Abstract
INTRODUCTION
CALGB 9633 was a Phase III trial that randomized patients with stage IB non-small cell lung cancer (NSCLC) to observation or four cycles of carboplatin and paclitaxel. A statistically significant effect in favor of adjuvant chemotherapy was seen for disease-free survival (DFS) and overall survival (OS) in the subgroup of patients with tumors ≥ 4 cm. A laboratory companion study was conducted to see if molecular and clinical factors could provide additional prognostic information.
METHODS
Formalin-fixed paraffin-embedded blocks were obtained for 250 of the 344 patients enrolled. Immunohistochemical staining for bcl-2, p53, blood group antigen A and mucin was correlated with DFS and OS.
RESULTS
The prevalence of the markers was bcl-2 17%, p53 47%, blood group antigen A 25% and mucin 45%. Univariate analysis for DFS showed a statistically significant effect for the presence of mucin (p=0.0005) and p53 (p=0.05) and for OS showed a significant effect for mucin (p=0.0005). In the multivariate Cox model, there was a statistically significant association between shorter DFS and presence of mucin (p=0.002; hazard ratio [HR] 2.05) and p53 (p=0.003; HR 1.95) and between shorter OS and presence of mucin (p=0.004; HR 2.03) and p53 (p=0.0005; HR 2.30). Of the clinical factors, male gender and larger tumor volume were also significant adverse prognostic factors (p<0.05).
CONCLUSIONS
A statistically significant association between shorter DFS and OS was seen for patients with p53 protein expression, mucin expression, male gender and larger tumors in this cohort of patients with stage IB NSCLC treated on CALGB 9633.
Keywords: Non-small cell lung cancer, prognostic factors, p53, mucin
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality for both men and women in the United States. In 2008, it is estimated that there will be 215,000 new cases and 162,000 deaths from lung cancer.1 The World Health Organization estimates 1.2 million new cases annually, making it the most common cancer worldwide.2 Non-small cell lung cancer (NSCLC) makes up about 85% of all newly diagnosed cases of lung cancer.
Recently reported randomized clinical trials have reported the benefit of cisplatin-based adjuvant chemotherapy for patients with early stage NSCLC.3,4,5 These trials have suggested survival benefit for stages II and IIIA, but the benefit for those with stage IB disease has been questioned.6 The randomized Phase III trial, CALGB 9633, is the only study that studied patients with stage IB disease exclusively.7 In this study, after surgical resection, patients were randomized to observation or 4 cycles of carboplatin/paclitaxel. The final results have recently been reported and while there were trends for superior disease-free and overall survival for the chemotherapy group, these did not reach statistical significance. A retrospective subset analysis did show a statistically significant survival advantage for patients with tumors ≥4 cm.
As part of this clinical trial, formalin-fixed paraffin-embedded tissue blocks were collected for a planned laboratory companion study to evaluate the ability of selected clinical and biological markers to add to the well-established prognostic value of tumor stage. At the time, several large single institution studies had suggested the potential prognostic value of a number of molecular and biological factors including microvessel density, tumor size, K-ras mutation, and expression of p53, blood group antigen A, bcl-2 and mucin.8,9,10
The purpose of this study was to investigate the prognostic significance of selected markers including p53 expression, loss of blood group antigen A, bcl-2 and mucin as determined at the time of protocol initiation.
MATERIALS AND METHODS
CALGB 9633 was a randomized Phase III trial of observation versus 4 cycles of carboplatin/paclitaxel after surgery for patients with stage IB NSCLC. Eligibility criteria included histologically proven NSCLC, T2 with pathologically negative lymph nodes at mediastinoscopy or at time of thoracotomy. Patients were required to undergo at least a lobectomy. Patients were randomized 4–8 weeks after surgery and chemotherapy was given every 3 weeks. Treatment and collection of paraffin-embedded blocks was approved by all involved institutional review boards.
Formalin-fixed paraffin-embedded tumour tissue blocks were obtained prospectively and sent to the CALGB Pathology Coordinating Office. Four micron sections were cut and placed on glass slides. One slide was stained with hematoxylin and eosin to facilitate interpretation. All studies were performed without knowledge of clinical outcome.
A modification of the avidin-biotin-peroxidase complex technique was used for immunohistochemical (IHC) studies.11,12 For p53, sections were treated with enzyme digestion and a high temperature antigen unmasking technique in order to facilitate labelling with p53 clones 240 and 1801 (Labvision Corporation, Fremont, CA) antibody cocktail. This antibody cocktail produces nuclear staining and detects p53 overexpression. Positive (strongly reactive colon cancer) and negative controls (normal colon) were used. For bcl-2, the monoclonal antibody clone 124 was used (Dako) and for blood group antigen A, the monoclonal antibody clone 81FR 2.2 was used (Biogenex Laboratories, San Ramon, CA). Details of the procedures employed are described in detail elsewhere.13,14 The results for p53, bcl-2, and blood group antigen A were graded from 0–4 according to intensity of the staining and from 0–4 by the percentage of positive cells: 0; 1, <25%; 2, 25 to <50%; 3, 50 to <75%; 4, 75–100%. Positives were considered ≥ 2 nuclear (p53 and bcl-2) or membrane (blood group antigen A) staining in any percentage of cells and slides were read by one pathologist (AHT).
The general histochemical technique to detect mucin was used and details are published elsewhere.15 Mucicarmine, which detects all mucins was employed. Briefly, slides were deparaffinized and hydrated in distilled water, exposed to Mayer’s hematoxylin for 10 minutes, washed in running tap water for 5 minutes and then exposed to Southgate’s Mucicarmine solution at room temperature for one hour. Slides were then rinsed quickly in distilled water and exposed to Metanil yellow stain for 30–60 seconds. Slides were then dehydrated quickly in three changes of absolute alcohol until clear and then coverslipped in Permount. The mucin stain was a deep rose color. A positive result was the presence of mucicarmine positive secretory vacuoles within tumor cells or within the lumens of tumor glands. In general, however, the mucicarmine result was kept independent from the subclassification of the tumor, which was determined by just the routine H&E stain. In other words, positive mucicarmine results were not used to re-classify tumors as adenocarcinomas, and negative mucicarmine results were not used to re-classify tumors as non-adenocarcinomas. Slides were interpreted by one pathologist (RTV).
The primary objective of CALGB 9633 was to determine if adjuvant chemotherapy improved overall survival after resection of stage IB NSCLC. The major endpoint of this laboratory study was to determine the prognostic importance of the specific markers on disease-free survival (DFS) and overall survival (OS). In addition, complete clinical information was available on each patient including sex, age, performance status, surgical procedure, tumor size (from pathology report), TNM staging, presence of symptoms (chest pain, cough, hemoptysis) and smoking history (ever smoker versus non-smoker).
Overall survival (OS) was defined as time from random assignment to death from any cause. Disease-free survival (DFS) was defined as time from random assignment to recurrence or death, whichever comes first. The Kaplan-Meier product-limit estimator was used to estimate DFS and OS for subgroups of patients with stage IB NSCLC as defined by the presence or absence of the various markers.16 The log rank test was used to compare subgroups.17 Cox proportional hazards model was used to examine the joint effect of markers on DFS and OS. Additional analyses using the Cox model were conducted to further examine the effect of markers on DFS and OS after adjusting for the effects of chemotherapy and other baseline prognostic factors, as well as whether there was an interaction between treatment and the presence of a marker.18 Statistical analyses were performed by CALGB statisticians (LG and XW) using SAS 9.1. All p values reported are two sided.
Patient registration and clinical data collection were managed by the CALGB Statistical Center. As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every three years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 136 patients (40%) of the 344 patients under this study.
RESULTS
CALGB 9633 was activated October 15, 1996 and closed on January 6, 2004. During that time period 344 subjects were registered to this protocol, and randomly assigned to one of the two arms – observation or adjuvant chemotherapy. Six patients were canceled or never treated (1 on observation and 5 on chemotherapy). Seven patients were retrospectively determined to be ineligible (5 on observation and 2 on chemotherapy).
Paraffin blocks were obtained for 250 of the 344 patients randomized to the clinical trial. In total, 250 patients had a least one biomarker for bcl-2, p53, blood group antigen A or mucin and are included in the analysis. However, because of ineligible patients, technical problems, insufficient tumor tissue, data is available for the following: bcl-2 222, p53 234, blood group antigen A 239 and mucin 207.
Tables 1 and 2 summarize baseline patient characteristics, initial clinical diagnosis, and the frequency distributions of biologic markers of bcl-2, p53, blood group antigen A, and mucin between the two arms at the time of registration. The median age was 62 with a range of 37 to 81. The two randomized arms were balanced in terms of the baseline characteristics. There was a statistically significant difference in mucin expression by histology (Table 2). Mucin expression was positive in 66% of adenocarcinomas, 15% of squamous cell carcinomas and 40% of others (p-value < 0.0001 for adenocarcinomas versus squamous cell carcinomas). Mucin expression was positive in 36 of 71 (51%) females and 58 of 136 (43%) males (p=0.27).
Table 1.
Baseline patients’ characteristics and initial clinical diagnosis*
| Characteristics | Placebo (N=126) | Chemo (N=124) | p-value | |
|---|---|---|---|---|
| Age – median (min, max) | 63(40,81) | 61(37,78) | 0.2180 | |
| Gender | Male | 84 (67%) | 77 (62%) | 0.4505 |
| Female | 42 (33%) | 47 (38%) | ||
| Race | White | 109 (87%) | 112 (91%) | 0.3296 |
| Non-white | 16 (13%) | 11 (9%) | ||
| Performance Status | Fully active | 71 (57%) | 68 (55%) | 0.7011 |
| Others | 53 (43%) | 56 (45%) | ||
| Weight loss in previous 6 months | < 5% | 96 (81%) | 92 (80%) | 0.7932 |
| ≥ 5% | 22 (19%) | 23 (20%) | ||
| Symptoms | Presence | 96 (76%) | 99 (80%) | 0.4863 |
| Absence | 30 (24%) | 25 (20%) | ||
| Diagnosis | Squamous cell | 40 (32%) | 46 (37%) | 0.6440 |
| Adenocarcinoma | 68 (54%) | 61 (50%) | ||
| Other types | 18 (14%) | 16 (13%) | ||
| Smoking History | Yes | 125 (99%) | 120 (97%) | 0.1696 |
| No | 1 (1%) | 4 (3%) | ||
The p-value for age was from the t-test, while Chi-square tests were used for the categorical variables.
Table 2.
Prevalence of molecular biologic markers by treatment arm and histology.
| Marker | % positive | ||||
|---|---|---|---|---|---|
| Placebo | Chemo | Overall (*) | Adenoca | Squamous (**) | |
| bcl-2 | 15.0 | 19.3 | 17.1 (0.40) | 15.5 | 20.5 (0.37) |
| p53 | 41.9 | 51.3 | 46.6 (0.15) | 37.5 | 56.0 (0.09) |
| Blood group antigen A | 27.5 | 22.7 | 25.1 (0.39) | 24.2 | 27.7 (0.57) |
| Mucin | 47.7 | 43.0 | 45.4 (0.50) | 65.5 | 15.2 (<0.0001) |
The p-value of Chi-square test for placebo versus chemotherapy
The p-value for Chi-square test for adenocarcinoma versus squamous
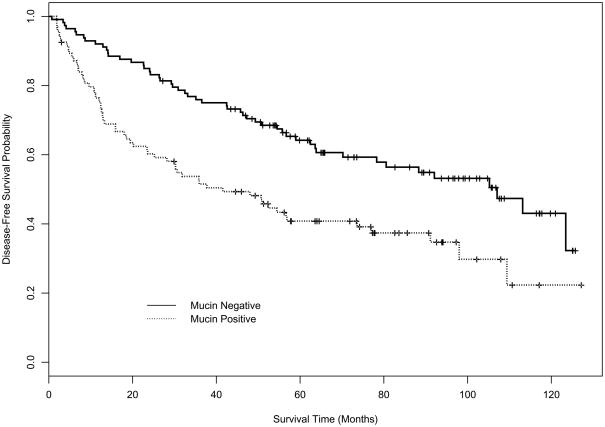
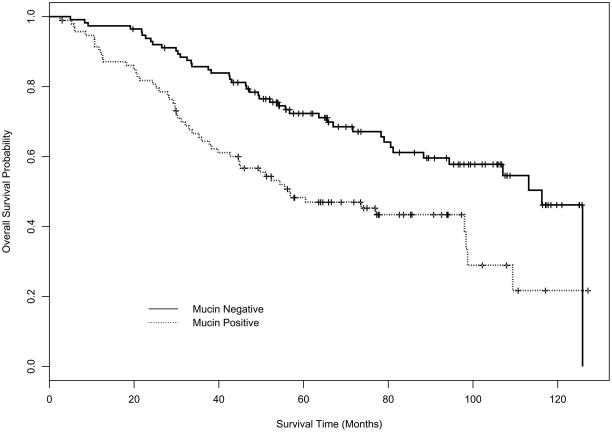
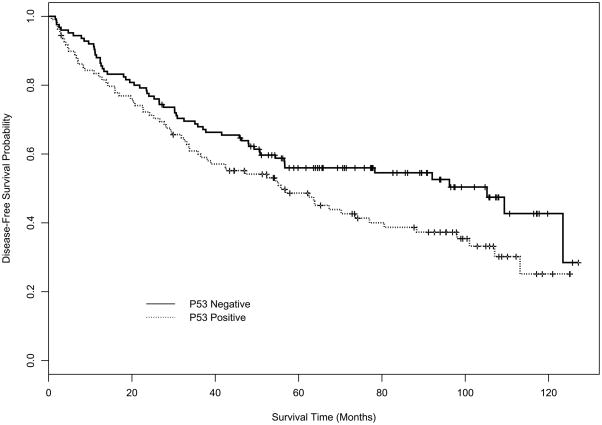
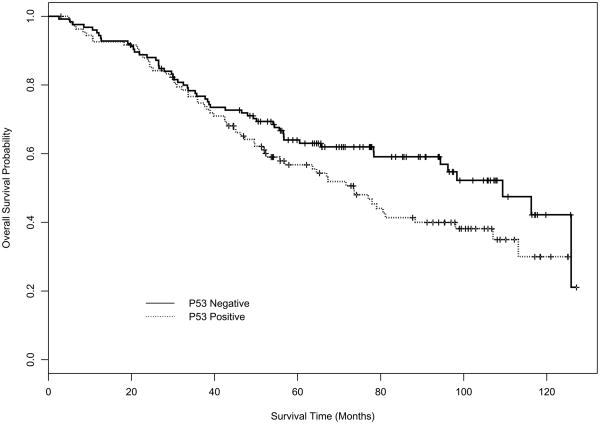
Figures 1 and 2 depict the disease-free survival (DFS) and overall survival (OS) curves for mucin and p53 expression using Kaplan-Meier (K-M) product-limit estimators. Table 3 displays the estimates of median DFS and median OS from the K-M product-limit estimator, and p-values from log rank tests of the four markers. Mucin expression (p=0.0005) and p53 expression (p=0.0485) had a statistically significant effect on DFS. For OS, only mucin (p=0.0005) had a statistically significant effect in univariate analysis.
Figure 1.
Figure 1A. Kaplan-Meier DFS curve for mucin.
Figure 1B. Kaplan-Meier OS curve for mucin.
Figure 2.
Figure 2A. Kaplan-Meier DFS curve for p53.
Figure 2B. Kaplan-Meier OS curve for p53.
Table 3.
Univariate analysis of disease-free survival (DFS) and overall survival (OS) using product-limit estimates.
| Marker | Median DFS in months (95% CI) | log rank test p-value | |
|---|---|---|---|
| negative / absent | positive / present | ||
| bcl-2 | 63.8 (49.3, 98.0) | 70.2 (32.5, NE*) | 0.7403 |
| p53 | 105.2 (56.6, NE) | 55.8 (36.5, 77.0) | 0.0485 |
| Blood group antigen A | 73.6 (47.2, NE) | 67.3 (50.8, 107.0) | 0.9207 |
| Mucin | 107.0 (70.2, NE) | 41.5 (25.2, 73.6) | 0.0005 |
| Marker | Median OS in months (95% CI) | log rank test p-value | |
| negative / absent | positive / present | ||
| bcl-2 | 79.0 (67.0, NE) | NE* (52.4, NE) | 0.2205 |
| p53 | 109.4 (78.4, NE) | 73.5 (52.4, 98.0) | 0.0633 |
| Blood group antigen A | 98.4 (73.6, NE) | 79.0 (65.6, 113.1) | 0.7399 |
| Mucin | 116.3 (88.3,125.9) | 56.7 (44.6, 98.4) | 0.0005 |
NE – Quantity is not estimable at this time.
Table 4 displays DFS and OS statistics from Cox proportional hazard regression analysis. Several multivariate Cox models were used to explore the relationships between DFS/OS and the biologic markers. Exploratory variables in the models with single markers are: Model (1) bcl-2; Model (2) p53; Model (3) blood group antigen A; and Model (4) mucin. Each marker was analyzed for its interaction with the study arms, and significant baseline covariates. Exploratory variables in Model 5 with multiple markers include the four markers (bcl-2, p53, blood group antigen A, and mucin), the pair-wise interactions of the study arms with the four markers, and significant baseline covariates. Baseline covariates include study arms, age, gender (male vs. female), race (white vs. non-white), performance status (PS 0 vs. 1), weight loss in previous 6 months (<5% of body weight vs. ≥5%), symptoms (chest pain/respiratory infection/cough etc. vs. none), initial diagnosis (adenocarcinoma/squamous cell carcinoma vs. others), smoking history (yes vs. no), estimated tumor volume, necrosis (presence vs. absence), and a binary variable for whether the number of nodal stations was less than 3 or not. Variables of the study arms and markers were forced into the models, while others were selected using stepwise method with entry level of 0.15 and stay level of 0.20.
Table 4.
Cox model for disease-free survival (DFS) and overall survival (OS) statistics of the molecular biologic markers
| Marker | p-value from Wald test for DFS HR (95% CI) * |
|
|---|---|---|
| single marker | multiple markers (Model 5) | |
| bcl-2 (positive vs. negative) |
(Model 1) | |
| 0.4812 | 0.3382 | |
| 0.81 (0.45, 1.46) | 0.79 (0.51, 1.24) | |
| p53 (positive vs. negative) |
(Model 2) | |
| 0.0385 | 0.0029 | |
| 1.56 (1.02, 2.37) | 1.95 (1.26, 3.02) | |
| Blood group antigen A (presence vs. absence) |
(Model 3) | |
| 0.6111 | 0.7463 | |
| 0.88 (0.55, 1.43) | 0.92 (0.55, 1.54) | |
| Mucin (positive vs. negative) |
(Model 4) | |
| 0.0021 | 0.0018 | |
| 1.88 (1.26, 2.81) | 2.05 (1.31, 3.21) | |
| p-value from Wald test for OS HR (95% CI) * |
||
| Marker | single marker | multiple markers (Model 5) |
| bcl-2 (positive vs. negative) |
(Model 1) | |
| 0.1017 | 0.1022 | |
| 0.57 (0.29, 1.12) | 0.54 (0.26, 1.13) | |
| p53 (positive vs. negative) |
(Model 2) | |
| 0.0190 | 0.0005 | |
| 1.71 (1.09, 2.67) | 2.30 (1.44, 3.68) | |
| Blood group antigen A (presence vs. absence) |
(Model 3) | |
| 0.9199 | 0.7981 | |
| 0.98 (0.59, 1.61) | 0.93 (0.55, 1.60) | |
| Mucin (positive vs. negative) |
(Model 4) | |
| 0.0040 | 0.0037 | |
| 1.88 (1.22, 2.89) | 2.03 (1.26, 3.26) | |
Hazard ratio and its 95% confidence interval.
The marker p53 was statistically significantly correlated with DFS/OS (Hazard Ratio [HR]: 1.56/1.71, log rank p-value = 0.0385/0.0190, respectively, see Model 2 in Table 4. Mucin in Model 4 was highly significant (HR: 1.88/1.88, p-value=0.0021/0.0040, respectively). The same conclusion could be reached in the multivariate Model 5; that is, p53 and mucin were detected to be highly significantly correlated with DFS/OS with p-value 0.0029/0.0005 and 0.0018/0.0037, and HR 1.95/2.30 and 2.05/2.03, respectively. Gender and estimated tumor volume were statistically significant (p-value < 0.05) in all models except in Model 2, where tumor volume was not significant. No other significant effects of covariates including study arms and interactions on DFS/OS were detected (p-value > 0.05).
The hazard ratio for OS of p53 positive versus negative patients was 1.92 (p=0.036) for placebo only patients and 1.40 (p=0.34) for the chemotherapy only patients. The hazard ratio for DFS of p53 positive versus negative patients was 2.05 (p=0.017) for placebo only patients and 1.36 (p=0.35) for chemotherapy only patients. However, there was no predictive value for these markers as evidenced by the lack of significant interactions between treatment and these markers (p-value > 0.05).
DISCUSSION
The well established negative prognostic factors for patients with NSCLC include higher TNM stage, weight loss and poor performance status.19 Many research efforts have investigated the utility of clinical, biologic and molecular markers to further refine prognosis. Studies have reported the possible prognostic significance of tumor size, gender, age, histologic subtype, degree of differentiation, aneuploidy, ras mutation, p53 expression and mutation, bcl-2, blood group antigens and angiogenesis, among others.12,13,20,21,22,23,24,25 While several of these factors have demonstrated prognostic value in selected series, only tumor size has been definitive enough to be incorporated into the newly proposed staging system.26,27 In CALGB 9633, a study of patients with exclusively stage IB NSCLC, the clinical factors that had a significant adverse impact on survival were male gender and larger tumor volume.
At the time of the initiation of CALGB 9633, several large single institution studies suggested the possible prognostic importance of p53 expression, bcl-2, blood group antigen A and mucin expression.7,8,9 The prospective randomized Phase III trial, CALGB 9633, provided a unique resource to study a well-staged group of patients with stage IB (T2N0) NSCLC. Patients on this study were randomized to observation or 4 cycles of carboplatin/paclitaxel with overall survival and disease-free survival as the major endpoints.6
Of the biological markers included in the current study, blood group antigen A and bcl-2 were not prognostic. However, several factors did have independent prognostic value including mucin, p53, gender and tumor size. The presence of mucin as detected by the mucicarmine stain was a strong negative prognostic factor. The Lung Cancer Study Group made an early observation that mucin expression was a negative prognostic factor in 237 surgical patients.28 Yu et al. found expression of sialomucin to be an independent predictor of early recurrence and survival in multivariate analysis.29 Kwiatkowski found that the subtype of adenocarcinoma, solid tumor with mucin, was associated with shorter survival.8 We also observed mucin to be associated with worse survival in a single institution study of 260 patients with stages I and II NSCLC.11 Overexpression of mucin in vitro has been shown to decrease tumor cell aggregation, promote tumor cell invasion, and block lymphocyte targeting, facilitating metastasis by escape from immuno-surveillance.28
p53 is a nuclear phosphoprotein that plays a key role in regulating the cell cycle, apoptosis, and initiation of DNA repair. Aberrant expression of p53 and p53 mutations occur in 50–60% of tumors and are among the most frequent molecular abnormalities in NSCLC. Meta-analyses have suggested a modest negative prognostic role for p53.30,31 In the current study, the pAb1801 antibody was used for the detection of p53 overexpression. In the meta-analysis by Mitusdomi et al., 11 studies used the DO7 antibody and 10 studies used the pAb1801 antibody with similar incidence of overexpression of p53, independent of the antibody employed.30 Because of the key role of p53 in DNA repair, it has been hypothesized that p53 may be a mediator of chemotherapy response.32,33 In addition, mutations in p53 have been associated with a higher incidence of invasive lung adenocarcinomas.34
In the current study, p53 protein expression as measured by an immunohistochemical (IHC) assay was positive in 47% and was associated with significantly worse overall survival with a hazard ratio of 2.30 in multivariate analysis. This compares closely to the results of p53 protein expression in the JBR.10 trial where 52% were IHC positive and was associated with inferior survival (HR 1.89) in patients on the observation arm. There was also a predictive role for p53, with p53 IHC positive patients showing a benefit from chemotherapy.35 We did not find a predictive value for p53 in our study, but small numbers limited the power to detect significant interactions between treatment and p53 expression. There was a trend towards a greater benefit from chemotherapy in the p53 IHC positive group, but it did not reach statistical significance.
While the current studies are of importance, before mucin and p53 can be accepted as having clinical utility, confirmatory studies are necessary. The Lung Adjuvant Cisplatin Evaluation (LACE) Collaborative group has performed a pooled analysis of the 5 largest trials of adjuvant cisplatin-based chemotherapy in NSCLC.5 Members from several of these trials have formed the LACE biological (LACE-Bio) collaborative group with the main objectives to study the prognostic and predictive value of tumor markers in patients treated with adjuvant chemotherapy. One of the goals of the LACE-Bio group is to perform cross-validation analyses for the predictive value of ERCC1. Olaussen and colleagues reported that low expression of ERCC1 was a negative prognostic factor but predicted for benefit from cisplatin-based chemotherapy.36 If confirmed in cross-validation studies, ERCC1 expression could help select patients who would benefit from cisplatin-based adjuvant chemotherapy.
The next generation of studies looking at markers such as ERCC1, ras and EGFR mutations, beta-tubulin, BRCA1, p27 and gene arrays have the potential to provide not only prognostic information but also may predict which chemotherapy agents would be most effective.37,38,39,40,41,42 CALGB is currently analyzing CALGB 9633 for the prognostic and predictive effect of K-ras and EGFR mutations. It is hoped that such studies will increase the understanding of the biology of lung cancer and will help individualize treatment and improve the outcome of patients with NSCLC.
Acknowledgments
The research for CALGB 9633 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Presented at the 12th World Conference on Lung Cancer, September 2–6, 2007, Seoul, Korea.
The following institutions participated in this study:
Dana-Farber Cancer Institute, Boston, MA–Eric P. Winer, M.D., supported by CA32291
Dartmouth Medical School - Norris Cotton Cancer Center, Lebanon, NH–Marc S. Ernstoff, M.D., supported by CA04326
Duke University Medical Center, Durham, NC–Jeffrey Crawford, M.D., supported by CA47577
Long Island Jewish Medical Center, Lake Success, NY–Kanti R. Rai, M.D., supported by CA11028
Massachusetts General Hospital, Boston, MA–Jeffrey W. Clark, M.D., supported by CA12449
Medical University of South Carolina, Charleston, SC–Mark Green, M.D., supported by CA03927
Mount Sinai Medical Center, Miami, FL–Rogerio C. Lilenbaum, M.D., supported by CA45564
Mount Sinai School of Medicine, New York, NY–Lewis R. Silverman, M.D., supported by CA04457
Northern Indiana Cancer Research Consortium CCOP, South Bend, IN–Rafat Ansari, M.D., supported by CA86726
Rhode Island Hospital, Providence, RI–William Sikov, M.D., supported by CA08025
Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, M.D., supported by CA02599
Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC–James N. Atkins, M.D., supported by CA45808
State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, M.D., supported by CA21060
The Ohio State University Medical Center, Columbus, OH–Clara D Bloomfield, M.D., supported by CA77658
University of California at San Diego, San Diego, CA–Barbara A. Parker, M.D., supported by CA11789
University of California at San Francisco, San Francisco, CA–Alan P. Venook, M.D., supported by CA60138
University of Chicago, Chicago, IL –Gini Fleming, M.D., supported by CA41287
University of Illinois MBCCOP, Chicago, IL–Lawrence E. Feldman, M.D., supported by CA74811
University of Iowa, Iowa City, IA–Daniel A. Vaena, M.D., supported by CA47642
University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, M.D., supported by CA31983
University of Massachusetts Medical School, Worcester, MA–William V. Walsh, M.D., supported by CA37135
University of Minnesota, Minneapolis, MN–Bruce A Peterson, M.D., supported by CA16450
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C Perry, M.D., supported by CA12046
University of Nebraska Medical Center, Omaha, NE–Anne Kessinger, M.D., supported by CA77298
University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, M.D., supported by CA47559
University of Tennessee Memphis, Memphis, TN–Harvey B. Niell, M.D., supported by CA47555
University of Vermont, Burlington, VT–Hyman B. Muss, M.D., supported by CA77406
Wake Forest University School of Medicine, Winston-Salem, NC–David D Hurd, M.D., supported by CA03927
Walter Reed Army Medical Center, Washington, DC–Thomas Reid, M.D., supported by CA26806
Washington University School of Medicine, St. Louis, MO–Nancy Bartlett, M.D., supported by CA77440
Weill Medical College of Cornell University, New York, NY–John Leonard, M.D., supported by CA07968
North Central Cancer Treatment Group, Rochester, MN-Jan Buckner, M.D., Chairman; supported by CA25224
Radiation Therapy Oncology Group, Philadelphia, PA-Walter J. Curran, Jr.; supported by CA21661
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global cancer rates could increase 50% to 15 million by 2020 [WHO Web site] Apr 3, 2003. [Accessed Jan 18, 2010]. Available at: http://www.who.int/mediacentre/news/releases/2003/pr27/en.
- 3.The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 4.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;52:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 6.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung Adjuvant Cisplatin Evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 7.Strauss GM, Herndon JE, II, Maddus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harpole DH, Jr, Herndon JE, II, Wolfe WG, et al. A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopathology and oncoprotein expression. Cancer Res. 1995;55:51–56. [PubMed] [Google Scholar]
- 9.Kwiatkowski DJ, Harpole DH, Godleski J, et al. Molecular pathologic substaging in 244 stage I non-small cell lung cancer patients: clinical implications. J Clin Oncol. 1998;16:2468–2477. doi: 10.1200/JCO.1998.16.7.2468. [DOI] [PubMed] [Google Scholar]
- 10.Mehdi SA, Tatum AH, Newman NB, et al. Prognostic markers in resected stage I and II non-small-cell lung cancer: an analysis of 260 patients with 5 year follow-up. Clinical Lung Cancer. 1999;1:59–67. doi: 10.3816/clc.1999.n.004. [DOI] [PubMed] [Google Scholar]
- 11.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 12.Graziano SL, Tatum AH, Newman NB, et al. The prognostic significance of neuroendocrine markers and carcinoembryonic antigen in patients with resected stage I and II non-small cell lung cancer. Cancer Res. 1994;54:2908–2913. [PubMed] [Google Scholar]
- 13.Pezzella F, Turley H, Kuzu I, et al. bcl-2 protein in non-small cell lung carcinoma. N Engl J Med. 1993;329:690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- 14.Graziano SL, Tatum AH, Gonchoroff NJ, et al. Blood-group antigen A and flow cytometric analysis in resected early stage non-small cell lung cancer. Clinical Cancer Res. 1997;3:87–93. [PubMed] [Google Scholar]
- 15.Bancroft J, Stevens A. Theory and Practice of Histological Techniques. 2. New York, NY: Churchill-Livingstone; 1982. pp. 201–202. [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Ass. 1958;53:457–481. [Google Scholar]
- 17.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 18.Cox DR. Regression models and life tables. J R Stat Soc [B] 1972;34:187–200. [Google Scholar]
- 19.Ginsberg RJ, Vokes EE, Rosenzweig K. Non-Small Cell Lung Cancer. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer, Principles and Practice of Oncology. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 925–983. [Google Scholar]
- 20.Gail MH, Eagan RT, Feld R, et al. Prognostic factors in patients with resected stage I non-small cell lung cancer: a report from the Lung Cancer Study Group. Cancer. 1984;54:1802–1813. doi: 10.1002/1097-0142(19841101)54:9<1802::aid-cncr2820540908>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Lipford EH, III, Eggleston JC, Lillemoe KD, et al. Prognostic factors in surgically resected limited stage NSCLC. Am J Surg Pathol. 1984;8:357–365. doi: 10.1097/00000478-198405000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Isobe H, Miyamoto H, Shimizu T, et al. Prognostic and therapeutic significance of flow cytometric nuclear DNA content in NSCLC. Cancer. 1990;65:1391–1395. doi: 10.1002/1097-0142(19900315)65:6<1391::aid-cncr2820650624>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 23.Slebos RJ, Kebbelaar RE, Dalesio O, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of lung. N Engl J Med. 1990;323:561–565. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Ro JY, Sahin AA, et al. Expression of blood-group antigen-A: a favorable prognostic factor in non-small cell lung cancer. N Engl J Med. 1991;324:1084–1090. doi: 10.1056/NEJM199104183241603. [DOI] [PubMed] [Google Scholar]
- 25.Fontanini G, Lucchi M, Vignati S, et al. Angiogenesis as a prognostic indicator of survival in non-small cell lung carcinoma. J Natl Cancer Inst. 1997;89:881–886. doi: 10.1093/jnci/89.12.881. [DOI] [PubMed] [Google Scholar]
- 26.Gajra A, Newman N, Gamble GP, et al. Impact of tumor size on survival in stage IA non-small cell lung cancer: a case for subdividing stage IA disease. Lung Cancer. 2003;42:51–57. doi: 10.1016/s0169-5002(03)00285-x. [DOI] [PubMed] [Google Scholar]
- 27.Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 28.Linnoila RI, Piantadosi S, Ruckdeschel JC. Impact of neuroendocrine differentiation in non-small cell lung cancer. The LCSG experience. Chest. 1994;106:367s–371s. [PubMed] [Google Scholar]
- 29.Yu C, Shun C, Yan P, et al. Sialomucin expression is associated with erbB-2 oncoprotein overexpression, early recurrence, and cancer death in non-small cell lung cancer. Am J Respir Crit Care Med. 1997;155:1419–1427. doi: 10.1164/ajrccm.155.4.9105088. [DOI] [PubMed] [Google Scholar]
- 30.Mitsudomi T, Hamajima H, Ogawa M, et al. Prognostic significance of p53 alterations in patients with non-small cell lung cancer: A meta-analysis. Clin Cancer Res. 2000;6:4055–4063. [PubMed] [Google Scholar]
- 31.Steels E, Paesmans M, Berghmans T, et al. Role of p53 as a prognostic factor for survival in lung cancer: A systematic review of the literature with a meta-analysis. Eur Respir J. 2001;18:705–719. doi: 10.1183/09031936.01.00062201. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta S, Harris CC. p53: Traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol. 2005;6:44–55. doi: 10.1038/nrm1546. [DOI] [PubMed] [Google Scholar]
- 33.Gajra A, Tatum AH, Newman N, et al. The predictive value of neuroendocrine markers and p53 for response to chemotherapy and survival in patients with advanced non-small cell lung cancer. Lung Cancer. 2002;36:159–165. doi: 10.1016/s0169-5002(01)00463-9. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi H, Matsumoto S, Iwakawa R, et al. Whole genome comparison of allelic imbalances between noninvasive and invasive small-sized lung adenocarcinomas. Cancer Res. 2009;69:1615–1623. doi: 10.1158/0008-5472.CAN-08-3218. [DOI] [PubMed] [Google Scholar]
- 35.Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and ras for adjuvant chemotherapy in non-small-cell lung cancer. J Clin Oncol. 2007;25:5240–5247. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 36.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–989. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 37.Filipits M, Pirker R, Dunant A, et al. Cell cycle regulators and outcome of adjuvant cisplatin-based chemotherapy in completely resected non-small-cell lung cancer: the International Adjuvant Lung Cancer Trial Biologic Program. J Clin Oncol. 2007;25:2735–2740. doi: 10.1200/JCO.2006.08.2867. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Z, Chen T, Li X, et al. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007;356:800–808. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 39.Potti A, Mukherjee S, Petersen R, et al. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N Engl J Med. 2006;355:570–580. doi: 10.1056/NEJMoa060467. [DOI] [PubMed] [Google Scholar]
- 40.Azzoli CG, Park BJ, Pao W, et al. Molecularly tailored adjuvant chemotherapy for resected non-small cell lung cancer; a time for excitement and equipoise. J Thorac Oncol. 2008;3:84–93. doi: 10.1097/JTO.0b013e31815efe24. [DOI] [PubMed] [Google Scholar]
- 41.Seve P, Lai R, Ding K, et al. Class III beta-tubulin expression and benefit from adjuvant cisplatin/vinorelbine chemotherapy in operable non-small cell lung cancer: analysis of NCIC JBR.10. Clin Cancer Res. 2007;13:994–999. doi: 10.1158/1078-0432.CCR-06-1503. [DOI] [PubMed] [Google Scholar]
- 42.Rosell R, Skrzypski M, Jassem E, et al. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS ONE. 2007;2:e1129. doi: 10.1371/journal.pone.0001129. [DOI] [PMC free article] [PubMed] [Google Scholar]