Abstract
Problem addressed
Maintenance of cognitive control is a major concern for many human disease condition, therefore a major goal of human neuroprosthetics is to facilitate and/or recover cognitive function when such circumstances impair appropriate decision making.
Methodology
Nonhuman primates trained to perform a delayed match to sample (DMS) were employed to record mini-columnar activity in the prefrontal cortex (PFC) via custom designed conformal multielectrode arrays that provided inter-laminar recordings from neurons in PFC layer 2/3 and layer 5. Such recordings were analyzed via a previously demonstrated nonlinear multi-input multi-output (MIMO) neuroprosthesis in rodents, which extracted and characterized multi-columnar firing patterns during DMS performance.
Results
The MIMO model verified that the conformal recorded individual PFC minicolumns responded to entrained target selections in patterns critical for successful DMS performance. This allowed substitution of task-related layer 5 neuron firing patterns with electrical stimulation in the same recording regions during columnar transmission from layer 2/3 at the time of target selection. Such stimulation facilitated normal task performance, but more importantly, recovered performance when applied as a neuroprosthesis following pharmacological disruption of decision making in the same task.
Significance and potential impact
These findings provide the first successful application of a neuroprosthesis in primate brain designed specifically to restore or repair disrupted cognitive function.
Keywords: cognitive neuroprosthesis, recovery of cognitive performance, prefrontal cortex, mini-column processing, decision making, nonhuman primates
INTRODUCTION
Cognitive deficits related to performance dysfunction are in many cases characterized by the inability to employ the appropriate behavioral response in circumstances in which choice changes routinely as a function of task-dependent complexity (Shallice and Burgess, 1991; Duncan et al., 1997; Beveridge et al., 2008; Buxhoeveden et al., 2006; Dobbs, 2010; Brennan and Arnsten, 2008; Wang et al., 2011). According to many theories of cognition, cortical executive decision mechanisms coordinate and control ‘online’ cognitive processes underlying behavioral selection, working memory, behavioral inhibition, and multi-tasking (Posner and Snyder, 1975; Shallice and Burgess, 1996; Goldman-Rakic, 1996; Miyaki et al., 2000; Baddeley, 2002; Miller and Cohen, 2001; Graybiel, 2008). Disruption of the normal functional status of the prefrontal cortex (PFC) which utilizes precise minicolumnar organization of neural firing to coordinate attention, decision making and movement selection, can lead to failure in cognitive performance for several reasons since this proposed microcircuitry consists of neurons in the supra-granular layers (L2/3), that integrate sensory signals and communicate directly with cells in the infra-granular layers (L5) in prefrontal (area 46) & frontal cortical regions (areas 6 and 8) (Buxhoeveden et al., 2006; Buxhoeveden and Casanova, 2002). However, since the microanatomic basis for information processing in this region is similar in terms of minicolumnar inter-laminar connectivity, restoration of lost function could be achieved by duplication of patterned layer 5 outputs based on previous inputs from layer 2/3 cells over the same minicolumnar structure (Kritzer and Goldman-Rakic, 1995; Hasselmo, 2005; Opris et al., 2011). In this report we provide evidence for the existence of such “executive microcircuitry” within PFC, featuring cortical minicolumns (Mountcastle, 1997; Weiler et al., 2008; Opris et al., 2011; Takeuchi et al., 2011) that coordinate activity in nonhuman primates (NHPs) required to make behavioral decisions to evaluate and respond to an appropriate target.
In order to implement a previously successful multi-input-multi-output (MIMO) dynamic nonlinear neural prostheses model for recovery of function and cognitive capability (Berger et al., 2012; Hampson et al., 2012a; Hampson et al., 2012b; Berger et al., 2011) a selective pharmacologic disruption of PFC columnar processing was produced which resulted in decreased cognitive performance, mimicking cortical malfunction similar to that characterized in many human disease states (Casanova et al., 2008; van Veluw et al., 2012; Tomasi et al., 2010; Buxhoeveden et al., 2006). Application under these conditions provided the means to test the MIMO model utilizing interposed delivery of device extracted patterns of task-successful multicolumnar firing via electrical stimulation to the same areas. Such stimulation was employed on a trial-by-trial basis to reverse the pharmacologic state of depressed inter-laminar transmission during the decision and selection stages of the task. The results provide the first instance of application of a neuroprosthesis designed specifically for recovery of cognitive processing in primate brain by restoring columnar activation patterns, and as such indicate a potential efficacy for application to cortical dysfunction related to many types of human disease (Casanova et al., 2008; van Veluw et al., 2012; Casanova et al., 2010; Kusunoki et al., 2010; Grabenhorst et al., 2008; Buxhoeveden et al., 2006).
METHODS
Experimental Subjects
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Wake Forest University, in accordance with U.S. Department of Agriculture, International Association for the Assessment and Accreditation of Laboratory Animal Care, and National Institutes of Health guidelines. Five nonhuman primate (NHP) subjects (rhesus, Macaca mulatta) were trained for at least 2 years to perform a version of delayed-match-to-sample (DMS) task (Hampson et al., 2004b) for juice rewards to a criterion performance level that was stable for at least 1 year.
Visual Delayed-Match-to-Sample (DMS) Task
NHPs utilized (n=5) were trained to perform the well characterized, custom-designed visual delayed-match-to-sample (DMS) task (Deadwyler et al., 2007; Porrino et al., 2005; Hampson et al., 2011; Hampson et al., 2004b) shown in Fig. 1a. During task performance animals were seated in a primate chair with a shelf-counter in front of them facing a large display screen (Fig 1a). Right arm position on the counter top was tracked via a UV-fluorescent reflector attached to the back of the wrist which was illuminated with a 15 W UV lamp and detected by a small LCD camera positioned 30 cm above the hand. Hand position and movement was digitized and displayed as a bright yellow cursor on the projection screen and horizontal positions of illuminated targets were computed from the video image using a Plexon Cineplex scanner connected to a behavioral control computer. Trials were initiated by the animal placing the cursor inside a yellow 3” diameter circle “Start signal” displayed randomly in one of the nine positions on the screen. This was followed by a clip-art image displayed in the Sample phase was to be selected in the Match phase among other images on that same trial. Trials were initiated by the animal placing the cursor inside a yellow “Start signal” consisting of a 3” diameter circle displayed randomly in one of the eight screen positions. Following trial initiation a single unique image was displayed randomly on the screen (Sample Image) and constituted the Sample phase of the task which required placement of the cursor into the image (Sample Response) to initiate the next phase, the Delay interval in which the screen was blanked for 1–90s on a trial-to-trial basis. Termination of the Delay interval was signaled by the onset of the screen display in the Match phase of the task in which 2–7 trial unique images, including the Sample image, were presented at separate randomly selected spatial locations on the screen as shown in Fig 1a. Placement of the cursor into the Sample image constituted the correct “Match Response” (MR) which blanked the screen and produced a drop of juice delivered via a sipper tube located near the animal’s mouth. Placement of the cursor into one of the non-match (distracter) images constituted a non-match-error response and caused the screen to blank without reward delivery. Trials were separated by a minimum of 10 sec in which the Start signal (circle) was presented following termination of the Match phase of the prior trial. All images (samples and distracters) were unique for each trial over the entire session (100–150 trials) and were selected randomly from an image reservoir (n=5000) updated every month (Hampson et al., 2004b). All subjects were trained to overall performance levels of 70–75% correct on the least difficult trials with graded declines in performance on trials with increased delays and number of images in the Match phase of the task (Fig 1a).
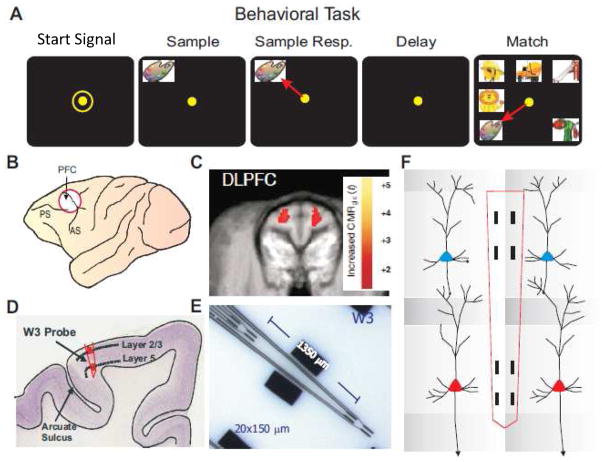
Figure 1.
Illustration of behavioral task and prefrontal cortical recording localization. A. Behavioral paradigm showing sequence of events in the DMS task: 1) ‘Focus Ring’ presentation and response to initiate the trial; commencing with 2) presentation of ‘Sample Target’ image; followed by 3) ‘Sample Response’ by cursor movement into the image; which initiates 4) variable ‘Delay’ period of 1–90 sec; prior to 5) presentation of the ‘Match’ Target (Sample image) accompanied by 1–6 Non-match (distracter) images on the same screen. Cursor movement into correct (Match target) image for 0.5s was rewarded by juice reward (0.5 ml) via a sipper tube next to the animal’s mouth. Placement of the cursor into a Non-match image for 0.5s caused the screen to blank without reward delivery. Intertrial interval (ITI): 10.0s. B. Diagram of NHP brain showing PFC recording locations (accessing cortical areas 46, 8, 6). C. Representative magnetic resonance image (MRI) of coronal section through dorsolateral prefrontal cortex (DLPFC) centered on the area in B. PET imaged localized cerebral metabolic rate (LCMRglu) activation (red blots) indicates metabolic activity of DLPFC during DMS task performance (Hampson et al., 2011; Porrino et al., 2007). D. Illustrated histologic section of DLPFC brain showing relative location of supra-granular layer 2/3 (L2/3) and infra-granular layer 5 (L5) with tract (in red) used for placement of conformal MEA recording (W3) probes shown in E. E. Ceramic conformal recording array custom designed (W3) for inter-laminar and inter-columnar cortical recording (diagram in F) consisting of dual sets of 4 recording pads vertically aligned and separated by 1350 μm the anatomic distance between L2/3 and L5 in primate brain. F. Dimensionally relevant illustration of the conformal MEA positioned for simultaneous recording from neurons in both layers in adjacent minicolumns (1&2), each minicolumn consisting of a “pair” of L2/3 and L5 PFC cells.
Surgery
Animals were surgically prepared with cylinders for attachment of a microelectrode manipulator over the specified brain regions of interest. During surgery animals were anesthetized with ketamine (10 mg/kg), then intubated and maintained with isoflurane (1–2 % in oxygen 6 l/min). Recording cylinders (Crist Instruments, Hagerstown, MD) were placed over 20 mm diameter craniotomies for electrode access (Hampson et al., 2011; Opris et al., 2011) to stereotaxic coordinates of the Frontal Cortex (25 mm anterior relative to interaural line and 12 mm lateral to midline/vertex) in the caudal region of the Principal Sulcus, the dorsal limb of Arcuate Sulcus in area 8 and the dorsal part of premotor area 6 (Fig. 1c,d), areas previously shown by PET imaging to become activated during task performance (Fig. 1e) (Porrino et al., 2005). Two titanium posts were secured to the skull for head restraint with titanium screws embedded in bone cement. Following surgery, animals were given 0.025 mg/kg buprenorphine for analgesia and penicillin to prevent infection. Recording cylinders were disinfected thrice weekly with Betadine during recovery and daily during recording. Vascular access ports (Norfolk Medical Products, Skokie, IL) for drug infusions were implanted subcutaneously in the mid-scapular region, the end of the catheter threaded subcutaneously, to a femoral incision, inserted into the femoral vein, and threaded for a distance calculated to terminate in the vena cava and flushed daily with 5 ml heparinized saline needed for IV drug administration.
Electrophysiological Recording
Electrophysiological procedures and analysis utilized the 64 channel MAP Spike Sorter by Plexon, Inc (Dallas, TX). Recordings from PFC were obtained with a custom designed conformal ceramic multielectrode array (MEAs) manufactured in collaboration with Dr. Greg Gerhardt (Center for Microelectrode Technology – CenMet, Lexington, KY) at the University of Kentucky (Moxon et al., 2004). MEAs consisted of etched platinum pads (Fig. 1e) constructed for recording isolated single neuron extracellular action potentials on specifically aligned recording pads (Fig. 2) during events within DMS trials {Opris, 2011 7758 /id}. The MEA probe (Fig. 1e) was specially designed to conform to the columnar anatomy of the PFC such that the top 4 recording pads (separated by 40 μm horizontally and 100 μm vertically) recorded activity from neurons in the supra-granular layer 2/3 (L2/3) while the lower set of four pads, separated vertically by 1300 μm in terms of probe orientation in PFC, simultaneously recorded neurons in infra-granular layer 5 (L5) as shown in Figure 1f. This pad configuration insured that only cells in L2/3 and L5 were isolated, since appearance of cells simultaneously on both vertically separated sets of pads required 0° angular orientation relative to both cell layers (Takeuchi et al., 2011) as shown in Figure 1d.
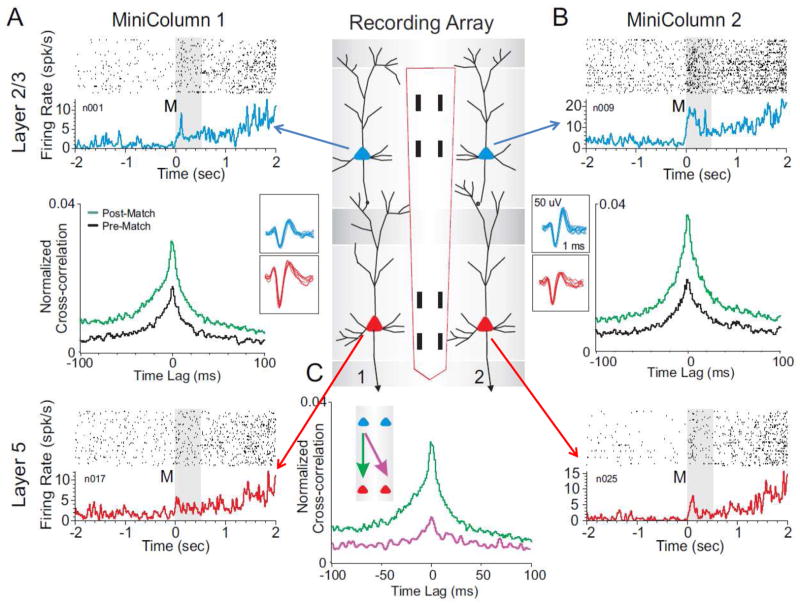
Figure 2.
Coherence of inter-laminar activity within but not between adjacent prefrontal minicolumns during DMS task performance. The center panel shows the conformal multielectrode recording array (MEA) positioned for simultaneous inter-laminar columnar recording from adjacent minicolumns 1&2 (40 μm separation) with L2/3 and L5 cell pairs (Fig. 1f) shown as the corresponding (blue and red) cell waveforms. A&B show corresponding cell data as individual trial rasters and average perievent histograms (PEHs) obtained from two cell pairs recorded simultaneously from L2/3 (blue) and L5 (red) within the respective minicolumns. Rasters and PEHs depict ± 2.0s relative to Match phase (Fig. 1A) onset (0.0s) within a single DMS session. Cross-correlation histograms (CCHs) for the same cell pairs within each minicolumn are shown in the middle of the raster-PEH displays. The CCHs display increased inter-laminar synchronization during target selection (green) during the Match phase (0.0+2,0s, post) relative to similar correlations between the same cell pairs constructed 2.0s prior to onset (−2.0s to 0.0s in PEHs) of the Match phase (black, pre). C: Examples of post Match phase CCHs in which L2/3 and L5 cell pairs were localized to 1) the same (minicolumn #1, green arrow) or diagonally via 2) different minicolumns (i.e. L2/3 minicolumn #1, L5 minicolumn #2 purple arrow) which was not significant (F(1,401)<3.22, p>0.10) show the specificity of MEA columnar orientation. Post-Match CCHs for cell pairs from all types of diagonal comparisons on the same MEA across different minicolumns were previously reported (Opris et al 2011).
Data Analysis
Task performance was determined for each animal (n=5) as % correct trials within and across sessions and related to simultaneous recordings of multiple MEA conformal single neuron firings during Match phase image selection on individual trials {Hampson, 2011 7757 /id} in the DMS task (Fig. 1a, Match phase). Cell types were identified as regular firing PFC cells in terms of baseline (nonevent) firing rate {Opris, 2011 7758 /id;Opris, 2009 7268 /id}and significant changes (z > 3.09, P < 0.001) in firing (see below) on single trials in perievent histograms (PEHs) derived for intervals of ± 2.0s relative to the onset of the screen image display (0.0s) in the Match phase of the task (Fig. 2). Task-related neural activity was classified according to locations on the conformal MEA positioned in L2/3 and L5 (Fig. 1f) upon insertion in PFC prior to the start of the DMS session. Standard scores, z = [peak - baseline firing rate]/SD baseline firing rate, were calculated for individual cell firing on each DMS task event (Hampson et al., 2004b). Firing rate for simultaneously recorded L2/3 and L5 neurons was analyzed in 100 ms bins over ± 2.0 s relative to the time of initiation (0.0s) of the Match phase (Fig. 2). Neurons were only included in the analysis if their firing rates were significantly elevated (Z- scores, ANOVA F test p < 0.01) relative to the same time interval prior to Match screen presentation (−2.0 to 0.0s).
Identification of Cortical layers and Minicolumns
The conformal MEA probe (Fig. 1e) was designed such that the two sets of vertical recording pads detected simultaneous activity from neurons separated by 1300μm, which given the perpendicular orientation of insertion to the cell layers in PFC (dorsal premotor gyrus in area 6, stereotactic coordinates AP:25 & ML:12) consisted of cells in supra-granular layer 2/3 (L2/3) and infra-granular layer 5 (L5) as shown in Figure 1f (Hampson et al., 2004a; Gold and Shadlen, 2007; Opris et al., 2005a; Opris et al., 2005b; Casanova et al., 2010). The MEAs (Hampson et al., 2010; Opris et al., 2011; Moxon et al., 2004) therefore provided recording of PFC columnar activity from two different adjacent sets of pads which allowed assessment and validation via correlation between interlaminar cell pairs recorded on the same probe in distinct minicolumns (Takeuchi et al., 2011; Hansen and Dragoi, 2011; Mo et al., 2011) as shown in Figure 2c. Statistical Analyses also determined whether there were inter-laminar differences in firing rates during activation in the Match Phase for cells in different layers (i.e. L2/3 vs. L5). Differences in cross-correlation were assessed using normalized distributions of coefficients extracted from firing of inter-laminar cell pairs under different conditions related to performance in the Match Phase of the task (Figs. 2,3,7). Normalized mean coefficients were used to construct cross-correlation histograms (CCHs) which satisfied 99% confidence limits requirement for comparison of the same cell pairs under different experimental conditions (Opris et al., 2011). The correspondence of firing between cells in different layers was tested via CCHs employing L2/3 cell firing to test synchronous discharge with simultaneously recorded L5 cells in 1.0 ms intervals over ±1.0–2.0s time of occurrence of task related events (Fig. 2c). CCHs for inter-laminar cell pairs (L2/3 & L5) were generated using a shift-predictor (http://www.neuroexplorer.com/), which computed random cross-correlation levels due to chance simultaneous spike occurrences and eliminated these from the true coefficients for CCHs to adjust for differences in cell firing rates and frequency of bursting (Opris et al., 2011; Takeuchi et al., 2011). Population CCHs were computed by averaging coefficients across multiple cell pairs and plotting the mean values (±SEM) in 1.0 ms bins (Figs. 3&7).
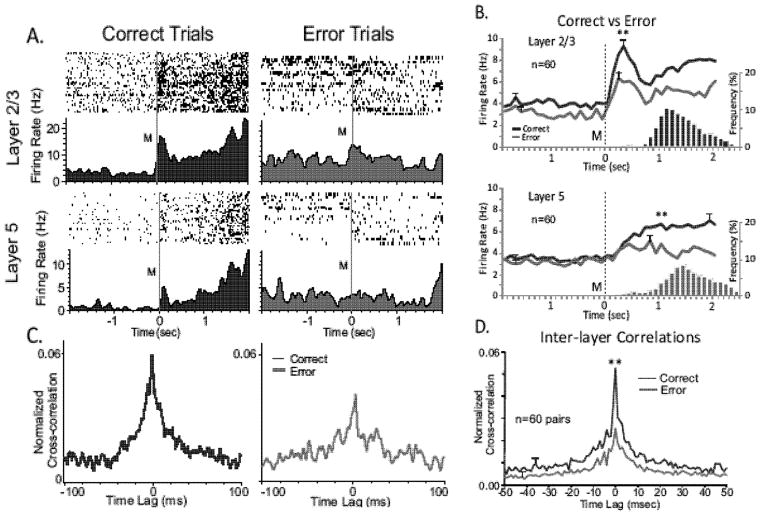
Figure 3.
Differences in PFC columnar processing on correct vs. error trials. A. Match phase rasters and PEHs recorded from a L2/3 (upper) and L5 (lower) cell pair within a single minicolumn, segregated as to correct (blue) and error (red) trials during a single session (n= 120 trials). B. Line graphs represent mean Match phase PEHs averaged over all recorded inter-laminar PFC cell pairs (n= 60), L2/3 (upper) & L5 (lower), on correct (blue) vs. error trials (red) summed across animals and sessions. No more than 2 cell pairs were recorded in same behavioral session from the same MEA. Blue (correct) and Red (error) bar graphs show the associated mean frequency distributions of Match Response latencies for the same associated correct and error trials plotted on the same time-base as the PEHs relative to Match phase onset (0.0s). C. Coherent intralaminar activity of L2/3 and L5 neuron pair segregated by correct and error trials as in A. CCHs indicate respective correct and error trial paired neural firing during first 1.0 second after Match presentation (M, time=0.0s above). D. Normalized cross-correlograms of Match phase firing (Fig. 2) between the same pair of (L2/3 and L5) cells for correct (blue) vs. error (red) trials during same session shown in A. D. Tuning plots (as in Fig. 3b) constructed for same pair of cells shown in A on correct (blue) vs. error (red) trials. Tuning bias= 135° for both cells. E. Mean cross-correlation histograms CCHs for the same inter-laminar cell pairs (n=60) shown in B constructed from correct (blue) and error (red) trials. **F(1,401) = 14.18, p < 0.001.
Figure 7.
Pharmacological interruption of DMS-dependent inter-laminar processing. Effects of midsession cocaine administration (0.40 mg/kg IV) on L2/3-L5 cell pair firing during performance of DMS task. A: Rasters and PEHs show Match phase L2/3 and L5 inter-laminar activity as in Figures 2&3 during the initial control (saline, blue) portion of the session and after cocaine administration (cocaine, red) midway through the same session. **F(1,958) > 19.72, p<0.001 vs. Control (saline). B. Average PEHs for control (upper) vs. cocaine trials (lower) summed over all inter-laminar L2/3 (blue) & L5 (red) PFC cell pairs (n=30) recorded in the same sessions with cocaine administered at the midpoint (trial #62) of the session. Black (control) and green (cocaine) histograms show mean frequency distributions of latencies to make the MR relative to Match phase onset (M, 0.0s). *F(1,958) = 13.43, p < 0.001 vs. Control. C. Cross-correlograms (CCHs) of firing between the L2/3 and L5 cell pair shown in A constructed from control trials (n=62) in the first half of the session (blue-left), and after cocaine administration during the second half of the same session (red-right). **F(1,401) = 17.22, p<0.001 vs. Control. D. Mean cross-correlation histograms (CCHs) for the same inter-laminar cell pairs (n=30) shown in B constructed from trials in the control (blue) vs. cocaine (red) halves of the session. **F(1,401) = 11.22, p < 0.001, vs. Control. E. Scatter plot of normalized cross-correlation coefficients from cell pairs shown in D for control (horizontal axis) and cocaine (vertical axis) halves of the same DMS sessions. Distribution of coefficients along the diagonal line would represent no change in correlation coefficients between the two halves of the session, whereas the demonstrated lack of coefficients distributed in that manner reflects a significant change in inter-laminar correlated cell firing after cocaine administration. F. Reduction in DMS (% correct) performance for all animals on trials with varying number of images (Figures 5&6) for control vs. cocaine segments of the same sessions (n=19). **F(1,239)>16.01, p<0.001 Cocaine vs. Control.
Application of MIMO Model to PFC Columnar Processing
In prior studies (Hampson et al., 2012b; Berger et al., 2011) it has been shown that a multi-input/multi-output (MIMO) nonlinear dynamic model applied to spatiotemporal patterns of multiple recordings from synaptically connected neurons is capable of extracting patterns of firing related to successful task performance which can then be used to facilitate and recover performance when administered to the same locations as patterns of electrical pulses(Hampson et al., 2012a; Berger et al., 2011). This type of general Volterra kernel-based nonlinear model has been applied in other formats which have also been shown to be effective in rodents (Marmarelis et al 2012) (Marmarelis et al., 2012). The MIMO version of the model was applied here to the minicolumnar data recorded by the conformal MEA probes in the animals performing the DMS task described here (Figs. 1&2). The MIMO model as applied here modeling strategy of the multi-input/multi-output (MIMO) nonlinear dynamics underlying spike train-to-spike train transformations between L2/3 and L5 which was established to predict L5 output firing patterns from input patterns of L2/3 neural activity as a representation of multicolumnar firing patterns (Berger et al., 2012; Hampson et al., 2012a; Berger et al., 2011; Song et al., 2009; Song et al., 2007; Berger et al., 2005). In this application, the identification of spatio-temporal pattern transformations from PFC layers 2/3 to layer 5 in MEA identified columns was formulated as the estimation of a MIMO model, decomposed into a series of multi-input, single-output (MISO) models with physiologically identifiable structure expressed by the following equations:
The variable x represents input spike trains; y represents output spike trains. The hidden variable w represents the pre-threshold membrane potential of the output neurons, and is equal to the summation of three components, i.e., post-synaptic potential u caused by input spike trains, the output spike-triggered after-potential a, and a Gaussian white noise ε with standard deviation σ. The noise term models both intrinsic noise of the output neuron and the contribution of unobserved inputs. When w exceeds threshold, θ, an output spike is generated and a feedback after-potential (a) is triggered and then added to w. Feedforward kernels k describe the transformation from x to u. The feedback kernel, h, describes the transformation from y to a. u can be expressed as a Volterra functional series of x, as in
The zeroth order kernel, k0, is the value of u when the input is absent. First order kernels, k1(n), describe the linear relation between the nth input xn and u. Second and third order self-kernels, k2s(n), and k3s(n), describe the 2nd and 3rd order nonlinear relation between nth input xn and u, respectively. Second order cross-kernels k2x(n1,n2) reflect the 2nd order nonlinear interactions between each unique pair of inputs (xn1 and xn2) as they affect u. N is the number of inputs. Mk denotes the memory length of the feedforward process. The feedback variable a can be expressed as:
where h is the linear feedback kernel. Mh is the memory length of the feedback process. In total, then, the model describes how temporal patterns of third-order (i.e., the effects of triplets) for each input, and second-order (i.e., the effects of pairs) for any of two interacting inputs, affect each output, taking into account differing noise level and output spike-triggered feedback (the latter due to circuitry and/or membrane biophysics), and neuron-specific differences in thresholds.
Analyses included extraction of first, second and third order temporal firing within at least two defined minicolumns on MEAs inserted repetitively on multiple recording sessions in order to extract relevant patterns of minicolumnar activity related to successful image selection during the Match phase of the task. The model defined inputs as intralaminar firing from neurons in L2/3 and outputs as intralaminar firing of L5 neurons but the manner in which they were recorded determined the multicolumnar nature of the output patterns extracted by the MIMO model. In this manner predictions of L5 output related to successful performance were monitored online during the task to define when successful trials were about to be completed by appropriate target selection as shown in Figure 4. This online monitoring by the MIMO model provided the basis for interposing the correct L5 firing pattern as electrical pulses delivered to the same L5 recording pads of the MEAs in the same temporal interval of occurrence during Match phase onset and completion of target selection (Figs. 2&4). Stimulation pulses consisted of biphasic constant-current square waves, 0.5 ms per phase, adjusted in intensity (10–50 μA) to produce local field potentials monitored on adjacent L5 recoding pads on the same and/or side-by-side implanted MEAs. Model derived stimulation was applied on 30–50% of trials in each session to compare stimulated and non-stimulated trials in terms of correct performance. Effective stimulation patterns that were determined to facilitate performance under normal conditions were also applied at the same time during the trial, irrespective of prior L2/3 activity, when employed for recovery of function when L2/3 to L5 activity (CCHs) were reduced pharmacologically as described below. In this manner it was demonstrated that the MIMO model served as a prostheses for recovering decision making that required appropriate PFC columnar processing related to successful target selection in the DMS task.
Figure 4.
Integration of MIMO model for calculating MR codes from L2/3 recordings and delivering output pulses to L5 recording pads to mimic strong codes during DMS task. Schematic shows prefrontal cortical (PFC L2/3) recording and NHP MIMO model with feedback stimulation applied to PFC L5. Neural recordings from Layer 2/3 are analyzed to predict Layer 5 neural activity, which is in turn used to generate stimulation patterns applied to Layer 5 recording sites. MIMO model coefficients applicable to PFC recordings distinguish different features of the DMS task. This has provided the means to test the specificity of the MIMO codes recorded in L2/3 that occur on different types of trials (with different cognitive load) when applied as stimulation patterns to L5.
Drug Administration and Dose
Animals were trained to perform the task with IV saline injections into the vascular access port or sapphenous vein of the left leg prior to and midway through DMS testing sessions. A drug that has been shown to disrupt cognitive processing by altering dopamine reuptake in PFC (Beveridge et al., 2008; Tomasi et al., 2010; Volkow et al., 2005), cocaine (0.40mg/kg) was administered (without cue) via IV injection midway through the session, replacing saline injections via the same route (Hampson et al., 2011). The dose of the drug was adjusted so that animals continued to perform the task the remainder of the session such that the effects of the drug could be assessed on the columnar recordings in the first half of the session. Control saline injections were also administered in the second half of the session.
RESULTS
The NHPs (n=5) were trained to perform the delayed-match-to-sample (DMS; Fig. 1a) task {Hampson, 2010 7760 /id; Hampson, 2011 7754 /id; Porrino, 2005 5828 /id} by making hand tracking movements to different positions on the screen in front of them to obtain a juice reward for selection of the correct (Sample) image, the location of which varied randomly in 1 of 7 spatial positions on the screen on each trial. Key variables in the task were the number of images (2–7) presented in the Match phase, the duration of the delay (1 to 90 sec), as well as the random placement of the Sample image in the Match phase (after the delay interval) that differed from the position responded to in the Sample phase. In addition to incorporating key cognitive features such as attention, short-term memory, cognitive workload and reward expectancy, subjects were also executing a “decision process” in the Match phase (Fig. 1a) which involved ‘target selection’ a process that was related directly to neuron firing in PFC {Hampson, 2011 7757 /id}.
Conformal Assessment of PFC Neural Processing during Match Phase Target Selection
The relevance of prefrontal cortical (PFC) activity to decision making has been investigated under several conditions in the past (Rao et al., 1999; Goldman-Rakic, 1996; Opris and Bruce, 2005; Pesaran et al., 2008; Resulaj et al., 2009; Heekeren et al., 2008; Opris et al., 2011). Many of the prior reports of neural correlations with executive function and decision making in a sensorimotor hierarchy (Miller and Cohen, 2001; Pesaran et al., 2008; Opris and Bruce, 2005; Sugrue et al., 2005) describe recordings from the area of dorsolateral PFC illustrated in Figure 1b-e, which have been shown to depend on the interaction between neurons in different layers within that same area of cortex (Weiler et al., 2008; Kritzer and Goldman-Rakic, 1995; Opris et al., 2011) In this study inter-laminar connectivity was sensed by conformal-designed MEAs (Fig. 1b-e) positioned to simultaneously record PFC L2/3 and L5 neurons in adjacent “minicolumns” during performance of the DMS task as shown in Figure 1f. A demonstration of this type of columnar processing is shown in Figure 2a&b for two PFC minicolumns recorded simultaneously with the MEA during the Match phase of the task. This relationship was assessed for all recordings from MEAs in this study and the overall analysis was significant (F(14,958) = 2.73, p<0.001). Raster/PEHs for the two simultaneously recorded cell pairs (L2/3 and L5) at the indicated locations on the conformal MEA array positioned strategically in PFC (illustration in middle) showed significant increases in overall mean firing rates at the onset of the Match phase (“M” onset = 0.0s; Pre vs. Post F(1,958) = 12.91, p<0.001) over the time (0.0 to +2.0s) in which arm movements involved in target selection were initiated {Hampson, 2011 7757 /id; Porrino, 2005 5828 /id}. In addition the L2/3 neurons shown in Figure 2a&b (upper raster/PEHs) exhibited significantly higher overall mean firing rates (F(1,958) = 11.17, p < 0.001) than the L5 neurons (lower raster/PEHs) recorded in minicolumns 1&2 on the MEA during the same phase of the task. However, to determine the fact that the firing of cell pairs in each vertical MEA recording array reflected columnar based inter-laminar communication, cross-correlations between vertically oriented cell pairs (minicolumns 1&2, Fig 2a&b) were constructed to determine firing synchrony relative to the time of target presentation and selection in the Match phase. The normalized CCHs in Figure 2 depict differences in correlation of L2/3 and L5 firing of the same cell pairs with respect to 1) the ‘Pre’ Match phase onset (M=0.0) baseline (−2.0 to 0.0s) vs. ‘Post’ Match phase onset (0.0 to +2.0s) after presentation of images on the screen (Fig. 1a). The specificity of the cross-correlations between both L2/3 and L5 cell pairs (minicolumns 1&2) is shown in Figure 2a&b by the overall significant increase in CCHs (Fig. 2a: F(1,401)=12.24, p<0.001; Fig. 2b: F(1,401)=15.14, p<0.001) during performance of the Match response (green, Post-Match) relative to when the animal was waiting for the Delay interval to time out prior to image presentation (black, Pre-Match). This was supported by the fact that CCHs which compared cell pairs within the same L2/3 layer (not shown) or L2/3 cells within the same on one side of the MEA with the L5 cells on the opposite, diagonal-noncolumnar, side (Fig. 2C), did not exhibit a significant correlation (F(1,401)< 3.22, p > 0.10).
Cognitive Specificity of PFC Columnar Processing
The relationship of columnar processing to cognitive performance of the DMS task was assessed by determining correlated firing between PFC L2/3 and L5 cell pairs in the Match phase of the task on correct vs. error trials as shown in Figure 3. Both the L2/3 and L5 cells showed increased firing during the Match phase on correct trials but reduced firing in that same task phase on trials in which the incorrect image was selected (Fig. 3a, correct vs. error trials). Figure 3b shows the mean (± SEM) firing rate change averaged over several PFC L2/3 and L5 cell pairs (n=60) on correct vs. error trials within the Match phase of trials in the same DMS sessions. Even though mean firing rates between 0.0 and +2.0s were significantly higher for L2/3 than L5 cells (F(1,958) = 6.27, p<0.01), rate increases for both L2/3 and L5 cells were significantly lower on error vs. correct trials (Layer 2/3: F(1,958) = 11.12, p < 0.001, Layer 5: F(1,958) = 6.67, p < 0.01). The distribution of Match Response latencies for both types of trial, correct (blue) vs. error (red) trials are shown in the histograms in Figure 3b with the respective mean PEHs, indicating a difference in speed of movement that could reflect an increase related to performance on trials with increases in # images (indicated below in Figures 5D&7F). The significance of columnar firing for correct trials is also indicated in Figure 3c by the decrease in correlated firing shown in the CCHs on error (red) vs. correct (blue) trials for the same cell pair and over the same trials shown in Figure 3a. This same decrease in synchronous firing for mean normalized CCHs constructed for all the cell pairs (n=60) in Figure 3b on error trials, is shown in Figure 3d which further validates the fact that firing between L2/3 and L5 cells was reduced on trials in which the wrong image was selected.
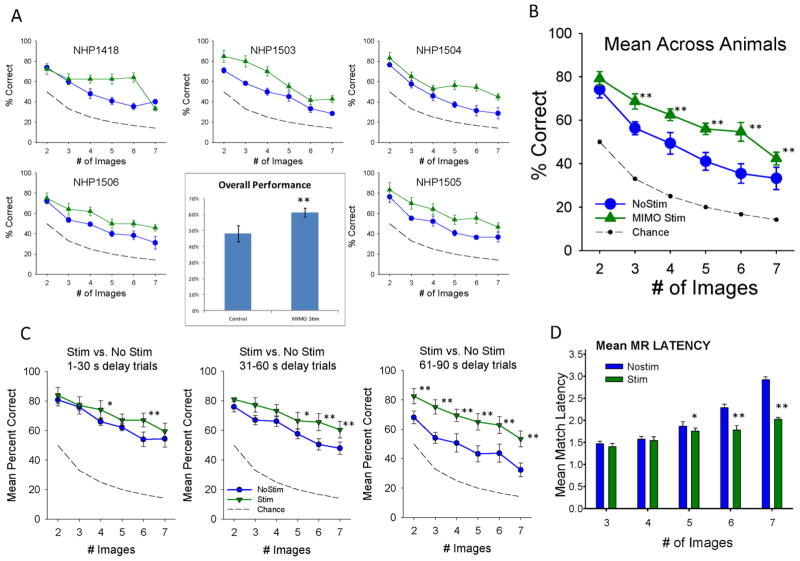
Figure 5.
Facilitation of DMS Performance by MIMO Stimulation A. MIMO stimulation applied to prefrontal cortex in five NHPs (indicated by number) performing the DMS task under normal conditions. MIMO model was adapted to utilize L2/3 input to predict L5 output patterns delivered as electrical stimulation (Fig. 4) during target image selection in the Match phase of the task (Fig. 1a). Each graph shows mean DMS performance (±S.E.M.) as a function of trial complexity indicated by number of images (2–7) over 3–5 sessions comprised of ≥120 trials per session. Performance is shown for normal trials in which stimulation was not delivered (No Stim) and trials in the same session in which MIMO model derived stimulation was delivered to the PFC L5 (MIMO Stim) recording sites on MEAs for 2.0 sec corresponding to limb movements associated with the Match Response. Dashed line indicates performance that could be achieved by random “chance” selection at each degree of Match difficulty related to the number of images to select from. Inset: Average overall performance summed across trials, animals and sessions for No Stim vs. Stim trials. **F(5,239) > 42.16, p<0.001 increase compared to No Stim. B. Mean DMS performance as a function of # Images across all animals shown in A for Stim vs. No Stim trials. **F(1,239)>18.34, p<0.001 increase compared to No Stim. C. Effect of MIMO Stimulation on DMS trials with differing delays as a function of number of images. Same results shown in B sorted by duration of trial delay prior to Match phase onset (Figure 1a) for No Stim vs. Stim trials. *F(1,239)>8.22, p<0.01; **F(1,239)>13.40, p<0.001 increase compared to No Stim. D. Effect of MIMO stimulation on mean Match Response (MR) latency to respond to the Match image on No Stim vs. Stim trials. Mean (±S.E.M.) latencies (in sec) across animals and sessions as a function of number of images presented in the Match phase. *F(1,239)=9.51, p<0.01; **F(1,239)>21.29, p<0.001 vs. No Stim.
Application of MIMO Model Facilitates Columnar Processing in PFC
Prior investigations applying MIMO model derived stimulation patterns to hippocampus in the rodent provided the means to enhance performance and overcome deficits induced by pharmacological treatments (Berger et al., 2011). The same MIMO model was applied in this study to multicolumnar PFC L2/3 and L5 cell pairs recorded during the Match phase of the DMS task. Spatiotemporal patterns associated with trials of correct performance were constructed from multiple (n=2–4) simultaneously recorded confirmed MEA cell pairs in all NHPs (n=5) tested. Derived L5 output firing associated with L2/3 input firing to the MIMO model, as shown in Figure 4, was used to a) predict performance on individual trials and b) interject electrical stimulation patterns in L5 onto the same MEA pads from which predicted firing was extracted by the MIMO model. The effects of interposed electrical stimulation patterns of several types including MIMO Stim (Fig. 5) and Control Stim (Fig. 6) averaged over all NHPs and trials were significant (F(59,239) = 20.94, p<0.001, overall ANOVA). There was a significant effect of # images (F(5,239)=181.04, p<0.001) and stimulation (F(9,239)=31.49, p<0.001) as well as the interaction (stim x images, F(45, 239)=1.65, p<0.01) shown in Figures 5&6. It is clear that MIMO stimulation facilitated performance above normal levels but was not consistent for the same types of trials in all animals (Fig. 5a, individual NHPs); however, Figure 5b shows the overall mean effect of MIMO stimulation was to facilitate performance across trials with increased # of images (F(5,239) = 42.16, p<0.001). Figure 5c shows the facilitatory effect of the MIMO derived stimulation on trials with different delays and number of images where performance on trials with longer delays (30–60s, 61–90s) was less impaired on stimulated vs. nonstimulated trials (F(2,239) = 13.49 p<0.001). Unlike the graded facilitation seen across trials as a function of the number of images (Fig. 5a), MIMO stimulation was equally effective (F(2,239) = 3.71, p>0.05) irrespective of the duration of delay (30–60s, 61–90s) prior to the onset of the Match phase in the task (Fig. 5b). Finally, the facilitatory effects of interposed MIMO stimulation on target selection and execution were strongly supported by a significant decrease (F(4,401) = 9.14, p<0.01) in the latency to make the Match Response (Fig. 5d) as a function of trial difficulty (# images) on MIMO stimulation vs. nonstimulation trials, which is consistent with Figure 3b for differences in time of response execution on correct vs. error trials.
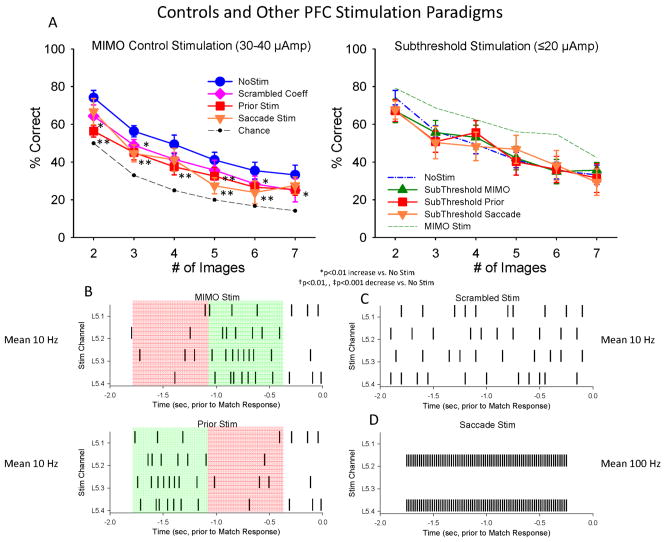
Figure 6.
Lack of DMS facilitation by Control stimulation paradigms which were not synchronized to MIMO-extracted PFC neuron firing. Mean (±S.E.M.) DMS performance summed across all animals (n=5) on trials with stimulation parameters and patterns (shown in B&C) that differed or were administered differently from those generated by the MIMO model (Figure 5). A. Left: Mean DMS performance on trials with three different types of non-MIMO Stim patterns (in B) delivered at the same intensity and pulse duration as MIMO Stim (30–40 μA, 1 ms pulses) was decreased compared to control (NoStim) performance. *F(1,239)>7.31, p<0.01; **F(1,239)>12.56, p<0.001 decrease vs. No Stim. Right: DMS performance across animals on trials receiving the same non-MIMO stimulation patterns shown at left with reduced current levels (≤ 20 μA). Dotted green curve shows performance on trials with MIMO Stim (Fig. 5b). B. PRIOR Stim pattern: The pattern consisted of the same stimulation channels and interstimulus intervals as the MIMO-derived Stim pattern (MIMO Stim, top), however the early and late epochs of stimulation were inverted temporally such that stimulation that normally occurred synchronous with the Match phase response was now delivered prior to the Match response (Prior Stim, bottom). The illustration shows stimulation on a single trial in the Match phase, contrasting MIMO Stim patterns at the top in green starting 1.0s after Match phase onset (−2.0s), with the control MIMO Stim pattern starting at 0.25s after Match phase onset (bottom, in green) and terminating 1.0 sec Prior to the Match Phase Response (0.0s). C. Scrambled Stim with randomized MIMO coefficients and the same overall frequency and number of stimulation pulses as MIMO Stim delivered in same Match phase time interval; D. Saccade Stim refers to stimulation associated with saccade generation with fixed frequency (100 Hz) delivered at the same intensity in the same Match phase interval as MIMO Stim (Fig. 5).
Effects of Control Stimulation Parameters Show Specificity of MIMO Facilitation
Because the effects of MIMO stimulation were so effective in facilitating performance (Fig. 5) several control measures were implemented to eliminate the possibility that mere addition of electrical pulses may have been the major factor for improved performance. One of the most obvious controls for the specificity of MIMO stimulation was to inject the same spatiotemporal stimulation pattern at a different time during the task to make sure that the derived multi-columnar L5 stimulation pattern was specific to when the decision to make the Match Response occurred on the trial. When the same MIMO pattern of stimulation at the same intensity was delivered in the 1.0 sec period prior to Match phase onset, performance was actually significantly reduced (F(5,239) =27.14, p<0.001) relative to non-stimulation levels as shown in Figures 6a&b. Another closely related control stimulation procedure involved delivery of stimulation pulses to L5 in the same temporal relation to the MR but in a pattern with randomly scrambled MIMO coefficients (Fig. 6c) derived from online L2/3 activity (Input Pattern-Layer 2, Fig. 4), which again, did not facilitate performance and actually decreased accuracy (F(5,239) = 13.95, p< 0.001) across all trials (Figures 6a scrambled). Another test for the specificity of MIMO stimulation was implemented using stimulation parameters previously shown to be effective for evoking saccades and improved performance in other cognitive tasks in NHPs (Opris et al., 2005a; Opris et al., 2005b). These saccade-type stimulation parameters (Fig. 6d) delivered at the same intensity and in the same temporal interval as MIMO stimulation during the trial, did not facilitate performance and produced slight decreases in accuracy (F(5,239) =19.13, p<0.001) similar to other control stimulation parameters shown in Figure 6a. As a final assessment of the relevance of stimulation intensity to these control stimulation patterns, pulses were reduced by 50% (<20 μA) and delivered in the same control stimulation patterns. Figure 6a (Subthreshold Stimulation) shows that not only did reducing stimulation intensity a) not produce facilitation of performance but also b) eliminated the reduction in performance produced at the higher simulation intensities (F(5,239) < 0.52, p > 0.10); which clearly indicates that the control stimulation parameters had an effect on neural tissue, but not the same facilitatory effect as columnar based, MIMO-derived stimulation.
Pharmacologic Impairment of PFC columnar processing and DMS Performance
Extensive prior investigation of features that affect cognitive processing in DMS tasks have shown that Match phase activation of PFC is altered by modulation of dopamine influences on task-related PFC cell firing by cocaine and other agents that alter dopamine uptake (Hampson et al., 2011; Porrino et al., 2005; Robbins and Arnsten, 2009). To determine similar actions on PFC columnar activity in this task firing was assessed in the same minicolumns before and after systemic injection of cocaine (0.40 mg/kg) midway through the DMS session. Figure 7a shows raster/PEHs for a PFC inter-laminar cell pair (L2/3 upper, L5 lower) recorded: 1) in the first 60 trials of a DMS session (Control) followed by, 2) the un-signaled administration (IV) of cocaine on trial 61 in the second half of the session for 60 more trials. It is clear that a significant reduction in Match phase firing occurred in L2/3 (F(1,958) = 24.17, p < 0.001) and L5 (F(1,958) =19.72 p<0.001) in the cocaine vs. control half of sessions (Fig. 7a), and that these changes in firing resembled closely the reductions shown for error trials in Figure 3a&b. The generality of this effect on Match phase mean firing rate over a large population of cell pairs (n=30) is shown in Figure 7b as a significant decrease in L2/3 cell activity (F1,958)=13.43, p <0.001) relative to the saline half of the session. The reduction in L5 average firing rates in the cocaine half of the session approached but did not reach significance (F(1,958) = 1.48, p > 0.10) which perhaps reflects decreased dopamine sensitive processes (Gulledge and Jaffe, 1998) relative to L2/3 cells as has been indicated in prior studies of dopamine receptor actions in these PFC layers in NHPs (Bordelon-Glausier et al., 2008; Robbins and Arnsten, 2009). However, the more specific columnar firing indicator, CCHs for the single cell pair in Figure 7a and for all cell pairs in Figure 7b (n=30) shown respectively in Figures 7c&d, indicate a clear decrease in correlated firing (F(1,401) > 11.22, p< 0.001) after cocaine administration. The significance of this change with respect to cocaine’s effect on columnar processing is shown in Figure 7e as a scatter plot of correlation coefficients for the initial (control) and second (cocaine) half of cocaine administered sessions. Clearly, the lack of points on the diagonal line in the scatter plot indicate an alteration in synchronized firing in cell pairs that showed high correlation in firing during the first half of the session which was reduced to low levels after cocaine administration in the second half of the session (Fig. 7e). Finally, it is an important coincidence that the alterations in columnar processing produced by cocaine also influenced DMS performance in a manner consistent with the cognitive demand of the task. Figure 7f shows reduced mean performance accuracy as a function of the number of images presented in the Match phase on trials presented during the cocaine half of the session (F(5,239) = 29.71, p<0.001) which confirms the graded effect as a function of trial difficulty and is also consistent with the lack of short latency MRs shown in Figure 6b. These data are supported by other findings showing impairment in cognitive function by agents which altered activity in PFC (Arnsten, 2000; Robbins and Arnsten, 2009; Wang et al., 2011) and agree with recent findings showing involvement of neural activity in PFC as a function of difficulty of the trial (Hampson et al., 2010).
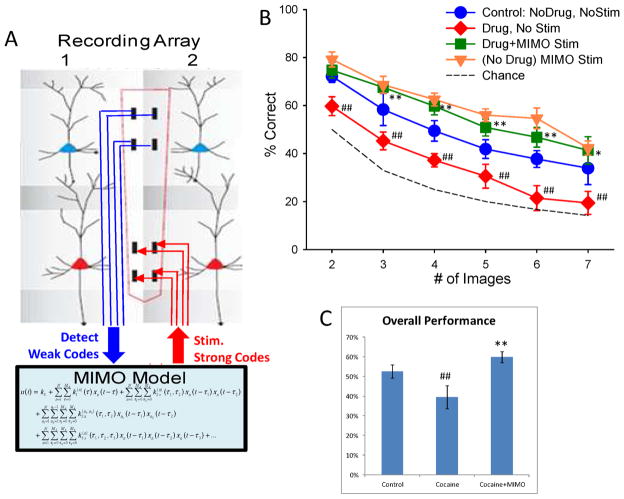
MIMO Stimulation-Induced Recovery of Impaired DMS Task Performance
Figures 5&6 show that interposed MIMO L5 stimulation was exceedingly effective in facilitating DMS-task performance under normal conditions. This provided the basis for testing the effects of the MIMO model as a neuroprosthesis by interpolating stimulation of L5 on trials disrupted by cocaine injections during the session. It is clear from Figure 7e that cocaine’s overall effect was to alter PFC columnar firing in the same minicolumns that processed information effectively in the first half of the same session (F(23,198) = 18.65, p < 0.001). Application of the MIMO model under these conditions (Fig. 8a) provided the means to detect non-effective trial specific firing in L2/3 cells which signaled the basis for concomitant delivery of L5 stimulation patterns associated with columnar firing patterns on successful trials shown to be facilitatory under normal conditions (Fig. 5). This online application, in which MIMO model controlled stimulation was delivered on trials during cocaine disrupted performance in the second half of the same session, was effective in reversing the detrimental effects of the drug (cocaine vs. cocaine+MIMO Stim; F(5,198)=15.05, p<0.001) across trials with 3–7 images (Fig 8b). MIMO stimulation not only re-established control responding but also increased performance to the level achieved on stimulated trials (control vs. drug+MIMO Stim, F(5,198) =8.18, p<0.001) under saline-control conditions (Figure 8b). Thus interpolated MIMO stimulation was more potent under conditions of cocaine-reduced columnar processing since it elevated mean performance from a markedly reduced level (relative to control performance) to a higher status that was slightly above control conditions (F(5,198) =1.86, p > 0.10, Fig. 8b); similar to the higher level achieved when the same stimulation was administered during baseline performance (Figure 5). These results provide the first evidence that a neural prostheses in the MIMO model format can effectively reverse externally induced impairments in cortical function that directly influence cognitive processing in primate brain. The findings clearly demonstrate that application of the MIMO model reversed the disruptive effects of cocaine on DMS performance (F(5,198) = 15.05, p<0.001) shown in Figure 8c. This performance change after cocaine administration was shown to result directly from reversal of reduced PFC inter-laminar processing in the same minicolumns to which successful MIMO L5 stimulation patterns were applied in the same session (Fig. 8a). Such inherent determinants as well as the demonstrated specificity of the MIMO stimulation patterns in altering DMS performance (Figs. 5&6) provide evidence that the MIMO model effectively mimicked columnar information processing in PFC necessary to perform the DMS task.
Figure 8.
MIMO-based Neural Prosthetic Recovery of PFC Dependent DMS Performance. A. Application of MIMO model detects more “weak code” L2/3 firing associated with error trials in DMS performance following cocaine exposure (Fig. 7). Output of MIMO model is then utilized to stimulate L5 with a “strong code” L5 pattern associated with correct (Control) performance at the time of target selection in the Match phase. B. DMS performance resulting from MIMO stimulation applied to prefrontal cortex in five nonhuman primates receiving split sessions in which each animal received saline injection prior to start of the behavioral sessions, and the received cocaine (0.4 mg/kg IV) at the midpoint of the behavioral session. DMS mean (±S.E.M.) performance during 1) control (no drug, no stim) half of the session, compared to 2) nonstimulated trials (Drug, No Stim) in the cocaine half of the session and 3) MIMO stimulated (Drug+MIMO Stim) trials in the cocaine half of the same session. Performance on MIMO Stim trials in the absence of drug (Fig. 5) is also shown for comparison (No drug MIMO Stim). ##F(1,239)>16.82, p<0.001 decrease vs. Control. *F(1,239)=7.22, p<0.01; **F(1,239)>10.63, p<0.001 increase vs. Control. C. Overall performance (mean ± S.E.M.) shown for all animals on trials in 1) non-drug half of session (Control), 2) Cocaine half of session on trials with no stimulation (Cocaine) and 3) Cocaine half of session on trials with MIMO stimulation (Cocaine+MIMO). ##F(5,239)=42.53, p<0.001; performance decrease vs. Control; **F(5,239)>15.05, p<0.001 performance increase vs. Control.
DISCUSSION
Cognitive Dependence on Columnar Processing in Prefrontal Cortex
The findings in this study (Figs. 2,3&5) are consistent with prior studies showing that neurons in the supra- and infra-granular layers of PFC form efficient mini-columnar circuits during the selection/decision process in different experimental contexts (Weiler et al., 2008; Takeuchi et al., 2011). This decision based columnar firing was demonstrated here during the Match phase as required for effective performance of this DMS task. The novel conformal ceramic recording probe (Fig. 1e&f) employed in this study allowed assessment of inter-laminar correlated firing that was validated across sessions and animals in which multiple recordings of L2/3 and L5 cell pairs showed similar relations with respect to task-related as well as correlated firing in the Match phase (Figs. 2,3&7). A key variable in the DMS decision/selection process was activation of multiple L5 neurons via minicolumnar related synchronous input from MEA associated L2/3 neurons (represented as increased correlation in CCHs in Figs. 2,3,7), the latter of which have been shown to participate in the integration of sensory information via “long-range” inputs from the dorsal visual stream in parietal/visual cortex (Resulaj et al., 2009; Pesaran et al., 2008; Opris and Bruce, 2005). The functional significance of this measure of processing reflected by increased L2/3 -to- L5 cross-correlation was validated directly via application of the nonlinear MIMO model to derive multicolumnar firing to define specific discharge patterns in L2/3 related to L5 firing under conditions of successful performance. Since this relationship was a direct spatiotemporal reflection of the increased inter-laminar firing shown by the same identified cell pairs, it provides independent validation that the extracted activity associated with correct decision making and target selection characterized by the MIMO model was in fact columnar specific. Further demonstration of the accuracy of this characterization of firing by the MIMO model was empirically demonstrated by the interposed stimulation of L5 recording areas with streams of pulses that mimicked the MIMO extracted L5 spatiotemporal firing patterns that 1) facilitated performance under normal testing conditions on stimulated vs. nonstimulated trials (Figs. 4&5) and 2) also recovered performance decreased by drug-reduced columnar firing following cocaine administration in the same session (Figs. 7&8).
Successful Performance Related to PFC Columnar Processing
Figure 3 shows that on correct vs. error trials processing of columnar information was related to significant increases in the cross-correlation of firing between L2/3 and L5 PFC cell pairs during image presentation on the screen in the Match phase of the DMS task. This close correspondence between columnar firing and cognitive processing was confirmed by administering cocaine midway through the test session which simultaneously decreased correlated inter-laminar firing in identified cell pairs (Fig. 7a–f) as well as reduced task performance (Fig. 7g) over the same time frame of the session. The scatter plot in Fig 7e shows that the reduced inter-laminar firing produced by cocaine was most extreme for cell pairs that exhibited a high degree of correlation in the normal (saline control) half of the session, while cell pairs with low initial normalized correlation coefficients (< 0.04), were relatively unaffected by cocaine administration. This supports the view that graded levels of cognitive output may be related to the degree or intensity of columnar activity on a given trial (van Veluw et al., 2012; Buxhoeveden et al., 2006). These findings are in complete agreement with prior studies showing marked influences of acute administered cocaine in altering task-related neural firing (Hampson et al., 2011; Opris et al., 2009; Stuber et al., 2005) and support the possibility that cocaine-influenced dopaminergic modulation of PFC neurons was responsible for regulating columnar activity in a manner that varies with decision-making in humans exposed to the same drug (Tomasi et al., 2010; Volkow et al., 2005).
Facilitation of Cognitive Performance via MIMO Model Columnar Stimulation
The effectiveness of MIMO derived multi-columnar stimulation of L5 neurons in this task provides important insight into the microcircuit nature of PFC activity related to decision making and target selection during the DMS task (Figs. 1a&2). The MIMO model derived columnar stimulation pattern (Fig. 4) was shown to be critical for facilitation of task performance (Figs. 5&8) because of the ineffectiveness of other forms of stimulation delivered to the same loci at the same or reduced intensities but in different patterns and frequencies during the execution of the Match Response (Fig. 6). Even more specificity was provided by showing that the same effective MIMO derived stimulation patterns, but delivered prior to the time at which movement initiation and target selection occurred within the Match phase of the task (Fig. 6b, ‘prior stimulation’). The specific nature of facilitatory MIMO stimulation patterns was also exhibited by the reduced latency to select the correct image, but, only on the more difficult trials in which latencies were increased more under nonstimulation conditions (Figure 5).
The neural prostheses presented here differs from prior studies to develop prostheses and brain-machine interfaces in humans, since neural interfaces have typically consisted of sensory input and/or motor output systems (Jackson and Fetz, 2011; Laczko, 2011; Lebedev and Nicolelis, 2011), each of which is “anchored” to a physical event of some type (i.e. receptor activation or limb movement). Basic sensory inputs, such as cochlear (Shannon, 2012) or retinal implants (Weiland et al., 2011) have employed neural stimulation as utilized here, but only as a replacement of sensory inputs, not for cognitive processing of that input. On the other hand, motor cortical prostheses are primarily output devices which typically utilize neural signals in motor and premotor areas for control of movement of a robotic limb (Judy, 2012; van Hemmen and Schwartz, 2008; Hochberg et al., 2012). More recently, attempts to integrate “haptic” feedback into limb prosthetics has resulted in devices which stimulate somatosensory and thalamic neurons to provide tactile and proprioceptive feedback information to the brain to “close the loop” on motor processing (Weber et al., 2011; Hatsopoulos and Suminski, 2011; O’Doherty et al., 2012). In contrast to these above approaches, the nonlinear MIMO neuroprostheses described here bypasses the cortico-spinal and thalamo-cortical systems for limb control by directly influencing motor control decisions via columnar stimulation in prefrontal cortex (Miller and Cohen, 2001; Graybiel, 2008; Laczko, 2011). In addition, the sensory information processed is neither primary nor proprioceptive since the input assessed by the MIMO model was from interlaminar layer 2 which receives pre-processed information from other brain regions (Weiler et al., 2008; Mountcastle, 1997). The results therefore demonstrate that prefrontal minicolumns represent and control specific cognitive decisions within the DMS task, and that MIMO-derived stimulation, delivered during the Match phase, selectively enhanced correct decisions at a higher cognitive level than strict motor control.
MIMO Model Induced Recovery from Cocaine Altered Cognitive Processing
Prior applications of MIMO models to disrupted neural processing in rodent hippocampus established the functional basis for employing this approach in the design and implementation of the cortical neuroprostheses demonstrated here (Berger et al., 2012; Hampson et al., 2012a; Hampson et al., 2012b; Berger et al., 2011). What is presented here is the first application of the MIMO model to primate brain via a conformal electrode MEA capable of extracting spatiotemporal neural firing patterns related to known underlying columnar microcircuitry in PFC, which not only extends application of the MIMO model to other brain areas but also to performance of human-like cognitive tasks. The recovery from cocaine-induced disruption shown in Figure 8 utilized the MIMO model to 1) extract, characterize and predict spatiotemporal patterns critical for effective performance, and 2) interpose those same patterns into layer 5 using multichannel electrical stimulation. This constitutes the direct application of a device that mimics local circuit (multi-columnar activation) operation to restore cognitive processing shown to depend on the same columnar-based firing under normal conditions (Fig 3). Also, in contrast to prior successful applications of MIMO model stimulation (Hampson et al., 2012a; Berger et al., 2011), the extent and range of effectiveness in improving and recovering performance (Figs. 5&7) was much greater in this task. However, what is of major importance in regard to this neuroprosthetics demonstration is the fact that effective L5 stimulation parameters had to mimic those derived by the MIMO model reflecting L2/3-L5 multi-columnar spatiotemporal firing sampled on the MEAs under normal circumstances. This was confirmed by stimulating the same MEA sites with different patterns or frequencies of pulses at the same intensities (Fig. 6), which were not only ineffective, but in most cases disrupted normal task performance. Therefore the stimulation patterns extracted by the MIMO model were not only specific, because they reflected firing on successful trials, they also increased performance above control levels, because the latter included more trials on which ineffective processing, (i.e. errors) occurred during the session (Figs. 3, 6&8).
Conclusions
These unique results show that columnar interactions between prefrontal neurons that encode and process information relevant to executive function and decision making (Goldman-Rakic, 1996; Opris and Bruce, 2005; Heekeren et al., 2008; Opris et al., 2005b) are necessary for successful performance of this DMS task (Figs. 2&3), and are capable of being simulated and interposed to facilitate and recover performance via application of a MIMO model-based neuroprosthesis, as demonstrated here. Since the possible neural basis for effective performance in this task relates to significant increased transmission within PFC minicolumns to select relevant cues during target presentation (Fig. 3), interposing a MIMO model to control this type of processing provides a means of reducing random fluctuations in performance under normal conditions. It was also possible to re-establish appropriate task-dependent processing under circumstances in which performance was impaired by factors that modulate the degree of columnar firing such as cocaine administration (Figs. 7&8). In addition to providing potential insight into other types of cognitive impairments involving decision making and executive function in human brain as a result of disease or injuries (Brennan and Arnsten, 2008; Dobbs, 2010; Duncan et al., 1997; Shallice and Burgess, 1991; Wang et al., 2011; Casanova et al., 2010) these results provide confirmation that a MIMO-based functional device, if properly integrated with normal brain operation, as provided by the conformal MEAs used here, can recover and even improve performance in complex tasks via simulation of columnar processing as a means of overcoming impaired cognitive function in primate brain.
Acknowledgments
We thank Joshua Long, Joseph Noto, Brian Parish, Joshua Fuqua, Mack Miller, and Shahina Kozhisseri for their assistance on this project. This work was supported by National Institutes of Health Grants DA06634, DA023573, DA026487 and by Defense Advanced Research Projects Agency (DARPA) contract N66601-09-C-2080 to S.A.D.
Footnotes
The authors declare no competing financial interests.
References
- Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res. 2000;126:183–92. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Fractionating the central executive. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 246–260. [Google Scholar]
- Berger TW, Ahuja A, Courellis SH, Deadwyler SA, Erinjippurath G, Gerhardt GA, Gholmieh G, Granacki JJ, Hampson R, Hsaio MC, LaCoss J, Marmarelis VZ, Nasiatka P, Srinivasan V, Song D, Tanguay AR, Wills J. Restoring lost cognitive function. IEEE Eng Med Biol Mag. 2005;24:30–44. doi: 10.1109/memb.2005.1511498. [DOI] [PubMed] [Google Scholar]
- Berger TW, Hampson RE, Song D, Goonawardena A, Marmarelis VZ, Deadwyler SA. A cortical neural prosthesis for restoring and enhancing memory. Journal of Neural Engineering. 2011;8:046017. doi: 10.1088/1741-2560/8/4/046017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger TW, Song D, Chan RHM, Marmarelis VZ, LaCoss J, Wills J, Hampson RE, Deadwyler SA, Granacki JJ. A hippocampal cognitive prosthesis: Multi-input, multi-output nonllinear modeling and VLSI instrumentation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2012;20:198–211. doi: 10.1109/TNSRE.2012.2189133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363:3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordelon-Glausier JR, Khan ZU, Muly EC. Quantification of D1 and D5 dopamine receptor Localization in layers I, III, and V of macaca mulatta prefrontal cortical area 9: Coexpression in dendritic spines and axon terminals. J Comp Neurol. 2008;508:893–905. doi: 10.1002/cne.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxhoeveden DP, Casanova MF. The minicolumn hypothesis in neuroscience. Brain. 2002;125:935–951. doi: 10.1093/brain/awf110. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Semendeferi K, Buckwalter J, Schenker N, Switzer R, Courchesne E. Reduced minicolumns in the frontal cortex of patients with autism. Neuropathol Appl Neurobiol. 2006;32:483–491. doi: 10.1111/j.1365-2990.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Vanbogaert E, Narahari P, Switala A. A topographic study of minicolumnar core width by lamina comparison between autistic subjects and controls: possible minicolumnar disruption due to an anatomical element in-common to multiple laminae. Brain Pathol. 2010;20:451–458. doi: 10.1111/j.1750-3639.2009.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Kreczmanski P, Trippe J, Switala A, Heinsen H, Steinbusch HW, Schmitz C. Neuronal distribution in the neocortex of schizophrenic patients. Psychiatry Res. 2008;158:267–277. doi: 10.1016/j.psychres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs D. Schizophrenia: The making of a troubled mind. Nature. 2010;468:154–156. doi: 10.1038/468154a. [DOI] [PubMed] [Google Scholar]
- Duncan J, Johnson R, Swales M, Freer C. Frontal lobe deficits after head injury: Unity and diversity of function. Cognitive Neuropsychology. 1997;14:713–741. [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Parris BA. From affective value to decision-making in the prefrontal cortex. Eur J Neurosci. 2008;28:1930–1939. doi: 10.1111/j.1460-9568.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 1998;18:9139–9151. doi: 10.1523/JNEUROSCI.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Coates TD, Jr, Gerhardt GA, Deadwyler SA. Ceramic-based microelectrode neuronal recordings in the rat and monkey. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and BIology Society (EMBS) 2004a;25:3700–3703. [Google Scholar]
- Hampson RE, Opris I, Deadwyler SA. Neural correlates of fast pupil dilation in nonhuman primates: relation to behavioral performance and cognitive workload. Behav Brain Res. 2010;212:1–11. doi: 10.1016/j.bbr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Pons TP, Stanford TR, Deadwyler SA. Categorization in the monkey hippocampus: A possible mechanism for encoding information into memory. Proc Natl Acad Sci U S A. 2004b;101:3184–3189. doi: 10.1073/pnas.0400162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Porrino LJ, Opris I, Stanford T, Deadwyler SA. Effects of cocaine rewards on neural representations of cognitive demand in nonhuman primates. Psychopharmacology (Berl) 2011;213:105–118. doi: 10.1007/s00213-010-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Song D, Chan RHM, Sweatt AJ, Fuqua J, Gerhardt GA, Shin D, Marmarelis VZ, Berger TW, Deadwyler SA. A nonlinear model for hippocampal cognitive prostheses: Memory facilitation by hippocampal ensemble stimulation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2012a;20:184–197. doi: 10.1109/TNSRE.2012.2189163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Song D, Chan RHM, Sweatt AJ, Riley MR, Goonawardena AV, Marmarelis VZ, Gerhardt GA, Berger TW, Deadwyler SA. Closing the loop for memory prosthesis: Detecting the role of hippocampal neural ensembles using nonlinear models. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2012b doi: 10.1109/TNSRE.2012.2190942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BJ, Dragoi V. Adaptation-induced synchronization in laminar cortical circuits. Proc Natl Acad Sci U S A. 2011;108:10720–10725. doi: 10.1073/pnas.1102017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. A model of prefrontal cortical mechanisms for goal-directed behavior. J Cogn Neurosci. 2005;17:1115–1129. doi: 10.1162/0898929054475190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsopoulos NG, Suminski AJ. Sensing with the motor cortex. Neuron. 2011;72:477–487. doi: 10.1016/j.neuron.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nat Rev Neurosci. 2008;9:467–479. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Fetz EE. Interfacing with the computational brain. IEEE Trans Neural Syst Rehabil Eng. 2011;19:534–541. doi: 10.1109/TNSRE.2011.2158586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judy JW. Neural interfaces for upper-limb prosthesis control: opportunities to improve long-term reliability. IEEE Pulse. 2012;3:57–60. doi: 10.1109/MPUL.2011.2181026. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol. 1995;359:131–143. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Sigala N, Nili H, Gaffan D, Duncan J. Target detection by opponent coding in monkey prefrontal cortex. J Cogn Neurosci. 2010;22:751–760. doi: 10.1162/jocn.2009.21216. [DOI] [PubMed] [Google Scholar]
- Laczko J. Modeling of human movements, neuroprostheses. Ideggyogy Sz. 2011;64:229–233. [PubMed] [Google Scholar]
- Lebedev MA, Nicolelis MA. Toward a whole-body neuroprosthetic. Prog Brain Res. 2011;194:47–60. doi: 10.1016/B978-0-444-53815-4.00018-2. [DOI] [PubMed] [Google Scholar]
- Marmarelis VZ, Shin DC, Hampson RE, Deadwyler SA, Song D, Berger TW. Design of optimal stimulation pattern for neuronal ensembles based on volterra-type hierarchical modeling. Trans Neural Sci Rehabil Eng. 2012 doi: 10.1088/1741-2560/9/6/066003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyaki A, Friedman N, Emerson M, Witzki A, Howerter A, Wagner T. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: A latent variable analysis. Cog Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mo J, Schroeder CE, Ding M. Attentional modulation of alpha oscillations in macaque inferotemporal cortex. J Neurosci. 2011;31:878–882. doi: 10.1523/JNEUROSCI.5295-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120 ( Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Moxon KA, Leiser SC, Gerhardt GA, Barbee KA, Chapin JK. Ceramic-based multisite electrode arrays for chronic single-neuron recording. IEEE Trans Biomed Eng. 2004;51:647–656. doi: 10.1109/TBME.2003.821037. [DOI] [PubMed] [Google Scholar]
- O’Doherty JE, Lebedev MA, Li Z, Nicolelis MA. Virtual active touch using randomly patterned intracortical microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2012;20:85–93. doi: 10.1109/TNSRE.2011.2166807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Barborica A, Ferrera VP. Effects of electrical microstimulation in monkey frontal eye field on saccades to remembered targets. Vision Res. 2005a;45:3414–3429. doi: 10.1016/j.visres.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Opris I, Barborica A, Ferrera VP. Microstimulation of the dorsolateral prefrontal cortex biases saccade target selection. J Cogn Neurosci. 2005b;17:893–904. doi: 10.1162/0898929054021120. [DOI] [PubMed] [Google Scholar]
- Opris I, Bruce CJ. Neural circuitry of judgment and decision mechanisms. Brain Res Brain Res Rev. 2005;48:509–526. doi: 10.1016/j.brainresrev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Opris I, Hampson RE, Deadwyler SA. The encoding of cocaine vs. natural rewards in the striatum of nonhuman primates: Categories with different activations. Neuroscience. 2009;163:195–204. doi: 10.1016/j.neuroscience.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Hampson RE, Stanford TR, Gerhardt GA, Deadwyler SA. Neural activity in frontal cortical cell layers: evidence for columnar sensorimotor processing. J Cogn Neurosci. 2011;23:1507–1521. doi: 10.1162/jocn.2010.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453:406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M, Snyder C. Attention and cognitive control. In: Solso R, editor. Information Processing and Cognition: The Loyola Symposium. Hillsdale, NJ: L. Erlbaum Assoc; 1975. [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature. 2009;461:263–266. doi: 10.1038/nature08275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philos Trans R Soc Lond B Biol Sci. 1996;351:1405–1411. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114 ( Pt 2):727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Advances in auditory prostheses. Curr Opin Neurol. 2012;25:61–66. doi: 10.1097/WCO.0b013e32834ef878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Chan RH, Marmarelis VZ, Hampson RE, Deadwyler SA, Berger TW. Nonlinear dynamic modeling of spike train transformations for hippocampal-cortical prostheses. IEEE Trans Biomed Eng. 2007;54:1053–1066. doi: 10.1109/TBME.2007.891948. [DOI] [PubMed] [Google Scholar]
- Song D, Chan RH, Marmarelis VZ, Hampson RE, Deadwyler SA, Berger TW. Nonlinear modeling of neural population dynamics for hippocampal prostheses. Neural Netw. 2009;22:1340–1351. doi: 10.1016/j.neunet.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: neural currencies for valuation and decision making. Nat Rev Neurosci. 2005;6:363–375. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- Takeuchi D, Hirabayashi T, Tamura K, Miyashita Y. Reversal of interlaminar signal between sensory and memory processing in monkey temporal cortex. Science. 2011;331:1443–1447. doi: 10.1126/science.1199967. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemmen JL, Schwartz AB. Population vector code: a geometric universal as actuator. Biol Cybern. 2008;98:509–518. doi: 10.1007/s00422-008-0215-3. [DOI] [PubMed] [Google Scholar]
- van Veluw SJ, Sawyer EK, Clover L, Cousijn H, De JC, Esiri MM, Chance SA. Prefrontal cortex cytoarchitecture in normal aging and Alzheimer’s disease: a relationship with IQ. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0381-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DJ, London BM, Hokanson JA, Ayers CA, Gaunt RA, Torres RR, Zaaimi B, Miller LE. Limb-state information encoded by peripheral and central somatosensory neurons: implications for an afferent interface. IEEE Trans Neural Syst Rehabil Eng. 2011;19:501–513. doi: 10.1109/TNSRE.2011.2163145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland JD, Cho AK, Humayun MS. Retinal prostheses: current clinical results and future needs. Ophthalmology. 2011;118:2227–2237. doi: 10.1016/j.ophtha.2011.08.042. [DOI] [PubMed] [Google Scholar]
- Weiler N, Wood L, Yu J, Solla SA, Shepherd GM. Top-down laminar organization of the excitatory network in motor cortex. Nat Neurosci. 2008;11:360–366. doi: 10.1038/nn2049. [DOI] [PMC free article] [PubMed] [Google Scholar]