Abstract
The need for effective targeted therapies for renal cell carcinomas (RCC) has fueled the interest for understanding molecular pathways involved in the oncogenesis of kidney tumors. Aiming to analyze the expression status and prognostic significance of mTOR and hypoxia-induced pathway members in patients with clear cell RCC (ccRCC) tissue microarrays were constructed from 135 primary and 41 metastatic ccRCC. Immunoexpression levels were compared and correlated with clinicopathologic parameters and outcome. PTEN levels were significantly lower in primary and metastatic ccRCC compared to benign tissues (P<0.001). Levels of phos-AKT, phos-S6, and 4EBP1 were higher in metastatic ccRCC (P≤0.001). For phos-S6 and 4EBP1, levels were higher in primary ccRCC compared to benign tissues (P<0.001). c-MYC levels were higher in metastatic ccRCC (P<0.0001) and incremental p27 levels were observed in benign, primary ccRCC, and metastatic ccRCC (P<0.0001). HIF-1α levels were significantly higher in primary and metastatic ccRCC compared to benign tissues (P<0.0001). In primary ccRCC, levels of all mTOR and hypoxia-induced pathway members were significantly associated with pT stage (P≤0.036), p27 levels with Fuhrman grade (P=0.031), and 4EBP1, p27, and HIF-1α levels with tumor size (P≤0.025). Tumor size, HIF-1α and phos-S6 levels were associated with disease-specific survival (P≤0.032) and tumor progression (P≤0.043). In conclusion, both mTOR and hypoxia-induced pathways were activated in primary and metastatic ccRCC. PTEN loss seems to be an early event during tumorigenesis. Tumor size, HIF-1α and phos-S6 expression were found to be independent predictors of both DSS and tumor progression in primary ccRCC.
Keywords: clear cell renal cell carcinoma, mammalian target of rapamycin pathway, hypoxia-induced pathway, PTEN, AKT, S6, 4EBP1, c-MYC, p27, HIF-1α, prognosis
INTRODUCTION
Renal cell carcinoma (RCC) accounts for only 3–4% of adult malignancies yet its incidence is rising steadily.20 Traditionally, RCC has been resistant to classic treatments (chemotherapy and radiotherapy), with only a small percentage of patients benefiting from cytokine therapy.39 Understanding the molecular pathways implicated in RCC oncogenesis has led to the development of more effective therapies such as drugs that target the vascular endothelial growth factor (VEGF) and the phosphatidylinositol 3-kinase/mammalian target of Rapamycin (PI3K/mTOR) pathways; both groups of inhibitors are now considered important first-line treatment options for patients with advanced RCC.18,25,36,41,49 In addition, loss of VHL gene, although characteristic of hereditary RCC, is detected in up to 50–60% of sporadic clear cell RCC (ccRCC). Some studies have found that VHL mutation and subsequent activation of hypoxia-inducible pathways is associated with tumor aggressiveness and poor survival34,37 although other have failed to show such association22 or even had detected an inverse relationship, with VHL positive tumors having a better cancer-related survival.48 Hypoxia-induced factor-1 (HIF-1) is a transcription factor that mediates responses to tissue oxygenation levels26,27 and has been shown to be required for the regulation of a number of proangiogenic factors, including VEGF receptors.11 As a result of VHL tumor suppressor gene inactivation the HIF-1α subunit is constitutively stabilized and highly expressed in VHL-related RCC.29,32,42 In addition, insulin-like growth factor (IGF) induced HIF-1α synthesis is dependent on the mTOR and the mitogen-activated protein kinase (MAPK) pathways14 and hypoxia itself modulates the mTOR pathway through accumulation of HIF-1α.4 Dysregulation of the mTOR pathway has been demonstrated in several types of malignancies, including RCC.33 Inactivation of PTEN tumor suppressor gene triggers the PIK3/AKT/mTOR pathway.17,21 AKT activation by phosphorylation (phos-AKT) promotes cell cycle progression through p27kip1 (p27) depletion,44 cell proliferation through c-MYC up-regulation,9 and protein translation through mTOR activation, which in turn regulates growth through its main downstream effectors: phosphorylated S6 protein (phos-S6) and eukaryotic translation initiation factor 4E-binding protein-1 (4EBP1).35 The aim of the current study is to analyze the expression status, reciprocal interplay, and prognostic significance of several members of mTOR pathway and related markers as well as the hypoxia-induced pathway through HIF-1α in a clinically well-characterized cohort of primary and metastatic ccRCC treated at a single tertiary academic center.
MATERIALS AND METHODS
The present study includes tissue samples from 176 patients with ccRCC treated at the Johns Hopkins Medical Institutions (Baltimore, MD). One-hundred and thirty-five cases correspond to primary ccRCC and the remainder 41 to unrelated metastatic lesions. All sections were retrieved and reviewed by two urologic pathologists (LS and GJN) for confirmation of the original diagnosis and stage of each case, in compliance with the American Joint Committee on Cancer 2002 Classification.2 Using a previously described procedure13 three sets of tissue microarrays (TMA) were constructed: the first consisted of 69 selected samples of primary ccRCC patients treated by either partial (3 cases) or radical (66 cases) nephrectomy between 1976 and 2002; the second consisted of 66 primary ccRCC patients treated by either partial (64 cases) or total (2 cases) nephrectomy during 2005; and the third one, included de novo metastatic ccRCC cases diagnosed between 2004 and 2006. Primary and metastatic ccRCC cases were not matched and they corresponded to unrelated patients. Triplicate tumor samples and paired benign kidney tissue were spotted from each primary ccRCC specimen, as well as for the metastatic ccRCC cases. Follow-up length from the moment of initial RCC diagnosis ranged from 3.5 to 208.5 months (mean 47.7 months, median 38.2 months) in the primary ccRCC cohort and from 1.5 to 144 months (mean 61.8 months, median 46 months) in the metastatic ccRCC group. In primary ccRCC cases pelvic recurrence and metastatic disease to distant sites were considered as indicative of tumor progression.
Immunohistochemistry
Standard immunohistochemistry (IHC) analysis was performed for the following mTOR pathway members: PTEN, phos-AKT, phos-S6, and 4EBP1. IHC analysis was also performed for AKT-regulated markers c-MYC and p27 and for the hypoxia-induced pathway member HIF-1α. Immunostaining was performed on formalin-fixed paraffin-embedded tissue sections using a Bond Max Leica autostainer (Leica Microsystems, Bannockburn, IL). Sections were deparaffinized, rehydrated, and subjected to heat induced antigen retrieval with a buffer solution using a steamer. Sections were then incubated with appropriate primary antibody. Following the application of a secondary polyclonal rabbit antibody (except for c-MYC, for which the Dako Catalyzed Signal Amplification System Kit was used), slides were developed using 3–3′-diaminobenzidine chromogen and counterstained with hematoxylin. Proper cell lines were used as external controls. For HIF-1α the protocol described by Tickoo et al was used.43 Table 1 lists all pertinent markers information.
TABLE 1.
Summary of Antibodies used for Immunohistochemical Analysis
| Vendor | Clone | Pre-treatment | Dilution | |
|---|---|---|---|---|
| PTEN | Cell Signaling | D4.3 | EDTA, 45 min | 1:50 |
| c-MYC | Epitomics | Y69 | EDTA, 45 min | 1:300 |
| p27 | Transduction Lab | 57 | Citrate, 25 min | 1:4000 |
| phos-AKT | Cell Signaling | 736E11* | EDTA, 45 min | 1:50 |
| phos-S6 | Cell Signaling | Polyclonal** | EDTA, 45 min | 1:200 |
| 4EBP-1 | ProSci | Polyclonal | Citrate, 25 min | 1:250 |
| HIF-1α | Novus Biologicals | NB100–123 | Heat (oven) at 62C, 60 min | 1:1600 |
Phosphorylation site at Ser473;
Phosphorylation site at Ser235/236
Scoring System
Internal controls were checked for negative and positive expression for validation of the assay. Tumor and benign TMA spots stained with each marker were evaluated for pattern of staining (nuclear vs. cytoplasmic), extent (percent of positive cells) and intensity (0 to 3+ score). PTEN positivity was evaluated as nuclear and/or cytoplasmic pattern while HIF-1α, phos-AKT, c-MYC and p27 were evaluated as exclusively nuclear, and phos-S6 and 4EBP1 as exclusively cytoplasmic. In order to evaluate not only the percentage of positive cells but also the staining intensity in the positive cells we devised an H-score, which was assigned to each TMA spot as the sum of the products of the intensity (0 for negative, 1 for weakly positive, 2 for moderately positive, and 3 for strongly positive) by the extent of immunoexpression (0–100), obtaining a value from 0 to 300. Final H-scores for each case were obtained as the average of all the individual H-scores of each TMA spot and these were used during statistical analyses for all markers. For multivariate analysis the biomarker expression was considered as positive (H-score>0) or negative (H-score=0) for all mTOR and hypoxia-induced pathway members.
Statistical Analysis
H-score means were compared using the 1-way ANOVA test with the Bonferroni’s post-hoc pairwise test when necessary. Cox regression models were constructed for multivariate analysis of both clinicopathologic parameters and biomarkers expression in predicting disease-specific survival (DSS) and tumor progression. Data was analyzed using the software STATA Version 9.2 (StataCorp LP, College Station, TX).
RESULTS
Clinicopathologic features of all patients are shown in Table 2. In primary ccRCC, the DSS and tumor progression rates were 75.4% and 29%, respectively. In metastatic ccRCC, the DSS rate was 36.6%.
TABLE 2.
Demographic and clinicopathological characteristics of 135 patients with primary ccRCC treated by nephrectomy
| Parameter | No cases (%) |
|---|---|
| Age at nephrectomy (years) | |
| Mean | 58.6 |
| Median | 59 |
| Range | 22–87 |
| Ethnicity | |
| African-American | 17 (13) |
| Caucasian | 110 (81) |
| Other | 8 (6) |
| Gender | |
| Female | 41 (30) |
| Male | 94 (70) |
| Pathologic Stage at Nephrectomy* | |
| pT1 | 67 (51.9) |
| pT2 | 8 (6.2) |
| pT3 | 50 (38.8) |
| pT4 | 4 (3.1) |
6 cases were not staged due to lack of information regarding gross findings;
ccRCC: clear cell renal cell carcinoma
Marker Expression Status in Primary and Metastatic ccRCC and Benign Tissues
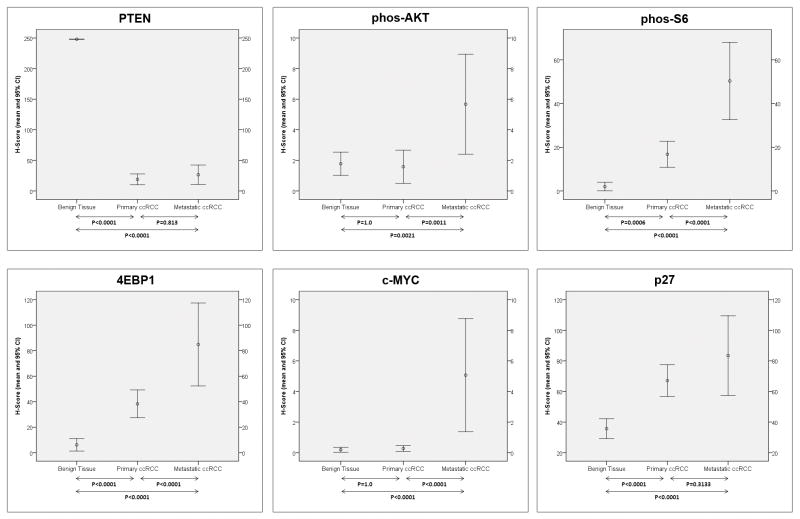
Patterns of immunoexpression are depicted in Figs 1. Overall, striking differences were noted in the levels of all biomarkers depending on the evaluated tissue (Table 3). Figures 2 and 3 depict the H-score levels of all biomarkers in benign tissues as well as in primary and metastatic ccRCC. PTEN levels in primary and metastatic ccRCC were significantly lower than in benign tissues. Levels of phos-AKT levels were significantly higher in metastatic ccRCC when compared to primary ccRCC and benign tissues. In metastatic ccRCC levels of phos-S6 were higher than in primary ccRCC and benign tissues. In addition, phos-S6 levels were significantly higher in primary ccRCC when compared to benign tissues. The same aforementioned pattern of expression was observed with 4EBP1, with significantly higher levels in metastatic ccRCC when compared to primary ccRCC and benign tissues and higher levels in primary ccRCC when compared to the latter. Levels of c-MYC in metastatic ccRCC were significantly higher compared to primary ccRCC and benign tissues. Regarding p27 H-score levels, a higher value was observed in metastatic ccRCC when compared to benign tissues and also higher in the latter when compared to primary ccRCC. Finally, levels of HIF-1α were significantly higher in primary and metastatic ccRCC when compared to benign tissues.
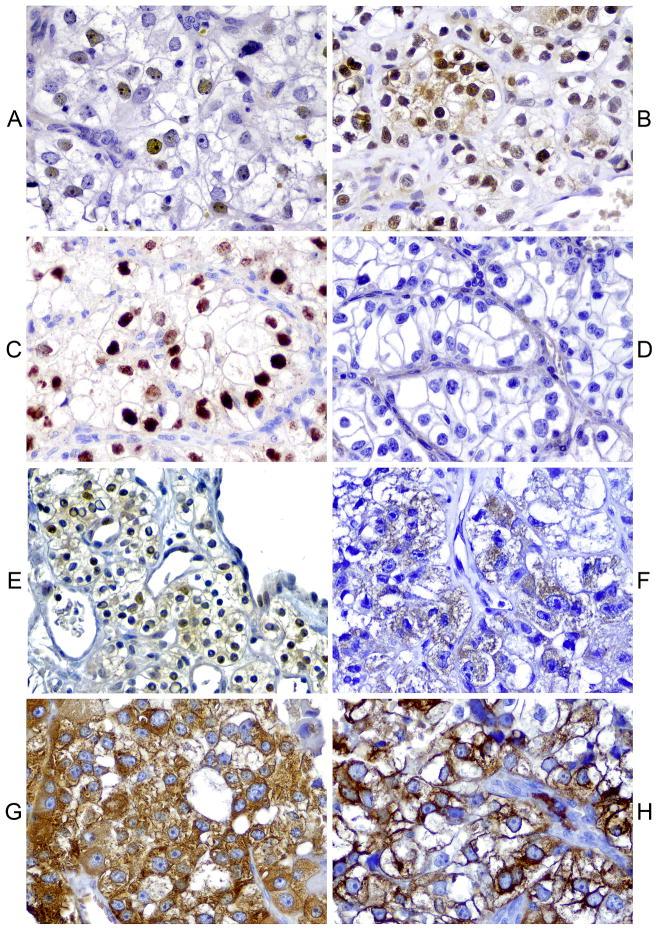
Figure 1. Patterns of immunoexpression in mTOR and hypoxia-induced pathway members.
A) Moderate nuclear c-MYC expression. B) Strong nuclear and focal cytoplasmic p27 expression. C) Diffuse strong nuclear HIF-1α expression. D) Absent PTEN expression. E) Moderate to strong nuclear phos-AKT expression. F) Diffuse cytoplasmic 4EBP1 expression. G & H) Diffuse and strong cytoplasmic and membranous phos-S6 expression.
TABLE 3.
Comparison of H-score means and 95% confidence intervals (CI) in primary ccRCC, metastatic ccRCC, and benign control tissues
| H-Score Mean (95% CI) | ||||
|---|---|---|---|---|
| Biomarker | Primary ccRCC | Metastatic ccRCC | Benign Tissues | P-value |
| PTEN | 18.9 (10.0–27.3) | 26.5 (10.7–42.4) | 248.0* | <0.0001 |
| phos-AKT | 1.6 (0.5–2.7) | 5.7 (2.4–8.9) | 1.8 (1.0–2.5) | 0.001 |
| phos-S6 | 16.8 (10.8–22.8) | 50.3 (32.7–68.0) | 2.0 (0.1–4.0) | <0.0001 |
| 4EBP1 | 38.3 (27.3–49.3) | 84.8 (52.3–117.4) | 6.13 (1.2–11.0) | <0.0001 |
| c-MYC | 0.3 (0.1–0.5) | 5.1 (1.4–8.8) | 0.2 (0.1–0.4) | <0.0001 |
| p27 | 67.1 (56.7–77.6) | 83.5 (57.4–109.5) | 35.7 (29.2–42.2) | <0.0001 |
| HIF-1α | 76.2 (56.8–95.6) | 99.8 (68.9–130.7) | 0.6 (−0.2–1.3) | <0.0001 |
Constant H-score;
ccRCC: clear cell renal cell carcinoma
Figure 2.
Mean H-scores and 95% confidence intervals (CI) for mTOR pathway (PTEN, phos-AKT, phos-S6, and 4EBP1) and related (c-MYC and p27) members in benign renal tissue and in primary and metastatic clear cell renal cell carcinomas. Arrows are used to provide the P-value (Bonferroni’s post-hoc test) between the H-score means.
Figure 3.
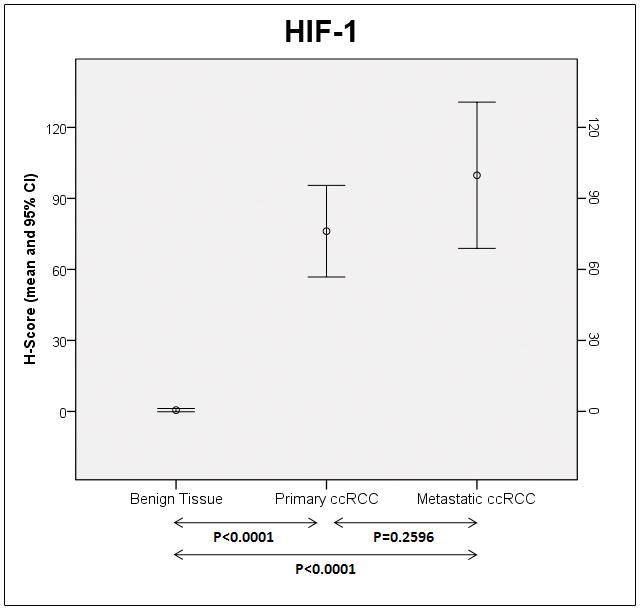
Mean H-scores and 95% confidence intervals (CI) for HIF-1α in benign renal tissue and in primary and metastatic clear cell renal cell carcinomas. Arrows are used to provide the P-value (Bonferroni’s post-hoc test) between the H-score means.
Predictors of Outcome in Primary ccRCC
Univariate analysis
On univariate analysis, pathologic stage and tumor size significantly predicted DSS and disease progression (Table 4). Fuhrman grade was of borderline significance in predicting outcome but when categorized as high (>2) vs. low (≤2) it significantly predicted both outcome parameters. All tested markers significantly correlated with pathological stage at nephrectomy (P≤0.036). Loss of p27 correlated with Fuhrman grade (P=0.031) and tumor size (P=0.0001). Additionally, higher levels of HIF-1α and 4EBP1 also correlated with tumor size (P=0.009 and P=0.025, respectively). Levels of p27, phos-S6, 4EBP1 and HIF-1α significantly correlated with disease progression (P≤0.031) and DSS (P≤0.025). Levels of phos-AKT correlated with OS (P=0.0326) but not with DSS.
TABLE 4.
Univariate Analysis Model including Clinicopathologic Parameters and Biomarker Expression Levels as Prognosticators of Outcome in Primary ccRCC
| Clinicopathologic parameters | Outcome | ||||
|---|---|---|---|---|---|
| pT | Fuhrman | Size | DSS | Progression | |
| pT | N/A | N/A | N/A | 0.0008 | 0.0001 |
| Fuhrman | N/A | N/A | N/A | NS | NS |
| Gender | N/A | N/A | N/A | NS | NS |
| Size | N/A | N/A | N/A | 0.0001 | 0.0001 |
|
| |||||
| Biomarkers expression | |||||
|
| |||||
| PTEN | 0.032 | NS | NS | NS | NS |
| phos-AKT | 0.004 | NS | NS | NS | NS |
| phos-S6 | 0.0004 | NS | NS | 0.0001 | 0.0001 |
| 4EBP1 | 0.036 | NS | 0.025 | 0.0152 | 0.0025 |
| c-MYC | 0.008 | NS | NS | NS | NS |
| p27 | 0.0001 | 0.031 | 0.0001 | 0.031 | 0.0093 |
| HIF-1α | 0.016 | NS | 0.009 | 0.0009 | 0.0007 |
ccRCC: clear cell renal cell carcinoma; DSS: Disease-Specific Survival; N/A: not applicable; NS: nonsignificant (P>0.05)
Multivariate Analysis
In a multivariate analysis model that included all four tested clinicopathologic parameters (pT stage, Fuhrman grade, gender, and tumor size) both pT stage and tumor size remained independent predictors of DSS and disease progression (P<0.001). In a second multivariate analysis model that included the previous clinicopathologic parameters and biomarkers that were significant prognosticators of outcome in the univariate model (phos-S6, HIF-1α, p27, and 4EBP1), only tumor size and levels of phos-S6 and HIF-1α remained independent predictors of DSS and progression (Table 5).
TABLE 5.
Multivariate Analysis Model including Clinicopathologic Parameters and Biomarker Expression Levels as Prognosticators of Outcome in Primary ccRCC
| Clinicopathologic Parameters | Outcome | |
|---|---|---|
| DSS | Progression | |
| pT Stage | NS | NS |
| Fuhrman Grade | NS | NS |
| Gender | NS | NS |
| Tumor Size | 0.005 | 0.043 |
|
| ||
| Biomarkers Expression | ||
|
| ||
| PTEN | NS | NS |
| phos-AKT | NS | NS |
| phos-S6 | 0.006 | 0.004 |
| 4EBP1 | NS | NS |
| c-MYC | NS | NS |
| p27 | NS | NS |
| HIF-1α | 0.032 | 0.008 |
ccRCC: clear cell renal cell carcinoma; DSS: Disease-Specific Survival; NS: nonsignificant (P>0.05)
Predictors of Outcome in Metastatic ccRCC
Expression levels of all analyzed markers failed to predict DSS in metastatic ccRCC on univariate and multivariate regression analysis.
DISCUSSION
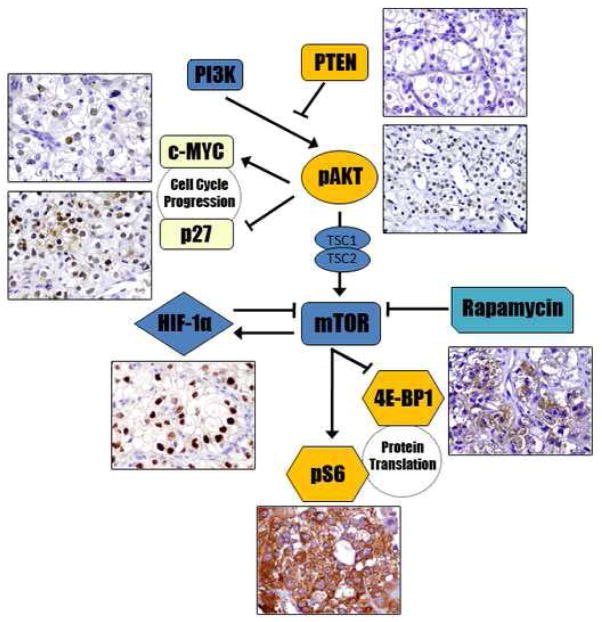
The PI3K/AKT/mTOR pathway plays a central role in regulating cell growth and participates in the control of other numerous cellular processes, including mRNA initiation and protein translation. PI3K, the first member of the pathway, can be directly activated by tyrosine kinase receptors, leading to phosphorylation and consequent activation of AKT; in turn, activation of AKT is negatively controlled by PTEN. Activated AKT (phos-AKT) exerts its downstream effects by phosphorylation of either proteins related to the tuberous sclerosus complex genes (TSC1/TSC2) or a proline-rich AKT substrate (PRAS40). A rapamycin-sensitive raptor complex (mTORC1) is then phosphorylated by any of these proteins, which in turns activates two downstream signaling pathways, S6 and 4EBP1. Phosphorylated 4EBP1 dissociates from eIF4E, allowing the latter to form a complex that facilitates cap-dependant translation of cell cycle-related proteins, such as c-MYC and cyclin D1, and hypoxia-induced factors, such as HIF-1α. On the other hand, phosphorylation of S6 ultimately leads to ribosomal protein translation and ribosome biogenesis. Figure 4 is a diagrammatic representation of the mTOR and hypoxia-induced pathways considering the members we evaluated in the present study.
Figure 4.
Diagrammatic representation of the mTOR and hypoxia-induced pathways. In tumors with activation of the mTOR pathway the use of mTOR inhibitors, such as sirolimus (Rapamycin) and derived drugs, may be beneficial. Blunt-ended arrows indicate inhibitory effects in the targeted molecule.
In our cohort, PTEN levels were markedly reduced in both primary and metastatic ccRCC relative to benign controls, suggesting that, when present, PTEN loss may represent an early step in ccRCC carcinogenesis. Our results are in agreement with previous studies which have showed a marked reduction in PTEN levels in ccRCC.3,15,33 Recently, using data mining in a set of ccRCC TMAs Dahinden et al demonstrated an adverse prognosis in patients with loss of cytoplasmic PTEN staining and suggested a crucial role of PTEN and p27 inactivation in tumor progression.6 All tested markers were significantly associated with the pT stage at nephrectomy. Additionally, expression levels of 4EBP1, p27, and HIF-1α were associated with tumor size, supporting the notion of a collaborative interplay between mTOR, hypoxia-induced pathways and cell cycle-related proteins in regulating tumor growth and local aggressiveness. We also observed significantly higher levels of phos-AKT, phos-S6, and 4EBP1 in metastatic ccRCC when compared to primary lesions, indicating that activation of the mTOR pathway might be associated with the acquisition of a more aggressive phenotype. The overall expression levels of c-MYC were low in all the samples but the higher levels we observed in metastatic tumors suggest that activation of this gene may play a role in tumor progression. Activation of the c-MYC pathway has been described previously in the majority of ccRCC,12,40 although other studies have failed in demonstrated c-MYC upregulation in this subset of renal tumors.24,38,45
Attempts to predict survival in renal cell carcinoma have traditionally relied on standard clinicopathologic variables such as performance status and other clinical variables, tumor histologic type, pTNM stage, Fuhrman grade, and size.28,31 In agreement with previous studies,7 in our cohort of patients with primary ccRCC, pT stage and tumor size were significant predictors of outcome when only clinicopathologic variables were considered; Fuhrman grade also showed a significant association with prognosis when categorized as low (Fuhrman 1 and 2) and high (Fuhrman 3 and 4) grades. However, when biomarkers were included into the multivariate model, pT stage and Fuhrman grade lost significance as prognosticators of DSS and tumor progression. Additional studies, preferably in prospective cohorts, are needed to support our current observation on the role of expression levels of mTOR and hypoxia-induced pathway members as predictors of outcome independent of pT stage or Fuhrman grade alone. It would also be of great interest to evaluate the predictive performance of these biomarkers in nonmetastatic RCC against prognostic tools currently in use such as the UCLA Integrated Staging System (UISS) or the Mayo Clinic’s SSIGN, among others.46 Finally, the design of nomograms considering clinicopathological features as well as expression levels of these biomarkers could be of value following confirmation of our findings in prospective series of cases.
In our cohort of primary ccRCC higher levels of phos-S6 predicted better outcome. Our results are consistent with those published by Pantuck et al33 who investigated 375 RCC, including 323 ccRCC, and found higher phos-S6 levels to be predictors of DSS. They also identified loss of PTEN and phos-AKT expression as significant prognosticators but only phos-S6 remained an independent predictor in their multivariate model. In our series, HIF-1α and 4EBP1 levels were also predictors of outcome in primary ccRCC. 4EBP1 has shown association with malignant progression and adverse prognosis in breast, ovarian, endometrial and prostate carcinomas1 yet there are no reports on its expression or prognostic significance in RCC. On the other hand, previous studies have shown that HIF-1α expression is an adverse prognostic marker in ccRCC.8,10,19,23,30 This is of particular interest since inactivation of the VHL tumor suppressor gene results in stabilization of the HIF-1α subunit.16 Furthermore, HIF-1α is thought to downregulate the mTOR pathway independent of a hypoxic microenvironment.47 Accordingly, both HIF-1α and phos-S6 were found to be independent predictors of DSS and tumor progression. Additionally, HIF-1α expression levels were elevated to a similar extent in both primary and metastatic ccRCC while metastatic tumors demonstrated significantly higher levels of phos-S6 compared to primary lesions. This finding may provide additional evidence for the importance of the interaction between the hypoxia-induced and the mTOR pathways in tumor progression of ccRCC.
A possible weakness of the present study includes the use of nonmatched tissue for primary and metastatic tumors. Metastatic ccRCC might had different tumor grades and stages in their primary setting compared to the ones observed in the evaluated primary ccRCC of our series, and this could have influenced the distribution of the biomarker levels of expression. However, we still deem worthwhile to evaluate the mTOR and hypoxia-inducible pathways in nonmatched metastatic ccRCC, especially considering that mTOR inhibitors, such as temsirolimus, have been recently FDA-approved for the treatment of advanced RCC.25 The fact that the expression levels of the analyzed biomarkers were not predictors of outcome in metastatic ccRCC suggest that the prognostic value of these might be limited to the evaluation of nonmetastatic ccRCC. Nevertheless, given the retrospective design of the current series, these findings require further prospective confirmation. Another potential limitation of the present study is the use of TMA sampling. Given the staining heterogeneity of some markers, the use of TMA instead of whole sections could have some impact on the detected expression levels. However, several studies have supported the high throughput value of the TMA usage and the adequate representation of the overall expression levels using multiple TMA spots.5
In summary, we found evidence of activation of both the mTOR and the hypoxia-induced pathways in primary and metastatic ccRCC. PTEN loss seems to be an early event during tumorigenesis. In addition, tumor size, HIF-1α and phos-S6 expression were found to be independent predictors of both DSS and tumor progression in primary ccRCC. Our results suggest that immunohistochemical evaluation of mTOR and hypoxia-induced pathway members might be useful in predicting the outcome of patients with primary ccRCC, complementing the information provided by routine gross pathologic and microscopic analysis.
Acknowledgments
Grant Information: Partially supported by The Brady Urological Institute – Johns Hopkins Medicine Patana Fund
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
Authors declare no conflicts of interest.
References
- 1.Armengol G, Rojo F, Castellvi J, et al. 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 2.Baldewijns MM, van Vlodrop IJ, Schouten LJ, et al. Genetics and epigenetics of renal cell cancer. Biochim Biophys Acta. 2008;1785:133–155. doi: 10.1016/j.bbcan.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Brenner W, Farber G, Herget T, et al. Loss of tumor suppressor protein PTEN during renal carcinogenesis. Int J Cancer. 2002;99:53–57. doi: 10.1002/ijc.10303. [DOI] [PubMed] [Google Scholar]
- 4.Brugarolas J, Lei K, Hurley RL, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol. 2008;26:5630–5637. doi: 10.1200/JCO.2008.17.3567. [DOI] [PubMed] [Google Scholar]
- 6.Dahinden C, Ingold B, Wild P, et al. Mining tissue microarray data to uncover combinations of biomarker expression patterns that improve intermediate staging and grading of clear cell renal cell cancer. Clin Cancer Res. 2010;16:88–98. doi: 10.1158/1078-0432.CCR-09-0260. [DOI] [PubMed] [Google Scholar]
- 7.Delahunt B, Bethwaite PB, Nacey JN. Outcome prediction for renal cell carcinoma: evaluation of prognostic factors for tumours divided according to histological subtype. Pathology. 2007;39:459–465. doi: 10.1080/00313020701570061. [DOI] [PubMed] [Google Scholar]
- 8.Di Cristofano C, Minervini A, Menicagli M, et al. Nuclear expression of hypoxia-inducible factor-1alpha in clear cell renal cell carcinoma is involved in tumor progression. Am J Surg Pathol. 2007;31:1875–1881. doi: 10.1097/PAS.0b013e318094fed8. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, et al. Prolactin induces c-Myc expression and cell survival through activation of Src/Akt pathway in lymphoid cells. Oncogene. 2004;23:7378–7390. doi: 10.1038/sj.onc.1208002. [DOI] [PubMed] [Google Scholar]
- 10.Dorevic G, Matusan-Ilijas K, Babarovic E, et al. Hypoxia inducible factor-1alpha correlates with vascular endothelial growth factor A and C indicating worse prognosis in clear cell renal cell carcinoma. J Exp Clin Cancer Res. 2009;28:40. doi: 10.1186/1756-9966-28-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y, Liu Z, Fang X, et al. Differential expression of full-length telomerase reverse transcriptase mRNA and telomerase activity between normal and malignant renal tissues. Clin Cancer Res. 2005;11:4331–4337. doi: 10.1158/1078-0432.CCR-05-0099. [DOI] [PubMed] [Google Scholar]
- 13.Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005;103:89–101. doi: 10.1385/1-59259-780-7:089. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda R, Hirota K, Fan F, et al. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 15.Hager M, Haufe H, Kemmerling R, et al. PTEN expression in renal cell carcinoma and oncocytoma and prognosis. Pathology. 2007;39:482–485. doi: 10.1080/00313020701570012. [DOI] [PubMed] [Google Scholar]
- 16.Hanna SC, Heathcote SA, Kim WY. mTOR pathway in renal cell carcinoma. Expert Rev Anticancer Ther. 2008;8:283–292. doi: 10.1586/14737140.8.2.283. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudes GR, Berkenblit A, Feingold J, et al. Clinical trial experience with temsirolimus in patients with advanced renal cell carcinoma. Semin Oncol. 2009;36 (Suppl 3):S26–36. doi: 10.1053/j.seminoncol.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen J, Rasmuson T, Grankvist K, et al. Vascular endothelial growth factor as prognostic factor in renal cell carcinoma. J Urol. 2000;163:343–347. [PubMed] [Google Scholar]
- 20.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 21.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784:150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Jung CW, Cho YH, et al. Somatic VHL alteration and its impact on prognosis in patients with clear cell renal cell carcinoma. Oncol Rep. 2005;13:859–864. [PubMed] [Google Scholar]
- 23.Klatte T, Seligson DB, Riggs SB, et al. Hypoxia-inducible factor 1 alpha in clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:7388–7393. doi: 10.1158/1078-0432.CCR-07-0411. [DOI] [PubMed] [Google Scholar]
- 24.Kozma L, Kiss I, Nagy A, et al. Investigation of c-myc and K-ras amplification in renal clear cell adenocarcinoma. Cancer Lett. 1997;111:127–131. doi: 10.1016/s0304-3835(96)04527-2. [DOI] [PubMed] [Google Scholar]
- 25.Kwitkowski VE, Prowell TM, Ibrahim A, et al. FDA approval summary: temsirolimus as treatment for advanced renal cell carcinoma. Oncologist. 2010;15:428–435. doi: 10.1634/theoncologist.2009-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lando D, Peet DJ, Gorman JJ, et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lando D, Peet DJ, Whelan DA, et al. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 28.Ljungberg B. Prognostic markers in renal cell carcinoma. Curr Opin Urol. 2007;17:303–308. doi: 10.1097/MOU.0b013e328277f180. [DOI] [PubMed] [Google Scholar]
- 29.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 30.Minardi D, Lucarini G, Filosa A, et al. Prognostic role of tumor necrosis, microvessel density, vascular endothelial growth factor and hypoxia inducible factor-1alpha in patients with clear cell renal carcinoma after radical nephrectomy in a long term follow-up. Int J Immunopathol Pharmacol. 2008;21:447–455. doi: 10.1177/039463200802100225. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 32.Ohh M, Park CW, Ivan M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 33.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 34.Patard JJ, Rioux-Leclercq N, Masson D, et al. Absence of VHL gene alteration and high VEGF expression are associated with tumour aggressiveness and poor survival of renal-cell carcinoma. Br J Cancer. 2009;101:1417–1424. doi: 10.1038/sj.bjc.6605298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 36.Schmidinger M, Zielinski CC. Novel agents for renal cell carcinoma require novel selection paradigms to optimise first-line therapy. Cancer Treat Rev. 2009;35:289–296. doi: 10.1016/j.ctrv.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Schraml P, Struckmann K, Hatz F, et al. VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J Pathol. 2002;196:186–193. doi: 10.1002/path.1034. [DOI] [PubMed] [Google Scholar]
- 38.Sitaram RT, Cairney CJ, Grabowski P, et al. The PTEN regulator DJ-1 is associated with hTERT expression in clear cell renal cell carcinoma. Int J Cancer. 2009;125:783–790. doi: 10.1002/ijc.24335. [DOI] [PubMed] [Google Scholar]
- 39.Suarez C, Morales R, Munoz E, et al. Molecular basis for the treatment of renal cell carcinoma. Clin Transl Oncol. 2010;12:15–21. doi: 10.1007/s12094-010-0461-4. [DOI] [PubMed] [Google Scholar]
- 40.Tang SW, Chang WH, Su YC, et al. MYC pathway is activated in clear cell renal cell carcinoma and essential for proliferation of clear cell renal cell carcinoma cells. Cancer Lett. 2009;273:35–43. doi: 10.1016/j.canlet.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 41.Thompson Coon J, Hoyle M, Green C, et al. Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation. Health Technol Assess. 2010;14:1–184. iii–iv. doi: 10.3310/hta14020. [DOI] [PubMed] [Google Scholar]
- 42.Thrash-Bingham CA, Tartof KD. aHIF: a natural antisense transcript overexpressed in human renal cancer and during hypoxia. J Natl Cancer Inst. 1999;91:143–151. doi: 10.1093/jnci/91.2.143. [DOI] [PubMed] [Google Scholar]
- 43.Tickoo SK, Alden D, Olgac S, et al. Immunohistochemical expression of hypoxia inducible factor-1alpha and its downstream molecules in sarcomatoid renal cell carcinoma. J Urol. 2007;177:1258–1263. doi: 10.1016/j.juro.2006.11.100. [DOI] [PubMed] [Google Scholar]
- 44.Viglietto G, Motti ML, Fusco A. Understanding p27(kip1) deregulation in cancer: down-regulation or mislocalization. Cell Cycle. 2002;1:394–400. doi: 10.4161/cc.1.6.263. [DOI] [PubMed] [Google Scholar]
- 45.Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol. 2006;16:318–330. doi: 10.1016/j.semcancer.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Volpe A, Patard JJ. Prognostic factors in renal cell carcinoma. World J Urol. 2010;28:319–327. doi: 10.1007/s00345-010-0540-8. [DOI] [PubMed] [Google Scholar]
- 47.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 48.Yao M, Yoshida M, Kishida T, et al. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002;94:1569–1575. doi: 10.1093/jnci/94.20.1569. [DOI] [PubMed] [Google Scholar]
- 49.Zbrozek AS, Hudes G, Levy D, et al. Q-TWiST analysis of patients receiving temsirolimus or interferon alpha for treatment of advanced renal cell carcinoma. Pharmacoeconomics. 2010;28:577–584. doi: 10.2165/11535290-000000000-00000. [DOI] [PubMed] [Google Scholar]