Abstract
Background
Arterial bypass graft implantation remains the primary therapy for patients with advanced cardiovascular disease; however, there is no available synthetic small-diameter vascular graft.
Methods
Tissue-engineered vessels were grown from human smooth muscle cells that were seeded on a biodegradable scaffold using a biomimetic perfusion system. The human tissue-engineered vessels (hTEV) were decellularized by a two-step process using a combination of detergents and hypertonic solutions. The mechanical characteristics were assessed by suture retention strength and burst pressure. The decellularized hTEV were implanted as aortic interpositional grafts in nude rats to evaluate in vivo performance as an arterial graft over a 6-week period.
Results
The human tissue-engineered structure formed a vessel composed of smooth muscle cells and the extracellular matrix proteins, including collagen. After decellularization, the collagen matrix remained intact while the cellular components were removed. The mechanical strength of the hTEV after decellularization was similar to human vein in vitro, with a burst pressure of 1,567 ± 384 mm Hg (n = 3) versus 1,680 ± 307 mm Hg for human saphenous vein. The hTEVs had a high patency rate (four of five grafts) without evidence of rupture or aneurysm over a 6-week period as an aortic interpositional graft in a nude rat model. Histologic analysis showed a thin neointima with a confluent endothelium and a subendothelial layer of smooth muscle cells on the explanted tissue-engineered vessels. Transmission electron microscopy on the explanted tissue demonstrated elastin formation in the neointima and intact residual collagen fibers from the tissue-engineered vessel.
Conclusions
The hTEV had a high patency rate and remained mechanically stable as an aortic interpositional graft in a nude rat. The vessel supported the growth of a neointima with endothelial cells and smooth muscle cells. The host remodeling suggested the engineered matrix had a positive effect to create a regenerated vascular graft.
Tissue engineering technology has the potential to generate engineered arterial grafts for multiple cardiovascular applications.1 Engineered vessels for arterial conduits have been developed that have similar structural and functional properties as native vessels.2,3 There are multiple approaches to biologic-based grafts that have been implanted in animal models.2,4–7 Examples of these techniques include collagen gel structures, decellularized native vessels, cell sheets to form a tubular structure, and synthetic biopolymers.2,4–7 Several of these types of grafts have been shown to maintain vessel dimensions during implantation with acceptable patency rates.
Despite successful proof of concept for tissue-engineered vessels in animal models, significant limitations exist that could prevent the widespread clinical implementation of completely autologous engineered arteries. Specifically, aged human smooth muscles produce vessels that have poor mechanical properties when used in vascular tissue engineering.8 Alternative strategies such as using human telomerase reverse transcriptase (hTERT)-infected aged cells or using a bone-marrow progenitor cell as the primary cell source also failed to produce a vessel with mechanics suitable for arterial implantation.8,9
Allogeneic tissues have been used for replacement tissues for cardiovascular therapies.10,11,12 Decellularized native vessels sustain mechanical properties as arterial bypass grafts, and have less immunologic response as compared to cryopreserved tissue.12,13 Engineered vessels have already been shown to retain their mechanical integrity after decellularization using animal cells.14 The advantages, including a decellularization step to produce an allogenic graft, are a reduced lead time and the opportunity to use a “younger” cell source to produce the mechanically robust matrix of the vessel wall. Using banked, nonautologous cells to produce engineered tissues would improve tissue consistency and the ability to screen banked cells for infectious contamination.14 Similar to synthetic grafts, the decellularized engineered graft would be available for immediate use.
In this study, we report the in vitro and in vivo properties of a decellularized tissue-engineered vessel (TEV) made from human vascular smooth muscle cells. This study characterizes the histologic and biomechanical properties of the human tissue-engineered vessel (hTEV) before and after decellularization, which are shown to be similar to native human vessels. We hypothesize that a completely TEV derived from human vascular smooth muscle cells can demonstrate suitable properties for implantation in vitro, and then remain mechanically stable in a rat aortic model over 6 weeks.
METHODS
Isolation of human smooth muscle cells and vessel culture
Human aortae from near the aortic arch were obtained from human transplant donors during organ harvest. The medial layer of the aorta was isolated using sterile instruments. The isolated media layer was then sectioned into pieces approximately 0.3 cm by 0.3 cm and placed lumen-side down in T75 falcon flasks coated in 1% bovine gelatin with M199 plus 20% bovine serum and 1% penicillin/streptomycin as media. The tissue was cultured in an incubator to allow the smooth muscle cells to migrate onto the flasks, and the media was changed every 3 days. After 2 to 3 weeks, smooth muscle cells reached confluency.
Poly-glycolic acid (PGA) mesh (Concordia, Mansfield, Mass) was sutured over a 1-mm diameter silicone tube (Saint Gobain, Valley Forge, Pa) using uncoated Dexon suture (Syneture, Mansfield, Mass). The silicone tubing and bioreactor equipment were sterilized and set up as previously described.2,3 The PGA mesh was sutured around the silicone tubing, and the silicone tubing was threaded through the side-arms of the bioreactor. A suspension of media containing 4.5 million human smooth muscle cells at passage 3 was seeded onto each of the PGA mesh tubes. The culture medium was Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, Calif) supplemented with 15% fetal bovine serum (Hyclone, Logan, Utah), 5% human serum (Gemini Bio-Products, Sacramento, Calif) 2.6 mg/mL HEPES (N-(2-Hydroxyethyl-)piperazine-N′-(2-ethanesulfonic acid); Sigma, St. Louis, Mo), 50 µg/mL proline (Sigma), 20 µg/mL alanine (Sigma), 50 µg/mL glycine (Sigma), 3 ng/mL CuSO4 (Sigma), 10 ng/mL basic fibroblast growth factor (R&D, Minneapolis, Minn), and 10 ng/mL platelet-derived growth factor-bb (R&D). Cultures were allowed to progress for 10 weeks with media changes every 5 days and vitamin C injections every 3 days.3
Decellularization of engineered vessel
The TEV were first treated in a solution containing 8 mM CHAPS (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate; Sigma), 1 M NaCl (Sigma), and 25 mM EDTA (Boston Bioproducts, Ashland, Mass) in phosphate-buffered saline (PBS; Gibco) for 1 hour on a stir plate at 37°C then washed three times in PBS for 10 minutes. The vessels were then treated with a second buffer containing 1.8 mM sodium dodecyl sulfate (Sigma), 1 M NaCl, and 25 mM EDTA in PBS for 1 hour on a stir plate at 37°C and washed three times in PBS. Vessels were treated overnight with EBM2 (without hydrocortisone; Lonza, Basel, Switzerland) with 12% fetal bovine serum and then stored in PBS with 1% penicillin streptomycin at 4°C.15
Vessel characterization
Intraluminal pressure was measured by attaching the vessel to a flow-loop system, and hydrostatic pressure was increased at a rate of approximately 50 mm Hg/sec until failure. Pressure was digitally recorded. Suture retention was measured by a single throw of 6-0 prolene suture (3.5 ± 0.3 mm bite) through the TEV, and then weights were sequentially added (in 5-gram increments). The weight at failure was recorded.2
Collagen was quantified by measuring hydroxyproline levels as previously described. A 1:10 w/w ratio of hydroxyproline and collagen was used to calculate the collagen content of the vessels. The collagen content was calculated as the percentage of dry weight.2
DNA contents of fresh and decellularized vessels were determined fluorometrically, as previously described. Tissue was digested in papain solution under high temperature (60°C for 24) and low pH conditions (for 24 hours), and PicoGreen (Invitrogen, Carlsbad, Calif) was added to the digest, causing the DNA fluorescence at an excitation wavelength of 485 nm and an emission wavelength of 535 nm.14
Histomorphological analysis included intima to media ratios for the native aorta compared with residual engineered vessel to neointima ratio. Using elastin stain (EVG), the area of the intima and media of the native aorta were calculated; in a similar fashion, the area of the residual engineered matrix and neointima were obtained.
Graft implantation
Six female nude rats (Crl: NIH-Foxn1rnu; Charles River Laboratories, Wilmington, Mass) 10- to 12-weeks old were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (10 mg/ kg). The abdominal hair was clipped, and the abdomen was prepared with Betadine and alcohol. A midline laparatomy incision was made, and the abdominal viscera were lateralized for exposure of the abdominal aorta. An 18× dissecting scope (Zeiss, Thornwood, NY) was used to dissect the abdominal aorta surrounding tissue. Proximal control was at the position of the infra-renal aorta, and distal control was superior to the aortic bifurcation. A 5-mm long, 1-mm diameter tissue-engineered graft was sutured as an interpostional end-to-end anastomosis with 10-0 monofilament nylon sutures using simple interrupted stitches. The proximal and distal clamps were removed, and flow was restored through the graft. Hemostasis was achieved prior to closure, and the abdomen was sutured with a running 5-0 prolene stitch. Animals recovered on warmed pads and were monitored for hind limb paralysis before returning to their preoperative cages. Postoperatively, the rats were treated with plavix (25 mg/kg) four times per week. This protocol was approved by the Institutional Animal Care and Use Committee of Yale University in compliance with animal care and handling with the Guide for the Care and Use of Laboratory Animals published by National Institute of Health.
In vivo ultrasound and microcomputed tomography
Rats were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (10 mg/kg). The grafts were evaluated along a longitudinal axis via transabdominal ultrasonography using the Vevo 770 ultrasound machine (VisualSonics, Toronto, Ontario). Lumens of the grafts were evaluated to assess for graft patency, stenosis/occlusion, or aneurismal dilation. One graft was also evaluated by high-resolution (50 µm) microcomputed tomography (micro-CT) imaging (Scanco Medical MicroCT 40, Southeastern, Pa). The rats were anesthetized at the time of sacrifice, and a thoracotomy incision was made. The thoracic aorta was cannulated for the administration of a bolus of contrast (Omnipaque; Amersham Biosciences, Piscataway, NJ). The residual TEV was highlighted using cross section images to determine when there was a transition from native aorta to engineered vessel.
Explant characterization
Grafts were explanted at 6 weeks by perfusion fixation and were analyzed by histology, immunohistology, and transmission electron microscopy. For histology and immunohistology, the explanted grafts were harvested and placed in 10% formalin (Sigma) overnight. The grafts were rinsed with PBS and paraffin embedded. The graft lumen and graft thickness were evaluated with hematoxylin and eosin (H&E) staining quantified by using ImageJ software (Image Processing and Analysis in Java; National Institute of Health, Bethesda, Md). Immunohistological staining included trichrome to assess collagen and elastic Van Gieson for elastin. Additional immunohistochemical staining was performed for smooth muscle cell α-actin (Dako, Carpinteria, Calif) to assess smooth muscle cell ingrowth and von Willebrand factor (Dako) for endothelial cell migration using a streptavidin-biotinylated peroxidase kit (Vector 6101 or 6102; Vector Labs, Burlingame, Calif). Using H&E staining, hoop stress was calculated for the explanted tissue engineered vessels and compared with the native aorta (τ = Pressure * radius/thickness).
Vessel segments were fixed for transmission electron microscopy (TEM) in 4% glutaraldehyde (EMD Chemicals Inc, Gibbstown, NJ) and 0.1 M sodium cacodylate trihydrate (Sigma). Fixed vessel segments were thin-sectioned into cross sections. A FEI Tenai Biotwin 80- to 120-kV transmission electron microscope was used to view the slide.
Statistical analysis
All quantitative results were obtained from three different cultivated vessels. Since the same vessels were used before and after decellularization, a paired t test was used for analysis. The data was expressed as the mean ± SEM. Statistical analysis was performed using Statview 6.0. A value of P < 0.05 was considered to be statistically significant.
RESULTS
Vessel morphology
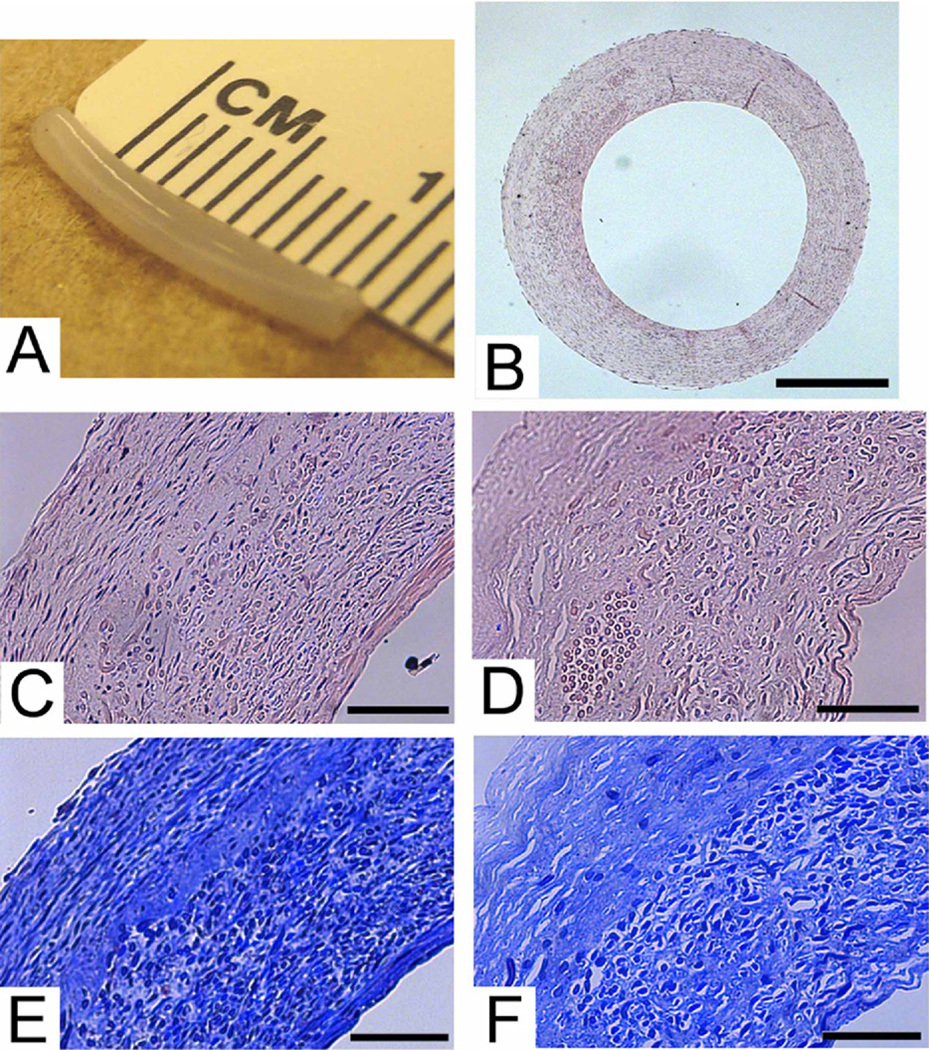
The TEV had a similar structure to a native artery. The hTEV had a tubular form after the culture period of 10 weeks under pulsatile conditions (Fig 1, A). The PGA scaffold rapidly degraded into nontoxic products, and allowed smooth muscle cells to migrate through the scaffold to form a tubular structure.16 On histology, smooth muscle cells distributed throughout the vessel wall (Fig 1, B). The wall thickness was on the same magnitude of a human saphenous vein at 300 to 400 µm.
Fig 1.
Human tissue-engineered vessel (hTEV). A, Gross picture of 1-mm hTEV. B, Cross section of hTEV, H&E, scale = 500 µm. C, Higher magnification H&E of hTEV, scale = 100 µm. D, Decellularized H&E of hTEV, scale = 100 µm. E, Human TEV, Masson’s Trichrome for collagen, scale = 100 µm. F, Decellularized hTEV, Masson’s Trichrome for collage, scale = 100 µm.
The decellularized TEV demonstrated stability of the extracellular matrix, while the cellular components were efficiently removed. On histology, the H&E staining showed the overall vessel structure intact after decellularization with minimal degradation of the extracellular matrix (Fig 1, C and D). After decellularization, there was a slight increase in diameter of the vessel because of a loss of muscle tone. The Masson’s Trichrome stain for collagen appeared similar before and after decellularization (Fig 1, E and F), wherein all the blue color was collagen matrix. Note that the PGA polymer fragments, visible as circles in the images, nonspecifically absorb the blue stain. The histologic findings were confirmed by quantitative evaluation of collagen and DNA content. There was no significant change in collagen content in the TEV after decellularization (Table; n = 3, P = .47). The fractional collagen content actually increased slightly and has been attributed to the loss of cells and other proteins in the decellularized group. The removal of cellular components was shown by a loss of nuclei in the H&E stain after decellularization, and verified by a significant decrease in DNA content as a percentage of dry weight after decellularization (Table; n = 3, P = .0014). Hence, the decellularization treatment of hTEV preserves the collagen matrix, yet removes cellular material.
Table.
Properties of fresh and decellularized human tissue-engineered vessels
| Fresh engineered vessel | Decellularized engineered vessel | |
|---|---|---|
| Collagen content (% per dry wt) | 31 ± 7 (n = 3) | 36 ± 8 (n = 3; P = .47) |
| DNA content (% per dry wt) | 0.52 ± 0.030 (n = 3) | 0.05 ± 0.013 (n = 3; P = .0014) |
| Suture retention strength (grams) | 47 ± 6 (n = 3) | 40 ± 8 (n = 3; P = .057) |
| Burst pressure (mm Hg) | 1722 ± 517 (n = 3) | 1567 ± 384 (n = 3; P = .41) |
Vessel mechanics
The mechanical properties of the hTEV are comparable to fresh tissues. The mechanical properties evaluated included suture strength for handling characteristics and burst pressure to assess vessel strength (Table). The suture strength was minimally reduced after decellularization (Table; n = 3, P = .0572). Although the suture strength of the engineered vessel was somewhat low, the vessels could be anastomosed to the native aorta without anastomotic bleeding or tearing at the suture line.2 As a comparison for suture retention, a decellularized porcine carotid artery has a suture retention of 300 ± 9 grams. We measured burst pressure of the engineered vessels by increasing intraluminal pressure until the vessel failed at its weakest point. The hTEV had a nonsignificant reduction in burst pressure after decellularization (Table; n = 3, P = .41). Even after decellularization, the burst pressure of 1567 ± 384 mm Hg (n = 3) was similar to reported values for saphenous vein (1680 ± 307 mm Hg).17 Therefore, the mechanical properties of the human tissue-engineered arteries were judged to be suitable for implantation.
Implantation study
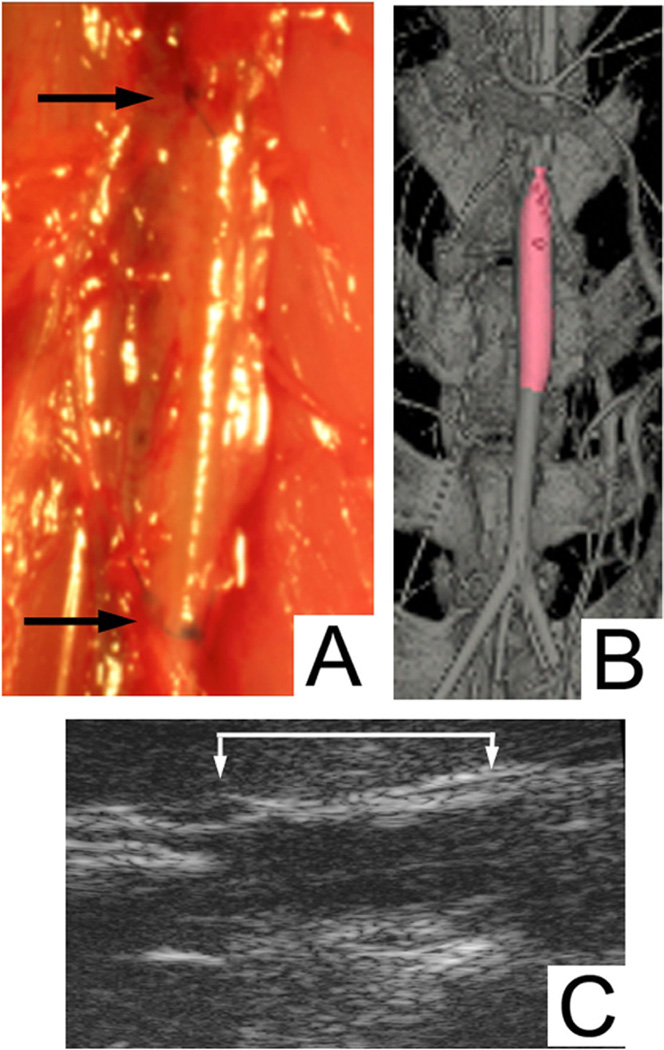
An animal model was used to evaluate the in vivo performance of the hTEV. A nude rat model was used to limit the adaptive immune-rejection response; however, the innate immune response that regulates inflammation was intact in these animals. An ideal location for implantation to match the graft diameter (1 mm) was an interpositional abdominal graft (Fig 2, A). The overall patency rate at 6 weeks in this study was 83% (n = 6). The grafts were evaluated for patency at 2 weeks by ultrasound (Fig 2, C), and one graft had occluded that suggested a technical failure at the time of implantation. The ultrasound at 2 weeks in the patent grafts did not suggest any significant dilation. At the time of sacrifice, one animal underwent micro-CT that demonstrated a widely patent graft without evidence of dilatation or aneurysm formation (Fig 2, B). The in vivo imaging results regarding patency correlated to explant histology.
Fig 2.
Implantation and in vivo imaging. A, Implantation of 1 mm decellularized human tissue-engineered vessel (hTEV) (arrows at the proximal and distal anastomosis). B, In vivo micro-computed tomography (micro-CT) imaging at the time at sacrifice at 6 weeks with patent decellularized hTEV (color highlight is the graft). C, In vivo ultrasound of decellularized hTEV at 2 weeks (brackets represent the borders of the graft).
Explanted grafts
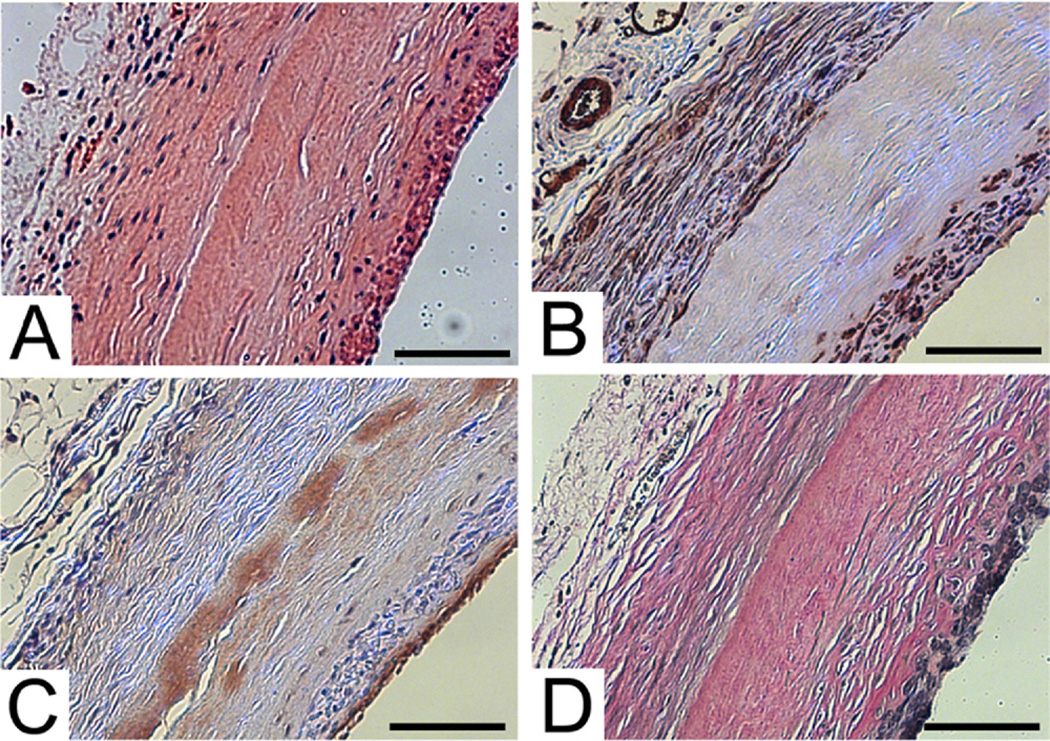
The explanted grafts by histology showed a high patency rate, no evidence of graft dilatation, and neotissue formation. By H&E (Fig 3, A), the imaging results were confirmed with a patency rate of 83% (n = 5) by histology. The explanted grafts were negative for evidence of graft dilation, with relatively uniform graft wall architecture. Although there was no significant dilation within each segment of the explanted vessels, only one time point at 6 weeks was obtained for histology. The decellularized human tissue-engineered graft exhibited neotissue integration on the luminal and outer side of the matrix. Host cells did not completely infiltrate through the entire wall of the graft, and there was a remaining acellular layer at 6 weeks (Fig 3, A). The one occluded vessel was likely related to technical failure with acute or delayed thrombosis that resulted in occlusion. The small size of the implanted graft into the rat aorta was a technically challenging procedure, and during the implantation the graft could have become narrowed related to suturing.
Fig 3.
Explant immunohistochemistry of decellularized human tissue-engineered vessel (hTEV) at 6 weeks stained with (A) H&E, (B) smooth muscle α-actin, (C) von Willebrand factor, and (D) elastin Van Gieson. Scale bar = 100 µm.
Histomorphology was used to determine the intimal area to the medial area and to obtain hoop stress. The intima-to-media ratio for native aorta was 0.14 ± 0.02, compared with the residual-engineered matrix-to-neointima ratio for the tissue-engineered vessel of 0.61 ± 0.14. The intima on the native aorta was a thin layer, and there was a more substantial neointima that was formed on the decellularized engineered vessel. Using the H&E slides for the tissue-engineered vessels at explants and adjacent native aorta as a reference, the hoop stress was 48,200 ± 5500 Pa compared with native aorta hoop stress of 77,000 ± 9100 Pa. The hoop stress of the TEV was less than the native aorta because, during the remodeling process, the engineered vessel has a greater thickness as compared with the native aorta.
The neotissue investing and adjacent to the grafts was characterized by immunohistochemistry for smooth muscle and endothelial cell markers. The cells on the outer side of the engineered vessels were predominately positive for smooth muscle α-actin (Fig 3, B), indicating a smooth muscle or myofibroblast phenotype. The neointima also had a thin layer of smooth muscle cells. The luminal surface displayed a confluent lining of endothelial cells on all samples, which were positive for von Willebrand factor (Fig 3, C). As an internal control for the endothelial staining on the engineered vessels, the same degree of endothelial staining was observed in the small vessels in the periphery of the adventitia. Since the decellularized TEV was acellular at the time of implantation, the endothelial cells were host derived. The TEM also showed flat cells on the luminal surface of the explanted graft that was consistent with endothelial cells. The endothelialized neointima likely aided in preventing graft thrombosis and was thin and did not lead to narrowing of the lumen.
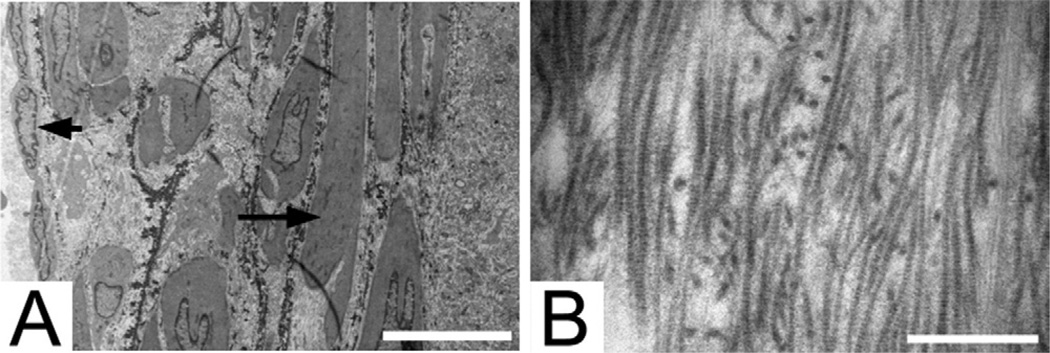
The decellularized hTEV provided a matrix that supported the host cellular infiltration and stimulated a functional neointima. Elastin formation within the neointima was shown on elastic van Gieson staining (Fig 3, D). The presence of elastin was confirmed by ultrastructural analysis using TEM. The characteristic dense elastin fibers were detected by TEM (Fig 4, A). The elastin fibers were seen adjacent to the smooth muscle cells in the neointima. The collagen fibers found in the acellular layer derived from the decellularized TEV remained intact after explanation at 6 weeks (Fig 4, B). The cellular ingrowth is demonstrated by the host rat through histology and immunohistochemistry as compared with the acellular engineered vessel at the time of implantation.
Fig 4.
Transmission electron microscopy of explanted decellularized human tissue-engineered vessel (hTEV) at 6 weeks. A, The luminal surface coated with endothelial cells (arrowhead) and elastin fibers (arrow) in the neointimal layer adjacent to smooth muscle cells. Scale bar = 10 µm. B, The accellular remnant decellularized hTEV demonstrating collagen fibers. Scale bar = 500 nm.
DISCUSSION
TEV may provide an alternative conduit over currently available synthetic grafts. A biologic-based structure that is decellularized could be tailored to a range of lengths and diameters, widely available, and easily transported. There are only two tissue-engineered blood vessels that are currently under clinical investigation, and both methods have shown promising results in early clinical trials.18,19 Only one method has been used for arterial conduits, and the process to generate a living engineered vessel takes 7 to 9 months.19 Although the use of autologous tissue is a theoretic ideal, the complexity and lead time required to develop individualized grafts will likely constrain completely autologous tissue-engineered vessels for therapeutic applications.
The immunodeficient rat model provided an appropriate model for mechanical evaluation of hTEV. The in vitro mechanical properties suggested the grafts were suitable for implantation, and the rat implantation study confirmed the mechanical stability of the vessels. The imaging and histology did not indicate the decellularized hTEV had dilation or stenosis after 6 weeks as an aortic interpositional graft. The graft appeared to stimulate remodeling, with the presence of smooth muscle cells and a luminal confluent layer of endothelial cells. Since collagen can be thrombogenic, antiplatelet therapy with plavix was given to the rats over the 6-week period to prevent thrombosis. An endothelial layer was present on the grafts after 6 weeks, and likely the antiplatelet therapy could have been discontinued at this time.
The integration of decellularization and tissue engineering concepts to form an allogeneic matrix could expand the therapeutic indications for these grafts. The only option to engineer vessels with cells intact would be to use autologous smooth muscle cells; however, the aged smooth muscle cells produced vessels with poor mechanics not suitable for implantation.8 The mechanism leading to poor vessel mechanics was an inverse relationship to donor age and the cellular factors of proliferative capacity and collagen production. Allogenic grafts are preferable to xenogenic grafts because of the presence of xenogeneic antigens, such as the Gal epitope, which can lead to failure as a result of biodegeneration and calcification.20 Allogeneic grafts can be processed with various treatments that lead to different mechanical and immunologic responses. Cryo-preservation was an early approach as a clinical peripheral bypass graft, although it has been shown to have low patency rates and to stimulate an immunogenic response. 10,21 Glutaraldehyde-fixed cardiovascular tissue has been utilized mostly in cardiac valves, but this approach also leads to late failures from inflammation and eventual calcification. 22,23 Decellularization with enzymatic or detergents methods maintain mechanical properties of a native artery as a conduit, but has less immunologic response when compared with cryopreservation.12,13 The advantage of using a decellularized TEV is that the majority of the immune response-generation is due to the cellular components, while the majority of the mechanical stability is from the extracellular matrix.14 The decellularized TEV may provide a model for clinical use of tissue-engineered products.
There is no ideal animal model to test how vascular grafts will perform in humans; each animal model has an associated set of limitations.24 None of the larger animal models offer an immunodeficient species, and rat implants are technically more feasible that mouse implants. The only animal models to test hTEV that will not generate a xenogenic inflammatory response are a nude mouse/rat model or an old world primate model. Although a nude rat model (Crl: NIH-Foxn1rnu) is immunocompromised, the rats are only T-cell deficient with an intact B-cell response and intact innate immune system. This is in contrast to a severe combined immunodeficiency mouse, for instance, that is T-cell and B-cell deficient. In addition to a hTEV, our laboratory has recently published a porcine TEV that was decellularized and implanted into a porcine carotid model and that did not generate a significant immunologic response. 25 A dog or large animal model is not an ideal animal model for testing hTEV because a xenogenic immune response will contribute to the inflammatory response. 26 Immunosuppressive medication could be used for a human vessel as a xenogenic implant, but this introduces other confounding factors. The only large animal model would be an old word primate, which is very expensive and presents ethical issues. Although limited, the nude rat/mouse models are the best models for preliminary evaluation of hTEV for in vivo graft implantation.
The primary drawbacks of a rat model are the high patency rates for short aortic interpositional grafts, despite the small diameter of the graft.24 The majority of prior interpositional aortic implants in the rat aorta are polytetrafluoroethylene or polyurethane; polytetrafluoroethylene grafts are associated with very high patency and endothelialization rates.27,28,29 Rat aorta studies have provided useful data on the assessment of cellular integration of synthetic polymer structures or TEV, although the patency rates are also very high.28,29
The limitations to this study include a small sample size and need for longer-term follow up. The patent grafts all had a similar appearance, with a widely patent lumen, and more animals would be expected to maintain a high patency rate. Longer-term results – up to 1 year – are needed to further understand the potential for late aneurismal formations or pathologic neointima that could cause stenosis. Since the response to implantation of other synthetic grafts has been well described, this study did not include other interpositional aortic grafts as a comparison. A control graft could have been a cellular engineered vessel, which has been implanted into other animal models, except this vessel would induce an inflammatory response in the rat model.
CONCLUSIONS
hTEV may provide an alternative option for small-diameter vascular grafts. Despite its limitations, the aortic implant in the nude rat can provide a screening tool to assess arterial graft performance and insight to the remodeling process.
Clinical Relevance.
The demand for alternative arterial conduits is due to the poor clinical efficacy of existing synthetic grafts for small-diameter artery applications, with many patients lacking adequate saphenous vein. We showed that a vessel culture system could produce a human vascular graft that could function as an arterial conduit in a small-diameter animal model. The decellularization process for the human tissue-engineered vessels expands the clinical potential by generating an allogeneic graft that is readily available for implantation.
Acknowledgments
Supported by National Institute of Health grants HL-083895 and EB-008366 (both to LEN).
The authors would like to thank Wawrzyniec Dobrucki for his technical assistance with the acquisition and data analysis of the micro-CT images.
Footnotes
Competition of interest: Dr Niklason has a financial interest in Humacyte, Inc, a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
Presented at the 2011 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, Ill, June 16-18, 2011.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
AUTHOR CONTRIBUTIONS
Conception and design: CQ, LN
Analysis and interpretation: CQ, MA, LN
Data collection: CQ, MA, AM
Writing the article: CQ, MA, LN
Critical revision of the article: CQ, AD, LN
Final approval of the article: CQ, MA, AM, AD, LN
Statistical analysis: CQ, MA, LN
Obtained funding: LN
Overall responsibility: CQ, MA, AM, AD, LN
REFERENCES
- 1.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 2.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 3.Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK, Ulubayram K, et al. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 4.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506–515. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He H, Shirota T, Yasui H, Matsuda T. Canine endothelial progenitor cell-lined hybrid vascular graft with nonthrombogenic potential. J Thorac Cardiovasc Surg. 2003;126:455–464. doi: 10.1016/s0022-5223(02)73264-9. [DOI] [PubMed] [Google Scholar]
- 8.Poh M, Boyer M, Solan A, Dahl SL, Pedrotty D, Banik SS, et al. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 9.Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635–1648. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RS, 3rd, Edwards WH, Mulherin JL, Jr, Edwards WH, Jr, Jenkins JM, Hoff SJ. Cryopreserved saphenous vein allografts for below-knee lower extremity revascularization. Ann Surg. 1994;219:664–670. doi: 10.1097/00000658-199406000-00009. Discussion: 70–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santini F, Dyke C, Edwards S, Stavri G, Feccia M, Khan H, et al. Pulmonary autograft versus homograft replacement of the aortic valve: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;113:894–899. doi: 10.1016/S0022-5223(97)70262-9. Discussion: 99–900. [DOI] [PubMed] [Google Scholar]
- 12.Murase Y, Narita Y, Kagami H, Miyamoto K, Ueda Y, Ueda M, et al. Evaluation of compliance and stiffness of decellularized tissues as scaffolds for tissue-engineered small caliber vascular grafts using intravascular ultrasound. ASAIO J. 2006;52:450–455. doi: 10.1097/01.mat.0000227727.87476.5e. [DOI] [PubMed] [Google Scholar]
- 13.Ketchedjian A, Kreuger P, Lukoff H, Robinson E, Linthurst-Jones A, Crouch K, et al. Ovine panel reactive antibody assay of HLA responsivity to allograft bioengineered vascular scaffolds. J Thorac Cardiovasc Surg. 2005;129:159–166. doi: 10.1016/j.jtcvs.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–666. [PubMed] [Google Scholar]
- 15.Gui L, Chan SA, Breuer CK, Niklason LE. Novel utilization of serum in tissue decellularization. Tissue Eng C Methods. 2010;16:173–184. doi: 10.1089/ten.tec.2009.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, et al. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (NY) 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 17.L’Heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139:431–436. doi: 10.1016/j.jtcvs.2009.09.057. 436.e1-2. [DOI] [PubMed] [Google Scholar]
- 19.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–1446. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 20.Konakci KZ, Bohle B, Blumer R, Hoetzenecker W, Roth G, Moser B, et al. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35:17–23. doi: 10.1111/j.1365-2362.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter JP, Tomaszewski JE. Human saphenous vein allograft bypass grafts: immune response. J Vasc Surg. 1998;27:492–499. doi: 10.1016/s0741-5214(98)70323-4. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths LG, Choe LH, Reardon KF, Dow SW, Christopher Orton E. Immunoproteomic identification of bovine pericardium xenoantigens. Biomaterials. 2008;29:3514–3520. doi: 10.1016/j.biomaterials.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manji RA, Zhu LF, Nijjar NK, Rayner DC, Korbutt GS, Churchill TA, et al. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation. 2006;114:318–327. doi: 10.1161/CIRCULATIONAHA.105.549311. [DOI] [PubMed] [Google Scholar]
- 24.Byrom MJ, Bannon PG, White GH, Ng MK. Animal models for the assessment of novel vascular conduits. J Vasc Surg. 2010;52:176–195. doi: 10.1016/j.jvs.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 25.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011;108:9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dardik A, Liu A, Ballermann BJ. Chronic in vitro shear stress stimulates endothelial cell retention on prosthetic vascular grafts and reduces subsequent in vivo neointimal thickness. J Vasc Surg. 1999;29:157–167. doi: 10.1016/s0741-5214(99)70357-5. [DOI] [PubMed] [Google Scholar]
- 28.Nieponice A, Soletti L, Guan J, Hong Y, Gharaibeh B, Maul TM, et al. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng A. 2010;16:1215–1223. doi: 10.1089/ten.tea.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pektok E, Nottelet B, Tille JC, Gurny R, Kalangos A, Moeller M, et al. Degradation and healing characteristics of small-diameter poly(epsiloncaprolactone) vascular grafts in the rat systemic arterial circulation. Circulation. 2008;118:2563–2570. doi: 10.1161/CIRCULATIONAHA.108.795732. [DOI] [PubMed] [Google Scholar]