Wikler 2006, 2007). This indicates that co-administration of these protective drugs would not be expected to interfere with the desired antibacterial properties of neomycin.
Discussion
Quinoline derivatives protect against aminoglycoside-induced hair cell loss
This study provides further validation of the utility of the zebrafish drug screening model. The eight quinoline derivatives described here were identified in a drug screen of the NINDS Custom Collection II and subsequently noted to have similar structural characteristics.
We found that all eight of the quinoline derivatives effectively protected against neomycin and short-term gentamicin. Significant protection against long-term gentamicin exposure was also found, albeit to a lesser extent. This may lend further support to previous findings suggesting that short- and long-term gentamicin damage occurs through different pathways (Owens et al. 2009). However, overall, these shared features between quinoline derivatives suggest a shared mechanism of action.
All of the protective quinoline derivatives reduce GTTR uptake
All eight of the protective quinoline compounds blocked uptake of GTTR when measured quantitatively after 3 and 18 min of exposure to GTTR. Interestingly, we found that protection against short-term gentamicin correlated significantly with a reduction in GTTR uptake and 18 min. We did not find a correlation of GTTR uptake with protection against neomycin or long-term gentamicin damage. This suggests that short-term gentamicin damage is more directly dependent on the rapid gentamicin uptake we observe with GTTR studies and is more evident that the mechanisms of damage between neomycin, short-term, and long-term gentamicin may differ. It also suggests that some of the protection that we see, in particular against long-term gentamicin by quinoline derivatives, may be through other mechanisms unrelated to blockade of gentamicin uptake.
A number of the quinoline derivatives do in fact have other potential mechanisms of protection, although our investigation into possible cholinergic or histaminergic involvement was not revealing. It is worth noting that quinoline derivatives have been noted to have some antioxidant properties in other systems (Detsi et al. 2007; Naik et al. 2009; Ghinet et al. 2012) although this has not been examined in hair cells.
Does the speed of aminoglycoside uptake affect toxicity?
One could hypothesize that reducing the rate of uptake of aminoglycoside into a hair cell would reduce the toxicity, by potentially allowing more time for scavengers of reactive oxygen species or other survival mechanisms to act. Alternatively, a reduction of the rate of aminoglycoside uptake might only “postpone” hair cell death until a certain critical level of intracellular gentamicin was reached. Our studies using longer durations of gentamicin (Fig. 5) suggested that the latter scenario was not the case. There was no increase of hair cell death with increased duration of exposure to gentamicin with any of the quinoline derivatives, including chloroquine and cinchonidine (both of which allowed measurable amounts of GTTR into the hair cell between 3 and 18 min). This finding supports the notion that the rate of aminoglycoside entry plays a role in the degree of toxicity. One can hypothesize that given enough time, a similar intracellular concentration of gentamicin could be reached in the presence of chloroquine and cinchonidine; however, the reduced rate of entry and possibly sequestration of gentamicin appeared to be sufficient to protect hair cells.
Inconsistencies with qualitative data
This study demonstrates the importance of studying aminoglycoside uptake in a quantitative fashion. This has been demonstrated by Alharazneh et al. (2011) in quantitative studies of GTTR uptake in mammalian cochlea in vitro and is echoed in this study. Previously, tacrine, based on qualitative testing, did not appear to significantly affect aminoglycoside uptake (Ou et al. 2009); however, in this study, it was found to significantly reduce uptake. Evaluating only qualitatively, complete blockade can easily be identified; however, it is extremely difficult to identify partial uptake blockade without careful quantitation.
Ototoxicity of some quinolines
It is interesting to note the antimalarial compounds contained within this group of protective drugs. In particular, quinine and mefloquine are known to have toxicity to hair cells. This was confirmed in our dose–response testing which revealed a reduction in hair cell survival at higher doses (Table 1) of these two drugs. This highlights the fact that all drugs inherently have multiple targets, and many protective drugs can have toxic effects at higher doses. It also demonstrates the importance of thorough dose testing to define the therapeutic window for any drug. The ease of this kind of extensive dose–response testing in the zebrafish lateral line makes it a particularly valuable model.
Structural observations
Of the four structural analogues, only indole demonstrated marginal but statistically significant protection (Fig. 6) against neomycin damage. Interestingly, the unmodified quinoline core structure and its isomer isoquinoline did partially protect against long-term but not short-term gentamicin damage. None of the structural analogues significantly blocked GTTR uptake, consistent with our hypothesis that protection occurred via blockade of aminoglycoside uptake. The small amount of protection afforded by indole against neomycin and quinoline and isoquinoline against long-term gentamicin could thus potentially be through alternative mechanisms unrelated to uptake.
The diastereomers cinchonine and cinchonidine interestingly have somewhat different protection profiles. Cinchonidine has a lower ED50, suggesting higher potency; however, cinchonine was more effective at inhibiting GTTR uptake. Of the two diastereomers, the more potent cinchonidine is also structurally more similar to quinine (which had the lowest ED50).
It is worth noting that the only class of medications currently recommended by the American Academy of Otolaryngology—Head and Neck Surgery for use in the middle ear are the fluoroquinolones (Roland et al. 2004), which also contain a quinoline-based structure. Since this would potentially be an attractive way of delivering protective drugs to the ear, we tested the fluoroquinolone ofloxacin in our model to determine whether it conferred protection in the lateral line, but found no significant protection (data not shown).
Caveats
As with all hair cell findings in the zebrafish lateral line, we must acknowledge that there are critical differences between lateral line hair cells and the mammalian inner ear. The lateral line has no distinction between inner or outer hair cells, nor is there a separation of fluid spaces. Furthermore, while we did not demonstrate an effect of cholinergic and histaminergic pathways, we cannot rule out a role for either pathway since it is possible that there was inadequate penetration of the drug into the lateral line hair cells to have an effect.
The quinoline ring structure is in fact a common building block for many drugs. In this case, in isolation, quinoline itself provided no protection (Fig. 6). Further studies and structural analysis may better characterize the specific structural element shared by these drugs that conferred protection against aminoglycoside-induced hair cell damage.
Summary
This study introduces a group of quinoline derivatives as a new class of protective drugs. The identification of new classes of protective drugs is critical, particularly in light of the fact that inhibition of certain death pathways can lead to upregulation of others. Acceptance of the hypothesis that hair cells will “find a way to die,” may necessitate the use of protective cocktails—mixtures of protective drugs from different classes to inhibit multiple death pathways. In some respects, blocking uptake of the hair cell toxicant may be the most effective method to prevent hair cell death by preventing the initiation of even the earliest steps in cell death. This group of protective compounds warrants further investigation in vivo in mammalian models.
Acknowledgments
This research was supported by grants from NIH/NIDCD: K08DC009631, P30-DC004661, R01-DC009807, and R01-DC005987-05.
References
- Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One. 2011;6(7):e22347. doi: 10.1371/journal.pone.0022347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SS, Larsen DL, Mitchell DL, El-Azizi M, Verhulst SJ, Hughes LF. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Cumming P, Reiner PB, Vincent SR. Inhibition of rat brain histamine-N-methyltransferase by 9-amino-1,2,3,4-tetrahydroacridine (THA) Biochem Pharmacol. 1990;40:1345–1350. doi: 10.1016/0006-2952(90)90402-7. [DOI] [PubMed] [Google Scholar]
- Detsi A, Bouloumbasi D, Prousis KC, Koufaki M, Athanasellis G, Melagraki G, Afantitis A, Igglessi-Markopoulou O, Kontogiorgis C, Hadjipavlou-Litina DJ. Design and synthesis of novel quinolinone-3-aminoamides and their alpha-lipoic acid adducts as antioxidant and anti-inflammatory agents. J Med Chem. 2007;50(10):2450–2458. doi: 10.1021/jm061173n. [DOI] [PubMed] [Google Scholar]
- Drukarch B, Kits KS, Van der Meer EG, Lodder JC, Stoof JC. 9-Amino-1,2,3,4-tetrahydroacridine (THA), an alleged drug for the treatment of Alzheimer's disease, inhibits acetylcholinesterase activity and slow outward K+ current. Eur J Pharmacol. 1987;141:153–157. doi: 10.1016/0014-2999(87)90424-9. [DOI] [PubMed] [Google Scholar]
- Farris HE, LeBlanc CL, Goswami J, Ricci AJ. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol. 2004;558:769–792. doi: 10.1113/jphysiol.2004.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinet A, Farce A, Oudir S, Pommery J, Vamecq J, Henichart JP, Rigo B, Gautret P. Antioxidant activity of new benzo[de]quinolines and lactams: 2D-quantitative structure–activity relationships. Med Chem. 2012;8(5):942–946. doi: 10.2174/157340612802084216. [DOI] [PubMed] [Google Scholar]
- Harle DG, Baldo BA. Structural features of potent inhibitors of rat kidney histamine N-methyltransferase. Biochem Pharmacol. 1988;37:385–388. doi: 10.1016/0006-2952(88)90203-1. [DOI] [PubMed] [Google Scholar]
- Horton JR, Sawada K, Nishibori M, Cheng X. Structural basis for inhibition of histamine N-methyltransferase by diverse drugs. J Mol Biol. 2005;353:334–344. doi: 10.1016/j.jmb.2005.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Ogilvie JM, Warchol ME. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–1227. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik HR, Naik HS, Naik TR, Naika HR, Gouthamchandra K, Mahmood R, Ahamed BM. Synthesis of novel benzo[h]quinolines: wound healing, antibacterial, DNA binding and in vitro antioxidant activity. Eur J Med Chem. 2009;44(3):981–989. doi: 10.1016/j.ejmech.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Ou HC, Cunningham LL, Francis SP, Brandon CS, Simon JA, Raible DW, Rubel EW. Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (Danio rerio) lateral line and mouse (Mus musculus) utricle. J Assoc Res Otolaryngol. 2009;10:191–203. doi: 10.1007/s10162-009-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 2008;4:e1000020. doi: 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Coffin AB, Hong LS, Bennett KO, Rubel EW, Raible DW. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct death pathways. Hear Res. 2009;253(1–2):32–41. doi: 10.1016/j.heares.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce BD, Potter LT. Effects of tetrahydroaminoacridine on M1 and M2 muscarine receptors. Neurosci Lett. 1988;88:281–285. doi: 10.1016/0304-3940(88)90224-8. [DOI] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. doi: 10.1002/(SICI)1096-9861(20000529)421:2<189::AID-CNE5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Roland PS, Stewart MG, Hannley M, Friedman R, Manolidis S, Matz G, Rybak L, Weber P, Owens F. Consensus panel on role of potentially ototoxic antibiotics for topical middle ear use: Introduction, methodology, and recommendations. Otolaryngol Head Neck Surg. 2004;130:S51–S56. doi: 10.1016/j.otohns.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Schmitt NC, Rubel EW, Nathanson NM. Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechine gallate. J Neurosci. 2009;29(12):3843–3851. doi: 10.1523/JNEUROSCI.5842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Qiu JH, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354:1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- Steyger PS, Peters SL, Rehling J, Hordichok A, Dai CF. Uptake of gentamicin by bullfrog saccular hair cells in vitro. J Assoc Res Otolaryngol. 2003;4(4):565–578. doi: 10.1007/s10162-003-4002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P, Vanden Berghe T, Festjens N (2006) Caspase inhibitors promote alternative cell death pathways. Sci STKE 2006:pe44 [DOI] [PubMed]
- Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004;64:9217–9224. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- Wikler M. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Wayne: Clinical and Laboratory Standards Institute; 2006. pp. 1–64. [Google Scholar]
- Wikler M. Performance standards for antimicrobial susceptibility testing. Wayne: Clinical and Laboratory Standards Institute; 2007. pp. 1–182. [Google Scholar]
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]