Abstract
Cardiac-specific expression of the N1325S mutation of SCN5A in transgenic mouse hearts (TG-NS) resulted in long QT syndrome (LQTS), ventricular arrhythmias (VT), and heart failure. In this study we carried out oligonucleotide mircoarray analysis to identify genes that are differentially expressed in the TG-NS mouse hearts. We identified 33 genes in five different functional groups that showed differential expression. None of the 33 genes are ion channel genes. STAT1, which encodes a transcription factor involved in apoptosis and interferon response, showed the most significant difference of expression between TG-NS and control mice (a nearly 10-fold increase in expression, P = 4 × 10−6). The results were further confirmed by quantitative real-time PCR and Western blot analyses. Accordingly, many interferon response genes also showed differential expression in TG-NS hearts. This study represents the first microarray analysis for LQTS and implicates STAT1 in the pathogenesis and progression of LQTS and heart failure.
Keywords: Cardiac sodium channel gene SCN5A mutation N1325S, Long QT syndrome (LQTS) and ventricular tachycardia, Microarray analysis, Dilated cardiomyopathy and heart failure, STAT1
The long QT syndrome (LQTS) is characterized by prolongation of the QT interval and T wave abnormalities on electrocardiograms (ECG) [1,2]. LQTS is associated with symptoms including syncope, seizures and sudden death caused by a specific ventricular arrhythmia, torsade de pointes [1,2]. One of the major genes identified for LQTS is the SCN5A gene on chromosome 3p21-23 (LQT3), which accounts for 10-20% LQTS cases [2,3]. SCN5A encodes a voltage-gated sodium channel Nav1.5, which is mainly expressed in the heart and responsible for the generation and rapid propagation of electrical signals (action potentials) in cardiomyocytes [4,5]. Besides gain-of-function mutations associated with LQTS, loss of function mutations in SCN5A were demonstrated to be involved in the pathogenesis of both Brugada syndrome [6] and progressive cardiac conduction defects (PCCD) [7]. Mutations of SCN5A have also been reported to be involved in dilated cardiomyopathy/heart failure [8,9].
The N1325S mutation in SCN5A is a substitution of an asparagine residue by a serine at position 1325 in the intercellular region of domain III S4-S5 of Nav1.5, and is one of the earliest mutations identified in LQT3 families [3]. It disrupts the Na+ channel inactivation and generates the late persistent INa inward current. Overexpression of the N1325S mutation in Xenopus oocytes and HEK293 cells induced dispersed reopening in the late inactivation phase, which produced a late persistent inward sodium current [10-12]. We have expressed the SCN5A N1325S mutation in the mouse heart (TG-NS mice) [13]. The TG-NS transgenic mice showed prolongation of the QT interval on ECG and high incidences of spontaneous polymorphic VT followed by sudden cardiac death [13,14]. The electrophysiological studies of cardiomyocytes from the transgenic mice showed that the N1325S mutation produced a late sodium current and prolonged the cardiac action potential duration, which is expected to prolong the QT interval on ECG [13,15]. Recent studies also detected the phenotype of dilated cardiomyopathy and heart failure in TG-NS mice [16] as well as in a human patient with the N1325S mutation of SCN5A [15]. Age-dependent apoptosis and abnormal calcium handling were also demonstrated in the TG-NS cardiomyocytes, and are the likely causes of dilated cardiomyopathy and heart failure [16]. However, the molecular mechanism for cardiomyocyte apoptosis in TG-NS mice is not known. In this study we found that the expression of the STAT1 gene was highly induced in TG-NS hearts, which may be a cause of apoptosis in these mice.
The STAT1 gene encodes one of the signal transduction and activator of transcription factors (STATs) which are involved in transduction of signals from various ligands (cytokines, growth factors, stress-induced stimuli) to the nucleus through Janus tyrosine kinases (JAKs) or mitogen-activated protein (MAP) kinases [17]. Seven different STAT family members have been identified, which are activated by different cytokines [18]. STAT1 mediates the response to interferon (IFN)-α and IFN-γ and has been shown to be pro-apoptotic [18]. STAT1-deficient mice are more susceptible to development of tumors, which implicates STAT1 in oncogenesis [17]. No transgenic mice with over-expression of STAT1 were developed, thus, the physiological effect for over-expression of STAT1 is unknown.
Microarray analysis is an unbiased approach to study expression of thousands of genes simultaneously in a system [19,20]. To date, no microarray analysis or other large-scale gene expression studies have been performed for LQTS, either in the humans or mice. Here, we took advantage of our mouse model for LQTS, the TG-NS mice, to explore global gene expression re-programming in these mice. We used mouse oligonucleotide microarrays with 22,690 unique genes to determine gene expression differences between TG-NS and non-transgenic control mice. A surprisingly large number of genes showed differential expression between the two types of mice, which may be partly caused by the marked up-regulation of transcription factor STAT1 as validated by RT-PCR and Western blot analyses. These results implicate STAT1 in the pathogenesis and progression of LQTS and heart failure and offer insights into the observation of cardiomyocyte apoptosis in TG-NS mice.
Materials and methods
Transgenic mice
Human mutant SCN5A gene with the LQTS-causing mutation N1325S was expressed in the mouse heart using a cardiac specific promoter, the mouse alpha-myosin heavy chain (α-mMHC) promoter, and we named this line of transgenic mice as TG-NS. Transgenic mice with cardiac-specific expression of wild type SCN5A, TG-WT, were also lately created. The creation of TG-NS and TG-WT mice was reported by us previously [13,21], and they carry the comparable number of the transgenes and have a comparable level of SCN5A expression. Genotyping of the positive TG-NS mice was performed by polymerase chain reactions (PCR) using genomic DNA isolated from mouse tails/toes using the tail lysis buffer (50 mM Tris-HCl, 100 mM EDTA, 100 mM NaCl, 1% SDS). We used PCR primers 5′-TGT CCG GCG CTG TCC CTG CTG-3′ and 5′-CTC ATG CCC TCA AAT CGT GAC AGA-3′ for specific amplification of the SCN5A transgene and primers 5′-GGC ACC TGC TGC AAC GCT CTT T-3′ and 5′-GGT GGG CAC TGG AGT GGC AAC TT-3′ for amplification of AGGF1 that serves as an internal control for quality of mouse genomic DNA. PCR was performed using standard procedures.
Microarray analysis
Total RNA was prepared from heart tissues of the non-transgenic control and TG-NS mice. First, heart tissues were homogenized by a polytron homogenizer (PT3100, Dispersing and Mixing Technology by Kinematica). Total RNA was then isolated using the TRIzol reagent (Invitrogen). The integrity and purity of the RNA was confirmed visually on a 1% denaturing agarose gel, and by measuring the optical density ratio (A260/A280). Double-stranded complementary DNA (ds-cDNA) was synthesized from 15 μg of total RNA using the Superscript Choice System (Invitrogen) with an HPLC-purified oligo-dT primer containing a T7 RNA polymerase promoter (GENSET, La Jolla, CA) as instructed by the manufacturer. The cDNA was extracted by the Phase Lock Gel (PLG) kit (Eppendorf) and purified by ethanol precipitation. In vitro transcription was performed with 1 μg of ds-cDNA using the ENZO BioArray RNA Transcript Labeling kit (ENZO Diagnostics). Fragmentation of biotinylated cRNA (20 μg), hybridization, washing, and staining were performed following the instructions by Affymetrix by the CWRU Gene Expression Core Facility. The Mouse Genome MOE430A arrays (Affymetrix) were used. Each array contains 22,690 genes.
Statistical Analysis
Microarray data was extracted from scanned images. GeneSpring 7.0 (Silicogenetics) was used to compare the data from three transgenic mice with those from three non-transgenic control littermates. All samples were considered as one group of replicates. The algorithm to generate a list of genes that showed a statistically significant difference between the two groups was described previously [20,22]. The median value for group comparisons was used. All raw data with a score less than zero were set to zero. Genes were further filtered by an absolute call: present (P) or marginally present (M) in the two groups for the up-regulated genes and down-regulated genes.
Quantitative real-time PCR (RT-PCR)
Quantitative RT-PCR was performed using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). Total RNA was extracted from hearts using TRIzol (Invitrogen). Reverse transcription was performed with 5 μg of RNA using the Superscript Choice System (Invitrogen). Primers spanning exon-intron junctions were designed to avoid amplification of genomic DNA. PCR conditions were 50°C for 2 minutes and 95°C for 10 minutes followed by 40 cycles of 95°C for 15s and 60°C for 1min. Fluorescence changes were monitored with SYBR Green PCR Supermix (VWR) after every cycle, and melting curve analysis was performed at the end of 40 cycles to verify PCR product (0.5°C/s increase from 55°C-99°C with continuous fluorescence reading). The 18S gene was used to normalize samples for comparison. To quantify changes in gene expression, the ΔΔCt method was used to calculate the relative fold changes as previously described [23].
Western blot analysis
To determine the expression level of the STAT1 protein, total proteins were extracted from mouse hearts. Hearts were homogenized with Polytron, and lysed on ice with the lysis buffer (0.5% NP-40, 20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA). The protein concentration was measured using the Bradford method (Bio-Rad). Equal amounts of protein extracts were separated on 10% SDS polyacrylamide gels by electrophoresis. Western blot analysis was performed as described previously [6]. The blots were incubated with agitation at room temperature in the presence of a rabbit polyclonal anti–STAT1 antibody (Santa Cruz Biotechnology) (diluted in 1:500 in 0.3% BSA in PBST). The signal was detected using enhanced chemiluminescence (ECL kit, Amersham Biosciences). An anti-β-actin antibody and an anti-GAPDH antibody (Sigma-Aldrich) were used as loading controls at 1:1,000 dilution in PBST.
Results
Identification of genes differentially expressed in the hearts from TG-NS mice
We investigated the gene expression profiles from three TG-NS mice and three age- and sex-matched control mice (6-8 months of age, male) using the Affymetrix Mouse Genome MOE430A Arrays. 2,492 of 22,690 genes showed significant expression differences between the two groups if P ≤ 0.05 (Welch t-test). Because of the large number of genes identified, filters exceeding 2- and 5-fold changes and several different P values were applied. The results are summarized in Table 1. With P ≤ 10−5, only two genes, STAT1 encoding signal transduction and activator of transcription factor 1 and DLM-1 encoding Z-DNA binding protein 1, showed an expression difference between two groups of mice. At P value of 10−4, 11 genes showed differential expression of ≥ 5 fold, 9 up-regulated and 2 down-regulated (9↑, 2↓). At P value of 10−3, 33 genes (31↑, 2↓) showed expression differences of ≥ 5 fold. The number of genes increased to 14 at P = 10−4 and 65 at P = 10−3 if the cut off expression difference was set to 2-fold (Table 1).
Table 1.
Summary data for the number of genes showing differential expression in TG-NS hearts
| Fold difference of expression |
P value | |||
|---|---|---|---|---|
| 0.01 | 0.001 | 0.0001 | 0.00001 | |
| Up-regulated genes | ||||
| 2-fold | 470 | 54 | 12 | 2 |
| 5-fold | 101 | 31 | 9 | 2 |
| Down-regulated genes | ||||
| 2-fold | 306 | 11 | 2 | 0 |
| 5-fold | 53 | 2 | 2 | 0 |
Our further analysis was focused on genes showing a large differential expression difference, i.e. those showing 5-fold expression differences and P value of ≤ 0.001. As a result, 31 genes were found to be up-regulated and 2 genes down-regulated in TG-NS mice (Table 1). These genes can be divided into five functional groups: 11 genes are involved in interferon-responses; 4 genes are related to apoptosis and inflammation; 4 genes may mediate other immune responses; 4 genes encode enzymes; 11 unknown genes (Table 2 and Supplementary Table 1).
Table 2.
Genes showing differential expression in TG-NS hearts by microarray analysis
| A. Genes involved in interferon (IFN)-signaling pathways | ||||
| Accession # | Symbol | Gene | P value |
Fold of
change |
|
| ||||
| NM_008332.1 | Ifit 2 | Interferon-induced protein with tetratricopeptide repeats 2 |
1.3 × 10−4 | 43.9 |
| NM_011909.1 | Usp18 | Ubiquitin specific protease 18 | 6.5 × 10−5 | 31.8 |
| NM_010501.1 | Ifit 3 | Interferon-induced protein with tetratricopeptide repeats 3 |
8.3 × 10−4 | 31.5 |
| NM_008331.1 | Ifit 1 | Interferon-induced protein with tetratricopeptide repeats 1 |
2.2 × 10−4 | 26.0 |
| NM_016850.1 | Irf 7 | Interferon regulatory factor 7 | 1.3 × 10−4 | 18.6 |
| NM_018734.1 | Gbp3 | Guanylate nucleotide binding protein 3 | 1.8 × 10−4 | 13.5 |
| AF136520 | Dlm-1 | Tumor stroma and activated macrophage protein DLM-1 |
8.6 × 10−6 | 12.9 |
| NM_009283 | STAT1 * | Signal transducer and activator of transcription 1 |
4.0 × 10−6 | 9.8 |
| AB067533.1 | Oasl 9 | 2,5-Oligoadenylate synthetase-like 9 | 9.8 × 10−4 | 6.6 |
| BC018470 | Oasl 1G | 2′-5′ Oligoadenylate synthetase 1G | 2.0 × 10−4 | 6.5 |
| AY090098.1 | Isg 12 | Interferon stimulated gene 12 | 2.7 × 10−4 | 6.2 |
|
| ||||
| B. Genes with a potential role in apoptosis and inflammation | ||||
| Accession # | Symbol | Gene | P value |
Fold of
change |
|
| ||||
| NM_009283 | STAT1 * | Signal transducer and activator of transcription 1 |
4.0 × 10−6 | 9.8 |
|
| ||||
| AY075132 | Helicard | Helicard | 2.1 × 10−5 | 7.9 |
| AF220142 |
Trim34
delta |
Tripartite motif protein 34 delta | 8.1 × 10−5 | 5.5 |
| NM_126166 | Tlr3 | Toll-like receptor 3 | 2.5 × 10−4 | 5.4 |
STAT1 is involved in both IFN signaling and apoptosis.
Validation of microarray results by real-time PCR (RT-PCR)
To verify the results from the microarray analysis, quantitative RT-PCR was performed with 9 additional TG-NS and 7 control mice (6-8 months of age). Results from RT-PCR analysis of 6 highly significant genes, including STAT1, Usp18, Oas1 1G, Helicard, Trim34 delta, and Phgdh, are shown in Table 3. The real-time PCR results generally confirmed the results from microarray analysis. Linage regression analysis demonstrated a strong positive correlation between the two technological platforms with R = 0.93.
Table 3.
Conformation of results from mircroarray analysis by RT-PCR
| Gene | Microarray1 | RT-PCR2 |
|---|---|---|
| STAT1: Signal transducer and activator of transcription 1 | 9.8 | 8.4 |
| Usp18: Ubiquitin specific protease 18 | 31.8 | 65.9 |
| Oas1 1G: 2′-5′ Oligoadenylate synthetase 1G | 6.5 | 14.9 |
| Helicard: Helicard | 7.9 | 3.4 |
| Trim34 delta: Tripartite motif protein Trim34 delta | 5.5 | 7.9 |
| Phgdh: 3-Phosphoglycerate dehydrogenase | 53.8 | 66.3 |
data of fold difference of expression between 3 TG-NS and 3 control mice
data of fold difference of expression between 9 TG-NS and 7 control mice; no overlapping of samples between the microarray and RT-PCR studies.
STAT1 showed the most significant differential expression in TG-NS mice
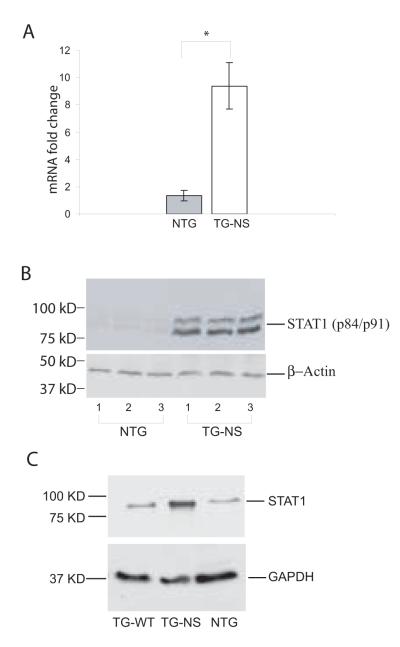
STAT1 is the gene showing the most significant expression difference between TG-NS and control mice with a P value of 4.0 × 10−6 and a fold difference of 9.8 (Table 2). Thus, in addition to the RT-PCR analysis described above (Fig. 1A, Table 3), Western blot analysis was also used to validate the finding of increased STAT1 expression in TG-NS mice compared to control littermate mice (Fig. 1B). Western blot analysis demonstrated that the basal expression level of the STAT1 protein in non-transgenic control mice was low, however, it dramatically increased in TG-NS hearts (Fig. 1B).
Fig. 1.
Markedly increased expression of STAT1 in TG-NS hearts. (A) Quantitative RT-PCR analysis with RNA isolated from 9 TG-NS and 7 NTG (non transgenic control) hearts (age = 8 months). Data were normalized to 18S RNA expression in the same samples and reported as fold changes (mean ± SE) from levels in control mice (*P =0.0016 0.05). (B) Western blot analysis of STAT1 from three NTG and three TG-NS hearts (age = 8 months). β-actin was used as loading control. The experiment was repeated 3 times and similar results were obtained. (C) Western blot analysis of STAT1 from non-transgenic control (NTG), TG-NS, and TG-WT hearts (age =6-8 months). The experiment was repeated twice and similar results were obtained. Note that TG-NS and TG-WT have the comparable copy number of the transgene and a similar level of expression of the cardiac sodium channel (see reference by Zhang et al. [23]). The only difference between these two types of mice is that TG-NS mice carry the mutant N1325S SCN5A and TG-WT mice carry the wild type SCN5A.
Recently, we created and studied transgenic mice with cardiac-specific expression of wild type SCN5A (TG-WT) (in contrast to TG-NS with mutant SCN5A containing the N1325S mutation) [21]. TG-WT mice did not develop LQTS, VT, or heart failure [21], thus they could serve as an excellent control for TG-NS mice (note that the TG-WT mice were not available when the microarray project started). Western blot analysis was used to compare the expression level of the STAT1 protein between TG-NS to TG-WT mice. As shown in Fig. 1C, the expression level of STAT1 in TG-WT heart was comparable to that in non-transgenic control hearts, but much lower than that in TG-NS hearts. These results suggest that induction of STAT1 expression is specific to TG-NS mice with the SCN5A mutation N1325S.
Discussion
Microarray analysis is a large scale study that can provide unbiased assessment of expression of thousands of genes in a cell or tissue simultaneously. To date, no microarray analysis has been reported for LQTS either in the humans or mice. We performed a microarray study using a mouse model for LQTS, the TG-NS mice with cardiac expression of the SCN5A mutation N1325S associated with LQTS. Microarray analysis revealed 33 genes showing marked differential expression between TG-NS and wild type mice (>5 fold difference, P < 0.001). The results from microarray analysis were almost identical to that from the follow-up RT-PCR analysis using independent samples for all selected genes. Our results suggest the involvement of expression remodeling in the progression of LQTS. Furthermore, the results from this study suggest that in addition to its effects on basic biophysical properties of the cardiac sodium channel, the N1325S mutation has a more profound effect on cardiomyocytes in vivo.
The gene that showed the most significant difference between TG-NS and wild type mice is STAT1. Follow-up RT-PCR and Western blot analysis confirmed that expression of STAT1 was markedly increased in TG-NS myocytes compared to non-transgenic control cells. Further analysis showed that STAT1 protein expression was also much higher in TG-NS hearts than in TG-WT hearts with cardiac expression of wild type SCN5A (Fig. 1C). STAT1 is a key signaling protein that functions as a transducer of cytokine signaling and as a sensor responding to cellular stresses, in particular, IFN response [18]. Thus, it was interesting to note that many genes involved in the IFN response also showed increased expression in TG-NS myocytes, for example, Usp18, Ifit1, Ifit2, Ifit3, Irf7, Gbp3, Dlm-1, Oasl 9, Oasl 1G, and Isg 12 that all showed many fold increases of expression (Table 2). The molecular mechanism for induction of high STAT1 expression in TG-NS hearts is not clear, however, abnormal handling of intracellular calcium transients detected in TG-NS cardiomyocytes [16] may be a likely cause.
STAT-1 has been shown to induce apoptosis. Human fibroblast cells deficient in STAT1 were resistant to TNF-α-induced apoptosis [24]. Neonatal rat cardiomyocytes subjected to ischemia for 4 hours showed increased expression of STAT1, and cardiomyocytes transfected with STAT1 showed increased apoptosis with exposure to ischemia [25]. A trend of increased apoptosis in the absence of exposure to ischemia was also detected in cardiomyocytes transfected with a STAT1 construct compared to control cells transfected with the vector (Fig. 3A in Stephanou et al. [25]). Because age-dependent apoptosis has been detected in TG-NS mouse hearts [16], we speculate that increased expression of STAT1 may be a cause for apoptosis in these mice.
In summary, our microarray analysis of a transgenic mouse model for LQTS, TG-NS, demonstrated that gene expression remodeling existed in these mice. Of a particular interest was the finding of highly increased STAT1 expression, which may provide insights into the findings of cardiomyocyte apoptosis in TG-NS mice and high risk of heart failure in these mice.
Supplementary Material
Acknowledgements
We gratefully acknowledge the expert technical assistance and advice from Yiqing Jiang, Xiaoli Tian, Lin Li and other members of Wang Laboratory. This study was supported by an NIH grant (R01 HL66251) and an AHA Established Investigator award (0440157N) to Q.K.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- [2].Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- [3].Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, Schwartz PJ, Keating MT. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum. Mol. Genet. 1995;4:1603–1607. doi: 10.1093/hmg/4.9.1603. [DOI] [PubMed] [Google Scholar]
- [4].Gellens ME, George AL, Jr., Chen LQ, Chahine M, Horn R, Barchi RL, Kallen RG. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc. Natl. Acad. Sci. U. S. A. 1992;89:554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu L, Nishiyama K, Hollyfield JG, Wang Q. Localization of Nav1.5 sodium channel protein in the mouse brain. NeuroReport. 2002;13:2547–2551. doi: 10.1097/01.wnr.0000052322.62862.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O’Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- [7].Tan HL, Bink-Boelkens MTE, Bezzina CR, Viswanathan PC, Beaufort-Krol GCM, van Tintelen PJ, van den Berg MP, Wilde AAM, Balser JR. A sodium-channel mutation causes isolated cardiac conduction disease. Nature. 2001;409:1043–1047. doi: 10.1038/35059090. [DOI] [PubMed] [Google Scholar]
- [8].Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ, Horton SC, Rodeheffer RJ, Anderson JL. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA. 2005;293:447–454. doi: 10.1001/jama.293.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zicha S, Maltsev VA, Nattel S, Sabbah HN, Undrovinas AI. Post-transcriptional alterations in the expression of cardiac Na+ channel subunits in chronic heart failure. J. Mol Cell Cardiol. 2004;37:91–100. doi: 10.1016/j.yjmcc.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bennett PB, Azawa KY, Makita N, George AL., Jr. Molecular mechanism for an inherited cardiac arrhythmia [see comments] Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- [11].Dumaine R, Wang Q, Keating MT, Hartmann HA, Schwartz PJ, Brown AM, Kirsch GE. Multiple mechanisms of Na+ channel--linked long-QT syndrome. Circ. Res. 1996;78:916–924. doi: 10.1161/01.res.78.5.916. [DOI] [PubMed] [Google Scholar]
- [12].Wang DW, Yazawa K, George AL, Jr., Bennett PB. Characterization of human cardiac Na+ channel mutations in the congenital long QT syndrome. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13200–13205. doi: 10.1073/pnas.93.23.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tian XL, Yong SL, Wan X, Wu L, Chung MK, Tchou PJ, Rosenbaum DS, Van Wagoner DR, Kirsch GE, Wang Q. Mechanisms by which SCN5A mutation causes cardiac arrhythmias and sudden death in vivo. Cardiovasc. Res. 2004;61:256–267. doi: 10.1016/j.cardiores.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tian XL, Cheng Y, Zhang T, Liao ML, Yong SL, Wang QK. Optical mapping of ventricular arrhythmias in LQTS mice with SCN5A mutation N1325S. Biochem. Biophys. Res. Commun. 2007;352:879–883. doi: 10.1016/j.bbrc.2006.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yong SL, Ni Y, Zhang T, Tester DJ, Ackerman MJ, Wang QK. Characterization of the cardiac sodium channel SCN5A mutation, N1325S, in single murine ventricular myocytes. Biochem. Biophys. Res. Commun. 2007;352:378–383. doi: 10.1016/j.bbrc.2006.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang T, Yong S, Drink J, Popvic Z, Wang Q. Late sodium currents generated by mutation N1325S in sodium channel gene SCN5A cause heart failure. Circulation. 2006;114:II–65. [Google Scholar]
- [17].Adamkova L, Souckova K, Kovarik J. Transcription protein STAT1: biology and relation to cancer. Folia Biol. (Praha) 2007;53:1–6. [PubMed] [Google Scholar]
- [18].Stephanou A, Latchman DS. STAT-1: a novel regulator of apoptosis. Int. J. Exp. Pathol. 2003;84:239–244. doi: 10.1111/j.0959-9673.2003.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Archacki S, Wang Q. Expression profiling of cardiovascular disease. Hum Genomics. 2004;1:355–370. doi: 10.1186/1479-7364-1-5-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Archacki SR, Angheloiu G, Tian XL, Tan FL, DiPaola N, Shen GQ, Moravec C, Ellis S, Topol EJ, Wang Q. Identification of new genes differentially expressed in coronary artery disease by expression profiling. Physiol Genomics. 2003;15:65–74. doi: 10.1152/physiolgenomics.00181.2002. [DOI] [PubMed] [Google Scholar]
- [21].Zhang T, Yong SL, Tian XL, Wang QK. Cardiac-specific overexpression of SCN5A gene leads to shorter P wave duration and PR interval in transgenic mice. Biochem. Biophys. Res. Commun. 2007;355:444–450. doi: 10.1016/j.bbrc.2007.01.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tan FL, Moravec CS, Li J, Pperson-Hansen C, McCarthy PM, Young JB, Bond M. The gene expression fingerprint of human heart failure. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11387–11392. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [24].Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278:1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- [25].Stephanou A, Brar BK, Scarabelli TM, Jonassen AK, Yellon DM, Marber MS, Knight RA, Latchman DS. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J. Biol. Chem. 2000;275:10002–10008. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.