Abstract
My lab investigates genetic control of autoimmune disease and autoimmune phenotypes using a series of nonobese diabetic (NOD) congenic mice. NOD congenic mice have regions from B6/B10 introgressed onto the NOD genetic background, which reduces the severity/incidence of autoimmune diabetes. We have demonstrated, however, that while diabetes is reduced, other autoimmune phenotypes and diseases arise in NOD congenic mice. Mapping the genomic regions responsible for these phenotypes has produced novel insights into genetic control of autoimmunity. This review will illustrate some of the genetically controlled phenotypes we have investigated, which shed light upon autoimmune features relevant to human type 1 diabetes, systemic lupus erythematosus, and primary biliary cirrhosis.
Keywords: Type 1 diabetes, Systemic lupus erythematosus, Primary biliary cirrhosis, T cells, Immunogenetics, Autoimmunity
Introduction
“Genetic control of autoimmunity” can manifest in many ways. In its simplest sense, it refers to the effect of a single nucleotide polymorphism (SNP) on quantitative gene expression. A higher level of complexity occurs when loci from one mouse strain bred onto a different genetic background changes an immune phenotype. In other words, immune phenotypes in congenic mice can vary from either parent strain. A third level of complexity occurs when congenic loci introgressed onto a parent strain results in an entirely different autoimmune disease from that of the parent strain. In other words, congenic loci can actually “switch” the disease state. We have proven that this highly complex form of genetic control of autoimmune disease occurs in NOD congenic mice. The implications of this finding are significant for human diseases, because it offers an explanation for increased incidence of phenotypically diverse autoimmune diseases distributed in human kindreds.
My lab works exclusively with NOD and NOD congenic mice. NOD mice spontaneously develop type 1 (autoimmune) diabetes (1). The NOD mouse is one of the most extensively studied autoimmune models from the standpoint of genetics. The first genomewide scan of NOD to detect disease associated loci (termed insulin dependent diabetes, or Idd, loci) was performed 15 yr ago (2), and over 20 Idd loci have been identified. The development of NOD Idd congenic mice provided proof that regions implicated by the genome scan actually prevented diabetes, because these regions bred onto the NOD background decreased incidence of diabetes (3). The disease-protective effect increased as more Idd loci were introgressed. This work produced two major questions. (1) What genes(s) in the Idd intervals were protective? (2) How and where did the genes act to prevent diabetes? What is the mechanism of Idd action?
Significant progress has been made in answering these questions. The Wicker lab has produced strong evidence suggesting that: (1) Idd3 is likely IL-2, (2) Idd5.1 is most likely CTLA4, and (3) CD137 is a strong candidate for Idd9.3 (4-6). The relevance of these studies is evident from genome-sequencing studies showing that many regions of the mouse genome are highly homologous to the human genome, i.e., in many areas the homologous genes occur in the same order across large stretches of mouse and human genome. Furthermore, functional studies of the above candidate genes suggest that some of the same genes may be acting in human and mouse type 1 diabetes. Particular human CTLA4 variants have been associated with increased incidence of diabetes just as in NOD mice (7). Of interest is the finding that while the same molecule may be implicated in both species, the mechanism of its disease-associated action can vary. In the case of CTLA4, in humans the associated allele affects levels of soluble CTLA4, while the NOD CTLA4 variant affects a novel ligand-independent form of the molecule (7,8).
The basic principle of congenic mapping is to retain the phenotype of interest while narrowing the genetic interval. This is illustrated by the above-mentioned studies: e.g., the Idd3 region retains its effect on diabetes incidence even when narrowed to an interval less than one megabase long (4). If a “favorite” candidate gene is outside the narrowed interval, but diabetes protection remains, that gene is excluded as a disease candidate gene. This is well illustrated by CD28, which is adjacent to CTLA4 on chromosome one and was a reasonable candidate gene, but was excluded by congenic mapping (9). The same reasoning applies to immune phenotypes in congenic mice. It must be realized that any phenotype expressed by an NOD congenic strain could be due to gene(s) in the interval that are different from the genes preventing diabetes. Hence work on the mechanism by which congenic loci protect from disease must proceed with great caution. My lab has studied immunophenotypes controlled by Idd regions and “followed” these phenotypes as the genetic interval is reduced via congenic mapping. We have also documented the modulation of these phenotypes in novel congenic mice that manifest diverse autoimmune disease states.
Immune Phenotypes Influenced by Idd Loci: General Effects of NOD non-MHC Regions
The original genome scan in NOD mice utilized B10.G7 congenic mice, so that all mice in the cross carried the NOD MHC II, I-Ag7, which is strongly implicated in disease pathogenesis. My lab took this as a starting point to investigate phenotypes in congenic mice, i.e., we have been concerned with the action of non-MHC loci. By comparing CD4+ T cells in NOD with B6.G7 MHC congenic mice, we demonstrated broad non-MHC genetic control of several immune phenotypes important to T cell function (10-12). Focusing on non-MHC control of T cell effector function, we showed that NOD peripheral lymphoid populations, when activated either by cognate antigen or polyclonally with Con A, produced significantly higher IFN-γ and less IL-4/IL-10 compared to B6.G7 CD4+ T cells (10). We proved that this was a feature of the non-MHC NOD genetic background by replicating this finding in a second NOD MHC congenic, comparing NOD-H2b to B6 mice (10). Next we demonstrated that this was a feature intrinsic to NOD CD4+ T cells, because purified naïve CD4+ T cells from NOD mice also produced more IFN-γ and less IL-4 compared to B6.G7 CD4+ T cells when stimulated with anti-CD3 and anti-CD28 (11). Moreover, this cytokine phenotype was entirely independent from both the cell cycle and the antigen-presenting-cell genetic background, thus truly reflecting an intrinsic, non-MHC controlled feature of NOD CD4+ T cells (11). Using these same NOD and B6.G7 mice, we next demonstrated that non-MHC regions, including some outside the MHC on chromosome 17, controlled the “set point” regulating CD4:CD8 ratios in mice. The mice have dramatically different, stable ratios of CD4:CD8, and these ratios were entirely genetically controlled (12).
These studies demonstrate both the strength and weakness of the genetic approach. The strength is to use mice that share distinct genetic regions, but differ at other regions, as internal controls to assess phenotypical differences. The weakness of the approach is revealed when the genetic differences are too great. It becomes impossible to determine if the phenotype, which is demonstrably genetically controlled, is in fact relevant to disease. Moreover, it is impossible to localize the genetic control—smaller intervals are needed. Realizing these drawbacks spurred our next set of investigations.
Genetic Control of Autoantibody Production: Effect of CD137
An example of a much smaller genetic region impacting a highly significant autoimmune phenotype is found in our work on Idd9.3 congenic mice (13). We first discovered that NOD.c3c4 mice, with B6/B10 genetic regions from chromosomes three and four on an NOD background, developed antinuclear and anti-Smith antibodies at a high penetrance not seen in NOD mice (14) (see below). We next investigated NOD.c3 vs NOD.c4 mice; only NOD.c4 mice developed the autoantibodies (14). The discovery of anti-Sm antibodies in high penetrance was important, because they are considered highly specific for human systemic lupus erythematosus (SLE). Hence, we proceeded to map this phenotype by a congenic approach. Using mice with progressively smaller regions on chromosome four, we showed that genetic control of anti-Smith autoantibody production in NOD congenic mice is mediated by a 5.6 mb region on chromosome four. Moreover, this region contains Idd9.3, which has been mapped to a 1.2 mb interval within the larger physical region (6,15).
Does this mean that an allelic variant of CD137, in a simple fashion “causes” anti-Smith antibody production? Absolutely not, since the B10 variant of CD137, when on a B10 background, does not result in anti-Sm antibodies. The B10 CD137 appears to have two effects: it reduces diabetes in NOD mice, but it also interacts with other NOD genes to amplify autoanti-body production, which is not present in NOD mice with the NOD CD137 allele. We decided to investigate the properties of NOD CD137 in further detail.
NOD CD137 Enhances Diabetes via Defective T Regulatory Costimulation
CD137 is an inducible member of the TNF receptor superfamily expressed on activated T cells (16). It is upregulated on T cells 48 h after activation in vitro, and functions to costimulate T cells (17,18). The CD137 ligand is expressed on activated antigen presenting cells (APCs), especially at the sites of inflammation (19-22). CD137 stimulation augments T cell proliferation, and can function as a T cell costimulatory molecule in the absence of CD28 (18,23). Cannons et al. had previously demonstrated that the NOD allele of CD137, compared to the B10 allele, had decreased T cell signaling; NOD T cells stimulated with a CD137L produced less IL-2 than NOD.Idd9.3 congenic T cells (15). They speculated that the NOD allele contributed to a defective regulatory response.
We investigated the mechanisms of CD137 action in autoimmune diabetes by using an agonistic anti-CD137 antibody, in collaboration with Robert Mittler (submitted). NOD mice treated with anti-CD137 had significantly reduced diabetes. Moreover, anti-CD137 did not prevent insulitis, nor did it eliminate autoreactive T cells. The mechanism of protection was via quantitative increase in CD4+CD25+ T cells, which we show express CD137. The induced CD25+ cells were Foxp3+ and exhibited suppressor function in vitro. Moreover, they strongly protected from diabetes in an NOD-scid transfer model (Ridgway lab, submitted).
These studies show another way to approach analysis of congenic intervals. When a strong candidate molecule emerges in a relatively small region, direct mechanistic studies can be preformed. To our knowledge this is one of the first demonstrations of a therapeutic mechanism mediated via an Idd candidate molecule.
Genetic Control of Autoimmune Disease: “Switching” the Autoimmune Disease Manifestation via Congenic Manipulation
We have illustrated how congenic intervals can affect immunophenotypes. Next we will illustrate underlying genetic control of what appeared to be entirely unrelated organ-specific autoimmune diseases. Single Idd congenic mice showed decreased diabetes incidence; double and triple congenic mice had even greater decrease (3). The NOD.c3c4 mouse was constructed with the hypothesis that multiple Idd loci should totally suppress disease, which in fact was demonstrated: NOD.c3c4 mice develop no diabetes and almost no insulitis (6). We demonstrated, however, that NOD.c3c4 mice develop an entirely different autoimmune disease: an aggressive autoimmune biliary disease (ABD), which leads to liver failure and death (14).
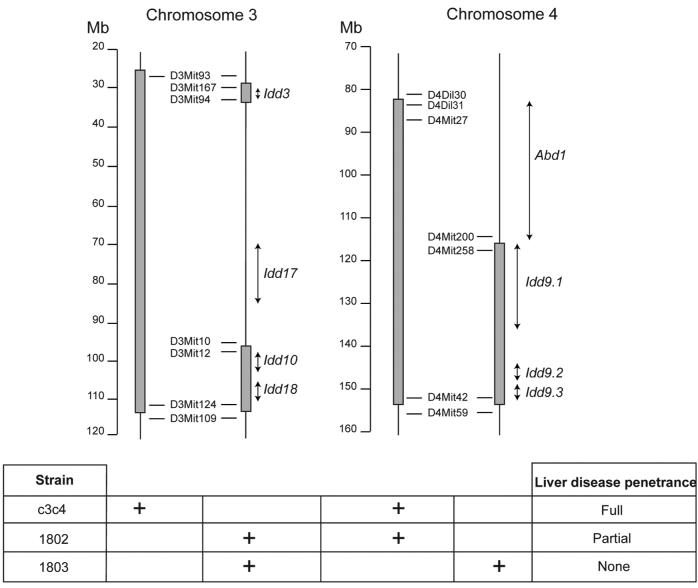
The ABD of NOD.c3c4 mice is a true autoimmune disease; splenocytes from diseased mice can transfer disease to irradiated mice (14). In conjunction with our close collaborator in this work, Dr. Linda Wicker, we have subsequently constructed an NOD.c3c4-scid mouse, and demonstrated that T cells can transfer disease into the c3c4-scid, again fulfilling the basic criteria to demonstrate an autoimmune disease (24). Moreover, we have mapped the first ABD locus, again by utilizing a congenic mapping approach (see Fig. 1). Mice with a highly truncated chromosome three interval, when combined with the c4 interval from c3c4 mice (strain 1802), also get ABD, although with decreased penetrance (Fig. 1). Moreover, mice differing from strain 1802 only by lacking the upper portion of the c4 interval (strain 1803) do not get liver disease. This region of chromosome four is thus not only essential to developing liver disease, but also does not contain any Idd loci. Because it uniquely contributes to the liver disease pathogenesis, it thus defines the first autoimmune biliary disease locus, abd1 (24).
Fig. 1.
A congenic mapping approach to analyzing genetic control of autoimmune disease. The dark vertical bars represent B6/B10 genetic intervals on the NOD background in NOD.c3c4 and related congenic mice. The scale is in megabases. Representative microsatellite markers demarcate the boundaries of the intervals. Positions of Insulin dependent diabetes (Idd) loci and the newly defined autoimmune biliary (abd) locus are shown in italics. The relative penetrance of ABD in each strain is shown in the chart on the bottom, which also depicts the genetic intervals carried in each strain.
Because NOD diabetes is of obvious relevance to human disease, is the ABD of NOD.c3c4 mice of any relevance to human autoimmunity? We addressed this question in conjunction with another major collaborator with our lab, Dr. Eric Gershwin. Primary biliary cirrhosis is a human disease manifest by small bile duct destruction and anti-pyruvate dehydrogenase autoantibodies. We demonstrated that NOD.c3c4 mice developed autoantibodies to the inner lipoyl domain of pyruvate dehydrogenase. Moreover, histologically we demonstrated that CD4+ cells were invading biliary epithelium and resulting in non-suppurative destructive cholangitis, giant cell formation, and macrophage aggregation—all histological features of human primary biliary cirrhosis (24). The combination of spontaneous anti-PDC-E2 antibodies and characteristic histological changes establish the NOD.c3c4 mouse as the first spontaneous mouse model of primary biliary cirrhosis (24).
The fundamental result of the NOD.c3c4 studies is that short genetic intervals (from a diabetes-protected strain) placed on the NOD background prevent diabetes, but result in an entirely different autoimmune disease. This result is both very strange and very important. It is strange because it boggles common sense: how can mice that are 95% genetically identical (NOD, NOD.c3c4) manifest completely different organ specific autoimmune diseases? What immune mechanisms are responsible for switching the disease pheno-type? Our approach to this will again utilize a congenic mapping scheme. The genetic intervals proven necessary for the liver disease (as shown in Fig. 1) will be reduced and rearranged to produce a minimal ABD congenic mouse, with the ultimate goal of identifying specific candidate genes in the intervals. This is a time- and resource-consuming approach; however, its importance lies in the demonstrated relevance of mouse autoimmunogenetic studies to human autoimmunity. Our ultimate goal is to continue to identify genetically controlled immune pathways in mice that will have human homologs, and thus shed light upon genetic control of autoimmunity in humans. The finding of entirely different autoimmune diseases emerging from genetically similar mice raises the possibility that apparently divergent autoimmune diseases in humans might have common underlying immunogenetic pathways amenable to analysis and remedy.
Acknowledgments
Dr. Ridgway is funded by grants NIH RO1 DK60714-01A1, NIH R01 DK074768, and NIH RFA A102-006.
Biography

References
- 1.Ridgway WM. The non obese diabetic (NOD) mouse: a unique model for understanding the interaction between genetics and T cell responses. Rev Endocr Metab Disord. 2003;4:263–269. doi: 10.1023/a:1025104429334. [DOI] [PubMed] [Google Scholar]
- 2.Todd JA, Aitman TJ, Cornall RJ, et al. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature. 1991;351:542–547. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- 3.Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 4.Lyons PA, Armitage N, Argentina F, et al. Congenic mapping of the type 1 diabetes locus, Idd3, to a 780-kb region of mouse chromosome 3: identification of a candidate segment of ancestral DNA by haplotype mapping. Genome Res. 2000;10:446–453. doi: 10.1101/gr.10.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill NJ, Lyons PA, Armitage N, Todd JA, Wicker LS, Peterson LB. NOD Idd5 locus controls insulitis and diabetes and overlaps the orthologous CTLA4/IDDM12 and NRAMP1 loci in humans. Diabetes. 2000;49:1744–1747. doi: 10.2337/diabetes.49.10.1744. [DOI] [PubMed] [Google Scholar]
- 6.Lyons PA, Hancock WW, Denny P, et al. The NOD Idd9 genetic interval influences the pathogenicity of insulitis and contains molecular variants of Cd30, Tnfr2, and Cd137. Immunity. 2000;13:107–115. doi: 10.1016/s1074-7613(00)00012-1. [DOI] [PubMed] [Google Scholar]
- 7.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 8.Vijayakrishnan L, Slavik JM, Illes Z, et al. An autoimmune disease-associated CTLA-4 splice variant lacking the B7 binding domain signals negatively in T cells. Immunity. 2004;20:563–575. doi: 10.1016/s1074-7613(04)00110-4. [DOI] [PubMed] [Google Scholar]
- 9.Wicker LS, Chamberlain G, Hunter K, et al. Fine mapping, gene content, comparative sequencing, and expression analyses support Ctla4 and Nramp1 as candidates for Idd5.1 and Idd5.2 in the nonobese diabetic mouse. J Immunol. 2004;173:164–173. doi: 10.4049/jimmunol.173.1.164. [DOI] [PubMed] [Google Scholar]
- 10.Koarada S, Wu Y, Ridgway WM. Increased entry into the interferon-gamma effector pathway by CD4+ T cells selected by I-Ag7 on a nonobese diabetic (NOD) versus C57B1/6 genetic background. Immunol. 2001;167(3):1693–1702. doi: 10.4049/jimmunol.167.3.1693. [DOI] [PubMed] [Google Scholar]
- 11.Koarada S, Wu Y, Olshansky G, Ridgway WM. Increased nonobese diabetic Th1:Th2 (IFN-gamma:IL-4) ratio is CD4+ T cell intrinsic and independent of APC genetic background. J Immunol. 2002;169:6580–6587. doi: 10.4049/jimmunol.169.11.6580. [DOI] [PubMed] [Google Scholar]
- 12.Koarada S, Wu Y, Yim YS, Wakeland EW, Ridgway WM. Nonobese diabetic CD4 lymphocytosis maps outside the MHC locus on chromosome 17. Immunogenetics. 2004;56:333–337. doi: 10.1007/s00251-004-0702-1. [DOI] [PubMed] [Google Scholar]
- 13.Irie J, Wu Y, Sass DA, Ridgway WM. Genetic control of anti-Sm antibody production in NOD congenic mice narrowed to the Idd9.3 region. Immunogenetics. 2006;58:9–14. doi: 10.1007/s00251-005-0066-1. [DOI] [PubMed] [Google Scholar]
- 14.Koarada S, Wu Y, Fertig N, Sass DA, Nalesnik M, Todd JA, Lyons PA, Fenyk-Melody J, Rainbow DB, Wicker LS, Peterson LB, Ridgway WM. Genetic control of autoimmunity: protection from diabetes, but spontaneous autoimmune biliary disease in a nonobese diabetic congenic strain. J Immunol. 2004;173:2315–2323. doi: 10.4049/jimmunol.173.4.2315. [DOI] [PubMed] [Google Scholar]
- 15.Cannons JL, Chamberlain G, Howson J, et al. Genetic and functional association of the immune signaling molecule 4-1BB (CD137/TNFRSF9) with type 1 diabetes. J Autoimmun. 2005;25:13–20. doi: 10.1016/j.jaut.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 17.Cannons JL, Lau P, Ghumman B, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;1167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 18.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 19.Futagawa T, Akiba H, Kodama T, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 20.Laderach D, Wesa A, Galy A. 4-1BB-ligand is regulated on human dendritic cells and induces the production of IL-12. Cell Immunol. 2003;226:37–44. doi: 10.1016/j.cellimm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Seko Y, Takahashi N, Oshima H, et al. Expression of tumour necrosis factor (TNF) ligand superfamily costimulatory molecules CD30L, CD27L, OX40L, and 4-1BBL in murine hearts with acute myocarditis caused by Coxsackievirus B3. J Pathol. 2001;195:593–603. doi: 10.1002/path.986. [DOI] [PubMed] [Google Scholar]
- 22.Seo SK, Choi JH, Kim YH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–1094. doi: 10.1038/nm1107. erratum 2004; 10:1261. [DOI] [PubMed] [Google Scholar]
- 23.Saoulli K, Lee SY, Cannons JL, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187:1849–1862. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irie J, Wu Y, Wicker LS, et al. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]