Abstract
Glucagon-like peptide-1 (GLP-1) is an intestinally derived insulinotropic hormone that is currently under investigation for use in the treatment of diabetes mellitus. To investigate the Ca2+ signaling pathways by which GLP-1 may stimulate the secretion of insulin from pancreatic β-cells, we examined its effects on the concentration of free intra-cellular Ca2+ ([Ca2+]i) while simultaneously determining what action it exerts on ion channel function. Measurements of [Ca2+]i were obtained from single rat β-cells and from βTC6 and HIT-T15 insulinoma cells loaded with the Ca2+ indicator fura-2, and changes in membrane potential and current were monitored using the perforated patch clamp technique. We report a previously undocumented action of GLP-1 and analogs of cAMP (8-bromo-cAMP, Sp- or Rp-adenosine 3′,5′-cyclic monophosphothionate tri-ethylamine) to raise [Ca2+]i that is attributable to the activation of a prolonged inward current designated here as IcAMP. Activation of IcAMP is associated with an increased membrane conductance, membrane depolarization, and triggers large increases of [Ca2+]i. IcAMP is primarily a Na+ current that is blocked by extracellularly applied La3+ or by intracellular administration of Ca2+ chelators (1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid/acetoxy-methyl, EGTA) and which exhibits a reversal potential of about −26 mV. We propose that IcAMP results from the opening of nonselective cation channels that are activated by intracellular Ca2+ and cAMP and which might play an important role in the regulation of insulin secretion from pancreatic β-cells.
GLP-11 is an intestinally derived hormone that plays an important role in systemic glucose homeostasis (1–3). GLP-1 is derived by tissue-specific post-translational processing of pro-glucagon (4, 5) and is secreted from enteroendocrine cells (L-cells) of the intestinal tract in response to a meal or an oral glucose challenge (1–3). GLP-1 is a gluco-incretin hormone because it mediates humoral communication among the intestine, the pancreas, and target sites of insulin action (the entero-insular axis), thereby exerting important influences on glucose uptake and disposal (1–3, 6, 7). GLP-1 is also an insulinotropic hormone by virtue of its ability to stimulate insulin gene expression (8, 9), proinsulin biosynthesis (9), and to potentiate glucose-induced insulin secretion from pancreatic β-cells (10–15).
These insulinotropic actions prompted interest in the potential therapeutic use of GLP-1 as an antidiabetogenic (blood glucose-lowering) agent. Intravenously administered GLP-1 raises circulating levels of insulin and lowers blood glucose in non-insulin-dependent diabetics (16–19). GLP-1 also offers several distinct advantages over sulfonylureas in the treatment of non-insulin-dependent diabetes. It exerts a stronger antidiabetogenic effect than is achieved following oral administration of glyburide, and it is effective even under conditions in which glyburide fails to control blood glucose levels adequately (18), a condition referred to as sulfonylurea failure (21). Unlike sulfonylureas, the antidiabetogenic effect of GLP-1 is glucose-dependent. GLP-1 fails to stimulate insulin secretion when blood glucose levels fall to below low normoglycaemic levels, approximately 5 mm (1–3, 7). Therefore, the action of GLP-1 to stimulate insulin secretion and to lower blood glucose is self-correcting, and the risk of hypoglycemia as an untoward side effect of GLP-1 treatment is reduced greatly.
The mechanisms by which GLP-1 potentiates glucose-induced insulin secretion from pancreatic β-cells have yet to be elucidated fully. The active forms of GLP-1 in vivo are the isopeptides GLP-1(7–37) and GLP-1(7–36)amide, both of which bind to G protein-coupled receptors on β-cells (22–24), and which stimulate production of cAMP (8, 14, 22–25). GLP-1 also enhances the inhibitory effect of glucose on the sulfonylurea-sensitive potassium current (IKATP), thereby augmenting glucose-induced membrane depolarization (26). This synergistic interaction results in activation of voltage-dependent calcium channels (VDCCs), thereby raising [Ca2+]i (26, 27). Since a rise in [Ca2+]i triggers insulin secretion (28, 29) and because GLP-1 increases [Ca2+]i in β-cells (30–32), such a sequence of events may contribute to the reported effects of GLP-1 on stimulus-secretion coupling.
GLP-1 stimulates the production of cAMP, a second messenger that has important effects on β-cell function. These effects include augmentation of electrical activity (26, 33–35), membrane depolarization (26, 34, 35), Ca2+ influx (35–39), and insulin secretion (34–37, 40, 41). GLP-1 also increases [Ca2+]i and potentiates glucose-induced insulin secretion in a Na+-dependent manner (32, 42), a requirement that suggests the involvement of a Na+ permeability change as an underlying feature of the action of GLP-1. In contrast, cAMP activates nonselective cation channels in the CRI-G1 insulinoma cell line (43), stimulates 45Ca2+ efflux from secretory granules in β-cells (44), and augments insulin secretion by facilitating fusion of secretory granules with the plasma membrane (45, 46). Therefore, no single mechanism of action accounts for the stimulatory effects of GLP-1 and cAMP in the β-cell system.
To further investigations of how GLP-1 influences insulin secretion, we examined its effects on [Ca2+]i while also determining what action it exerts on ion channel function. Measurements were obtained from rat β-cells maintained in short term primary cell culture. Also studied were βTC6 and HIT-T15 insulinoma cells, two cell lines that secrete insulin in response to glucose (47–51) and which express GLP-1 receptors that couple to cAMP production (8,14, 31, 53). We report that GLP-1 and analogs of cAMP raise [Ca2+]i by activating an inward current designated here as IcAMP. Activation of IcAMP is accompanied by an increased membrane conductance, membrane depolarization, and large increases of [Ca2+]i which are dependent on extracellular Ca2+ and Na+. These findings document a novel GLP-1-sensitive Ca2+-signaling pathway that might play an important role in the regulation of insulin secretion from β-cells.
MATERIALS AND METHODS
Preparation of Cell Cultures
HIT-T15 cells were obtained from the American Type Culture Collection. βTC6 cells were obtained from Dr. Shimon Efrat (Albert Einstein College of Medicine, New York). HIT-T15 cells were maintained in Ham’s F-12 medium containing 10 mm glucose, 10% heat-inactivated horse serum, and 2.5% fetal bovine serum. βTC6 cells were maintained in Dulbecco’s modified Eagle’s medium containing 25 mm glucose, 15% horse serum, and 2.5% fetal bovine serum. Culture media also contained 100 units/ml penicillin G and 100 µg/ml streptomycin. Primary cultures of rat β-cells were prepared and maintained as described previously (26). Cells were plated onto glass coverslips coated with 1 mg/ml type V concanavalin A (Sigma), which facilitates their adherence to glass. Cultures were maintained at 37 °C in a 5% CO2 atmosphere incubator, and experiments were conducted 1—5 days postplating.
Measurement of Intracellular Calcium
Cells were prepared for measurement of [Ca2+]i by incubation in fura-2 acetoxymethyl ester (fura-2/AM; Molecular Probes, Inc., Eugene, OR). Cells were loaded in saline containing 2% fetal bovine serum, 0.03% pluronic F-127, and 0.5–5 µm fura-2/AM for 10–30 min at 20–22 °C. Under these conditions, 91% of the fluorescence emission is attributable to cytosolic fura-2 (54). Coverslips with fura-loaded adherent cells formed the base of a recording chamber mounted on a temperature-controlled stage (MicroDevices, Jenkintown, PA). Cells were visualized using a Zeiss IM35 microscope equipped with a Nikon UVF100 100X objective. Measurements of [Ca2+]i were performed at 1-s intervals using a dual excitation wave-length video imaging system (IonOptix Corp., Milton, MA). Experiments were conducted at 32 °C. [Ca2+]i was estimated from the ratio of 510 nm emission fluorescences due to excitation by 350 nm and 380 nm wavelength light from Equation 1 (55)
| (Eq. 1) |
where Kd is the dissociation constant of fura-2 (225 nm), β is the ratio of 380 nm induced fluorescences of free/bound fura-2, R is the measured ratio of 350 nm/380 nm fluorescences, and Rmin and Rmax are 350 nm/380 nm fluorescence ratios in zero [Ca2+] and saturating [Ca2+], respectively. Values of β, Rmin, and Rmax were determined using fura-2 pentapotassium salt and calibration solutions from Molecular Probes, Inc.
Preparation of Test Solutions
Cells for fura-2 loading and patch clamp recording were bathed in a standard extracellular buffered saline containing 138 mm NaCl, 5.6 mm KCl, 2.6 mm CaCl2, 1.2 mm MgCl2, 10 mm HEPES (295 mosm; pH adjusted to 7.4 with NaOH). The concentration of d-glucose was adjusted to be near threshold for stimulation of insulin secretion (7.5 mm for rat β-cells (28), 0.8 mm for insulinoma cells (47, 58)). Na+-free solutions were prepared using 138 mm N-methyl-d-glucamine (NMG) substituted for NaCl/NaOH and adjusted to pH 7.4 with HCL. In some experiments 138 mm Tris-HCl (pH 7.4) was used as a substitute for Na+ in place of NMG. Solutions to which no Ca2+ was added were prepared by substituting MgCl2 for CaCl2.
Test solutions containing GLP-1, pituitary adenylyl cyclase-activating peptide-27 (PACAP-27), glucagon, forskolin, Sp- and Rp-cAMP-S, IBMX, or 8-Br-cAMP, were applied to individual cells by focal application from “puffer” micropipettes (26) using a PicoSpritzer II pressure ejection system (General Valve, Fairfield, NJ). For experiments examining the effects of peptides, the test solutions also contained 0.05% human serum albumin (fraction V; Sigma) added to protect against absorption of the peptides to borosilicate glass culture tubes in which the solutions were prepared. Human serum albumin was found to be without effect on the measurements reported here. A superfusion system driven by a Ismatec direct current-powered peristaltic pump (Cole-Palmer Instrument Co., Chicago) applied known concentrations of Ca2+ and Na+ channel blockers to the superfusate.
GLP-1(7—37) was obtained from Peninsula Laboratories (Belmont, CA). GLP-1(7–36)amide, PACAP-27, glucagon, forskolin, 8-Br-cAMP, IBMX, nifedipine, nimodipine, verapamil, and diazoxide were obtained from Sigma. Sp- and Rp-cAMP-S were obtained from BioLog Life Sciences Institute (Bremen, FRG). Tetrodotoxin and ryanodine were obtained from Calbiochem. ω-Conotoxin GVIA was from Bachem California (Torrance, CA).
Patch Clamp Recording Techniques
The resting potential and holding current were measured under current clamp or voltage clamp using the tight seal, whole cell, perforated patch configuration (56, 57). Patch pipettes pulled from borosilicate glass (Kimax-51, tip resistance 2—3 megohms) were fire polished and tip dipped in 95 mm K2SO4, 7 mm MgCl2, 5 mm HEPES (300 mosm; pH adjusted to 7.4 with NaOH; final concentration of Na+ equal to about 2 mm), and back filled with the same solution containing nystatin (240 µg/ml). A limited number of experiments utilized the standard whole cell recording configuration in which the patch membrane was ruptured, and diffusional exchange was allowed to occur between the pipette solution and the cytosol. Under these conditions the pipette solution contained either a “Ca2+-free” intracellular solution containing (in mm): 140 KC1, 2 mm MgCl2, 10 HEPES/KOH (pH 7.4), and 5 EGTA, or an intracellular solution containing 160 nm Ca2+ and composed of (in mm): 140 KC1, 2 mm MgCl2, 10 HEPES/KOH (pH 7.4), 0.1 CaCl2, and 1.1 EGTA.
The patch pipette was connected to an Heka Electronik EPC-9 patch clamp amplifier (Instrutech Corp., Mineola, NY) interfaced with a Macintosh Quadra 840AV computer running Pulse software (Instrutech Corp.). The series resistance (Rs) and cell capacitance (Cm) were monitored following seal formation, and experiments were conducted when Rs declined to 12–25 megohms and Cm increased to 10–40 picofarads. Electrical access was confirmed by noting a −50 to −70 mV resting potential and by noting a rise in [Ca2+]i in response to a depolarizing voltage step. The experiment was rejected if such a [Ca2+]i response was not noted. In voltage clamp experiments, Rs was compensated for by 60–80%. The pipette solutions did not contain fura-2 and were nominally Ca2+-free unless otherwise noted. A sudden decrease in Rs accompanied by a rise in [Ca2+]i and decrease in fluorescence intensity provided a useful marker for unintended rupture of the cell membrane during perforated patch recordings, whereupon the experiment was terminated.
GLP-1 Receptor Binding Assays
The specific binding of 125I-GLP(7–37) or 125I-GLP(7–36)amide to the GLP-1 receptor was determined by a rapid filtration binding assay in which the binding of ligand to receptor was monitored using intact COS-7 cells transiently transfected by the DEAE-dextran method with the rat GLP-1 receptor cDNA encoded within the pcDNA-1 expression vector (22). 125I-GLP(7–37) and 125I-GLP(7–36)amide were prepared by the chloramine-T method of iodination, and the monoiodinated form of the peptide was purified by high performance liquid chromatography. Bound radioligand was separated from unbound by filtration through Whatman GF/C filters pretreated with Krebs bicarbonate buffer containing 6% fetal bovine serum and 0.8% Tween 20. Total binding was determined after equilibration of cells with iodinated peptide for 60 min at 22 °C in HEPES-buffered saline containing 2% human serum albumin. Nonspecific binding was defined as binding of the radioligand observed following pretreatment of cells with a 1 µm concentration of the corresponding nonradioactive peptide. Specific binding was calculated as the difference between total and nonspecific binding. Using this approach, ≤1% of the radioligand bound nonspecifically, and 10–20% bound specifically.
Radioimmunoassay for cAMP
βTC6 cells were cultured in 24-well tissue culture plates until reaching 70–80% confluence. Extracellular solution (with 138 mm Na+ or 138 mm NMG substituted for Na+) with 100 µm IBMX ± 10 nm GLP-1 (a concentration shown previously (53) to be saturating for cAMP production in βTC1 cells) was substituted for the culture medium, and the exposure to GLP-1 was allowed to progress for 30 min at room temperature. Ice-cold absolute ethanol (1 ml) was then added to each well, and the cells were subjected to three rounds of freeze-thawing. The lysed cells and extracellular solution were collected, and the total content of cAMP was measured by a specific radioimmunoassay as described previously (53).
RESULTS
GLP-1 Induces a Sustained Rise of[Ca2+]i Which Is Glucose-dependent
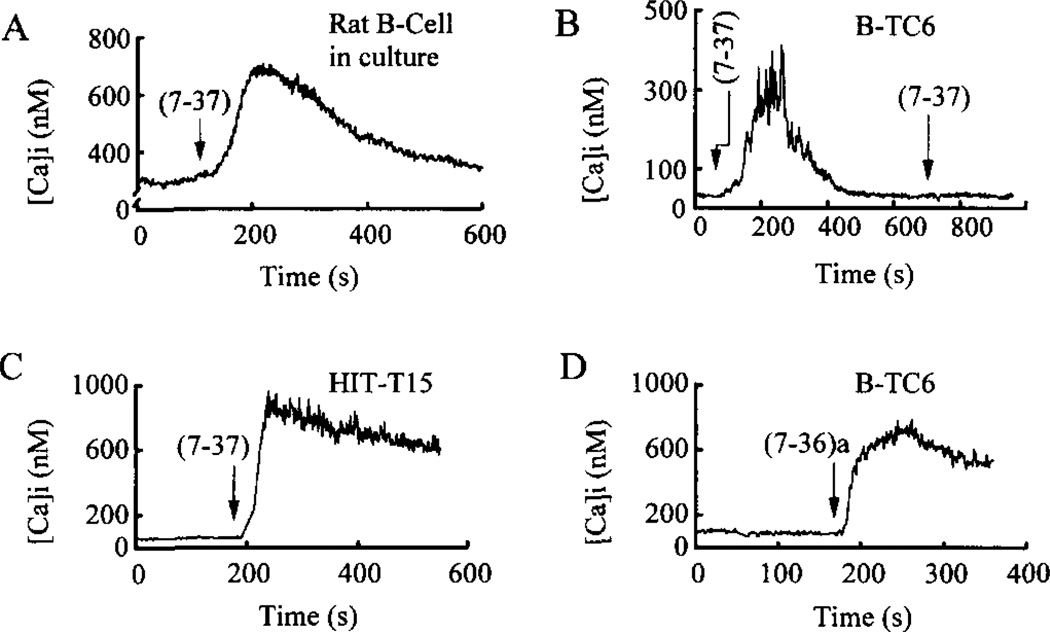
To determine in what manner GLP-1 influences cytosolic Ca2+ levels, measurements of [Ca2+]i were obtained from single rat β-cells and insulinoma cells equilibrated in buffer containing 7.5 and 0.8 mm d-glucose, respectively. Under these conditions the cells exhibited stable membrane potentials (−62 ± 7 mV; mean ± S.D.; n = 10 βTC6 cells) and stable, low levels of [Ca2+]i (91 ± 8 nm; n = 50 βTC6 cells). An increase of [Ca2+]i was observed in response to a 10-s focal application of GLP-1(7-37) to single rat β-cells (Fig. 1A, n = 20 cells), βTC6 cells (Fig. 1B, n = 40), and HIT-T15 cells (Fig. 1C; n = 20). The sustained nature of these responses is not explained by the kinetics of the drug delivery system used (26) since the super-fusion system ensures that GLP-1 is eliminated from the solution surrounding the cells within 30 s. An increase of [Ca2+]i was also observed in response to GLP-1(7–36)amide, not only in βTC6 cells (Fig. 1D; n = 20) but also in rat β-cells (n = 20) and HIT-T15 cells (n = 20). These responses were variable with respect to reversibility. In some cells [Ca2+]i recovered to pre-stimulus levels (Fig. 1, A and B), whereas in others the [Ca2+]i exhibited only partial recovery (Fig 1, C and D). No consistent differences were noted in the amplitude, kinetics, or reversibility of responses when comparing effects of GLP-1(7–37) versus GLP-1(7–36)amide.
Fig. 1. GLP-1 increases [Ca2+]i, in rat β-cells and pancreatic insulinoma cells.
Each of the two isoforms of GLP-1, abbreviated as (7-37) or (7-36)a, were administered to individual cells at a concentration of 10 nm for 10 s (arrows indicate the start of application). The GLP-1 was then removed within 30 s by a superfusion system. A sustained rise of [Ca2+]i in response to GLP-1 was observed in a rat β-cell maintained in primary cell culture (panel A), in βTC6 cells (panels B and D), and an HIT-T15 cell (panel C). In panel A the extracellular solution contained 7.5 mm glucose, whereas in panels B-D it contained 0.8 mm glucose.
In βTC6 cells the GLP-1(7-37)-induced rise of [Ca2+]i was dose-dependent and was diminished by removal of extracellular d-glucose (Table I). These responses also exhibited desensitization (Fig. 1B) to repeated application of concentrations of the peptides (≥10 nm) reported to induce homologous desensitization of GLP-1-induced insulin secretion (53). The action of GLP-1 to raise [Ca2+]i was mimicked by activators of cAMP signaling pathways, including PACAP-27, glucagon, forskolin, IBMX, Sp-cAMP-S, and 8-Br-cAMP (Table II). Notably, the cAMP antagonist Rp-cAMP-S was an effective agonist in this system (Table II).
Table 1. Glucose dependence of three GLP-1 concentrations on [Ca2+]i.
Only cells exhibiting a ≥ 75 nm increase of [Ca2+]i were counted as exhibiting a response to GLP-1. The increase of [Ca2+]i was measured at the peak of the response. Base-line levels of [Ca2+]i were 91 ± 8 nm (n = 50). Except where indicated, the extracellular solution contained 0.8 mm glucose. To deprive the cells of glucose, cultures were preincubated in glucose-free recording solution for 1 h at 37 °C. GLP-1 was applied directly to individual cells for 30 s using a puffer pipette. Data summarize results obtained in two experiments using βTC6 cells from two different platings.
| [GLP-1(7-37)] | Fraction of βTC6 cells responding to GLP-1(7-37) |
Mean ± S.D. increase of [Ca2+]i |
|---|---|---|
| nm | nm | |
| With 0.8 mm | ||
| d-glucose | ||
| 0.1 | 3/10 | 207 ± 55 |
| 1.0 | 6/10 | 295 ± 87 |
| 10 | 8/10 | 386 ± 73 |
| Without | ||
| d-glucose | ||
| 0.1 | 0/10 | |
| 1.0 | 0/10 | |
| 10 | 2/10 | 267 ± 69 |
Table II. Effect of GLP-1 on [Ca2+]i mimicked by activators of cAMP signaling.
Counting and measurements were as described in Table I. All test substances were applied for 30 s to individual cells using a puffer pipette. Data summarize results obtained in six experiments using βTC6 cells from six different platings. Statistical significance was evaluated by the t test.
| Pharmacological treatment |
Fraction of βTC6 cells responding to test substance |
Mean ± S.D. increase of [Ca2+]ia |
|---|---|---|
| nm | ||
| 10 nm GLP-1(7-37) | 8/10 | 410 ± 52 |
| 10 nm PACAP-27 | 10/10 | 492 ± 43 |
| 10 nm glucagon | 6/10 | 422 ± 50 |
| 10 µm forskolin | 10/10 | 1,050 ± 240** |
| 100 µm IBMX | 5/10 | 575 ± 109 |
| 200 µm Sp-cAMP-S | 8/10 | 840 ± 175* |
| 200 µm Rp-cAMP-S | 7/10 | 885 ± 166* |
| 1 mm 8-Br-cAMP | 10/10 | 1,209 ± 347** |
*(p ≤ 0.01) and ** (p ≤ 0.005) relative to GLP-1 alone.
The GLP-1-induced Rise of [Ca2+]i Requires Extracellular Na+
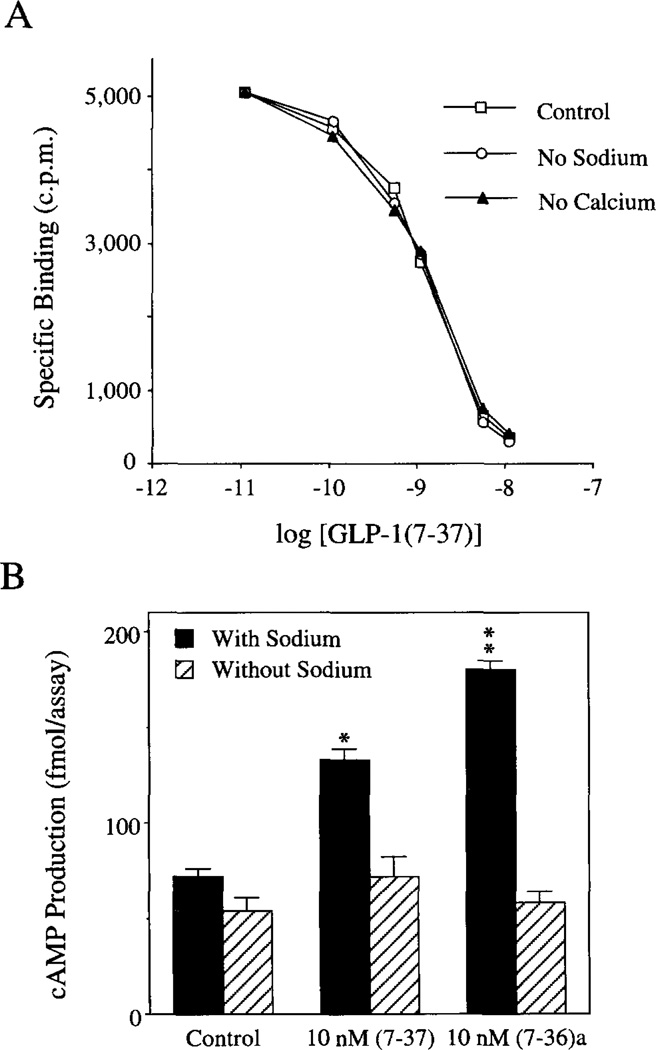
The GLP-1-(7-37)-induced rise of [Ca2+]i in βTC6 cells was blocked by omission of Na+ from the extracellular bathing and test solutions (Table III). This was observed when Na+ was replaced by either NMG or Tris-HCl. The failure of GLP-1 to raise [Ca2+]i in the absence of Na+ was not due to the inability of GLP-1 to bind to its receptor. Radioligand binding assays using 125I-GLP-1(7-37) or (7–36)amide confirmed that the binding of the peptides to the rat islet GLP-1 receptor expressed in transfected COS-7 cells was unaffected by omission of Na+ from the extracellular solution (Fig. 2A).
Table III. Na+ and Ca2+ requirement of GLP-1-induced rise of [Ca2+]i.
Summarized are results obtained in three experiments using βTC6 cells from three different platings. The compositions of the extracellular solutions were as indicated under “Materials and Methods” except that 138 mm NMG or 138 mm Tris was substituted for Na+ when Na+ was omitted. Note that Mg2+ was substituted for Ca2+ to obtain a nominally Ca2+-free solution and that the βTC6 cells were exposed to this solution for 10–30 min, during which the action of GLP-1 was tested.
| Composition of extracellular Solutiona |
Fraction of βTC6 cells responding to 10 nm GLP-1(7-37) |
Mean ± S.D. increase of [Ca2+]i |
|---|---|---|
| nm | ||
| (+) Ca/(+) Na | 18/20 | 326 ± 43 |
| (+) Ca/(−) Na/(+) NMG | 2/20 | 149 ± 65 (n = 2) |
| (+) Ca/(−) Na/(+) Tris-HCl | 1/10 | 120 (n = 1) |
| (−) Ca/(+) Na | 0/20 |
The extracellular solution contained 0.8 mm glucose.
Fig. 2. Effects of ion substitution on GLP-1 binding and cAMP production.
Panel A, omission of Na+ or Ca2+ from the extracellular solution had no effect on the binding of GLP-1 to its receptor. COS-7 cells were transiently transfected with the rat islet GLP-1 receptor cDNA, and the binding of 125I-GLP-1(7-37) was determined 3 days post-transfection. Illustrated are displacement curves generated under conditions in which the binding of l25I-GLP-1(7-37) was competed for by prior equilibration of cells with the indicated concentration of non-radioactive GLP-1(7-37). Under control conditions in which the extra-cellular solution contained 138 mm Na+ and 2.6 mm Ca2+, approximately 20% of added tracer was bound specifically, and the IC50 for displacement of radioligand by nonradioactive peptide was determined to be approximately 2 nm. Neither Na+ omission (by replacement of Na+ with NMG) nor Ca2+ omission (by replacement of Ca2+ with Mg2+) influenced either the total binding activity or the IC50 value for displacement of radioligand. Nearly identical results were obtained using 125I-GLP-1(7-36)amide as radioligand and nonradioactive GLP-1(7-36)amide as displacer (data not shown). Panel B, the stimulation of βTC6 cell cAMP production by GLP-1 was dependent on extracellular Na+. Cells were incubated without (Control) or with GLP-1 for 30 min at 23 °C in extracellular solution containing 0.8 mm glucose, 100 µm IBMX, 2.6 mm CaCl2, and either 138 mm NaCl or 138 mm NMG (no Na+). Total cAMP production was determined by radioimmunoassay. Statistical significance relative to the control was evaluated by the t test (*,p ≤ 0.05; **,p ≤ 0.01).
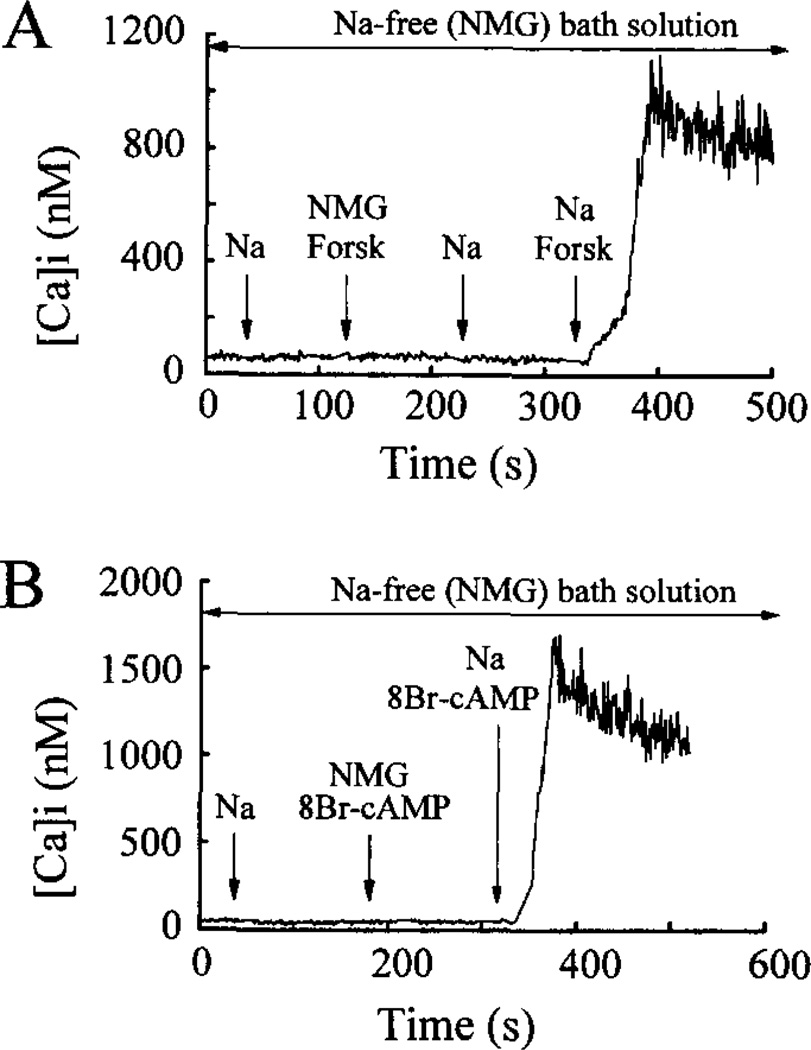
In contrast, omission of extracellular Na+ markedly attenuated GLP-1-induced production of cAMP in βTC6 cells (Fig. 2B). This observation suggested that the failure of GLP-1 to influence [Ca2+]i under conditions of Na+ omission might be simply attributed to its failure to generate cAMP, a requisite second messenger. It was noted, however, that omission of extracellular Na+ also blocked the ability of forskolin (Fig. 3A) and 8-Br-cAMP (Fig. 3B) to increase [Ca2+]i in βTC6 cells. These findings suggested that Na+ is an obligatory cation, acting to influence multiple steps in the GLP-1 signaling system.
Fig. 3. The rise of [Ca2+]i evoked by forskolin and 8-Br-cAMP requires extracellular Na+.
Illustrated in panels A and B are measurements of [Ca2+]i obtained from βTC6 cells equilibrated in Na+-free extracellular solution that contained 0.8 mm glucose and 138 mm NMG substituted for Na+. Focal application for 30 s of extracellular solution containing 138 mm Na+ had no effect on [Ca2+]i (panels A and B). Similarly, [Ca2+]i was not influenced by a 30-s application of Na+-free extracellular solution containing 10 µm forskolin (panel A) or 1 mm 8-Br-cAMP (panel B). In contrast, an increase of [Ca2+]i was observed in response to simultaneous application of Na+ and forskolin (panel A) or Na+ and 8-Br-cAMP (panel B). Data presented are representative of findings obtained in five experiments using 20 cells (n = 5 forskolin; n = 15 8-Br-cAMP).
The GLP-1 and 8-Br-cAMP-induced Rise of [Ca2+]i Also Requires Extracellular Ca2+
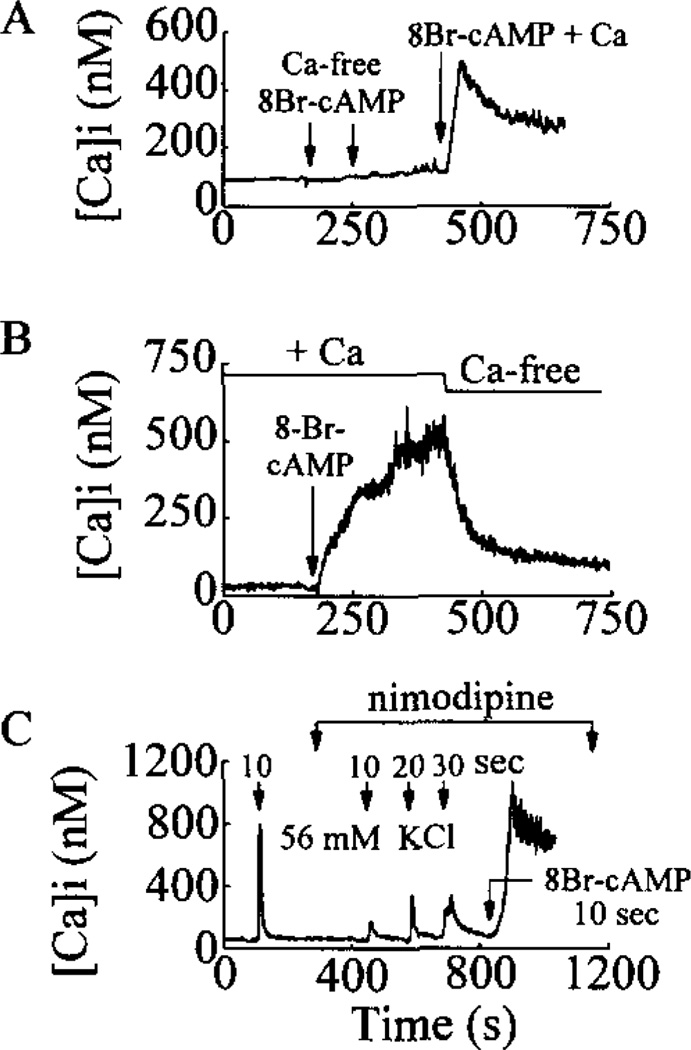
The response of βTC6 cells to GLP-1 could not be initiated under conditions in which Ca2+ was omitted from the extracellular bathing and test solutions (Table III). Moreover, when βTC6 cells were bathed in a solution containing Ca2+, no response was observed when the cells were challenged for 10 s with a Ca2+-free test solution containing 8-Br-cAMP and 50 µm EGTA, whereas a large rise of [Ca2+]i was observed upon exposure to a test solution containing Ca2+ and 8-Br-cAMP (Fig. 4A). Furthermore, the rise of [Ca2+]i observed after treatment with GLP-1 or 8-Br-cAMP was reversed within 1 min following superfusion of the cells with a nominally Ca2+-free bath solution (Fig. 4B). This requirement for Ca2+ was observed even though the binding of 125I-GLP-1(7-37) to its receptor appeared normal under conditions of the omission of extracellular Ca2+ (Fig. 2A).
Fig. 4. The rise of [Ca2+]i evoked by 8-Br-cAMP requires extra-cellular Ca2+ and is not blocked by nimodipine.
Illustrated in panels A-C are measurements of [Ca2+]i obtained from βTC6 cells equilibrated in the standard extracellular solution containing Na+, Ca2+, and 0.8 mm glucose. Panel A, the cell failed to respond to repeated 10-s applications of 1 mm 8-Br-cAMP dissolved in a Ca2+-free extracellular solution containing 50 µm EGTA. A subsequent 10-s application of 1 mm 8-Br-cAMP dissolved in the standard extracellular solution containing Ca2+ resulted in a large increase of [Ca2+]i. Panel B, the cell was challenged with a 10-s application of 1 mm 8-Br-cAMP dissolved in the standard extracellular solution containing Ca2+. A large increase of [Ca2+]i was observed which was reversed by equilibration of the cell in a nominally Ca2+-free extracellular solution introduced into the recording chamber by supervision. Panel C, a 10-s application of a depolarizing concentration (56 mm) of KCl resulted in a large rise of [Ca2+]i due to activation of VDCCs. Bath application of 1 µm nimodipine substantially reduced the rise of [Ca2+]i evoked by a test solution containing 56 mm KCl and 1 µm nimodipine applied for 10, 20, and 30 s, as indicated. A large rise in [Ca2+]i was then observed in response to a 10-s application of a test solution containing 1 mm 8-Br-cAMP and 1 µm nimodipine.
Pharmacological Characterization of the GLP-1-induced Rise of [Ca2+]i
In βTC6 cells the response to GLP-1 (and 8-Br-cAMP; n = 5; data not shown) was abrogated by extracellular application of 10 µm La3+ (Table IV), a broad spectrum antagonist of VDCCs which at this concentration also blocks nonselective cation channels. In contrast, nifedipine and verapamil, two specific antagonists of L-type VDCCs, did not block the response to GLP-1 in either cell type, although a statistically significant inhibitory trend was noted (Table IV). As was the case for GLP-1, the rise of [Ca2+]i in response to 8-Br-cAMP was also not blocked by the L-type VDCC antagonist nimodipine, whereas the rise of [Ca2+]i observed during exposure to a depolarizing concentration (56 mm) of KCl was markedly attenuated (Fig. 4C).
Table IV. La3+ blockade of GLP-1-induced rise of[Ca2+]i.
Summarized are results obtained in eight experiments using cells from eight different platings. Lanthanum, nifedipine, verapamil, cono-toxin, tetrodotoxin (TTX) diazoxide, and ryanodine were applied by superfusion at equilibrium concentrations. GLP-1 ± channel modulators was then applied for 30 s using a puffer pipette. The extracellular solution contained 0.8 mm glucose. Statistical significance was evaluated by the t test.
| Pharmacological treatment | Fraction of βTC6 cells responding to 10 nm GLP-1(7-37) |
Mean ± S.D. increase of [Ca2+]ia |
|---|---|---|
| nm | ||
| 10 nm GLP-1(7-37) | 8/10 | 410 ± 52 |
| 10 µm La3+ and 10 nm GLP-1(7-37) | 0/10 | |
| 5 µm nifedipine and 10 nm GLP-1(7-37) | 7/10 | 224 ± 48* |
| 50 µm verapamil and 10 nm GLP-1(7-37) | 8/10 | 305 ± 25* |
| 1 µm omega-conotoxin GVIA and 10 nm GLP-1(7-37) | 8/10 | 379 ± 61 |
| 5 µm TTX and 10 nm GLP-1(7-37) | 8/10 | 445 ± 70 |
| 100 µm diazoxide and 10 nm GLP-1(7-37) | 7/10 | 275 ± 60* |
| 100 µm ryanodine and 10 nm GLP-1(7-37) | 10/10 | 426 ± 19 |
*p ≤ 0.05 relative to GLP-1 alone.
The rise of [Ca2+]i, in response to GLP-1 (and 8-Br-cAMP; n = 5; data not shown) was significantly reduced but not blocked by diazoxide, an activator of IKATP which chemically clamps the β-cell membrane potential to a value close to the K+ equilibrium potential, thereby preventing activation of VDCCs (Table IV). In contrast, the response to GLP-1 was not blocked by ω-conotoxin GVIA, a blocker of N-type VDCCs, nor was it eliminated by ryanodine, a blocker of intracellular Ca2+ release channels (Table IV). Furthermore, tetrodotoxin, a blocker of voltage-dependent Na+ channels, was also without effect (Table IV).
Effects of GLP-1 and 8-Br-cAMP on Membrane Potential
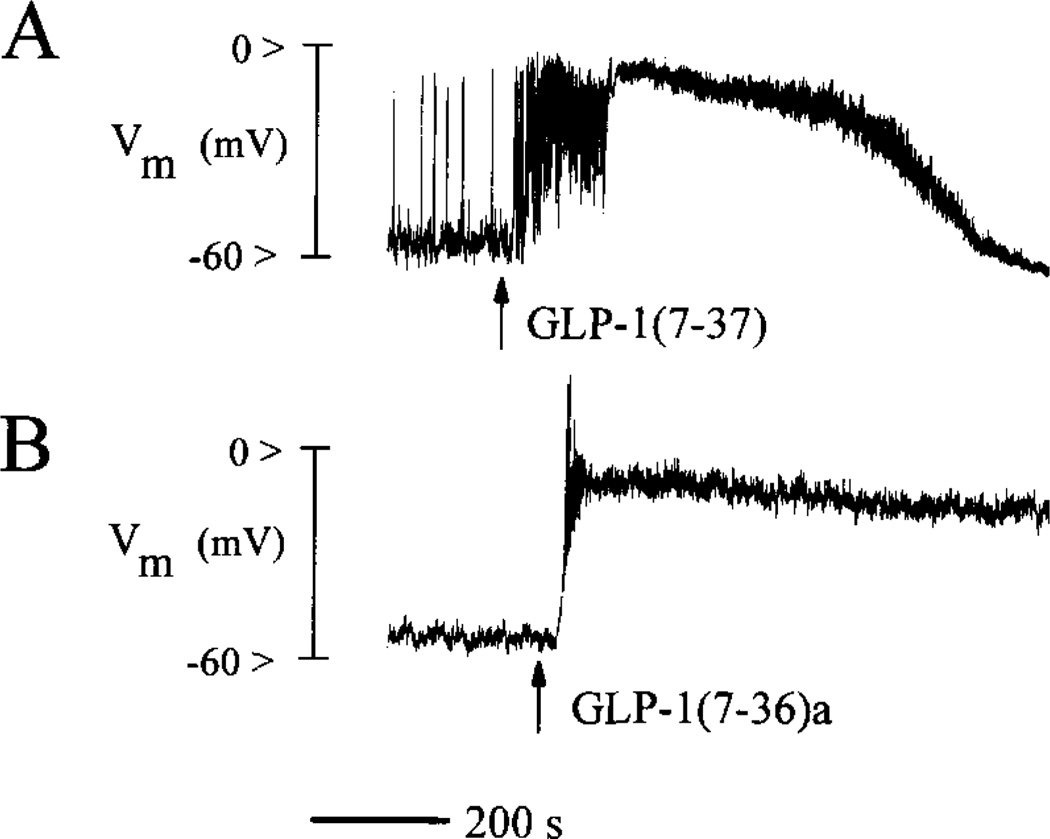
Perforated patch clamp measurements obtained from rat β-cells equilibrated in a steady-state concentration of glucose (7.5 mm) revealed that a 10-s exposure to GLP-1 produced a prolonged and reversible depolarizing shift in membrane potential from an initial value of about −60 mV to a plateau value of ca. −10 mV (Fig. 5A, n = 10). Membrane depolarization in response to GLP-1 was also observed in HIT-T15 cells (Fig. 5B, n = 10) and βTC6 cells (n = 5; data not shown). The onset of the depolarization was accompanied by the generation of numerous action potentials, as evidenced by spike-like phenomena on the rising phase of the response (Fig. 5, A and B).
Fig. 5. Effects of GLP-1 on membrane potential (Vm).
Panel A, illustrated is a perforated patch current clamp measurement of membrane potential obtained from a rat β-cell equilibrated in buffer containing 7.5 mm glucose. Application for 10 s of 10 nm GLP-1(7-37) produced sustained membrane depolarization and the generation of action potentials (spike-like phenomena). Panel B, illustrated are the sustained membrane depolarization and generation of action potentials observed in response to 10 nm GLP-1(7-36)amide applied for 10 s to an HIT-T15 cell equilibrated in buffer containing 0.8 mm glucose.
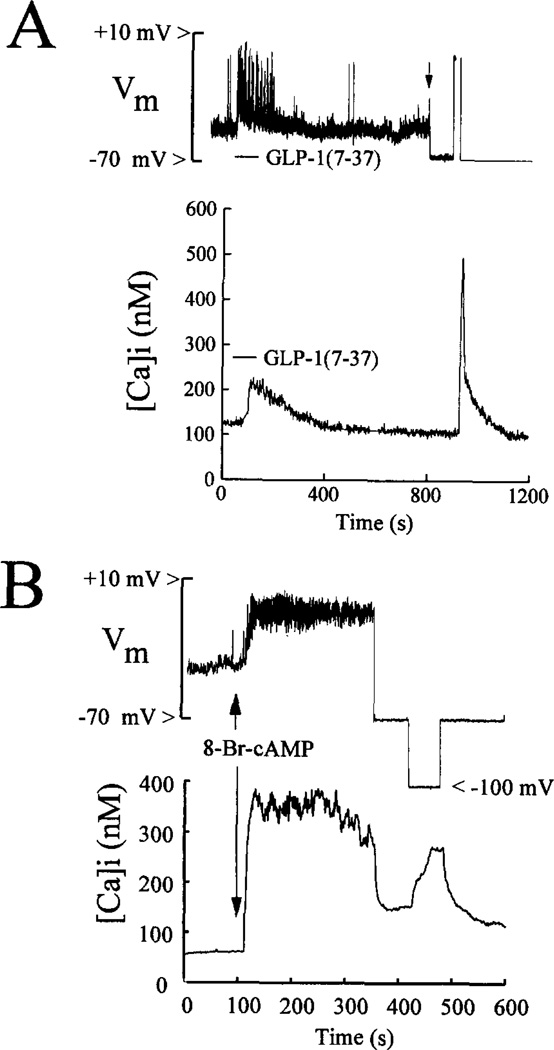
Simultaneous measurements of membrane potential and [Ca2+]i obtained from an HIT-T15 cell demonstrated that a rise of [Ca2+]i was associated with the increased excitability and membrane depolarization observed during exposure to GLP-1 (Fig. 6A). In voltage-clamped cells, a rise of [Ca2+]i was also observed in response to a stepwise shift of the membrane potential from −70 to 0 mV for 10 s, thereby confirming the presence of VDCCs in this cell type (Fig. 6A). Such responses to GLP-1 were also obtained in rat β-cells (n = 5) and βTC6 cells (n = 5).
Fig. 6. Simultaneous measurements of membrane potential and [Ca2+]i.
Panel A, a 10-s application of 10 nm GLP-1(7-37) to an HIT-T15 cell equilibrated in 0.8 mm glucose resulted in membrane depolarization and the generation of action potentials (top trace) accompanied by rise of [Ca2+]i (bottom trace). The cell was then placed under voltage clamp (indicated by an arrow in the top trace), and the membrane potential was held at −70 mV. A step wise shift of the membrane potential from −70 to 0 mV for 10 s produced a large rise of [Ca2+]i. Panel B, application of 1 mm 8-Br-cAMP for 10 s to a βTC6 cell equilibrated in 0.8 mm glucose produced membrane depolarization and the generation of action potentials (top trace) and a rise of [Ca2+]i (bottom trace). The 8-Br-cAMP-induced rise of [Ca2+]i was reduced by voltage clamping the membrane to −70 mV and was augmented by stepping the membrane potential from −70 to −100 mV.
Application of 8-Br-cAMP to βTC6 cells also resulted in membrane depolarization and a rise of [Ca2+]i to a new steady-state level (Fig. 6B; n = 5). Voltage clamping the membrane to −70 mV at the peak of the response resulted in a rapid reduction of [Ca2+]i, as expected if the initial rise of [Ca2+]i resulted, at least in part, from the opening of VDCCs. A subsequent shift of the membrane potential from −70 to −100 mV resulted in a large increment of [Ca2+]i, suggesting an additional mechanism of Ca2+ entry distinct from VDCCs (Fig. 6B). Similar responses were also observed in rat β-cells (n = 5) and HIT-T15 cells (n = 10), not only in response to 8-Br-cAMP but also in response to GLP-1 (n = 5 HIT-T15 cells).
Activation of IcAMP by GLP-1 and 8-Br-cAMP
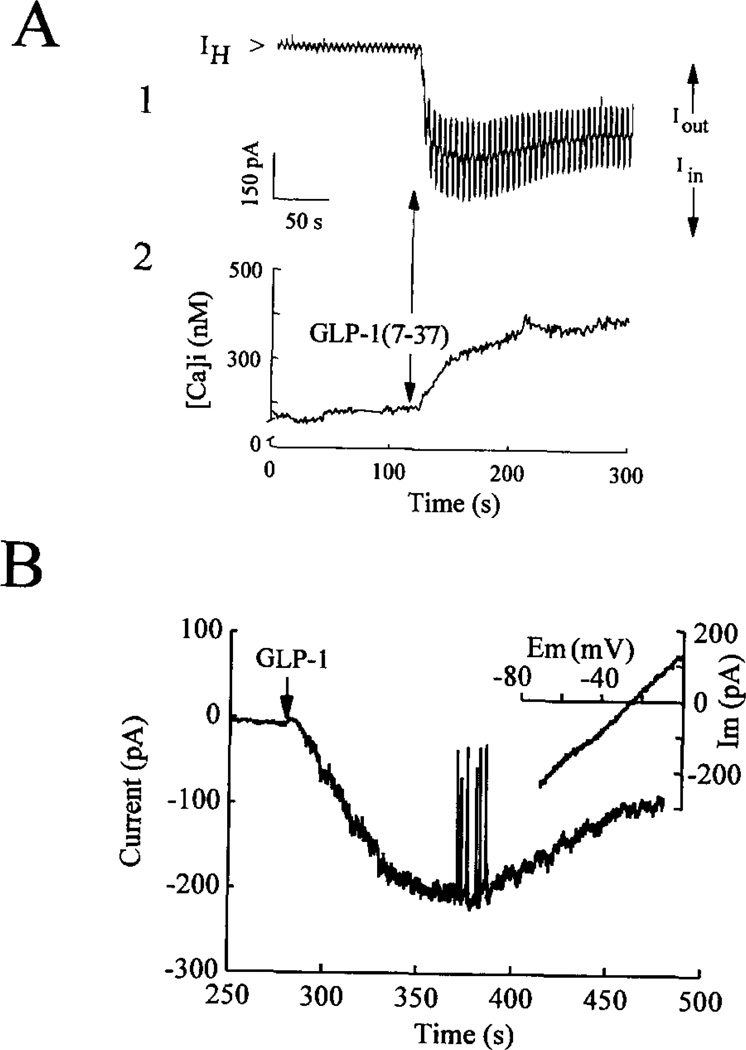
Measurements obtained from voltage-clamped βTC6 cells revealed that the actions of GLP-1 were associated with the appearance of IcAMP indicated by an inward shift of the holding current (IH, Fig. 7A, trace 1). IcAMP outlasted the transient (10–s) application of the peptide and was accompanied by a gradual rise of [Ca2+]i (Fig. 7A, trace 2). Such inward current responses (typically 200-400 pA; −70 mV holding potential) were observed in 10/10 (βTC6, 7/10 HIT-T15, and 4/5 rat β-cells tested.
Fig. 7. Activation of IcAMP and a rise of [Ca2+]i by GLP-1(7-37).
Panel A, simultaneous measurements of membrane current (trace 1) and [Ca2+]i (trace 2) were obtained from a βTC6 cell equilibrated in 0.8 mm glucose. IH was monitored while voltage clamping the membrane at −70 mV in the perforated patch configuration. Measurements of membrane conductance were obtained by applying ±10-mV shifts in the holding potential at 5-s intervals. These shifts in membrane potential evoked outward (Iout) and inward (Iin) current responses (upward and downward deflections superimposed on the holding current), the amplitudes of which are directly proportional to the membrane conductance. Application of 10 nm GLP-1(7-37) for 10 s induced an inward shift of the holding current (IH) and a large increase of membrane conductance (trace 1), the time course of which matched the rise of [Ca2+]i (trace 2). Panel B, perforated patch measurements of membrane current obtained from a rat β-cell equilibrated in 7.5 mm glucose and held under voltage clamp at −70 mV. Application of 10 nm GLP-1(7-36)amide for 10 s produced an inward shift of the holding current (IH). Deflections superimposed on the inward current are membrane current transients evoked by shifting (1 mV/ms) the membrane potential from −70 to 0 mV. This ramp stimulus protocol was used to evaluate the current as a function of voltage (I – V) relationship for IcAMP (inset; see “Results”). Not illustrated are control current transients evoked prior to application of GLP-1. IcAMP exhibited a reversal potential of −26 mV (inset).
IcAMP resulted from the activation of ion channels, as demonstrated by measurements of membrane conductance. Changes in conductance associated with the appearance of IcAMP were monitored by determining the amplitude of steady-state inward (Iin) and outward (Iout) currents generated by ± 10-mV shifts in membrane potential of 1-s duration evoked from a holding potential of −70 mV. A large conductance increase in response to GLP-1 was observed (Fig. 7A, trace 1), as indicated by the increased amplitude of the evoked inward and outward currents). The time course of the conductance change matched that of the inward current and the associated rise in [Ca2+]i, suggesting a causal relationship between these phenomena.
An estimate of the reversal potential for the GLP-1-induced inward current was determined for a rat β-cell by using a ramp stimulus protocol to obtain a current-voltage relationship (Fig. 7B). Cells were bathed in an extracellular solution containing 5 mm tetraethylammonium, 1 µm tetrodotoxin, and 1 µm nifedipine to block voltage-dependent K+, Na+, and Ca2+ channels. Voltage ramps of 70-ms duration from −70 to 0 mV were applied prior to and during the application of GLP-1. The membrane current generated before stimulation with GLP-1 was subtracted from the current evoked during exposure to the peptide, and the difference current was plotted as a function of membrane potential (inset of Fig. 7B). The resultant current-voltage relationship indicated an apparent reversal potential of −26 mV. The mean reversal potential based on four such experiments was −26 ± 4 mV (n = 4 HIT-T15 cells).
Measurements obtained from a voltage-clamped HIT-T15 cell demonstrated that 8-Br-cAMP also induced an inward current and an increased membrane conductance (Fig. 8A). This was accompanied by a large rise of [Ca2+]i even though the membrane potential was maintained at −70 mV (data not shown). These actions of 8-Br-cAMP were also observed in rat β-cells (n = 5) and βTC6 cells (n = 10, data not shown). Furthermore, the reversal potential for the 8-Br-cAMP-induced inward current (−28 ± 2.3 mV, n = 4) measured in HIT-T15 cells approximated that of GLP-1, as expected if both agents activate the same type(s) of ion channels.
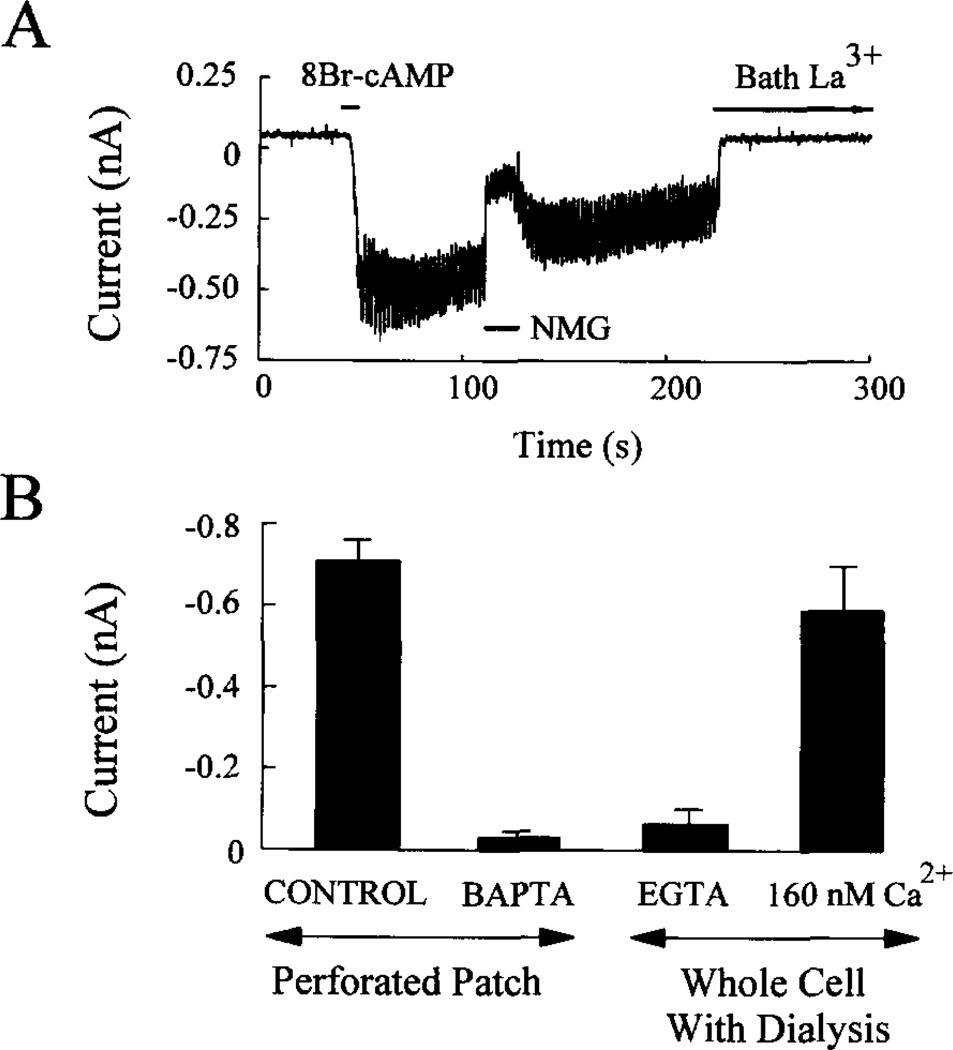
Fig. 8. IcAMP represents a La3+-blockable Na+ current, the activation of which is sensitive to intracellular Ca2+.
Panel A, the inward current and increased membrane conductance observed in response to 1 mm 8-Br-cAMP applied to an HIT-T15 cell for 10 s was blocked by focal application of Na+-free extracellular solution (NMG) or by bath application of 100 µm La3+. The membrane was voltage clamped at −70 mV in the perforated patch configuration, and the membrane conductance was monitored by applying ±10-mV voltage steps. Panel B, intracellular administration of Ca2+ chelators to HIT-T15 cells blocked the inward current response to 1 mm 8-Br-cAMP. Prior to initiating perforated patch recordings, cells were incubated for 1 h in 20 µm BAPTA/AM. For whole cell recordings, the cells were dialyzed with an intracellular solution containing 5 mm EGTA (Ca2+-free) or 160 nm Ca2+. Each bar of the histogram represents measurements obtained from five cells. Error bars indicate mean ± S.D. The membrane was voltage clamped at −70 mV. In panels A and B the extracellular solution contained 0.8 mm glucose.
IcAMP May Result from Activation of Nonselective Cation Channels by cAMP and Ca2+
The inward current observed in response to GLP-1 could result from activation of two different signaling mechanisms. Such a current might be secondary to the GLP-1-induced rise of [Ca2+]i since, in insulinoma cells, Ca2+ is reported to activate nonselective cation channels (Ca-NS channels; terminology of Ref. 59), the properties of which conform in some ways to the findings presented here. Alternatively, GLP-1 might exert a more immediate stimulatory effect, possibly involving the direct activation of nonselective cation channels by cAMP, as has also been reported for insulinoma cells (43). Both concepts are consistent with three additional sets of observations obtained in HIT-T15 cells.
First, IcAMP was inhibited by exposure of cells to an extracellular solution containing either NMG (an impermeant cation substituted for Na+) or the cation channel blocker La3+ (Fig. 8A). Second, activation of IcAMP was blocked by prior incubation of the cells for 1 h in a 20 µM concentration of the membrane-permeant Ca2+ chelator BAPTA/AM (n = 5), which will buffer cytosolic Ca2+, thereby blocking activation of nonselective cation channels by a rise of intracellular Ca2+ (Fig. 8B). Third, activation of IcAMP was not observed when cells were dialyzed in the standard whole cell configuration (no nystatin; ruptured membrane patch) using a pipette solution that was Ca2+-free and which contained 5 mm EGTA (Fig. 8B). In contrast, IcAMP was observed when the cells were dialyzed with a pipette solution containing 160 nm Ca2+ (Fig. 8B). These findings suggest that IcAMP represents a La3+-blockable Na+ current, the appearance of which results from the opening of nonselective cation channels activated not only by cAMP, but also by intracellular Ca2+.
DISCUSSION
The two isopeptides of GLP-1, namely GLP-1(7-37) and GLP-1(7-36)amide, have attracted considerable attention because of their ability to stimulate insulin gene expression and proinsulin biosynthesis and to potentiate glucose-induced insulin secretion (1,7). Since these actions of GLP-1 are known to be glucose-dependent (1–3) and since GLP-1 is a potent stimulator of cAMP production (8, 14, 22–25, 31, 53), it has been proposed that GLP-1 is a modulator of the pancreatic β-cell glucose signaling system, acting to regulate enzymes and ion channels that normally mediate the stimulatory actions of glucose on insulin synthesis and secretion (26, 27). Here we demonstrate that a novel Ca2+ signaling pathway is likely to mediate some of these actions of GLP-1 and that this pathway may serve as an important effector mechanism at which signal transduction cross-talk occurs between the cAMP and glucose signaling systems.
The ability of GLP-1 to raise [Ca2+]i in β-cells exhibits several unusual features. The response to GLP-1 is augmented by d-glucose, is dependent on extracellular Na+ and Ca2+, and is mimicked by forskolin, IBMX, and cAMP analogs. Moreover, the GLP-1-induced rise of [Ca2+]i is associated with, but is not dependent on, membrane depolarization and appears to be the result of IcAMP. IcAMP is associated with an increased membrane conductance and represents, at least in part, a Na+ current that is blocked by extracellular La3+ or by intracellular application of the Ca2+ chelators EGTA or BAPTA/AM.
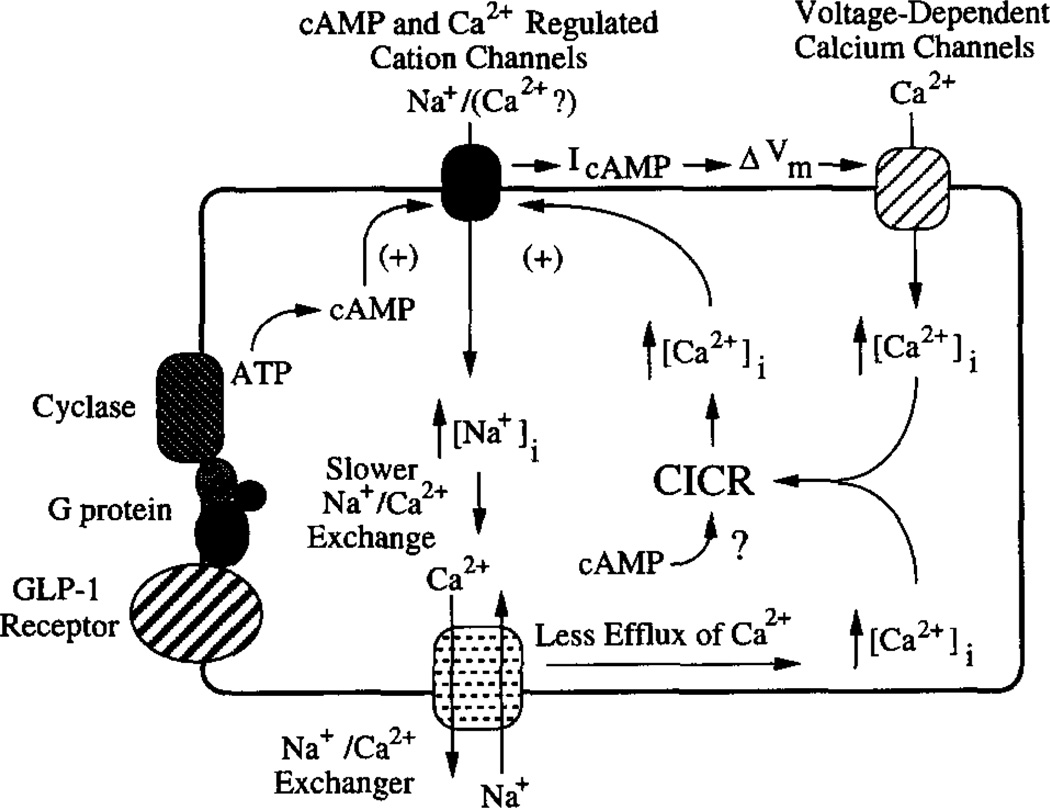
Fig. 9 provides a model with which to evaluate these diverse actions of GLP-1. Receptor occupancy by GLP-1 activates Gsα proteins (60) and stimulates adenylyl cyclase, thereby accelerating conversion of ATP to cAMP. We propose that this catalytic process is dependent on extracellular Na+ and that the subsequent binding of cAMP to cyclic nucleotide-regulated non-selective cation channels (or a protein closely associated with the channel) results in channel activation, thereby generating IcAMP. Activation of these channels by cAMP is also proposed to require intracellular Ca2+. The rise of [Ca2+]i which accompanies IcAMP is achieved by stimulation of at least two distinct Ca2+ signaling pathways. First, the membrane depolarization that is a direct consequence of IcAMP results in activation of VDCCs, thereby raising [Ca2+]i. Second, a rise of [Ca2+]i is observed even under conditions in which the membrane potential is voltage-clamped at values (−100 to −70 mV) negative to the activation threshold of VDCCs. Although the nature of this additional rise of [Ca2+]i remains to be determined, it may signify the mobilization of Ca2+ from intracellular stores, as well as Ca2+ influx via nonselective cation channels and/or membrane transporters (see below). Acting in concert, these Ca2+ signaling pathways are proposed to contribute to the stimulatory actions of GLP-1 on insulin secretion from β-cells.
Fig. 9. Signaling pathways that may mediate the stimulatory effects of GLP-1 on [Ca2+]i in β-cells.
Receptor occupancy by GLP-1 leads to activation of Gsα proteins and stimulation of adenylyl cyclase. This step results in cAMP production and requires extracellular Na+. The cAMP activates Ca-NS channels that are also activated by cytosolic Ca2+ and which fail to respond to cAMP when [Ca2+]i is very low. The opening of these channels generates IcAMP, an inward Na+ current. IcAMP results in membrane depolarization (ΔVm) and raises [Ca2+]i by activation of VDCCs. IcAMP may also promote a rise of [Na+]i which slows or reverses Na+/Ca2+ exchange. The rise of [Ca2+]i which is secondary to the slowing of Na+/Ca2+ exchange may then, in turn, promote Ca2+-induced Ca2+ release (CICR) from intracellular Ca2+ stores. Ca2+-induced Ca2+ release may also result from the initial rise of [Ca2+]i due to the opening of VDCCs. Release of Ca2+ from intracellular stores may also be favored by a more direct effect of cAMP on them.
A distinguishing feature of the GLP-1 signaling system is its absolute dependence on extracellular Na+. As summarized above, omission of extracellular Na+ blocks the ability of GLP-1 to stimulate cAMP production even though the binding of GLP-1 to its receptor remains unaffected. This observation is reminiscent of a previous report that Na+ (5–100 mm) enhances β-adrenergic receptor-mediated stimulation of adenylyl cyclase (61). Furthermore, the stimulatory action of forskolin on adenylyl cyclase is also facilitated by Na+ (61). Therefore, Na+ can act as a positive regulator of the receptor-Gs-cyclase complex, as was reported previously for receptor-Gi-cyclase interactions (62, 63). Na+ enhances receptor-mediated stimulation of GTPase activity (63, 64), and it amplifies receptor-mediated inhibition of adenylyl cyclase (65–68). A similar regulatory action of Na+ on receptor-Gs-cyclase coupling in β-cells might then explain why GLP-1 fails to stimulate cAMP production in the absence of Na+.
We also find that omission of extracellular Na+ blocks the actions of GLP-1, forskolin, and 8-Br-cAMP to activate IcAMP and to raise [Ca2+]i. Such observations are not unique to single, isolated β-cells since Fridolf and Ahren reported that GLP-1 increased [Ca2+]i and stimulated insulin secretion from rat islets in a Na+-dependent manner (32, 42), actions that clearly resemble the effects of GLP-1 reported here. However, since these previous studies did not include an electrophysiological analysis, no correlation was made between the GLP-1-induced rise of [Ca2+]i and the effects of GLP-1 on membrane potential or current. Based on our own findings, the most likely explanation for these observations is that Na+ is required not only for stimulation of cAMP production by GLP-1 but also for generation of IcAMP, which represents, at least in part, a Na+ current.
The exact mechanism by which GLP-1 activates IcAMP remains to be determined. The rise of [Ca2+]i which accompanies IcAMP is observed not only in response to the protein kinase A agonists 8-Br-cAMP and Sp-cAMP-S, but also in response to Rp-cAMP-S, a protein kinase A antagonist. Therefore, it appears likely that this response is initiated by the binding of cAMP to a target (possibly the channel itself) other than protein kinase A. Precedent for such a conclusion exists given that the effects of cAMP on cyclic nucleotide-regulated Ca-NS channels in excised membrane patches of CRI-G1 insulinoma cells are reportedly independent of protein kinase A (43). Furthermore, we find that activation of IcAMP by 8-Br-cAMP is observed even under conditions in which diffusional exchange occurs between the patch pipette (containing 160 nm Ca2+) and the cytosol, a configuration that disrupts protein kinase A-mediated signaling by favoring “wash out” of enzymes and cofactors.
As indicated above, Ca-NS channels that are activated by cAMP and which are permeant to both Na+ and K+ are known to be expressed in the CRI-G1 insulinoma cell line (43). To date, the existence of such channels has yet to be explored in β-cells or other insulinoma cell types. In rat β-cells and HIT-T15 cells we find that IcAMP exhibits a reversal potential of −26 mV, a value indicative of a permeation pathway that does not strongly differentiate between Na+ and K+, as is the case for Ca-NS channels. The Ca-NS channels expressed in CRI-G1 cells are also noteworthy in that they are stimulated by intracellular Ca2+ (59). This may also be the case for HIT-T15 cells since intracellular application of the Ca2+ chelators EGTA or BAPTA/AM blocks the activation of IcAMP by 8-Br-cAMP. In marked contrast, the response to 8-Br-cAMP is supported by intracellular application of a solution containing 160 nm free Ca2+. Evidently, there is a requirement for some minimal level of cytosolic Ca2+ for activation of IcAMP to occur. These findings are as expected if IcAMP represents an inward current carried by Na+ through cation channels activated not only by cAMP but also by intracellular Ca2+.
The activation of IcAMP results in membrane depolarization and an increase of [Ca2+]i due, in part, to activation of VDCCs (Fig. 9). On the basis of pharmacological criteria, it was suggested previously that L-type VDCCs play a dominant role in determining the magnitudes of the [Ca2+]i responses to GLP-1 and 8-Br-cAMP in the β-cell system (30, 31, 37). Surprisingly, we find that the actions of GLP-1 and 8-Br-cAMP on [Ca2+]i are only partially diminished by the L-type Ca2+ channel antagonists nifedipine, nimodipine, and verapamil. Moreover, the ability of GLP-1 and 8-Br-cAMP to raise [Ca2+]i is reduced but not blocked by voltage clamping the membrane to potentials negative to the activation threshold of VDCCs. These findings are clear indications that the effects of GLP-1 and cAMP analogs on [Ca2+]i are mediated not only by VDCCs, but also by an as yet to be fully characterized voltage-independent process that plays a major role in determining the magnitude of the [Ca2+]i response.
A clue as to the nature of this voltage-independent process is provided by previous studies demonstrating that nonselective cation channels can allow permeation by Ca2+ as well as by monovalent cations (69). Therefore, under conditions of hyper-polarizing voltage clamp, Ca2+ entry might contribute to the generation of IcAMP and the ensuing rise of [Ca2+]i. Consistent with this concept, we find that GLP-1 and 8-Br-cAMP are without effect on [Ca2+]i when the extracellular solution does not contain Ca2+. However, 8-Br-cAMP also fails to raise [Ca2+]i when the extracellular solution contains Ca2+ but not Na+. It is conceivable that Na+ serves to support permeation of such channels by Ca2+ so that under Na+-free conditions, no Ca2+ current is generated. If, however, Ca2+ entry does not contribute to IcAMP, the question then arises as to how GLP-1 or 8-Br-cAMP raises [Ca2+]i under conditions in which the cells are voltage-clamped at membrane potentials negative to the activation range of VDCCs. One possibility is that IcAMP, a Na+ current, produces a rise of [Na+]i, which then indirectly raises [Ca2+]i, by slowing or reversing the process of plasma membrane Na+/Ca2+ exchange (Fig. 9).
It is important to point out that our findings do not rule out the additional possibility that GLP-1 and cAMP raise [Ca2+]i by stimulating the release of Ca2+ from intracellular stores. Such a process might be blocked by transient exposure to a Ca2+-free extracellular solution (as, for example, Fig. 4A) which disrupts Ca2+-dependent mobilization of intracellular Ca2+ stores by cAMP. Furthermore, the release of Ca2+ from such stores might also be disrupted by removal of extracellular Na+ (as, for example, Fig. 3B). Under Na+ -free conditions, activation of IcAMP will not slow Na+/Ca2+ exchange since no increase of [Na+]i is likely. Therefore, the increase of [Ca2+]i which results from a slowing of the exchanger will not occur, and the process of Ca2+-induced Ca2+ release will not be initiated (Fig. 9). Whatever the exact mechanism, it appears that initiation of the rise of [Ca2+]i by GLP-1 does not require Ca2+ release from ryanodine-sensitive intracellular Ca2+ stores since we find that pretreatment of cells with ryanodine fails to block the response.
From a functional standpoint, the ability of GLP-1 to raise [Ca2+]i through activation of a signaling system not involving effects on IKATP has at least one important ramification. GLP-1 augments insulin secretion in non-insulin-dependent diabetics, even under conditions in which the sulfonylurea drugs such as glyburide (which inhibits IKATP) fail to stimulate insulin secretion (sulfonylurea failure) (18, 21). This observation suggests that one therapeutic advantage of GLP-1 relative to that of sulfonylureas in the treatment of non-insulin-dependent diabetes is that GLP-1 triggers a rise of [Ca2+]i, insulin secretion, and a lowering of blood glucose, even under conditions in which sulfonylurea receptors and ATP-sensitive potassium channels no longer play a dominant role in the regulation of β-cell stimulus-secretion coupling. Therefore, activation of IcAMP by GLP-1 may serve as a reserve mechanism of action, one that complements its previously reported inhibitory effects on IKATP (26). This would then explain why the glucagon-like peptides retain their biological activity and augment insulin secretion even under conditions in which sulfonylureas are no longer effective.
Acknowledgments
We thank Dr. Bernard Thorens for the receptor cDNA, Dr. Shimon Efrat for the βTC6 cells, Dr. Douglas Tillotson of IonOptix Corp. for excellent technical support, Maurice Castonguay for preparation of cell cultures, and Heather Herman for assistance with the cAMP assays.
Footnotes
This work was supported by United States Public Health Service Grants DK45817 (to G. G. H.) and DK30834 (to J. F. H.).
The abbreviations used are: GLP-1, glucagon-like peptide-1; IKATP, ATP-sensitive potassium current; VDCC(s), voltage-dependent Ca2+ channel(s); [Ca2+]i, free intracellular Ca2+; IcAMP, inward current activated by cAMP and GLP-1; NMG, N-methyl-d-glucamine; PACAP-27, pituitary adenylyl cyclase-activating peptide-27; Sp- and Rp-cAMP-S, Sp- or Rp-adenosine 3′,5′-cyclic monophosphothionate triethylamine; IBMX, isobutylmethylxanthine; 8-Br-cAMP, 8-bromo-cAMP; IH, holding current; Ca-NS, Ca2+-activated nonselective cation channel; BAPTA/AM, 1,2-bis(2-aminophenoxy)ethane N,N,N′,N′-tetraacetic acid/acetoxymethyl.
Contributor Information
George G. Holz, IV, Diabetes Unit, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts 02114.
Colin A. Leech, Laboratory of Molecular Endocrinology, Howard Hughes Medical Institute, Harvard Medical School, Boston, Massachusetts 02114
Joel F. Habener, Laboratory of Molecular Endocrinology, Howard Hughes Medical Institute, Harvard Medical School, Boston, Massachusetts 02114.
References
- 1.Fehmann H-C, Habener JF. Trends Endocrinol. Metab. 1992;3:158–163. doi: 10.1016/1043-2760(92)90165-w. [DOI] [PubMed] [Google Scholar]
- 2.Kreymann B, Ghater MA, Williams G, Bloom SR. Lancet. 1987;2:1300–1303. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 3.Orskov C. Diabetologia. 1992;35:701–711. [PubMed] [Google Scholar]
- 4.Bell GI, Santerre RF, Mullenbach GT. Nature. 1983;302:716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- 5.Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. J. Biol. Chem. 1986;261(11):880–889. [PubMed] [Google Scholar]
- 6.Creutzfeldt W. Diabetologia. 1979;16:75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- 7.Habener JF. Endocrinol. Metabol. Clin. North Am. 1993;22:775–794. [PubMed] [Google Scholar]
- 8.Drucker DJ, Philippe H, Mojsov S, Chick WL, Habener JF. Proc. Natl. Acad. Sci. U.S.A. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehmann HC, Habener JF. Endocrinology. 1992;130:159–166. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- 10.Holst JJ, Orskov C, Nielsen OV, Schwartz TW. FEBS Lett. 1987;211:169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- 11.Mojsov S, Weir GC, Habener JF. J. Clin. Invest. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orskov C, Holst JJ, Nielsen OV. Endocrinology. 1988;123:2009–2013. doi: 10.1210/endo-123-4-2009. [DOI] [PubMed] [Google Scholar]
- 13.Kawai K, Suzuki S, Ohashi S, Mukai H, Ohmori H, Murayama Y, Yamashita K. Endocrinology. 1989;124:1768–1773. doi: 10.1210/endo-124-4-1768. [DOI] [PubMed] [Google Scholar]
- 14.Gefel D, Hendrick GK, Mojsov S, Habener JF, Weir GC. Endocrinology. 1990;126:2164–2168. doi: 10.1210/endo-126-4-2164. [DOI] [PubMed] [Google Scholar]
- 15.D’Allesio DA, Fujimoto WY, Ensinck JW. Diabetes. 1989;38:1534–1538. doi: 10.2337/diab.38.12.1534. [DOI] [PubMed] [Google Scholar]
- 16.Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Diabetes Care. 1992;15:270–276. doi: 10.2337/diacare.15.2.270. [DOI] [PubMed] [Google Scholar]
- 17.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. N. Engl. J. Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 18.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 19.Nauck MA, Heimesaat MA, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. J. Clin. Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deleted in proof
- 21.Gerich JE. N. Engl. J. Med. 1989;321:1231–1245. doi: 10.1056/NEJM198911023211805. [DOI] [PubMed] [Google Scholar]
- 22.Thorens B. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dillon J, Tanizawa Y, Wheeler MB, Leng X-H, Lignon BB, Rabin DU, Yoo-Warren H, Permutt MA, Boyd AE., III Endocrinology. 1993;133:1907–1910. doi: 10.1210/endo.133.4.8404634. [DOI] [PubMed] [Google Scholar]
- 24.Thorens B, Porret A, Buhler L, Deng SP, Morel P, Widmann C. Diabetes. 1993;42:1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler MB, Lu M, Dillon JS, Leng X-H, Chen C, Boyd AE., III Endocrinology. 1993;133:57–62. doi: 10.1210/endo.133.1.8391428. [DOI] [PubMed] [Google Scholar]
- 26.Holz GG, IV, Kuhtreiber WM, Habener JF. Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holz GG, IV, Habener JF. Trends Biochem. Sci. 1992;17:388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollheim CB, Sharp GWG. Physiol. Rev. 1981;61:914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 29.Zawalich WS, Rasmussen H. Mol. Cell. Endocrinol. 1990;70:119–137. doi: 10.1016/0303-7207(90)90152-x. [DOI] [PubMed] [Google Scholar]
- 30.Yada T, Itoh K, Nakata M. Endocrinology. 1993;133:1685–1692. doi: 10.1210/endo.133.4.8404610. [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Wheeler MB, Leng X-H, Boyd AE., III Endocrinology. 1993;132:94–100. doi: 10.1210/endo.132.1.8380389. [DOI] [PubMed] [Google Scholar]
- 32.Fridolf T, Ahren B. Mol. Cell. Endocrinol. 1993;96:85–90. doi: 10.1016/0303-7207(93)90098-5. [DOI] [PubMed] [Google Scholar]
- 33.Ikeuchi M, Cook DL. Life Sci. 1984;35:685–691. doi: 10.1016/0024-3205(84)90264-9. [DOI] [PubMed] [Google Scholar]
- 34.Eddlestone GT, Oldham SB, Lipson LG, Premdas FH, Beigelman PM. Am. J. Physiol. 1985;248:C145–C153. doi: 10.1152/ajpcell.1985.248.1.C145. [DOI] [PubMed] [Google Scholar]
- 35.Henquin JC, Meissner HP. Endocrinology. 1984;115:1125–1134. doi: 10.1210/endo-115-3-1125. [DOI] [PubMed] [Google Scholar]
- 36.Prentki M, Glennon MC, Geschwind JF, Matschinsky FM, Corkey BE. FEBS Lett. 1987;220:103–107. doi: 10.1016/0014-5793(87)80884-0. [DOI] [PubMed] [Google Scholar]
- 37.Rajan AS, Aguilar-Bryan L, Nelson DA, Yaney GC, Hsu WH, Kunze DL, Boyd AE., III Diabetes Care. 1990;13:340–363. doi: 10.2337/diacare.13.3.340. [DOI] [PubMed] [Google Scholar]
- 38.Fournier L, Whitfield JF, Schwartz JL, Begin-Heick N. J. Biol. Chem. 1994;269:1120–1124. [PubMed] [Google Scholar]
- 39.Grapengiesser E, Gylfe E, Hellman B. J. Biol. Chem. 1991;266:12207–12210. [PubMed] [Google Scholar]
- 40.Malaisse WJ, Garcia-Morales P, Dufrane SP, Sener A, Valverde I. Endocrinology. 1984;115:2015–2020. doi: 10.1210/endo-115-5-2015. [DOI] [PubMed] [Google Scholar]
- 41.Wang JL, Corbett JA, Marshall CA, McDaniel ML. J. Biol. Chem. 1993;268:7785–7791. [PubMed] [Google Scholar]
- 42.Fridolf T, Ahren B. Biochem. Biophys. Res. Commun. 1991;179:701–706. doi: 10.1016/0006-291x(91)91429-g. [DOI] [PubMed] [Google Scholar]
- 43.Reale V, Hales CN, Ashford MLJ. J. Membr. Biol. 1994;141:101–112. doi: 10.1007/BF00238244. [DOI] [PubMed] [Google Scholar]
- 44.Hahn HJ, Gylfe E, Hellman B. Biochim. Biophys. Acta. 1980;630:425–432. doi: 10.1016/0304-4165(80)90291-3. [DOI] [PubMed] [Google Scholar]
- 45.Ammala C, Ashcroft FM, Rorsman P. Nature. 1993;363:356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- 46.Gillis KD, Misler S. Pflügers Arch. Euro. J. Physiol. 1993;424:195–197. doi: 10.1007/BF00374612. [DOI] [PubMed] [Google Scholar]
- 47.Efrat S, Linde S, Kodof H, Spector D, Delannoy M, Grant S, Hanahan D, Baekkeskov S. Proc. Natl. Acad. Sci. U.S.A. 1988;85:9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Efrat S, Leiser M, Surana M, Tal M, Fusco-Demane D, Fleischer N. Diabetes. 1993;42:901–907. doi: 10.2337/diab.42.6.901. [DOI] [PubMed] [Google Scholar]
- 49.D’Ambra R, Surana M, Efrat S, Starr RG, Fleischer N. Endocrinology. 1990;126:2815–2822. doi: 10.1210/endo-126-6-2815. [DOI] [PubMed] [Google Scholar]
- 50.Efrat S, Tal M, Lodish HF. Trends Biochem. Sci. 1994;19:535–538. doi: 10.1016/0968-0004(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 51.Santerre RF, Cook RA, Crisel RMD, Sharp JD, Schidt RJ, Williams DC, Wilson CP. Proc. Natl. Acad. Sci. U.S.A. 1981;78:4339–4342. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deleted in proof
- 53.Fehmann HC, Habener JF. Endocrinology. 1991;128:2880–2888. doi: 10.1210/endo-128-6-2880. [DOI] [PubMed] [Google Scholar]
- 54.Leech CA, Holz GG, IV, Habener JF. Endocrinology. 1994;135:365–372. doi: 10.1210/endo.135.1.8013370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grynkiewicz G, Poenie M, Tsien RY. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 56.Horn R, Marty A. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sala S, Parsey R, Cohen AS, Matteson DR. J. Membr. Biol. 1991;122:177–187. doi: 10.1007/BF01872640. [DOI] [PubMed] [Google Scholar]
- 58.Regazzi R, Li G, Deshusses J, Wollheim CB. J. Biol. Chem. 1990;265:15003–15009. [PubMed] [Google Scholar]
- 59.Sturgess NC, Hales CN, Ashford MLJ. FEBS Lett. 1986;208:397–400. doi: 10.1016/0014-5793(86)81056-0. [DOI] [PubMed] [Google Scholar]
- 60.Schmidtler J, Dehne K, Offermanns S, Rosenthal W, Classen M, Schepp W. Am. J. Physiol. 1994;266:G775–G782. doi: 10.1152/ajpgi.1994.266.5.G775. [DOI] [PubMed] [Google Scholar]
- 61.Watson EL, Jacobson KL, Singh JC. Biochem. Pharmacol. 1989;38:1069–1074. doi: 10.1016/0006-2952(89)90250-5. [DOI] [PubMed] [Google Scholar]
- 62.Jacobs KH, Aktories K, Schultz G. Adv. Cyclic Nucleotide. Protein Phosphorylation Res. 1984;17:135–143. [PubMed] [Google Scholar]
- 63.Klee WA, Koski G, Tocque B, Simonds WF. Adv. Cyclic. Nucleotide Protein Phosphorylation Res. 1984;17:153–159. [PubMed] [Google Scholar]
- 64.Koski G, Streaty RA, Klee WA. J. Biol. Chem. 1982;257:14035–14040. [PubMed] [Google Scholar]
- 65.Blume AJ, Lichtshtein D, Boone G. Proc. Natl. Acad. Sci. U.S.A. 1979;76:5626–5630. doi: 10.1073/pnas.76.11.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cooper DMF. FEBS Lett. 1982;138:157–163. doi: 10.1016/0014-5793(82)80431-6. [DOI] [PubMed] [Google Scholar]
- 67.Jacobs KH, Aktories K, Schultz G. Adv. Cyclic Nucleotide Res. 1981;14:173–187. [PubMed] [Google Scholar]
- 68.Jacobs KH, Aktories K, Schultz G. J. Rec. Res. 1983;3:137–149. doi: 10.3109/10799898309041929. [DOI] [PubMed] [Google Scholar]
- 69.Yau KY. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3481–3483. doi: 10.1073/pnas.91.9.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]