INTRODUCTION
Pituitary adenylyl cyclase-activating polypeptide (PACAP) and glucagon-like peptide-1 (GLP-1) are members of a family of structurally related peptides that also include vasoactive intestinal peptide (VIP), glucose-dependent insulinotropic peptide (GIP), growth hormone releasing hormone (GHRH), secretin, and glucagon. PACAP and GLP-1 are noteworthy in that each is a potent stimulator of insulin secretion from pancreatic β cells. These insulinotropic actions have prompted interest in the potential therapeutic usefulness of the peptides as blood glucose-lowering (antidiabetogenic) agents. PACAP is a neuropeptide that may mediate stimulatory effects of the autonomic nervous system on insulin secretion, whereas GLP-1 is an intestinally derived hormone that is released into the systemic circulation in response to a meal. PACAP and GLP-1 are derived by tissue-specific alternative posttranslational processing of their corresponding prohormones. This generates two biologically active forms of each peptide. PACAP-27 and PACAP-38 stimulate insulin secretion by binding to type 3 PACAP receptors (PACAP-R3)1 that are homologous in structure to the type 2 VIP receptors.2 GLP-1(7–37) and GLP-1(7–36)amide bind to the GLP-1 receptor,3,4 of which only a single isoform has been identified. These PACAP and GLP-1 receptors are G protein-coupled receptors that are expressed on β cells and that stimulate the production of CAMP. The recombinant PACAP and GLP-1 receptors can also stimulate inositol phosphate production when expressed in Xenopus oocytes or in transfected mammalian cells.1,5,6 However, direct effects of PACAP or GLP-1 on inositol phosphate production have yet to be demonstrated for β cells where the major effector appears to be cAMP.7
The insulin secretagogue actions of PACAP and GLP-1 are dependent on simultaneous exposure of β cells to d-glucose.8–10 This observation prompted speculation that the peptides are neural and hormonal regulators of the β cell glucose signaling system. They may act to influence enzymes and ion channels that normally mediate the stimulatory effect of blood glucose on insulin secretion.11–13 PACAP potentiates glucose-dependent insulin secretion at subpicomolar concentrations (10−14-10−13 M),14 and PACAP immunoreactivity was demonstrated in nerve terminals within the pancreas and within the islets of Langerhans.14,15 In contrast, GLP-1 is synthesized by enteroendocrine L cells of the distal intestine, and is secreted into the blood in response to ingestion of nutrients. Therefore, PACAP and GLP-1 may play a role in a neuro-entero-endocrine loop whereby feeding initiates release of the peptides. Under these conditions, PACAP and GLP-1 are proposed to synergize with blood glucose and to induce pancreatic insulin secretion.9,10,16
GLP-1 has attracted much interest due to its potential as a therapeutic agent in the control of non-insulin-dependent diabetes mellitus (NIDDM). It may serve as a useful alternative to conventional oral hypoglycemic agents (sulfonylureas), as it not only potentiates insulin secretion but also stimulates insulin gene expression and proinsulin biosynthesis.17,18 Interestingly, GLP-1 maintains its insulinotropic activity in NIDDM, whereas the related intestinally derived peptide, GIP, does not.19 GLP-1 was also reported to reduce blood glucose levels in insulin-dependent diabetics.20 This effect suggests that GLP-1 may exert an additional extrapancreatic mechanism of action.
A model to explain glucose-dependent insulin secretion was developed from experiments in which β cells were challenged with a stepwise increase of extracellular glucose concentration from a low level (usually below the normal, resting blood glucose level) to a high value. The β cells within intact islets show a membrane depolarization, a rise of [Ca2+]i, and secrete insulin in response to such a challenge.21 The model proposes that glucose uptake and metabolism lead to an increase of intracellular [ATP] (or of the ATP/ADP ratio) that blocks ATP-sensitive K+ channels (K-ATP). These channels are largely responsible for the resting K+ permeability of β cells, and their closure leads to membrane depolarization, activation of voltage-dependent Ca2+ channels (VDCCs), and an influx of Ca2+, Ca2+ influx raises [Ca2+]i, which is an important factor in triggering insulin secretion.21,22 When single, isolated β cells are challenged in similar experiments, the cells exhibit heterogeneous responses and only a fraction respond to glucose.12,23,24 This difference in responsiveness between whole islets and isolated β cells might be explained by the effects of endogenous glucagon, secreted from α cells within the islet, to maintain some tonic activity of the cAMP signaling system. Such a possibility is suggested by observations that isolated β cells, which did not respond to glucose alone, were rendered glucose competent (i.e., capable of responding to glucose) by treatment with GLP-1.12,24
This report focuses on the signal transduction pathways by which PACAP and GLP-1 stimulate insulin secretion from pancreatic β cells. It is demonstrated that these peptides modulate several target sites to increase the electrical excitability of β cells and to potentiate glucose-induced insulin secretion.
ELEVATION OF [cAMP]i RESULTS IN MEMBRANE DEPOLARIZATION AND A RISE OF [Ca+2]i
Stimulation of β cells and insulinoma cells with agents that elevate [cAMP], leads to membrane depolarization and a rise of [Ca2+]i (Fig. 1). This response is observed when the cells are preequilibrated in a constant concentration of glucose that is close to the threshold for glucose-induced insulin secretion. Such depolarizing responses have been observed in response to both forms of PACAP16 and GLP-1,13 and also to 8-Br-cAMP,13 Sp-cAMP-S,13 IBMX,25 and forskolin.26 These observations led to the suggestion that cAMP tightens the link between glucose metabolism, membrane depolarization, and insulin secretion.12,25
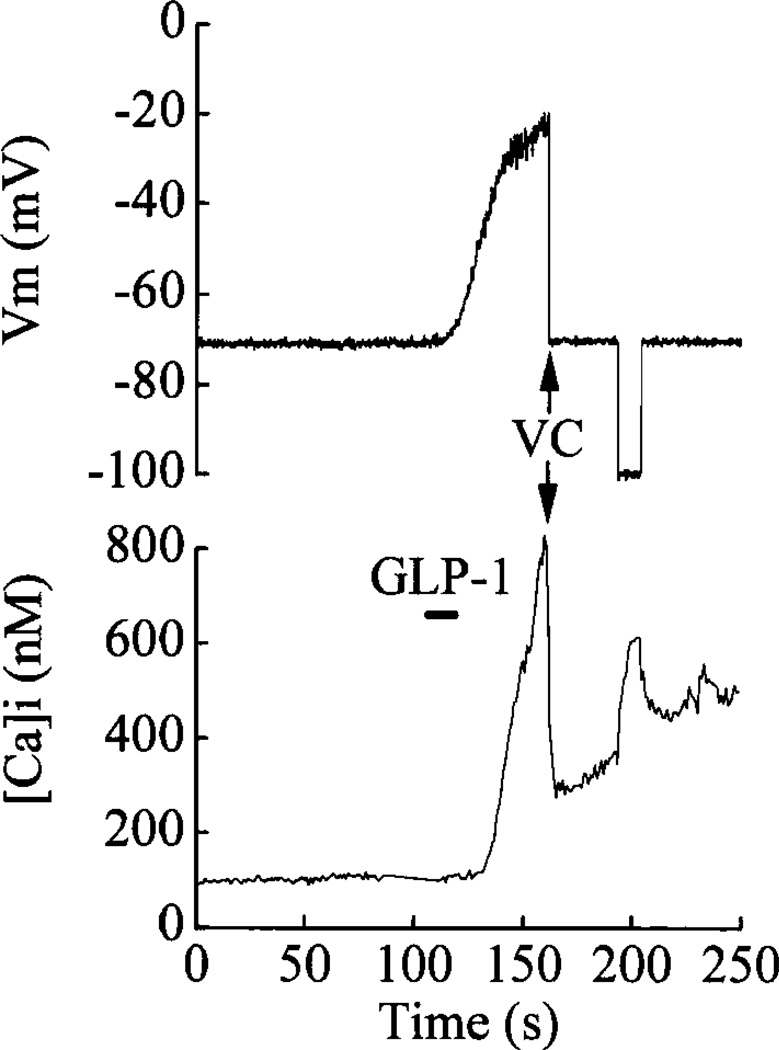
FIGURE 1.
GLP-1-induced membrane depolarization and elevation of [Ca2+]i. Stimulation of a βTC-6 cell with 5 nM GLP-1(7–37) (indicated by bar) resulted in membrane depolarization (top truce) and rise in [Ca2+]i (lower trace). The cell was then voltage clamped at −70 mV (arrows, VC), which resulted in a rapid fall of [Ca2+]i. Hyperpolarization of the cell from −70 mV to −100 mV resulted in a reversible increase of [Ca2+]i. Methods: Cells were loaded with fura 2 to measure [Ca2+]i, and the membrane potential was recorded simultaneously using the perforated patch technique. Cells were bathed in a standard extracellular saline containing 140 mM NaC1, 5.6 mM KCl, 2.6 mM CaC12, 1.2 mM MgCl2, 10 mM HEPES/NaOH (pH 7.4), and 0.8 mM glucose. Cells were loaded with fura 2 by incubation at 21°C in extracellular saline supplemented with 2% fetal bovine serum, 0.03% pluronic F-127, and 1 µM fura 2-AM for 15–20 min. The pipette solution contained 95 mM K2SO4, 7 mM MgCl2, 5 mM HEPES/NaOH (ca. 2 mM Na+, pH 7.4), and 240 µg/mL nystatin to permeabilize the membrane and to obtain electrical access. Experiments were performed in a Peltier temperature-controlled chamber at 32°C.
Figure 1 illustrates a depolarizing response and a rise of [Ca2+]i in a βTC-6 insulinoma cell exposed to GLP-1. A substantial component of the rise of [Ca2+]i appears to be due to Ca2+ influx through VDCCs. This is indicated by the rapid fall of [Ca2+]i when the cell was voltage clamped at −70 mV, a membrane potential at which VDCCs close. However, [Ca2+]i did not recover completely and continued to rise slowly with the membrane potential fixed at −70 mV. Further, on hyperpolarizing the cell to −100 mV, which will increase the electrochemical driving force for Ca2+ influx, [Ca2+]i increased. This rise of [Ca2+]i reversed when the membrane potential was returned to −70 mV. These observations suggest that an additional Ca2+ influx pathway may be operating and that this pathway may represent the opening of voltage-independent Ca2+ channels.
THE MECHANISM OF PACAP AND GLP-1-INDUCED DEPOLARIZATION
There are two mechanisms by which PACAP and GLP-1 depolarize the β cell membrane: (1) PACAP and GLP-1 close K-ATP channels, and (2) PACAP and GLP-1 activate nonselective cation channels. The inhibitory effect of PACAP on K-ATP was observed in studies of HIT-T15 insulinoma cells,16 whereas GLP-1 was shown to produce such an effect in rat β cells.12 Support for this concept was also provided by studies in which isobutylmethylxanthine (IBMX, a phosphodiesterase inhibitor) and glucagon were reported to inhibit K-ATP in canine β-cells25 and in HIT-T15 cells,27 respectively. Interestingly, it was proposed that the effect of glucagon on K-ATP might result not only from cAMP production, but from a membrane-delimited G protein signaling system.27
If closure of K-ATP was the sole mechanism acting to produce membrane depolarization, and if the influx of Ca2+ through VDCCs was the only source of the rise of [Ca2+]i, then it would be predicted that no rise of [Ca2+]i would be observed when the membrane potential was maintained at −70 mV, a value negative to the activation threshold of VDCCs. This would be consistent with reports that Ca2+ channel blockers attenuate the rise of [Ca2+]i induced by PACAP14 or GLP-1.7,28 We studied the effects of PACAP and GLP-1 in β cells in which the membrane potential was voltage clamped at −70 mV.13,16 Under these conditions, an inward current and a rise of [Ca2+]i are observed (Fig. 2). The inward currents activated by PACAP and GLP-1 appear to be identical and can also be activated by 8Br-cAMP. IBMX, and forskolin. These observations suggest that the current is activated by cAMP and led to it being designated IcAMP.13 IcAMP is predominantly a Na+ current, as indicated by its reversible inhibition by extracellular N-methyl d-glucamine (NMG).13,16 As expected, the inward currents and the rise of [Ca2+]i induced by PACAP16 or GLP-128 are also dependent on extracellular Na+. However, more detailed analysis revealed that the requirement for Na+ is manifest not only at the level of inward current activation, but also at the level of cAMP production. GLP-1 failed to stimulate a rise of cAMP levels in the absence of extracellular Na+.13
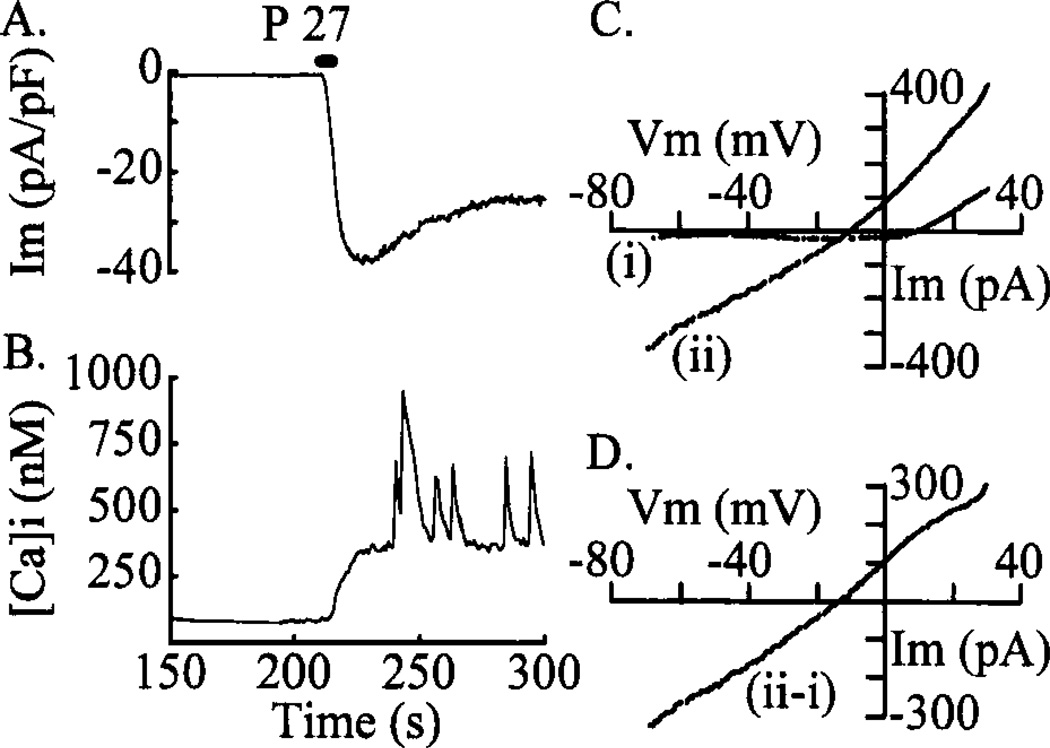
FIGURE 2.
PACAP activates an inward membrane current and raises [Ca2+]i. A βTC-6 cell was voltage clamped at −70 mV and a 5-s pulse of 10 nM PACAP-27 (P-27) was applied to the cell as indicated by the arrow. PACAP generated a pronounced inward current (IcAMP) and a simultaneous rise of [Ca2+]i. The inward current was partially and reversibly inhibited by 25 µM SKF 96365. The bath contained normal extracellular saline (Fig. 1, legend) and the pipette solution contained 95 mM Cs2SO4, (to block K+ currents), 7 mM MgSO4, 5 mM HEPES/NaOH (ca. 2 mM, pH 7.4) with 240 µg/mL nystatin. The magnitude of the inward current is normalized relative to the membrane capacitance (32 pF for this cell).
Activation of IcAMP is dependent on intracellular Ca2+, its activation being blocked by loading cells with the Ca2+ chelator BAPTA or by dialyzing the cell, in the whole cell recording mode, with Ca2+-free salines.13 However, IcAMP was observed when cells were dialyzed with a solution containing 160 nM Ca2+.13 This finding implies that activation of IcAMP requires some minimal, steady level of [Ca2+]i or a rise of [Ca2+]i.
IcAMP is not generated by the opening of voltage-dependent Na+ channels. The current is not affected by tetrodotoxin (TTX, 5 µM),13 a potent blocker of these channels in β cells.21 IcAMP is, however, blocked by high concentrations of La3+,13,16 and is partially and reversibly blocked by SKF 9636.5 (2.5 µM, Fig. 2), an inhibitor of both receptor-mediated Ca2+ entry and of voltage-dependent Ca2+ channels.29
It was recently reported that a class of nonselective cation channels (NSCCs) found in insulinoma cells can be activated by cAMP.30,31 Support for the idea that the current activated by PACAP and GLP-1 (IcAMP) may be carried through NSCCs comes from estimates of the reversal potential of the current (Fig. 3). Figure 3 illustrates an inward membrane current (Fig. 3(A)) and the associated rise of [Ca2+]i (Fig. 3(B)) measured from a voltage-clamped (−70 mV) βTC-6 cell activated by stimulation with 10 nM PACAP-27. This cell exhibited a number of large [Ca2+]i oscillations superimposed on the rise of [Ca2+]i (Fig. 3(B)), which do not appear to be associated with coincident inward current spikes (Fig. 3(A)). This absence of current spikes suggests that the [Ca2+]i oscillations may be due to intracellular Ca2+ release. To determine the reversal potential for the inward current, a series of four I–V/s voltage ramps from −70 mV to +30 mV were applied to this cell before (Fig. 3(C(i))) and after (Fig. 3(C(ii))) PACAP stimulation. The subtracted current (Fig. 3(D)), which mainly represents the current activated by PACAP (assuming that other, voltage-dependent currents are not changed), reversed at about −14 mV in this cell. Reversal potentials in this range are consistent with IcAMP being generated by the opening of NSCCs.
FIGURE 3.
PACAP activates a nonselective cation channel. A series of records from a βTC-6 cell voltage clamped at −70 mV using the perforated-patch technique is shown. A 5-s pulse of 10 nM PACAP-27 (P-27) induced an inward current (A) associated with a rise of [Ca2+]i (B). Averaged currents from four 1 V/s voltage ramps from −70 mV to +30 mV before (C(i)) and after (C(ii)) stimulation with PACAP are shown in (C) and the difference current ((ii)-(i)) is shown in (D). This subtracted current, which predominantly represents the PACAP-activated current, reversed at −14 mV and exhibited a linear current-voltage relationship. The bath and pipette solutions were as described in Figure 1 and Figure 2, respectively.
THE VOLTAGE DEPENDENCE OF Ca CHANNEL ACTIVATION IS NOT ALTERED BY cAMP
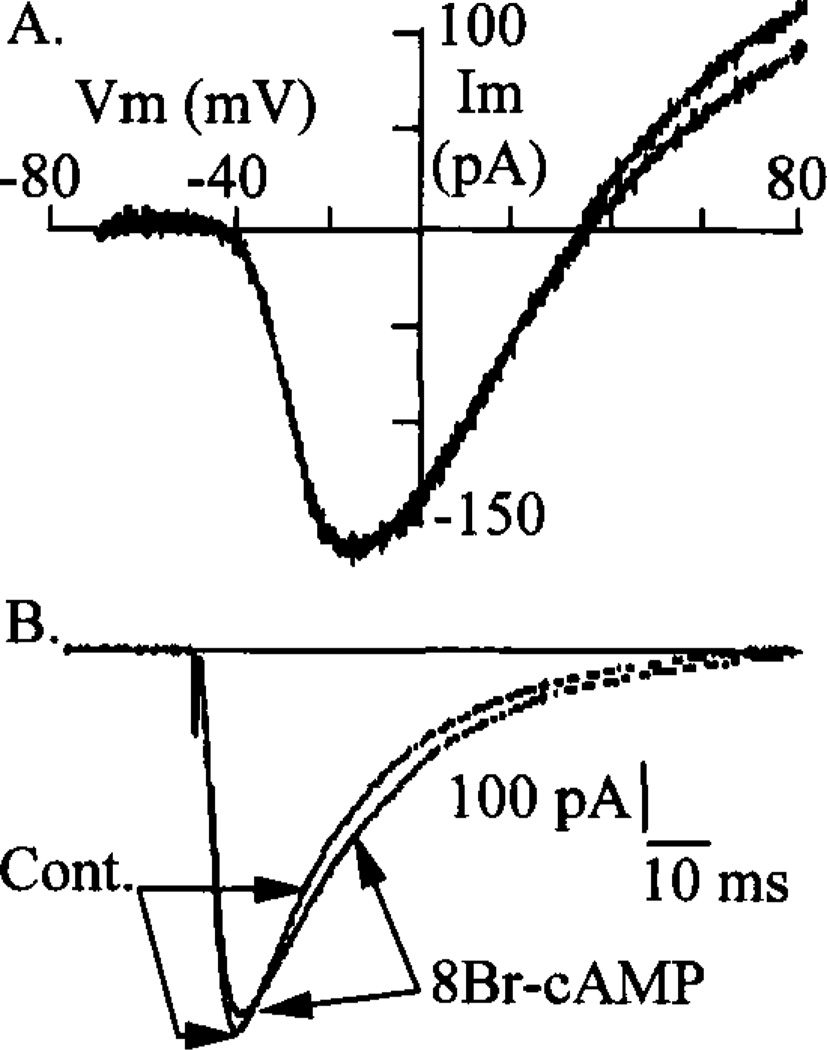
Voltage-dependent Ca2+ channels can show detectable activity at −70 mV under physiological conditions,32 and the blocking effects of La3+ or SKF 96365 on IcAMP might be explained if the elevation of [cAMP]i shifted the threshold for activation of VDCCs in the hyperpolarizing direction, thereby increasing the channel open probability at negative potentials. Such a shift was observed in RINm5F cells in response to glyceraldehyde stimulation,33 but these effects were small. In order to test for a similar effect of cAMP on calcium channel function, we measured depolarization-induced Ca2+ currents in voltage-clamped HIT-T15 cells using the perforated-patch technique. Ca2+ currents were evoked by shifting the membrane potential using a ramp stimulus protocol to examine the current-voltage (I–V) relationship, or by stepwise changes in membrane potential to investigate the time course of the currents (Fig. 4). Figure 4(A) shows the I–V relationship for the Ca2+ current before and during a 1-min exposure to 1 mM 8Br-cAMP (note that the peak current during exposure to 8-Br-CAMP was scaled up to match the peak current of the control response). The amplitude of the peak current and the threshold for current activation were not significantly altered during treatment with 8-Br-cAMP (Table 1). Figure 4(B) shows Ca2+ currents from a different cell in response to voltage steps from −70 mV to 0 mV. Following treatment with 8Br-cAMP, the current appears to inactivate more slowly, as previously described, and the integrated Ca2+ current is increased34,35 (Table 1). These findings indicate that alterations in the voltage-dependence of Ca2+ channel activation are unlikely to explain the rise of [Ca2+]i measured in response to PACAP, GLP-1, or CAMP.
FIGURE 4.
Effects of cAMP on Ca2+ currents. Ca currents were measured in HIT-T15 cells before and during a 1 min exposure to 1 mM 8-Br-cAMP using the perforated-patch technique. The current-voltage relationship was examined using a series of four 1.5 V/s voltage ramps from −70 mV to +80 mV. The averaged currents from two such series of ramps, before and during treatment with 8-Br-cAMP, are shown in (A). These currents represent the total membrane current minus the leak current. The currents have been scaled so that the peak inward currents are superimposed. The extracellular solution was as described in Figure 1 and was supplemented with 5 mM tetraethylammonium and 2 µM TTX to block voltage-dependent K+ and Na+ channels, respectively. The pipette solution was as described in Figure 2. (B) shows Ca2+ currents measured from another HIT-T15 cell. The bath and pipette solutions were as in (A). These currents were evoked by a voltage step from −70 mV to 0 mV. After stimulation with 8Br-CAMP, the currents appear to inactivate more slowly than the prestimulus, control (cont.) currents, as previously reported.34,35
TABLE 1.
Effect of 8Br-cAMP on HIT-T15 Cell Ca2+ Current Parametersa
| Parameter | Control | 8Br-cAMP | Recovery |
|---|---|---|---|
| Activation threshold (mV) | −40.2 ± 2.5 (5) | −39.8 ± 3.8 (5) | −42.0 ± 3.6 (4) |
| Peak current potential (mV) | −8.2 ± 2.6 | −8.8 ± 3.1 | −9.7 ± 3.1 |
| Peak current (pA/pF) | −14.2 ± 2.5 | −12.9 ± 2.0 | −14.5 ± 1.7 |
| Reversal potential (mV) | +39.0 ± 5.1 | +39.6 ± 5.3 | +39.0 ± 5.7 |
| Integrated current (pC/pF) | −0.61 ± 0.10 | −0.80 ± 0.13 | −0.67 ± 0.25 |
Ca2+ currents were recorded in the perforated patch configuration with bath and pipette solutions as described in Figure 4. The activation threshold, peak current potential, peak current amplitude, and reversal potential were estimated from currents evoked by 1.5 V/s voltage ramps from −70 mV to +80 mV, as shown in Figure 4(A). These parameters for control and 8Br-cAMP-stimulated currents were obtained from 5 cells. The recovery parameters were obtained from 4 of these cells. The integrated current values during voltage steps from −70 mV to 0 mV were obtained from 5 different cells and were measured using Pulsefit software (Instrutech Corp.). Values are given as the mean ± SEM.
THE ROLE OF INTRACELLULAR Ca2+ RELEASE
The role of intracellular Ca2+ release in the regulation of insulin secretion from β cells remains controversial. It has been suggested that ryanodine-sensitive intracellular Ca2+ release plays an important role in the GLP-1-induced rise of [Ca2+]i.36 The most likely interpretation of the literature is that intracellular Ca2+ release alone is not a very effective stimulus for secretion, but that it potentiates release in response to glucose. In the context of PACAP and GLP-1 signaling, a direct stimulation of inositol trisphosphate (IP3) production by the peptides has not been described. However, it was reported that GLP-1 indirectly stimulates production of inositol phosphates, an action attributed to the GLP-1-induced rise of [Ca2+]i.36 This concept is consistent with reports that a rise of [Ca2+]i, can activate phospholipase C (PLC) in the β cell,37 and that activation of the PLC system in these cells is blocked by removal of extracellular Ca2+,36 or by SKF 96365.38 Furthermore, exposure of β cells to maitotoxin stimulates Ca2+ influx and the activation of PLC.38,39 Taken together, these findings suggest that Ca2+ release from intracellular stores might play a significant role in determining the magnitude of the rise of [Ca2+]i in response to PACAP and GLP-1.
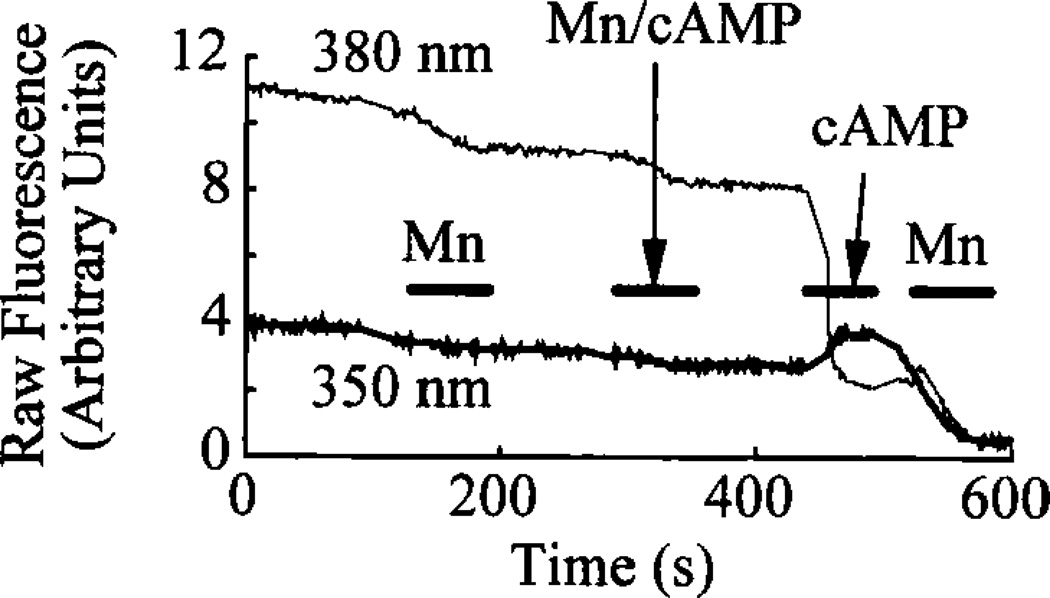
The release of intracellular Ca2+ may also be an important regulator of the membrane conductance. Ca2+-activated K+ channels are expressed in β-cells21 and are activated by a rise of [Ca2+]i. Recently, a Ca2+-release-activated divalent cation influx pathway has been described.40–43 Ca2+ influx via this route may play an important role in refilling Ca2+ stores and in regulating oscillatory changes of membrane potential (bursting) within the islet.44 Ca2+-release-activated Ca2+ channels may contribute to this Ca2+ influx pathway, as suggested by Mn2+ quenching of intracellular fura 2 fluorescence following depletion of intracellular Ca2+ stores. Consistent with this concept, Mn2+ quenching is observed after stimulation of β-cells with PACAP16 or 8Br-cAMP (Fig. 5). Furthermore, a component of the PACAP-induced inward membrane current is inhibited by SKF 96365 (Fig. 2), a blocker of depletion-activated Ca2+ influx pathways in some cell types.29
FIGURE 5.
The cAMP-induced rise of [Ca2+]i activates Mn2+ quenching of fura 2. Shown are fura 2 records from a βTC-6 cell bathed in normal extracellular saline (see Fig. 1). Raw fluorescence emission values (arbitrary units) at 510 nm in response to excitation at 380 nm and 350 nm are shown. The extracellular Mn2+ had no effect on [Ca2+]i (not shown) and a negligible effect on the raw fluorescence values. A pulse of 1 mM 8Br-cAMP in the Mn2+ saline also had little or no effect on [Ca2+]i or raw fluorescence. In contrast, a pulse of 1 mM 8Br-cAMP in normal, Ca2+-containing saline caused a large rise in [Ca2+]i with the raw fluorescence values changing appropriately. A subsequent pulse of Mn2+ then markedly reduced [Ca2+]i and quenched the intracellular fura 2 fluorescence. The failure of 8Br-cAMP to increase [Ca2+]i in the Mn2+ saline may be due to the ability of Mn2+ to block L-type VDCCs,21 or may have been an effect of this saline being (nominally) Ca2+ free. Following the rise in [Ca2+]i induced by cAMP, the extent of quenching of the fura signal increased markedly. In view of the known blocking effect of Mn2+ on VDCCs, this quenching may reflect the activation of a Ca-release-activated Ca2+ current.
SUMMARY
PACAP and GLP-1 depolarize pancreatic β cells and stimulate insulin secretion in the presence of glucose. Depolarization occurs through at least two distinct mechanisms: (1) closure of ATP-sensitive K+ channels, and (2) activation of nonselective cation channels (NSCCs). Under physiological conditions the NSCCs carry a predominantly Na+-dependent current. The current may also have a Ca2+ component, but this remains to be determined. Acting together, these two signaling systems reinforce each other and serve to promote membrane depolarization, a rise of [Ca2+]i, and exocytosis of insulin-containing secretory granules.
The NSCCs in β cells are dually regulated by intracellular cAMP and [Ca2+]i.13 In view of this dual regulation, it appears likely that NSCC channel activation results from signaling events occurring not only at the plasma membrane (gating of channels by cAMP; protein kinase A-mediated phosphorylation of channels) but also at intracellular sites (mobilization of calcium stores by an as yet to be determined process). It is noteworthy that activation of NSCCs has also been reported following stimulation of β-cells with maitotoxin, or after depletion of intracellular Ca2+ stores.43 Therefore, the possibility arises that PACAP, GLP-1, and maitotoxin all act on the same types of ion channels in these cells, and that these channels are sensitive to alterations in the content of intracellular calcium. Figure 6 summarizes our current knowledge concerning the properties of the PACAP and GLP-1 signaling systems as they pertain to the regulation of NSCCs and intracellular calcium homeostasis in the β cell.
FIGURE 6.
Signaling systems that may mediate effects of PACAP and GLP-1. Receptor occupancy leads to a stimulation of cAMP production and the activation of NSCCs. This step may result from a direct effect of cAMP on the channels, or it may be mediated by protein kinase A (PKA). Activation of NSCCs leads to generation of IcAMP, membrane depolarization, and the opening of voltage-dependent calcium channels (VDCCs). Ca2+ influx through VDCCs produces a rise of [Ca2]i and a stimulation of phospholipase C (PLC). PLC catalyzes hydrolysis of polyphosphoinositides (PIP2) to form inositol trisphosphate (IP3). IP3 synergizes with cytosolic Ca2+ to mobilize intracellular Ca2+ stores, thereby providing an additional rise of [Ca2+]i. A rise of [Ca2+]i may also result from Na+ entry via NSCCs since this would be expected to slow the Na+/Ca2+ exchange mechanism. A more direct effect of cAMP on Ca2+ stores may also occur, although this remains to be demonstrated.
Given that PACAP and GLP-1 are proven to be exceptionally potent insulin secretagogues, it is of considerable interest to determine their usefulness as blood glucose-lowering agents. Initial evaluations of the therapeutic effectiveness of GLP-1 indicate a role for this peptide in the treatment of NIDDM, and also possibly insulin-dependent diabetes mellitus (IDDM). A very attractive feature of such a strategy is the demonstrated lack of hypoglycemic side effects attendant to administration of GLP-1 to diabetic subjects. These observations reinforce the notion that peptides of the PACAP/glucagon/VIP family represent important pharmacological tools for use in experimental therapeutics.
ACKNOWLEGEMENTS
We would like to thank M. Castonguay for maintenance of cell cultures. One of the authors (J. F. H.) is an investigator of the Howard Hughes Medical Institute.
DISCUSSION OF THE PAPER
Gabriel M. Makhlouf (Medical College of Virginia, Richmond, VA): Have you considered the possibility that chloride channels might be activated and that they may be responsible for depolarization?
George G. Holz (Massachusetts General Hospital, Harvard Medical School, Boston): Yes, we have considered that, and we have adjusted the solutions in our patch clamp experiments so that activation of chloride channels cannot produce a chloride current. The fact that we see currents therefore indicates that it has to be something other than a chloride current.
Makhlouf: You raise the possibility that what you see as oscillations may be due to calcium-induced calcium release. Have you tested this with ruthenium red or cyclic ADP ribose inhibitor?
Holz: I have tried ryanodine and been unable to get it to work as an antagonist of this response. However, there is one report in the literature suggesting that some of the effects of GLP may be blocked by ryanodine. I don’t know of any reports looking at ruthenium red or cyclic ADP ribose.
Emanuel DiCicco-Bloom (UMDNJ/Robert Wood Johnson Medical School, Piscataway, NJ): Is cyclic AMP and PKA required for these effects?
Holz: Well, you would have to use a cyclic AMP antagonist or PKA antagonist. In fact, what we have observed with, for example, Rp cyclic AMPs, which is thought in some ways to antagonize PKA signaling, is agonist-like actions of Rp cyclic AMPs. This might be interpreted as suggesting that these are cyclic-nucleotide gated ion channels that recognize both cyclic AMP agonists and antagonists. But that’s the only type of investigation of this sort that we’ve made.
DiCicco-Bloom: In light of the other pathways that are being thought about, might there be other ways that these peptides might elicit changes in calcium?
Holz: Yes.
Toshihiko Yada (Kagoshima University School of Medicine, Kagoshima, Japan): You showed the effect of GLP1 to increase cytosolic calcium in a cell that has a membrane potential of about −70 mV, which is a typical membrane potential of the resting β cell. On the other hand, it is well known that the GLP1 effect is glucose dependent and that glucose elevated to 5 mM is known to depolarize the β cell membrane from about −70 mV, the resting level, to about −50 mV. From this we might think that the membrane potential should be depolarized to allow the GLP-1 effect. How can you then get the effect of the GLP-1 to increase the calcium at the resting level?
Holz: What we’re suggesting is that there’s the activation of this new type of ion channel, which we think is a nonselective cation channel, and which could produce a rise of intracellular calcium, either by letting calcium through the channel in a voltage-independent manner, or by the entry of sodium into the cell, which might slow the sodium-calcium exchange mechanism.
Dominique Bataille (INSERM, Montpellier, France): Are you able to entirely block the rise of calcium?
Holz: We’ve done a rather extensive pharmacological analysis, and found that the rise of calcium in response to PACAP, GLP, or 8 bromo is somewhat attenuated by dihydropyridine calcium channel antagonists, but is definitely not blocked. It’s not blocked by Verapamil, nor is it blocked by peptide toxins of calcium channels.
Footnotes
This work was supported by U.S. Public Health Service Grants DK45817 (G.G.H.), DK30834, and DK30457 (J.F.H.).
REFERENCES
- 1.Inagaki N, Yoshida H, Mizuta N, Fujii Y, Gonoi T, Miyazaki JI, Seino S. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2679–2683. doi: 10.1073/pnas.91.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutz EM, Sheward WJ, West KM, Morrow LA, Fink G, Harmar AJ. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- 3.Thorens B. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Y, Mojsov S. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler MB, Lu M, Dillon JS, Leng XH, Chen C, Boyd AE. Endocrinology. 1993;133:57–62. doi: 10.1210/endo.133.1.8391428. [DOI] [PubMed] [Google Scholar]
- 6.Dillon JS, Tanizawa Y, Wheeler MB, Leng XH, Ligon BB, Rabin DU, Yoo-Warren H, Permutt MA, Boyd AE. Endocrinology. 1993;133:1907–1910. doi: 10.1210/endo.133.4.8404634. [DOI] [PubMed] [Google Scholar]
- 7.Lu M, Wheeler MB, Leng XH, Boyd AE. Endocrinology. 1993;132:94–100. doi: 10.1210/endo.132.1.8380389. [DOI] [PubMed] [Google Scholar]
- 8.Yokota C, Kawai K, Ohashi S, Susuki S, Yamashita K. Acta Endocrinol. 1993;129:473–479. doi: 10.1530/acta.0.1290473. [DOI] [PubMed] [Google Scholar]
- 9.Fehmann HC, Habener JF. Trends Endocrinol. Metab. 1992;3:158–163. doi: 10.1016/1043-2760(92)90165-w. [DOI] [PubMed] [Google Scholar]
- 10.Fehmann HC, Goke R, Goke B. Endocrine Rev. 1995;16:390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- 11.Holz GG, Habener JF. Trends Biochem. Sci. 1992;17:388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holz GG, Kuhtreiber WM, Habener JF. Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holz GG, Leech CA, Habener JF. J. Biol. Chem. 1995;270:17749–17757. [PMC free article] [PubMed] [Google Scholar]
- 14.Yada T, Sakurada M, Ihida K, Nakata M, Murata F, Arimura A, Kikuchi M. J. Biol. Chem. 1994;269:1290–1293. [PubMed] [Google Scholar]
- 15.Fridolf T, Sundler F, Ahren B. Cell Tissue Res. 1992;269:275–279. doi: 10.1007/BF00319618. [DOI] [PubMed] [Google Scholar]
- 16.Leech CA, Holz GG, Habener JF. Endocrinology. 1995;136:1530–1536. doi: 10.1210/endo.136.4.7895663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehmann HC, Habener JF. Endocrinology. 1992;130:159–166. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- 18.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Proc. Natl. Acad. Sci. U.S.A. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. J. Clin. Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. New Eng. J. Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 21.Ashcroft FM, Rorsman P. Prog. Biophys. Molec. Biol. 1991;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 22.Prentki M, Matschinsky FM. Physiol. Rev. 1987;67:1185–1249. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 23.Wang JL, Corbett JA, Marshall CA, McDaniel ML. J. Biol. Chem. 1993;268:7786–7791. [PubMed] [Google Scholar]
- 24.Holz GG, Leech CA. Glucagon-like Peptide-1 and the Glucose Competence Concept of Pancreatic Beta-Cell Function. Basel: Karger; 1996. In press. [Google Scholar]
- 25.Barnett DW, Pressel DM, Chern HT, Scharp DW, Misler S. J. Membrane Biol. 1994;138:113–120. doi: 10.1007/BF00232639. [DOI] [PubMed] [Google Scholar]
- 26.Henquin JC, Meissner HP. Endocrinology. 1984;115:1125–1134. doi: 10.1210/endo-115-3-1125. [DOI] [PubMed] [Google Scholar]
- 27.Ribalet B, Ciani S. J. Membrane Biol. 1994;142:395–408. doi: 10.1007/BF00233444. [DOI] [PubMed] [Google Scholar]
- 28.Fridolf T, Ahren B. Mol. Cell. Endocrinol. 1993;96:85–90. doi: 10.1016/0303-7207(93)90098-5. [DOI] [PubMed] [Google Scholar]
- 29.Rink TJ. FEBS Lett. 1990;268:381–385. doi: 10.1016/0014-5793(90)81290-5. [DOI] [PubMed] [Google Scholar]
- 30.Reale V, Hales CN, Ashford MLJ. J. Membrane Biol. 1994;141:101–112. doi: 10.1007/BF00238244. [DOI] [PubMed] [Google Scholar]
- 31.Reale V, Hales CN, Ashford MLJ. J. Membrane Biol. 1995;145:267–278. doi: 10.1007/BF00232718. [DOI] [PubMed] [Google Scholar]
- 32.Smith PA, Ashcroft FM, Fewtrell CMS. J. Gen. Physiol. 1993;101:767–797. doi: 10.1085/jgp.101.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velasco JM, Petersen JUH, Petersen OH. FEBS Lett. 1988;123:366–370. doi: 10.1016/0014-5793(88)80851-2. [DOI] [PubMed] [Google Scholar]
- 34.Ammala C, Ashcroft FM, Rorsman P. Nature. 1993;363:356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- 35.Britsch S, Krippeit-Drews P, Lang F, Gregor M, Drews G. Biochem. Biophys. Res. Commun. 1995;207:33–39. doi: 10.1006/bbrc.1995.1149. [DOI] [PubMed] [Google Scholar]
- 36.Gromada J, Dissing S, Bokvist K, Renstrom E, Frokjaer-Jensen J, Wulff BS, Rorsman P. Diabetes. 1995;44:767–774. doi: 10.2337/diab.44.7.767. [DOI] [PubMed] [Google Scholar]
- 37.Biden TJ, Peter-Riesch B, Schlegel W, Wollheim CB. J. Biol. Chem. 1987;262:3567–3571. [PubMed] [Google Scholar]
- 38.Soergel DG, Yasumoto T, Daly JW, Gusovsky F. Mol. Pharmacol. 1992;41:487–493. [PubMed] [Google Scholar]
- 39.Soergel DG, Gusovsky F, Yasumoto T, Daly JW. J. Pharmacol. Exp. Therap. 1990;255:1360–1365. [PubMed] [Google Scholar]
- 40.Leech CA, Holz GG, Habener JF. Endocrinology. 1994;135:365–372. doi: 10.1210/endo.135.1.8013370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bode HP, Goke B. FEBS Lett. 1994;339:307–311. doi: 10.1016/0014-5793(94)80436-2. [DOI] [PubMed] [Google Scholar]
- 42.Worley JF, McIntyre MS, Spencer B, Mertz RJ, Roe MW, Dukes ID. J. Biol. Chem. 1994;269:14359–14362. [PubMed] [Google Scholar]
- 43.Worley JF, McIntyre MS, Spencer B, Dukes ID. J. Biol. Chem. 1994;269:32055–32058. [PubMed] [Google Scholar]
- 44.Bertram R, Smolen P, Sherman A, Mears D, Atwater I, Martin F, Soria B. Biophys. J. 1995;68:1–10. doi: 10.1016/S0006-3495(95)80414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]