Summary
Epidermal growth factor receptor (EGFR) is a member of the erbB tyrosine kinase family reported to be overexpressed in a variety of solid malignancies. Mutations in exons 19 to 21 of the tyrosine kinase domain have been detected in a subset of these tumors and its presence associated with a better response to EGFR inhibitors. Several clinical trials are currently underway to evaluate the performance of such drugs in patients with bladder cancer, but data on EGFR mutation status are limited. The current study assesses EGFR immunohistochemical expression and the presence of mutations in exons 19 and 21 by polymerase chain reaction in 19 bladder urothelial carcinomas from formalin-fixed, paraffin-embedded tissues. Representative paraffin sections were microdissected for DNA extraction using a pinpoint isolation system. Parallel sections were immunostained using a monoclonal anti-EGFR antibody. No mutations in exons 19 and 21 of EGFR were identified in any of the cases. Immunohistochemical EGFR positivity was observed in 14 of 19 cases. In summary, we found EGFR protein expression in 74% of urothelial carcinomas, but we failed to detect EGFR mutations at exons 19 to 21, suggesting that EGFR overexpression is not related to the presence of mutations in the tyrosine kinase domain of the gene. Mutation analysis of EGFR exons 19 and 21 is feasible in microdissected paraffin sections from archival tissues. Immunohistochemical expression of EGFR may not be useful to predict therapeutic response to EGFR inhibitors in patients with urothelial carcinomas. To explain EGFR immunohistochemical overexpression, other mechanisms besides mutations in the EGFR kinase domain should be investigated in future studies.
Keywords: EGFR expression, EGFR mutation, Exon 19 deletion, Exon 21 L858R substitution, Urothelial carcinoma
1. Introduction
Bladder cancer is the fourth most common cancer in males, with an estimated 52 760 new cases and 10 410 cancer-related deaths for 2010 in the United States [1]. For patients with muscle-invasive disease, radical cystectomy is considered the standard treatment, and prognosis is mainly related to the extent of local invasion and the lymph nodes status. About one quarter of patients treated by radical cystectomy present with lymph node metastases. The recurrence-free survival is significantly lower in these patients when compared with those without nodal involvement [2,3]. Cisplatin-based chemotherapy is considered the first-line treatment option for patients with advanced bladder cancer. In the metastatic setting, the response rates are initially very high when compared with other epithelial tumors [4]. Nevertheless, the survival rates of patients in whom tumor progression ensues are low, with a median survival of only 15 months [5]. Aiming to improve the outcome of patients with advanced bladder cancer, several inhibitors and monoclonal antibodies directed against specific molecular targets are currently under evaluation, either as single agents or in combination with cytotoxic chemotherapy [4]. One of the most promising targets in bladder cancer therapy is the epidermal growth factor receptor (EGFR).
EGFR is a 170-kDa membrane glycoprotein with intrinsic tyrosine kinase activity that mediates the cellular response to several proliferation signals. EGFR activation has been implicated in the pathogenesis of several malignant tumors including urothelial carcinomas [6]. Various mechanisms have been proposed to explain EGFR activation, including gene amplification, activating mutation, increased transcription, loss of inhibitory signals, and decreased protein recycling [7]. In gliomas, the most common mechanism involves a deletion of exons 2 to 7 of the EGFR extracellular domain, yielding a mutant form (EGFRvIII) that is constitutively active. EGFRvIII has been also identified in tumors of the breast, lung, ovary, and prostate [6]. Mutations of EGFR in exons 19 to 21 have been reported in several solid malignancies, mainly, non–small cell lung carcinomas, in which the detection rate approaches 40% of all cases [8]. EGFR mutations involving exons 18 to 21 have been reported in a variety of tumors including colorectal, head and neck, bile duct and gallbladder, prostate, esophageal, pancreatic, and non–small cell lung carcinomas [8,9]. However, EGFR mutations in urothelial carcinomas seem to be rare events. Villares et al [10] and Blehm et al [11] explore the kinase domain of EGFR (exons 18-21) from 11 bladder cancer lines and 75 tissue samples of urothelial carcinomas. No mutations were detected in all the samples tested.
Despite this apparent absence of EGFR mutations in bladder cancer, various investigators have found a significant association between EGFR immunohistochemical overexpression, recurrence rate, and survival in patients with urothelial carcinoma [12,13]. In addition, the reported response rates to EGFR inhibitors are higher in patients harboring tumors with activating mutations. Particularly, a point mutation in exon 21 (L858R) and a deletion mutation in exon 19 (del E746-T751) offer a predictive marker for improved therapeutics with gefitinib or erlotinib [7]. These findings underscore the potential use that the identification of these mutations might have for therapeutic planning. However, immunohistochemical overexpression of EGFR might not necessarily be indicative of the presence of activating mutations and therefore cannot reliably be used to predict EGFR mutation status [14].
Considering the feasibility of immunohistochemistry in routine specimens and the putative impact of EGFR inhibitors in the outcome of patients with tumors showing activation of the EGFR pathway, we designed this study to evaluate EGFR immunoexpression levels and the presence of mutations in exons 19 and 21 of the EGFR gene by polymerase chain reaction (PCR) in formalin-fixed, paraffin-embedded tumor samples of bladder urothelial carcinomas.
2. Materials and methods
2.1. Tissue collection
Twenty-one tissue samples of patients with urothelial carcinoma of the urinary bladder treated by radical cystectomy without neoadjuvant chemotherapy were selected from the pathology files of the Johns Hopkins Medical Institutions (Baltimore, MD). Representative formalin-fixed, paraffin-embedded sections from 17 invasive high-grade urothelial carcinomas, 2 noninvasive high-grade papillary urothelial carcinomas, 1 urothelial carcinoma in situ, and 1 urothelial carcinoma metastatic to lymph nodes were available for microdissection. DNA extraction and determination of EGFR mutation status failed in 2 cases (both invasive high-grade urothelial carcinomas); these cases were excluded from the study.
2.2. Immunohistochemistry for EGFR expression
EGFR immunohistochemistry was carried out on unstained 4-μm-thick formalin-fixed, paraffin-embedded tissue sections using a monoclonal mouse anti-EGFR antibody (DAKO, Carpinteria, CA) on a Dako autostainer. After baking and deparaffinization, the sections were hydrated and incubated for 30 minutes with the primary antibody. The reaction was developed with an EGFR polymer detection kit and visualized with DAB (DAKO). Negative and positive controls provided by the manufacturer were used, and reactions were observed appropriately. Membranous staining of EGFR was evaluated in tumor and adjacent normal urothelium. EGFR expression was evaluated by intensity and distribution of the staining, using the following categories: negative staining; 1+, weak partial staining; 2+, weak complete staining; and 3+, intense complete staining. Tumors were classified as Low EGFR expression when scored 1+ in more than 5% of tumor cells and as High EGFR expression when scored either 2+ or 3+ membranous staining, regardless of the extent. Tumors with 5% or less of 1+ staining were considered as Negative for EGFR expression. The highest score obtained among different areas of the same tumor was used as the final EGFR immunoexpression status for each urothelial carcinoma. The same approach was used for evaluating EGFR expression in normal urothelium.
2.3. DNA extraction from formalin-fixed, paraffin-embedded tissue
Tumor areas were identified in routine sections stained with hematoxylin and eosin, and 10 unstained sections (10 μm thick) from each paraffin-embedded specimen were obtained. DNA isolation of the targeted tissue area on tissue sections was done using DNA Isolation System. A drop of pinpoint solution (Pinpoint Slide DNA Isolation System; Zymo Research, Orange, CA) was applied to the mapped area of the tumor (approximately 5 × 5 mm2). Next, the targeted tumor tissue was microdissected with a scalpel and placed in a PCR tube. The excised tissues were digested in proteinase K buffer solution at 55°C for 8 hours, then at 97°C for 10 minutes. Exons 19 to 21 from the tyrosine kinase domain of EGFR were sequenced in each tumor to assess mutation status. Two cell lines were used as controls for EGFR mutation status, NCI-H1650, which has a deletion of amino acids 749 to 750, and NCI-H1975, which has 2 point mutations, L858R and T790M.
2.4. EGFR exon 19 deletion assay
PCR reactions were prepared with 1× PCR buffer, 1.5 mmol/L MgCl2, 500 μmol/L dNTPs (Applied Biosystems, Foster City, CA), 1.5 U AmpliTaq Gold (Applied Biosystems), and 500 nmol/L of each primer (Table 1) in a 50-μL reaction. Reactions were heated to 95°C for 9 minutes, followed by 35 cycles of 95°C for 30 seconds, 60°C for 1 minute, and 72°C for 1 minute and by a final extension at 72°C for 7 minutes. Eight microliters of amplification product was separated by agarose (2%) gel electrophoresis to verify the product. One microliter of PCR product was added to 9 μL formamide and 1 μL ROX size standard (Applied Biosystems). The samples were denatured at 98°C for 2 minutes and cooled on ice for 1 minute. The samples were detected on an ABI 3100 Avant genetic analyzer and analyzed using GeneScan 3.7 Software (Applied Biosystems).
Table 1.
PCR primers for EGFR mutation analysis
| Exon | Primer | Sequence (5′-3′) |
|---|---|---|
| 19 | EGFR-Ex19-FWD1 | GCACCATCTCACAATTGCCAGTTA |
| EGFR-Ex19-REV1 | FAM-AAAAGGTGGGCCTGAGGTTCA | |
| 21 | EGFR-Ex21-FWD1 | CCTCACAGCAGGGTCTTCTCTGT |
| EGFR-Ex21-REV1 | FAM-TCAGGAAAATGCTGGCTGACCTA |
2.5. EGFR exon 21 L858R mutation assay
PCR reactions were prepared with 1× PCR buffer, 15 mmol/L MgCl2, 500 μmol/L dNTPs (Applied Biosystems), 1.5 U AmpliTaq Gold (Applied Biosystems), and 500 nmol/L of each primer (see Table 1) in a 50-μL reaction. Reactions were heated to 95°C for 9 minutes, followed by 35 cycles of 95°C for 30 seconds, 60°C for 1 minute, and 72°C for 1 minute and by a final extension at 72°C for 7 minutes. Eight microliters of amplification products was separated by agarose (2%) gel electrophoresis to verify the product. Amplification products were purified using QIAquick Spin Columns according to the manufacturer’s protocol (Qiagen, Valencia, CA). Five microliters of the purified product was added to a 20-μL digest reaction containing 2 U Sau96I enzyme and 1× NEB4 buffer (New England Biolabs, Ipswich, MA), as well as a mock reaction without enzyme. Digestion was performed at 37°C for 2 hours. One microliter of digest reactions was added to 9 μL formamide and 1 μL ROX size standard (Applied Biosystems). The samples were denatured at 98°C for 2 minutes and cooled on ice for 1 minute. The samples were detected on an ABI 3100 Avant genetic analyzer and analyzed using GeneScan 3.7 Software (Applied Biosystems).
3. Results
3.1. Clinicopathologic data
Patient cohort consisted of 14 men and 5 women, with a mean age of 65.5 years (range, 45–76 years). Thirteen patients had advanced disease (≥pT2) at the time of the diagnosis, 3 cases were staged as pTa/pTis, and 3 were staged as pT1.
3.2. Expression of EGFR by immunohistochemistry
EGFR positivity was observed in 14 tumors (74%), with high levels in 10 tumors (53%) and low levels in 4 tumors (21%). The remaining 5 tumors (26%) were negative for EGFR. Immunohistochemical results are summarized in Table 2. Patterns of EGFR immunoexpression in urothelial carcinomas are shown in Fig. 1. In adjacent urothelium, EGFR expression was negative in 14 cases (78%) and low in the remaining 4 cases (22%). In all cases, EGFR expression was lower or equal in the urothelium compared with the tumor. In the 14 cases with negative EGFR expression in the urothelium, EGFR expression remained negative in 5 tumors (36%), low in 2 tumors (14%), and high in the remaining 7 tumors (50%). In the 4 cases with low EGFR expression in the urothelium, high EGFR expression was observed in 2 tumors (50%) and low EGFR expression in the remaining 2 tumors (50%). Fig. 2 shows the distribution of EGFR expression in urothelial carcinoma and adjacent urothelium.
Table 2.
EGFR immunoexpression in urothelial carcinomas of the urinary bladder distributed by pT stage
| No. of cases | EGFR immunoexpression
|
|||
|---|---|---|---|---|
| High | Low | Negative | ||
| pTis | 1 | 1 | 0 | 0 |
| pTa | 2 | 0 | 2 | 0 |
| pT1 | 3 | 1 | 1 | 1 |
| pT2 | 4 | 3 | 1 | 0 |
| pT3 | 6 | 4 | 0 | 2 |
| pT4 | 3 | 1 | 0 | 2 |
| Total | 19 | 10 | 4 | 5 |
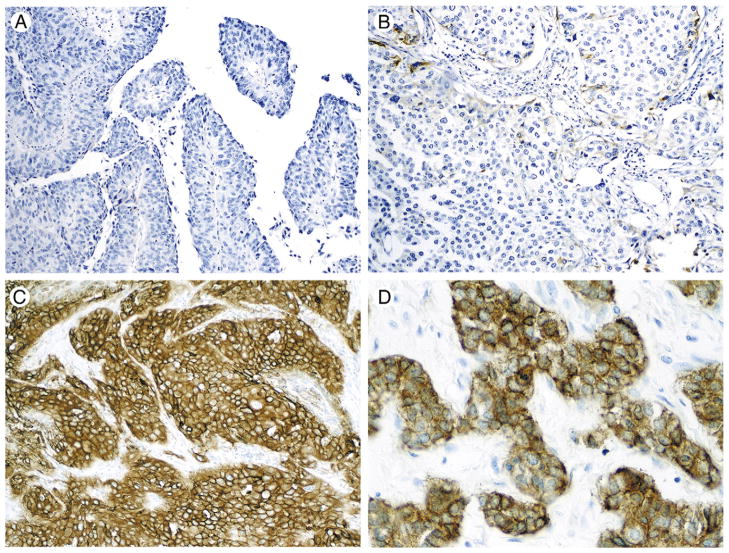
Fig. 1.
Patterns of EGFR immunoexpression. A, Invasive high-grade papillary urothelial carcinoma with no EGFR expression. B, Invasive high-grade urothelial carcinoma with focal positivity; cases that showed this staining pattern were considered as “EGFR positive, low expression level.” C, High-grade invasive urothelial carcinoma with strong and diffuse EGFR immunoexpression. Cases showing this staining pattern were considered as “EGFR positive, high expression level.” D, A higher magnification is depicted here.
Fig. 2.
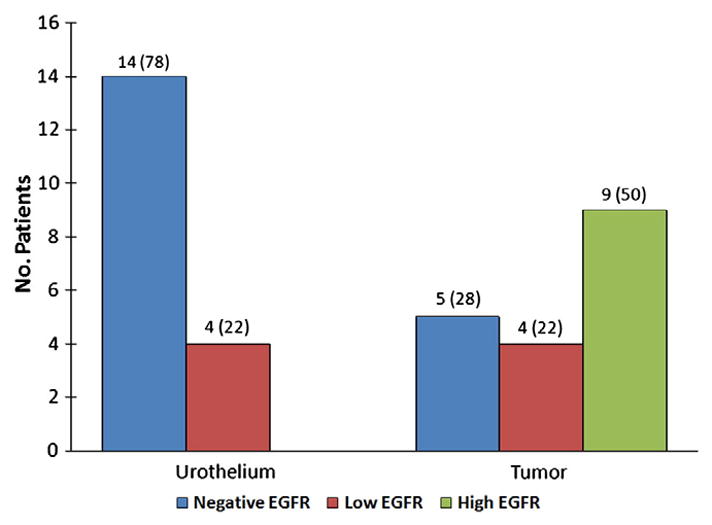
EGFR expression in urothelial carcinoma and adjacent urothelium. High levels of EGFR were only observed in urothelial carcinoma. EGFR immunoexpression was consistently lower in urothelium compared with urothelial carcinoma. Comparison excluded 1 case of urothelial carcinoma metastatic to lymph node.
3.3 EGFR mutation analysis of exons 19 and 21
No deletions at exon 19 or L858R substitutions at exon 21 of EGFR were found in any of the 19 informative cases.
4. Discussion
In this study, most urothelial carcinomas showed EGFR expression by immunohistochemistry. Nevertheless, its occurrence was not associated with mutations within the tyrosine kinase domain (exons 19 and 21) of the EGFR gene. Our detection rate of 74% is in agreement with previous reports and further confirms that EGFR overexpression is a frequent event in bladder cancer [13]. The prognostic value of EGFR in bladder and upper urinary tract urothelial carcinomas has been previously established by various recent studies that found a significant association between its expression and the presence of aggressive phenotype, advanced stage, likelihood of tumor recurrence, and survival [12,13,15,16]. However, other studies suggest that the predictive value of EGFR expression is not entirely independent of tumor stage and grade [15]. Nonetheless, because the potential therapeutic use of EGFR inhibitors has been firmly established in preclinical models [17], several clinical trials are currently evaluating the benefits of these drugs in the treatment of bladder cancer [4].
EGFR is a member of the erbB family of receptor tyrosine kinase proteins involved in cell growth, differentiation, cell survival, cell cycle progression, and angiogenesis. It is composed of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain with tyrosine kinase activity. Growth factors such as transforming growth factor α and epidermal growth factor induce EGFR homodimerization or heterodimerization with other members of the erbB family, including HER2/neu (erbB2), HER3 (erbB3), and HER4 (erbB4), followed by EGFR autophosphorylation at specific tyrosine sites. This, in turn, activates several downstream pathways, including the Ras/Raf/mitogen-activated protein kinase pathway, the phosphatidylinositol 3-kinase/AKT pathway, and the signal transduction and activator of transcription pathway. The mitogen-activated protein kinase pathway is involved in cell growth and proliferation, whereas the latter 2 pathways regulate cell survival and apoptosis. Phosphatidylinositol 3-kinase/AKT activation also mediates angiogenesis via up-regulation of angiogenic factors such as vascular endothelial growth factor and interleukin-8 [7]. Emerging evidence suggests that along this traditional transduction pathway, there is also a nuclear signaling pathway involving direct transport of EGFR to the cell nucleus where it mediates the activation of a number of genes including cyclin D1, inducible nitric oxide synthase, B-myb, aurora A, and cyclooxygen-ase-2, as well as phosphorylates the proliferating cell nuclear antigen to promote cell proliferation and DNA repair [18].
Our study confirms earlier results [10,11] by showing that EGFR mutations in exons 19 and 21 are rare events in urothelial carcinomas. Although the kinase domain of EGFR also includes other exons (ie, 18 and 20), we decided to target only exons 19 and 21. This decision was based on previous studies linking mutations in these specific exons to improved therapeutic response to EGFR inhibitors [7]. We hypothesize that either mutations in exons 19 and 21 of the EGFR gene are not involved in the oncogenesis of urothelial carcinomas or mutation rates were too low to be detected in samples we and others [10,11] have sequenced. It seems that, with the exception of non–small cell carcinomas and glioblastomas, the overall prevalence of EGFR activating mutations in solid malignancies is very low. Other mechanisms besides mutations in the EGFR kinase domain should be investigated to explain the EGFR immunohistochemical overexpression. These mechanisms include EGFR gene amplification, increased coexpression of receptor ligands (eg, transforming growth factor α and amphiregulin), heterodimerization and cross-talk with HER2 or other members of the erbB family, and interactions with heterologous receptor systems [7,19,20]. Induction of EGFR expression by the hypoxic neoplastic microenvironment, via hypoxia-inducible factor 2α, and modulation of the proteasome-mediated degradation of receptor tyrosine kinases could also explain EGFR overexpression in the absence of mutations [21,22]. As suggested by this and other similar studies [14], mutations of the tyrosine kinase domain of the EGFR gene are not required for EGFR protein overexpression. Therefore, immunohistochemistry for EGFR expression cannot be used as a surrogate for defining EGFR mutation status.
By using pinpoint slide DNA isolation techniques, we demonstrated that evaluation of EGFR mutation status is possible in formalin-fixed, paraffin-embedded archive tissue samples. The advantages of using archival blocks instead of frozen samples for detecting such mutations are evident.
In summary, we found evidence of EGFR protein expression in 74% of urothelial carcinomas. In contrast, using formalin-fixed, paraffin-embedded tissue samples, we did not detect EGFR mutations at exons 19 to 21. Our findings indicate that EGFR overexpression is not necessarily related to the presence of mutations in the tyrosine kinase domain of the gene. Immunohistochemical expression of EGFR may not be useful in predicting therapeutic response to EGFR inhibitors in patients with urothelial carcinomas. To explain EGFR immunohistochemical overexpression, other mechanisms besides mutations in the EGFR kinase domain should be investigated in future studies.
Footnotes
Disclosure: This study was supported by The Johns Hopkins Medicine–Patana Fund for Research, Baltimore, MD, PO1# CA077664 National Cancer Institute/National Institutes of Health Grant, Bethesda, MD, David H. Koch Prostate Cancer Fund, Santa Monica, CA, and the Flight Attendant Medical Research Institute Clinical Innovator Award, Miami, FL.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 3.Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today—a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21:690–6. doi: 10.1200/JCO.2003.05.101. [DOI] [PubMed] [Google Scholar]
- 4.Zachos I, Konstantinopoulos PA, Tzortzis V, et al. Systemic therapy of metastatic bladder cancer in the molecular era: current status and future promise. Expert Opin Investig Drugs. 2010;19:875–87. doi: 10.1517/13543784.2010.496450. [DOI] [PubMed] [Google Scholar]
- 5.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 6.Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Ono M, Kuwano M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin Cancer Res. 2006;12:7242–51. doi: 10.1158/1078-0432.CCR-06-0646. [DOI] [PubMed] [Google Scholar]
- 8.Chintala L, Kurzrock R. Epidermal growth factor receptor mutation and diverse tumors: case report and concise literature review. Mol Oncol. 2010;4:306–8. doi: 10.1016/j.molonc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leone F, Cavalloni G, Pignochino Y, et al. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin Cancer Res. 2006;12:1680–5. doi: 10.1158/1078-0432.CCR-05-1692. [DOI] [PubMed] [Google Scholar]
- 10.Villares GJ, Zigler M, Blehm K, et al. Targeting EGFR in bladder cancer. World J Urol. 2007;25:573–9. doi: 10.1007/s00345-007-0202-7. [DOI] [PubMed] [Google Scholar]
- 11.Blehm KN, Spiess PE, Bondaruk JE, et al. Mutations within the kinase domain and truncations of the epidermal growth factor receptor are rare events in bladder cancer: implications for therapy. Clin Cancer Res. 2006;12:4671–7. doi: 10.1158/1078-0432.CCR-06-0407. [DOI] [PubMed] [Google Scholar]
- 12.Kassouf W, Black PC, Tuziak T, et al. Distinctive expression pattern of ErbB family receptors signifies an aggressive variant of bladder cancer. J Urol. 2008;179:353–8. doi: 10.1016/j.juro.2007.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow NH, Chan SH, Tzai TS, Ho CL, Liu HS. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7:1957–62. [PubMed] [Google Scholar]
- 14.Li AR, Chitale D, Riely GJ, et al. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. J Mol Diagn. 2008;10:242–8. doi: 10.2353/jmoldx.2008.070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popov Z, Gil-Diez-De-Medina S, Ravery V, et al. Prognostic value of EGF receptor and tumor cell proliferation in bladder cancer: therapeutic implications. Urol Oncol. 2004;22:93–101. doi: 10.1016/j.urolonc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Leibl S, Zigeuner R, Hutterer G, Chromecki T, Rehak P, Langner C. EGFR expression in urothelial carcinoma of the upper urinary tract is associated with disease progression and metaplastic morphology. APMIS. 2008;116:27–32. doi: 10.1111/j.1600-0463.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez-Escrig JL, Kelly JD, Neal DE, King SM, Davies BR. Evaluation of the therapeutic potential of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in preclinical models of bladder cancer. Clin Cancer Res. 2004;10:4874–84. doi: 10.1158/1078-0432.CCR-04-0034. [DOI] [PubMed] [Google Scholar]
- 18.Lo HW. Nuclear mode of the EGFR signaling network: biology, prognostic value, and therapeutic implications. Discov Med. 2010;10:44–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Ciardiello F, Tortora G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer. 2003;39:1348–54. doi: 10.1016/s0959-8049(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 20.Jarvela S, Helin H, Haapasalo J, et al. Amplification of the epidermal growth factor receptor in astrocytic tumours by chromogenic in situ hybridization: association with clinicopathological features and patient survival. Neuropathol Appl Neurobiol. 2006;32:441–50. doi: 10.1111/j.1365-2990.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 21.Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, Lee S. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci U S A. 2007;104:13092–7. doi: 10.1073/pnas.0702387104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–70. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]