Abstract
Objective
Recent observations have revealed an interaction between inflammation and angiogenesis, which may be mediated by angiopoietins and chemokines. Given the importance of inflammation in parturition, we sought to determine whether angiopoietin-2 (Ang-2) is present in amniotic fluid (AF) and if its concentration changes with gestational age, labor, and in intra-amniotic infection/inflammation (IAI) in patients with spontaneous preterm labor and intact membranes.
Study design
This cross-sectional study included 486 patients in the following groups: 1) women in the midtrimester of pregnancy (14–18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=52); 2) normal pregnant women at term with (n=48) and without (n=45) spontaneous labor; 3) patients with an episode of spontaneous preterm labor (PTL) and intact membranes who were classified into: a) PTL without IAI who delivered at term (n=152); b) PTL without IAI who delivered preterm (<37 weeks gestation; n=107); and c) PTL with IAI (n=82). Ang-2 concentration in AF was determined by enzyme-linked immunoassay. Non-parametric statistics were used for analysis.
Results
1) Ang-2 was detected in all AF samples; 2) the median AF Ang-2 concentration at term was significantly lower than that in the mid-trimester (1877.4 pg/mL vs. 3525.2 pg/mL; P<0.001); 3) among patients with PTL, the median AF Ang-2 concentration was significantly higher in patients with IAI than in those without IAI (4031.3 pg/mL vs. 2599.4 pg/mL; P<0.001) and those with PTL without IAI who delivered at term (4031.3 pg/mL vs. 2707.3 pg/mL; P<0.001); and 4) no significant differences were observed in the median AF Ang-2 concentration between patients with spontaneous labor at term and those at term not in labor (1722.9 pg/mL vs. 1877.4 pg/mL; P=0.6).
Conclusions
1) Ang-2, a protein involved in the process of vascular remodeling, is a physiologic constituent of the amniotic fluid and its concentration decreased with advancing gestation; 2) the median Ang-2 concentration in amniotic fluid is higher in patients with IAI than in those without; and 3) spontaneous parturition at term is not associated with changes in the AF concentration of Ang-2. These findings support the view of a link between angiopoietins and inflammation.
Keywords: Angiogenesis; chorioamnionitis; microbial invasion of the amniotic cavity; pregnancy; preterm delivery, preterm labor; preterm parturition syndrome, Tie-2; vasculogenesis
INTRODUCTION
Angiogenesis is the process by which new vessels are formed from pre-existing vasculature. This process is tightly regulated by different families of growth factors. Along with the well-characterized vascular endothelial growth factor (VEGF) family, angiopoietins have been shown to be critical in orchestrating blood vessel formation [8]. Angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2) are structurally-related endothelial growth factors. They bind with similar specificity and affinity to the tyrosine kinase receptor with immunoglobulin and epidermal growth factor homology domain 2 (Tie-2), which is expressed on endothelial cells and hemopoietic cells [12, 33, 60]. Studies in knockout mice have shown that the Ang-1/Tie-2 system plays an essential role in embryonic vascular remodeling [60, 63]. Under physiologic conditions, Ang-1, through Tie-2 signaling, mediates vessel maturation and maintains vessel integrity by the recruitment of periendothelial cells [31].
Ang-2 is a competitive inhibitor of Ang-1 because both bind to Tie-2 tyrokinase receptor [12, 33, 60]. Ang-1 induces phosphorylation of Tie-2, whereas Ang-2 binding does not activate the receptor w33x. Remarkably, Ang-2 acts only as an antagonist of Ang-1 on endothelial cells, leads to loosening of cell/cell interactions and allows access to angiogenic inducers such as VEGF [33]. Ang- 2 may act with VEGF to promote angiogenic sprouting from established vasculature [18, 33]. Thus, Ang-2 is specifically required for normal postnatal vessel remodeling [18].
Parturition is considered to be an inflammatory process [24, 49, 52, 54]. In addition, the expression of angiogenesis-related genes is significantly increased in the mouse uterus during spontaneous labor at term, as well as induced preterm labor by either bacteria or ovariectomy [23]. These findings suggest that changes in gene expression of angiogenic-related genes are part of the common pathway of parturition.
Since there is a paucity of information about the role of Ang-2 in human amniotic fluid, we determined whether Ang-2 is present in human amniotic fluid and if its concentration changes with gestational age, in the presence of labor (term and preterm), and in intra-amniotic infection/inflammation (IAI) in patients with spontaneous preterm labor and intact membranes.
MATERIAL AND METHODS
Study design and population
A cross-sectional study was designed by searching our clinical database and bank of biological samples including 486 patients in the following groups: 1) women in the mid-trimester of pregnancy (14–18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=52); 2) normal pregnant women at term with (n=48) and without (n=45) spontaneous labor; and 3) patients with an episode of spontaneous preterm labor (PTL) and intact membranes who were classified into: a) PTL without IAI who delivered at term (n=152); b) PTL without IAI who delivered preterm (<37 weeks’ gestation; n=107); and c) PTL with IAI (n=82).
Definitions
Patients were considered to have a normal pregnancy outcome if they did not have any medical, obstetrical, or surgical complications and delivered a term neonate (≥37 weeks) of greater than the 10th percentile for gestational age [1, 22] without complications. Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 min associated with cervical changes before 37 completed weeks of gestation that required hospitalization. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms. Intra-amniotic inflammation was diagnosed by an amniotic fluid interleukin (IL)-6 concentration ≥2.6 pg/mL [68]. Histologic chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes. Acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton’s jelly, using criteria previously described [41].
Sample collection
Amniotic fluid samples were obtained from transabdominal amniocentesis performed for genetic indications, evaluation of microbial status of the amniotic cavity, and/or assessment of fetal lung maturity in patients approaching term. Women at term in labor consisted of women who were admitted for suspected preterm labor because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity. The criteria for determining that these patients were at term was derived retrospectively, if the following criteria were met: 1) spontaneous labor; 2) delivery within 24 hours from amniocentesis; 3) analysis of amniotic fluid consistent with fetal lung maturity; 4) birthweight >2500 g; 5) absence of respiratory distress syndrome or other complications of prematurity; and 6) physical examination of the newborn by pediatricians which was consistent with a term neonate. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell (WBC) count, glucose concentration and Gram-stain were also performed shortly after collection, as previously described [48, 53, 55]. The results of these tests were used for clinical management. Amniotic fluid IL-6 concentrations were used only for research purposes. Amniotic fluid not required for clinical assessment was centrifuged for 10 min at 4°C, and the supernatant was aliquoted and stored at −70°C until analysis. Among patients with spontaneous preterm labor with intact membranes who delivered within 72 hours of amniocentesis, placenta, umbilical cord, and chorioamniotic membranes were collected and the presence or absence of histologic chorioamnionitis and funisitis was assessed. This period of time was selected to preserve a meaningful temporal relationship between amniotic fluid Ang-2 concentration and placental pathologic findings.
All women provided written informed consent prior to the collection of amniotic fluid. The collection of amniotic fluid and its utilization for research purposes was approved by the Institutional Review Boards of Wayne State University (Detroit, Michigan, USA), Sotero del Rio Hospital (Santiago de Chile, Chile) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Many of these samples have been previously used to study the biology of inflammation, hemostasis, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Determination of human Ang-2 concentration in amniotic fluid
Specific and sensitive enzyme-linked immunoassay (R&D System, Inc. Minneapolis, MN, USA) was used to determine concentrations of Ang-2 in human amniotic fluid. The concentrations of Ang-2 in amniotic fluid samples were determined by interpolation from individual standard curves composed of recombinant human Ang-2. The calculated inter- and intra-assay coefficients of variation for Ang-2 immunoassays in our laboratory were 3.1% and 3.8%, respectively. The lower limit of detection of the Ang-2 immunoassay was calculated to be 17.9 pg/mL.
Statistical analysis
The normality of the data was tested using the Shapiro–Wilk and Kolmogorov–Smirnov tests. Because amniotic fluid Ang-2 concentrations were not normally distributed, non-parametric tests were used for analyses. Comparisons between proportions were performed with X2-test. Kruskal–Wallis with post-hoc analysis and Mann–Whitney U-tests were employed for continuous variables. Adjustment for multiple comparisons was performed using the Bonferroni method [5]. Spearman rank correlation was utilized to assess correlations between amniotic fluid concentrations of Ang-2, glucose, IL-6 and WBC count. A P<0.05 was considered statistically significant. The statistical package used was SPSS v.15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and clinical characteristics of the study population
Four hundred and eighty-six patients were included in the study. Table 1 shows the demographic and clinical characteristics of the patients in the mid-trimester, term not in labor and term in labor groups. Table 2 displays the demographic and clinical characteristics of the patients with spontaneous preterm labor and intact membranes. The median body mass index (BMI), and the rates of smoking and obesity (BMI ≥30 kg/m2) were significantly higher in patients with PTL with IAI and preterm delivery than in those without IAI and preterm delivery and those without IAI and term delivery. In addition, the gestational age at amniocentesis, the gestational age at delivery and birthweight were significantly lower in patients with PTL with IAI and preterm delivery than that of those without IAI and preterm delivery and those without IAI and term delivery.
Table 1.
Demographic and clinical characteristics of patients in the mid-trimester and those at term with and without spontaneous labor.
| Mid-trimester | pa | Term no labor | Term in labor | pb | |
|---|---|---|---|---|---|
| (n=52) | (n=45) | (n=48) | |||
| Maternal age (years) | 36.5 (35–38) | <0.001 | 36 (21–31) | 22 (20–28) | <0.05 |
| Gestational age at amniocentesis (weeks) | 16 (16.0–17.0) | <0.001 | 38.7 (38.0–39.0) | 38.5 (38.0–39.3) | NS |
| Gestational age at delivery (weeks) | 39 (38.0–40.0) | NS | 38.7 (38.0–39.0) | 38.5 (38.0–39.3) | NS |
| Birthweight (grams) | 3344 (3128–3596) | NS | 3260 (3110–3630) | 3375 (3100–3550) | NS |
Values are expressed as median (inter-quartile range).
NS: not significant.
pa: comparison between patients in the mid-trimester and those at term not in labor.
pb: comparison between patients at term not in labor and those at term in labor.
Table 2.
Demographic and clinical characteristics of patients presenting with spontaneous preterm labor with intact membranes.
| PTL without IAI term delivery |
p | PTL without IAI preterm delivery |
pa | PTL with IAI preterm delivery |
pb | |
|---|---|---|---|---|---|---|
| (n=152) | (n=107) | (n=82) | ||||
| Maternal age (years) | 23 (19–30) | NS | 22 (19–30) | NS | 23 (19–29) | NS |
| Smoking | 18.4 (28/151) | NS | 10.3 (11/105) | <0.01 | 29.3 (24/78) | <0.05 |
| BMI (kg/m2) | 22.7 (20.1–25.6) | NS | 22.1 (20.0–25.7) | <0.01 | 25 (21.1–30.1) | <0.01 |
| Obesity (BMI ≥30 kg/m2) |
8.7 (12/138) | NS | 11.4 (11/96) | <0.05 | 25.3 (16–63) | <0.01 |
| GA at amniocentesis (weeks) |
31.8 (29.6–33.3) | NS | 31.9 (29.7–33.0) | <0.01 | 28.8 (25.2–33.0) | <0.001 |
| GA at delivery (weeks) |
38.7 (37.9–39.7) | <0.001 | 34.5 (33.1–35.6) | <0.001 | 30.8 (25.8–33.2) | <0.001 |
| Birthweight (grams) | 3160 (2880–3530) | <0.001 | 2320 (1940–2670) | <0.001 | 1240 (750–2110) | <0.001 |
Values expressed as percentage (number) or median (inter-quartile range).
p: comparison between PTL who delivered at term and PTL without IAI.
pa: comparison between PTL who delivered preterm without IAI and PTL with IAI.
pb: comparison between PTL who delivered at term and PTL with IAI.
PTL, preterm labor; GA, gestational age; BMI, body mass index; IAI, intra-amniotic infection/inflammation; NS, not significant.
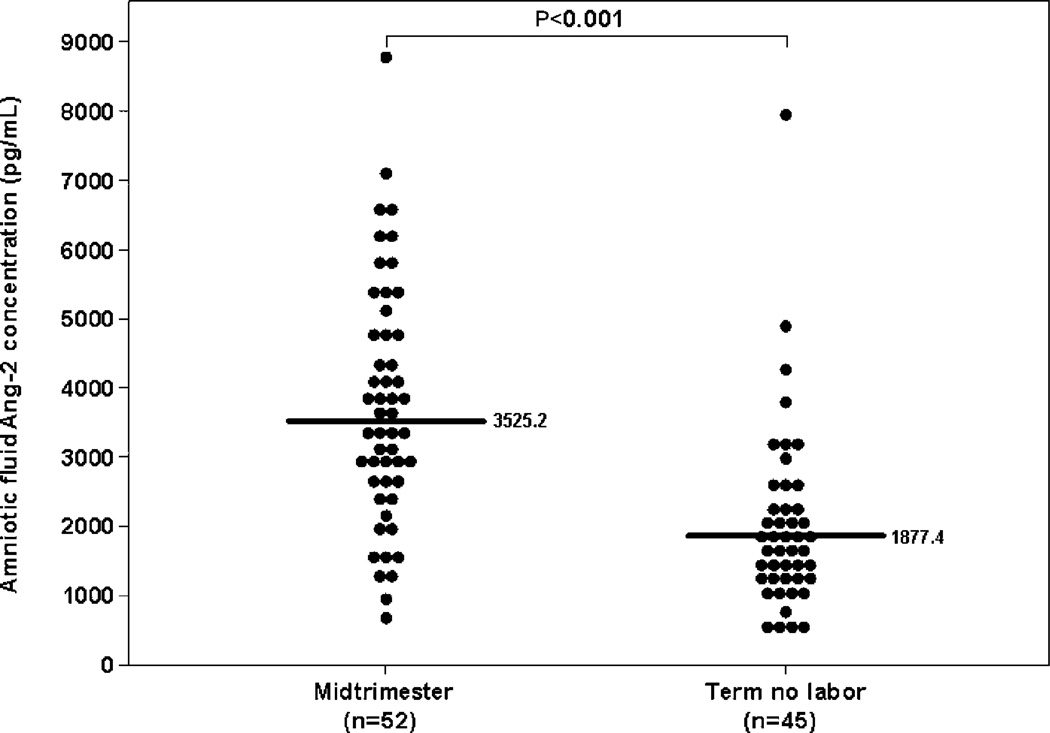
Amniotic fluid Ang-2 concentration decreases with gestational age and does not change in spontaneous labor at term
Ang-2 was detected in all amniotic fluid samples. Women with a normal pregnancy at term not in labor had a significantly lower median Ang-2 concentration in amniotic fluid than those in the mid-trimester [term not in labor: 1877.4 pg/mL, inter-quartile range (IQR) 1322.6–2434.1 vs. mid-trimester: 3525.2 pg/mL, IQR 2574.3–4852.2; P<0.001] (Figure 1). In contrast, no significant differences were observed in the median amniotic fluid Ang-2 concentration between patients with spontaneous labor at term and those at term not in labor (term in labor: 1722.6 pg/mL, IQR 1293.5–2499.3 vs. term not in labor: 1877.4 pg/mL, IQR 1322.6–2434.1; P=0.6).
Figure 1.
Amniotic fluid concentration of angiopoietin-2 (Ang-2) in normal pregnancies in the mid-trimester and in those at term without labor. The median amniotic fluid concentration of Ang-2 was significantly higher in the mid-trimester than in pregnancies at term not in labor [term not in labor: 1877.4 pg/mL, inter-quartile range (IQR) 1322.6–2434.1 vs. mid-trimester: 3525.2 pg/mL, IQR 2574.3–4852.2; P-0.001x\].
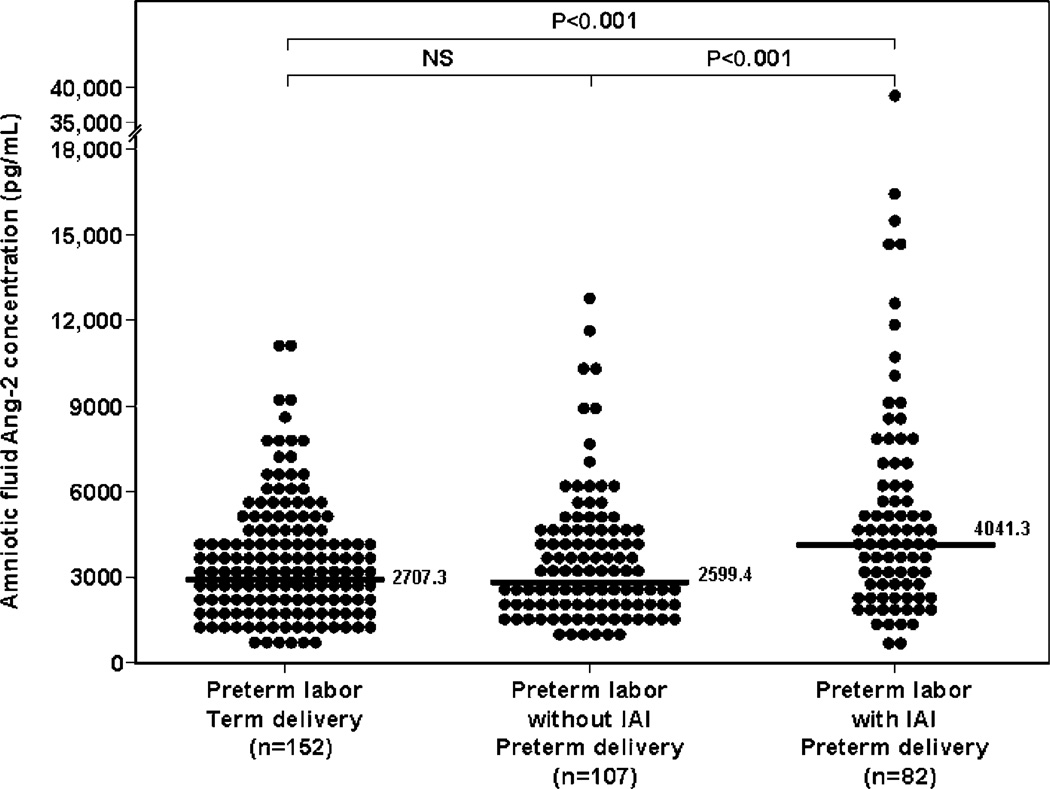
Ang-2 concentration is increased in the amniotic fluid of women with spontaneous preterm labor with intact membranes with intra-amniotic infection/inflammation
Patients with spontaneous preterm labor with IAI had a significantly higher median amniotic fluid concentration of Ang-2 than those who delivered preterm without IAI (PTL with IAI: 4041.3 pg/mL, IQR 2463.0–6385.3 vs. PTL without IAI: 2599.4 pg/mL, IQR 1822.3–4145.8; P<0.001) and than patients with spontaneous preterm labor who delivered at term (PTL delivered at term: 2707.3 pg/mL, IQR 1791.9–4274.4; P<0.001) (Figure 2). After adjusting for gestational age at amniocentesis and storage time, similar results were obtained (ANCOVA, P=0.02 among subgroups comparisons). There were no differences in the median amniotic fluid Ang-2 concentration between patients with spontaneous preterm labor without IAI who delivered preterm and those who delivered at term (PTL without IAI: 2599.4 pg/mL, IQR 1822.3–4145.8 vs. PTL delivered at term: 2707.3 pg/mL, IQR 1791.9–4274.4; P=0.6) (Figure 2).
Figure 2.
Amniotic fluid concentration of angiopoietin-2 (Ang-2) among women with intra-amniotic infection/inflammation in spontaneous preterm labor with intact membranes. The median amniotic fluid concentration of Ang-2 in patients with spontaneous preterm labor with IAI was significantly higher than that of those who delivered preterm without IAI (PTL with IAI: 4041.3 pg/mL, IQR 2463.0–6385.3 vs. PTL without IAI: 2599.4 pg/mL, IQR 1822.3–4145.8; P<0.001) and that of patients with spontaneous preterm labor who delivered at term (PTL delivered at term: 2707.3 pg/mL, IQR 1791.9–4274.4; P<0.001). There were no differences in the median amniotic fluid Ang-2 concentration between patients with spontaneous preterm labor without IAI who delivered preterm and those who delivered at term (PTL without IAI: 2599.4 pg/mL, IQR 1822.3–4145.8 vs. PTL delivered at term: 2707.3 pg/mL, IQR 1791.9–4274.4; P=0.6).
When the analysis was restricted to patients with spontaneous preterm labor without IAI who delivered within 72 h from the amniocentesis (n=18), this subgroup had a similar median amniotic fluid concentration of Ang-2 compared to those with spontaneous preterm labor with intact membranes who delivered at term (PTL without IAI with delivery within 72 h: 2460.5 pg/mL, IQR 1648.7–4162.8 vs. PTL delivered at term: 2707.3 pg/mL, IQR 1791.9–4274.4; P=0.4).
Amniotic fluid Ang-2 concentrations in patients with histologic chorioamnionitis and funisitis
Placental histopathologic diagnoses were available in 77% (54/70) of patients with spontaneous preterm labor who delivered within 72 h of amniocentesis. Of those, 52% (28/54) had evidence of placental inflammation. Patients with histologic chorioamnionitis and/or funisitis (n=28) had higher median Ang-2 concentration in amniotic fluid than those (n=26) without histologic chorioamnionitis, but this difference was not statistically significant (histologic chorioamnionitis: median 4693.3 pg/mL, IQR 2242.3–8054.5 vs. non-histological choriamnionitis: median 3180.9 pg/mL, IQR 1690.1–5310.1, P=0.09).
Amniotic fluid Ang-2 concentration correlated with IL-6 in spontaneous preterm labor
In patients with spontaneous preterm labor, a weak but significant correlation was observed between amniotic fluid concentration of Ang-2 and those of IL-6 (Spearman’s rho coefficient: IL-6: 0.27, P<0.001), but not with amniotic fluid glucose concentration or WBC count (glucose: –0.09, P=0.09 and WBC count: 0.08, P=0.1).
DISCUSSION
Principal findings of the study
1) Ang-2 is a physiologic constituent of the amniotic fluid; 2) Ang-2 concentration in amniotic fluid is significantly higher in the presence of IAI in patients with preterm labor with intact membranes; 3) amniotic fluid Ang-2 concentrations correlated with those of IL-6 in patients with IAI; and 4) the amniotic fluid Ang-2 concentration decreased with advancing gestation, and did not change in spontaneous labor at term.
What is Ang-2?
Ang-2 is a 66 kDa polypeptide that contains 496 amino acids, is 60% homologous to Ang-1, and promotes angiogenesis [33]. Although, both Ang-1 and Ang-2 are encoded by genes localized on chromosome 8 [9] and share a similar protein structure, their biological activities are different. Ang-2 is classically considered as a Tie-2 antagonist, and it is accepted that at sites of vascular remodeling, Ang-2 counteracts the stabilizing action of Ang-1 by exposing the endothelium to pro-angiogenic factors such as VEGF. This antagonistic role of Ang-2 was first suggested when its overexpression resulted in the impairment of blood vessel formation in transgenic mice, a phenotype similar to the one obtained in Ang-1 and Tie-2 knockout mice [33]. However, studies with Ang-2 knockout mice suggest that this role would not be restricted to counteracting Ang-1 activities. Ang-2 may also act as a Tie-2 agonist, being involved in postnatal vascular remodeling events [18], because Ang-2 can activate Tie-2 and stimulate both endothelial cell migration and endothelial cell capillary-like tube formation in vitro [38, 64]. In addition to their roles in angiogenesis, angiogenic factors increase vascular permeability [3, 42] and can be expressed during the course of inflammation.
Ang-2 concentration in amniotic fluid decreases with advancing gestational age in normal human pregnancy
Human pregnancy is characterized by angiogenesis, tissue development and remodeling [19, 67, 70]. In the early phase of development, vascular endothelial growth factor-A (VEGF-A) and placental growth factor (PlGF) play an important role in angiogenesis [13, 15]. Human trophoblast expresses PlGF, but VEGF-A is expressed in villous and decidual macrophages [11]. Vascular endothelial growth factor interacts with vascular endothelial growth factor receptor 1 (VEGFR-1; Flt-1) and vascular endothelial growth factor receptor 2 (VEGFR-2; KDR) to promote endothelial cell proliferation, cell migration, and vascular permeability [67, 70]. In a later phase, Ang-1, Ang-2 participate in angiogenesis [44, 70].
Ang-1 and Ang-2 bind with equal affinity to Tie-2, but have different functions. Ang-1 maintains vessel integrity and plays a role in the later stages of vascular remodeling [20]. Ang-2 is a functional antagonist of Ang-1, and leads to loosening of cell/cell interactions and allows access to angiogenic inducers such as VEGF [33]. When VEGF is present, Ang-2 promotes vascular growth, but when VEGF is absent, vascular regression occurs [2, 33]. Ang-2 is selectively expressed at sites of active angiogenesis, such as the ovary, uterus, and placenta [2, 33].
Our finding of higher amniotic fluid concentration of Ang-2 in mid-trimester than that of term gestation are consistent with those of Zhang et al. [70] who, performing in situ hybridization studies, have shown that Ang-2 mRNA was readily detected in the syncytiotrophoblast in the first trimester human placenta, but at term, only low levels of Ang-2 mRNA were detected [70]. Both findings suggest that the amniotic cavity is one of the most active sites of angiogenesis during the first half of pregnancy. We propose two explanations for the decrease of amniotic fluid Ang-2 concentration with gestational age. First, normal term pregnancy is characterized by a lower angiogenesis activity than that of early pregnancy. For instance, the median amniotic fluid concentration of soluble fms-like tyrosine kinase-1 (sFlt-1 or soluble receptor- 1 of VEGF: sVEGFR-1), an inhibitor of the activity of VEGF and PlGF, at mid-trimester is one-third of that at normal term pregnancy (10,236 pg/mL vs. 33,490 pg/mL) [43, 62]. As normal pregnancy approach to term, a switch off of the angiogenesis process might occur. Second, there might be a dynamic transfer of Ang-2 into the fetal vessels through an increase in intramembranous absorption in the amniotic cavity that occurs at term gestation [7, 14]. We have detected Ang-2 mRNA expression in human amnion, which might be a source of amniotic fluid Ang-2 (unpublished data). Since there is a vascular pathway in the fetal surface of the placenta and within the fetal membranes which serves as the primary site for transfer of water and solutes from the amniotic compartment across the amnion into fetal blood within the placenta and fetal membranes [6, 10, 57], the decreased amniotic fluid Ang-2 concentration at term might be an effect of the dilution of the protein in the amniotic cavity because there is an increased placental water flux with advancing gestation from the mother to the fetus [4]. Aquaporin-1, a cell membrane water channel that regulates the flow of water across a variety of cell membranes, has been detected in the epithelium of the chorionic plate amnion, suggesting that aquaporin-1 rapidly facilitates water transport between the amniotic cavity and the fetal circulation [35].
Ang-2 in amniotic fluid is increased in intra-amniotic infection and inflammation
A novel observation of this study is that the median amniotic fluid Ang-2 concentration was increased in patients with IAI who delivered preterm. This finding supports the view that Ang-2 plays a role in inflammation, and suggests that Ang-2 participates in the host response to intrauterine infection. In addition, a significant correlation was observed between the amniotic fluid concentrations of Ang-2 and that of IL-6. IL-6 is considered a sensitive and specific marker of intra-amniotic infection [56] and/or inflammation [68].
Unlike Ang-1, which protects adult peripheral vasculature from vascular leakage and inhibits the effects of proinflammatory cytokines on endothelial cells [29, 30, 66], the effect of Ang-2 on angiogenesis is more complex and is context dependent; when Ang-2 concentrations are elevated, angiogenesis is enhanced when VEGF is present, whereas vascular regression has been observed in its absence [27].
The concept that Ang-2 may promote inflammation is based on the following findings: 1) pro-inflammatory cytokines strongly activate Ang-2 transcription in endothelial cells [26, 28, 33, 34]; 2) elevated serum concentrations of Ang-2 are present in patients with sepsis [21, 39, 42]; 3) the injection of Ang-2 protein in vivo elicits a significant increase in edema formation in the mouse paw [58]; and 4) Ang-2 deficient mice have an impaired ability to express cytokine-inducible adhesion molecules on endothelial cell surfaces after inflammatory activation [16].
Since intrauterine inflammation/infection is characterized by elevated pro-inflammatory cytokine concentration in amniotic fluid [49, 50, 56, 68], the findings of our study support a role of amniotic fluid Ang-2 in the pathologic inflammation elicited by microbial agents or its products in the process of preterm parturition. Ang-2 acts by an autocrine mechanism w17x and is stored in endothelial Weibel-Palade bodies from where it can be rapidly released upon stimulation w16x. Inflammation exists in a mutually dependent association with angiogenesis [37, 61]. Indeed, during an inflammatory process, newly formed vessels supply the inflamed tissues with nutrients and oxygen allowing the transport of inflammatory cells. Among these, neutrophils are the first cells recruited in the angiogenic bed and provide cytokines, growth factors, and proteolytic enzymes, which contribute to regulate angiogenesis [32, 45, 59]. It has been shown also that neutrophil granules contain a variety of preformed pro-angiogenic proteins and may promote the development of laser induced choroidal neovascularization in the retina at the early stage by providing preformed pro-angiogenic factors including VEGF, Ang-1, Ang-2, and MMP-9 [71].
Since we did not find a significant correlation between amniotic fluid Ang-2 concentration and the number of amniotic fluid WBC, Ang-2 found in the amniotic fluid is most likely to originate from other tissues, such as amnion. We could detect angiopoiein-2 mRNA expression in human amnion, and its expression was increased in the presence of chorioamnionitis (unpublished data). Our preliminary observations strongly suggest that Ang-2 could be released into the amniotic cavity from the amnion.
Ang-2 in amniotic fluid and parturition
Human parturition has a common pathway characterized by increased uterine contractility, cervical ripening/dilatation, and membrane/decidual activation, culminating in membrane rupture [46, 47, 51]. This activation is generally a coordinated inflammatory phenomenon in normal spontaneous labor at term [40, 65, 69]. In addition, labor at term induces gene expression changes in chorioamniotic membranes consistent with a localized acute inflammatory response, despite the absence of histologic evidence of inflammation [23]. Moreover, spontaneous labor at term, as well as pathologically induced preterm labor, result in greatly increased expression of angiogenesis- related genes in the mouse uterus [23].
Our finding that Ang-2 did not change in the presence of human term labor is unexpected and may have three possible explanations. First, physiological human parturition at term may not be associated with changes in the amniotic fluid Ang-2 concentrations because, as normal pregnancy approaches to term, a switch off of the angiogenesis process might occur [43, 62]. This explanation agrees with our finding that amniotic fluid Ang-2 increases only when there is IAI. Indeed, the mean concentration of amniotic fluid Ang-2 of patients without IAI who delivered preterm was not different than that of patients with preterm labor without IAI who delivered at term (Figure 2). A physiologic inflammatory process might be different than that of pathologic inflammation. Second, the increased concentration of the angiopoietin receptor Tie-2 in term spontaneous labor might have dampened the amniotic fluid Ang-2 concentration during labor, because the expression of Tie-2 in amnion and choriodecidua of patients with spontaneous term labor is greater than that of those at term not in labor [35]. Third, human amnion is a biologically heterogenous and compartmentalized tissue [25] and, therefore, amniotic fluid Ang-2 production in the upper compartment may not change before the onset of labor. Further research is warranted to test these hypotheses.
CONCLUSIONS
We report an association between Ang-2 concentrations in amniotic fluid and intra-amniotic infection and/or inflammation. These results suggest that Ang-2 plays a role in normal gestation, as well as in preterm labor with IAI.
Acknowledgements
This research was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
REFERENCES
- 1.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 3.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2002;39:225–237. doi: 10.1016/s1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 4.Beall MH, Wang S, Yang B, Chaudhri N, Amidi F, Ross MG. Placental and membrane aquaporin water channels: correlation with amniotic fluid volume and composition. Placenta. 2007;28:421–428. doi: 10.1016/j.placenta.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Bonferroni C. Il calcolo delle assicurazioni su gruppi di teste. 1935:13–60. [Google Scholar]
- 6.Brace RA. Progress toward understanding the regulation of amniotic fluid volume: water and solute fluxes in and through the fetal membranes. Placenta. 1995;16:1–18. doi: 10.1016/0143-4004(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 7.Brace RA, Vermin ML, Huijssoon E. Regulation of amniotic fluid volume: intramembranous solute and volume fluxes in late gestation fetal sheep. Am J Obstet Gynecol. 2004;191:837–846. doi: 10.1016/j.ajog.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Manipulating angiogenesis in medicine. J Intern Med. 2004;255:538–561. doi: 10.1111/j.1365-2796.2003.01297.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheung AH, Stewart RJ, Marsden PA. Endothelial Tie2/Tek ligands angiopoietin-1 (ANGPT1) and angiopoietin-2 (ANGPT2): regional localization of the human genes to 8q22.3-q23 and 8p23. Genomics. 1998;48:389–391. doi: 10.1006/geno.1997.5207. [DOI] [PubMed] [Google Scholar]
- 10.Cheung CY. Vascular endothelial growth factor activation of intramembranous absorption: a critical pathway for amniotic fluid volume regulation. J Soc Gynecol Investig. 2004;11:63–74. doi: 10.1016/j.jsgi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Clark DE, Smith SK, Licence D, Evans AL, Charnock-Jones DS. Comparison of expression patterns for placenta growth factor, vascular endothelial growth factor (VEGF), VEGF-B and VEGF-C in the human placenta throughout gestation. J Endocrinol. 1998;159:459–467. doi: 10.1677/joe.0.1590459. [DOI] [PubMed] [Google Scholar]
- 12.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 13.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 14.El-Haddad MA, Desai M, Gayle D, Ross MG. In utero development of fetal thirst and appetite: potential for programming. J Soc Gynecol Investig. 2004;11:123–130. doi: 10.1016/j.jsgi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 16.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 17.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 18.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 19.Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB. Human placental vascular development: vasculogenic and angiogenic (branching and non-branching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab. 2002;87:4213–4224. doi: 10.1210/jc.2002-020195. [DOI] [PubMed] [Google Scholar]
- 20.Geva E, Jaffe RB. Role of angiopoietins in reproductive tract angiogenesis. Obstet Gynecol Surv. 2000;55:511–519. doi: 10.1097/00006254-200008000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano JSJ, Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, et al. Admission angiopoietin levels in children with septic shock. Shock. 2007;28:650–654. [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, et al. wA national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 23.Haddad R, Romero R, Gould BR, Tromp G, Gotsch F, Edwin SS, et al. Angiogenesis gene expression in mouse uterus during the common pathway of parturition. Am J Obstet Gynecol. 2008;198:539–538. doi: 10.1016/j.ajog.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394.e1–394.e24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han YM, Romero R, Kim JS, Tarca AL, Kim SK, Draghici S, et al. Region-specific gene expression profiling: novel evidence for biological heterogeneity of the human amnion. Biol Reprod. 2008;79:954–961. doi: 10.1095/biolreprod.108.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hangai M, He S, Hoffmann S, Lim JI, Ryan SJ, Hinton DR. Sequential induction of angiogenic growth factors by TNFalpha in choroidal endothelial cells. J Neuroimmunol. 2006;171:45–56. doi: 10.1016/j.jneuroim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 28.Kim I, Kim JH, Ryu YS, Liu M, Koh GY. Tumor necrosis factor-alpha upregulates angiopoietin-2 in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2000;269:361–365. doi: 10.1006/bbrc.2000.2296. [DOI] [PubMed] [Google Scholar]
- 29.Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin- 1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and Eselectin expression. Circ Res. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 30.Kim I, Oh JL, Ryu YS, So JN, Sessa WC, Walsh K, et al. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. FASEB J. 2002;16:126–128. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- 31.Lemieux C, Maliba R, Favier J, Theoret JF, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–1530. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 32.Lorant DE, Patel KD, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991;115:223–224. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 34.Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Cir Res. 1998;83:852–859. doi: 10.1161/01.res.83.8.852. [DOI] [PubMed] [Google Scholar]
- 35.Mann SE, Ricke EA, Yang BA, Verkman AS, Taylor RN. Expression and localization of aquaporin 1 and 3 in human fetal membranes. Am J Obstet Gynecol. 2002;187:902–907. doi: 10.1067/mob.2002.127168. [DOI] [PubMed] [Google Scholar]
- 36.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Expression of angiogenic and neurotrophic factors in the human amnion and choriodecidua. Am J Obstet Gynecol. 2002;187:728–734. [PubMed] [Google Scholar]
- 37.McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134:1325–1331. doi: 10.1001/archsurg.134.12.1325. [DOI] [PubMed] [Google Scholar]
- 38.Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci. 2002;115:175–183. doi: 10.1242/jcs.115.1.175. [DOI] [PubMed] [Google Scholar]
- 39.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, et al. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35:199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 40.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 41.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 42.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of midtrimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193:984–989. doi: 10.1016/j.ajog.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 44.Plaisier M, Rodrigues S, Willems F, Koolwijk P, van Hinsbergh VM, Helmerhorst FM. Different degrees of vascularization and their relationship to the expression of vascular endothelial growth factor, placental growth factor, angiopoietins, and their receptors in first-trimester decidual tissues. Fertil Steril. 2007;88:176–187. doi: 10.1016/j.fertnstert.2006.11.102. [DOI] [PubMed] [Google Scholar]
- 45.Rollin S, Lemieux C, Maliba R, Favier J, Villeneuve LR, Allen BG, et al. VEGF-mediated endothelial P-selectin translocation: role of VEGF receptors and endogenous PAF synthesis. Blood. 2004;103:3789–3797. doi: 10.1182/blood-2003-07-2272. [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. 2004:28–60. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. 1997:29–49. [Google Scholar]
- 48.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 49.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. Br J Obstet Gynecol. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 53.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–238. [PubMed] [Google Scholar]
- 55.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 56.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–220. doi: 10.1002/9780470514269.ch13. [DOI] [PubMed] [Google Scholar]
- 57.Ross MG, Brace RA. National Institute of Child Health and Development Conference summary: amniotic fluid biology – basic and clinical aspects. J Matern Fetal Med. 2001;10:2–19. doi: 10.1080/714904292. [DOI] [PubMed] [Google Scholar]
- 58.Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314:738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 59.Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 60.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 61.Shaw JP, Chuang N, Yee H, Shamamian P. Polymorphonuclear neutrophils promote rFGF-2-induced angiogenesis in vivo. J Surg Res. 2003;109:37–42. doi: 10.1016/s0022-4804(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 62.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;122:33–39. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 63.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 64.Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AI, Master Z, Bendeck MP, et al. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49:659–670. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 65.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 66.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 67.Wulff C, Wilson H, Dickson SE, Wiegand SJ, Fraser HM. Hemochorial placentation in the primate: expression of vascular endothelial growth factor, angiopoietins, and their receptors throughout pregnancy. Biol Reprod. 2002;66:802–812. doi: 10.1095/biolreprod66.3.802. [DOI] [PubMed] [Google Scholar]
- 68.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 69.Young A, Jordan F, Ledingham M, Thomson A, Jordan F, Norna J, et al. Quantification of pro-inflammatory cytokines in myometrium, cervix and fetal membranes during human parturition. J Soc Gynecol Investigation. 2002;9:137. [Google Scholar]
- 70.Zhang EG, Smith SK, Baker PN, Charnock-Jones DS. The regulation and localization of angiopoietin-1-2, and their receptor Tie2 in normal and pathologic human placentae. Mol Med. 2001;7:624–635. [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou J, Pham L, Zhang N, He S, Gamulescu MA, Spee C, et al. Neutrophils promote experimental choroidal neovascularization. Mol Vis. 2005;11:414–424. [PubMed] [Google Scholar]