Abstract
The amygdala is important in emotion, but it remains unknown whether it is specialized for certain stimulus categories. We analyzed responses recorded from 489 single neurons in the amygdalae of 41 neurosurgical patients and found a categorical selectivity for pictures of animals in the right amygdala. This selectivity appeared to be independent of emotional valence or arousal and may reflect the importance that animals held throughout our evolutionary past.
The amygdala is involved in processing both aversive and appetitive stimuli1,2. Earlier notions that the amygdala might be specialized to mediate fear responses have been supplemented by accounts in which the amygdala processes more abstract attributes, such as stimulus unpredictability3. Electrophysiological recordings in monkeys have found single neurons that respond to faces4,5, as well as to the reward value of conditioned and unconditioned stimuli6,7. In humans, neurons have been reported that are selective for a variety of visual stimuli8, and neuroimaging studies of the amygdala argue for a broad role in processing stimuli that are strongly rewarding or punishing9. This diversity of findings has left it unclear exactly what stimulus categories or dimensions the amygdala might process.
To test whether neurons in the human amygdala have preferential responses to particular stimulus categories, we recorded from the medial temporal lobe (MTL) in 41 neurosurgical patients undergoing epilepsy monitoring. Informed written consent was obtained from each subject. Subjects were sitting in bed while they viewed approximately 100 images per session on an LCD monitor (1 s each with six repetitions in pseudorandom order as described previously10). Stimulus sets contained images of persons, animals, landmarks or objects. During 111 experimental sessions, we recorded from a total of 1,445 single neurons in the amygdala (489 neurons), hippocampus (549 neurons) and entorhinal cortex (407 neurons) (Supplementary Fig. 1). Of these, 183 single units (57 in amygdala, 86 in hippocampus and 40 in entorhinal cortex) responded significantly to one or more of the presented stimuli (see below).
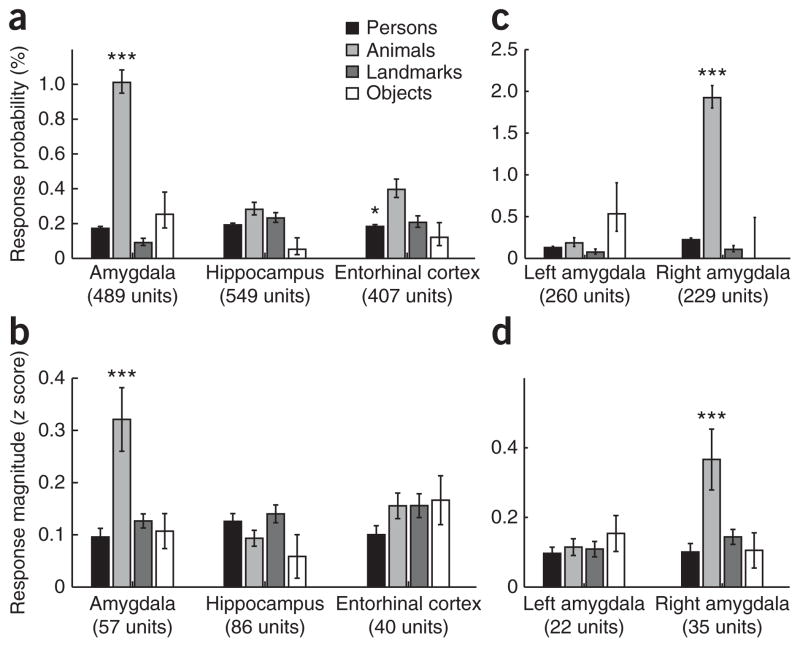
Neurons in the amygdala responded preferentially to pictures of animals rather than to pictures of other stimulus categories (Supplementary Figs. 2 and 3). To statistically compare neuronal selectivity across regions and categories, we calculated the probability that any neuron from a given MTL region responds to any stimulus from a given category. Comparison of response probabilities for different stimulus categories in the three MTL regions showed a highly significant selectivity (P < 10−15) in the responses of amygdala neurons for animal images, whereas responsiveness in hippocampus exhibited no significant difference (P = 0.9) between any stimulus categories and entorhinal cortex showed a significantly decreased (P < 0.03) response to persons compared with other categories (Fig. 1a). For all of the neurons that showed a significant response to at least one stimulus in the entire set, we compared the average response magnitudes to each of the four stimulus categories; this analysis confirmed selectivity for animals in the amygdala (P < 10−5) and revealed that there was no significant preference (P > 0.05) in the other two regions (Fig. 1b). To test for laterality effects, we repeated the above analysis separately for the left and right amygdala. We found that neuronal responses selective for animals arose exclusively from the right amygdala (Fig. 1c, d). This pattern of results remained unchanged when we included multi-unit data in the analysis (Supplementary Figs. 4 and 5; also see Supplementary Results).
Figure 1.
Amygdala neurons respond preferentially to animal pictures. (a) Response probabilities of neurons in different MTL regions to different stimulus categories revealed significant preferences in the amygdala (P < 10−15, main effect of increased responses to animals at ~1%) and entorhinal cortex (P < 0.03, main effect of decreased responses to persons), but not in the hippocampus. (b) Mean response magnitudes of all responsive neurons showed increased response activity of amygdala neurons to animals (P < 10−5). (c, d) The animal preference in both response probability and magnitude was seen only in the right amygdala (P < 10−15 and P < 0.0005, respectively). Error bars denote binomial 68% confidence intervals (a, b) and s.e.m. (c, d). *P < 0.05, ***P < 0.001.
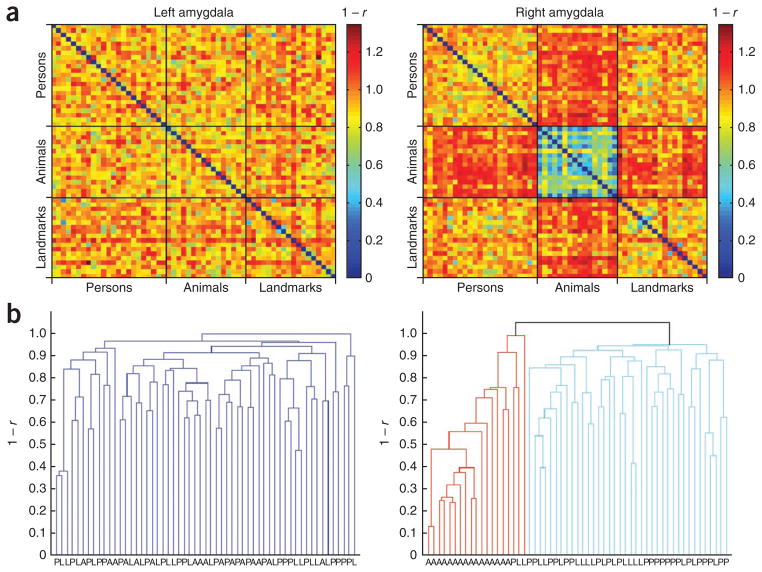
To test whether specific response patterns of amygdala neurons to animals are also present at the population level, we analyzed how images are segregated by response patterns using a categorization technique that has been applied to neurons in monkey inferotemporal cortex11. To apply this technique, we used an objective algorithm to select the largest subset of units from a total of 1,239 single and multi-units in the amygdala that had all been presented with the largest subset of stimuli. This subset consisted of 201 amygdala units (96 left, 105 right) that had all been presented with an identical subset of 57 stimuli from three different categories (Supplementary Fig. 6; see Supplementary Methods for details about the selection procedure). Representational dissimilarity matrices reflecting the dissimilarity in response between all pairs of these 57 stimuli revealed a specific response pattern to animals in the right amygdala that differed from the response patterns to persons and landmarks (Fig. 2a and Supplementary Fig. 7a). Hierarchical clustering analysis, which groups the stimuli objectively based on similar response patterns, further confirmed that animals and non-animals formed distinguishable clusters in the population code of the right, but not left, amygdala (Fig. 2b and Supplementary Fig. 7b). This pattern of results remained unchanged when we restricted the analysis to single units (data not shown), and no such category effects were found in the hippocampus or entorhinal cortex (Supplementary Fig. 8).
Figure 2.
A specific category response to animals in the right amygdala at the population level. (a) For a set of 201 amygdala units (96 left, 105 right) that were all presented with the same 57 stimuli (23 persons, 16 animals, 18 landmarks), we constructed representational dissimilarity matrices by determining the dissimilarity in evoked response patterns for each pair of stimuli (as 1 – r from the Pearson correlation across units). (b) Hierarchical cluster analysis automatically grouped stimuli with similar response patterns together into clusters. In the right amygdala, this unsupervised procedure yielded a cluster that contained all animal stimuli, whereas no such category effect was found in the left amygdala.
The mean response latency of amygdala units that responded to animal pictures (324 ms) was significantly shorter (P = 0.006) than the latency to stimuli from all other categories (398 ms; Supplementary Fig. 9). This effect was statistically significant in the amygdala, but not in the hippocampus or entorhinal cortex. Although this expedited processing of animal pictures may reflect their biological importance and category selectivity, the observed amygdala latencies are nevertheless similar to those found in other regions in the temporal lobe10, and thus seem more likely to be generated along the cortical object recognition pathway than via a rapid subcortical route12.
Previous studies have implicated the human amygdala in fear- and threat-related processing9. The animal images that elicited neuronal responses in the amygdala contained both aversive and cute animals, and we found no relationship between amygdala responses and either the valence or arousal of the animal stimuli (Supplementary Fig. 10). Furthermore, we reproduced an animal versus non-animal category effect in the right amygdala in an additional functional magnetic resonance imaging (fMRI) control experiment using stimuli that were controlled for emotional valence and arousal (Supplementary Fig. 11). In addition, we ruled out an influence of confounding stimulus features (Supplementary Control Analyses and Supplementary Fig. 12) such as sharpness of contours that have been shown to affect amygdala activity13.
Taken together, our results demonstrate that the right amygdala is specialized for processing visual information about animals. The selectivity appears to be truly categorical and argues in favor of a domain-specific mechanism for processing this biologically important class of stimuli. A plausible evolutionary explanation is that the phylogenetic importance of animals, which could represent either predators or prey, has resulted in neural adaptations for the dedicated processing of these biologically salient stimuli. This idea is consistent with recent findings that animals can be detected preferentially in change-blindness tasks14. The right-lateralized effect that we found is consistent with previous findings that support the notion that, early on in vertebrate evolution, the right hemisphere became specialized for detecting and responding to unexpected and behaviorally relevant stimuli15. In the future, it will be important to replicate our finding in species other than humans and to investigate the mechanisms that generate this functional laterality (see Supplementary Discussion). It will be particularly interesting to see whether such a hemispheric asymmetry can also be found at earlier stages of the cortical object recognition pathway that constitutes the visual input to the amygdala.
Supplementary Material
Acknowledgments
We thank all of our subjects for their participation, E. Behnke, T. Fields, E. Ho, V. Isiaka, E. Isham, K. Laird, N. Parikshak and A. Postolova for technical assistance with the electrophysiological recordings, and D. Tsao, I. Riedel-Kruse, U. Rutishauser and K. Fliessbach for useful discussion and comments on the manuscript. This research was supported by grants from the European Commission (Marie Curie OIF 040445, to F.M.), World Class University program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-10008, to C.K.), the US National Institute of Neurological Disorders and Stroke, the G. Harold and Leila Y. Mathers Foundation, the Gimbel Discovery Fund, and the Dana Foundation.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
F.M., S.K., R.Q.Q., I.F. and C.K. designed the electrophysiology study. I.F. carried out all of the neurosurgical procedures. F.M., M.C., M.I., R.Q.Q., A.K. and I.F. collected the electrophysiological data, and S.K. and F.M. analyzed the electrophysiological data. F.M., N.T., M.M., C.K. and R.A. designed the fMRI control experiment, F.M., M.M., J.D. and N.T. collected the fMRI data, and J.D. and F.M. analyzed the fMRI data. F.M., R.A. and C.K. wrote the paper. All of the authors discussed the results and commented on the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Phelps EA, LeDoux JE. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Murray EA. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Herry C, et al. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard CM, Rolls ET, Wilson FA, Baylis GC. Behav Brain Res. 1985;15:159–176. doi: 10.1016/0166-4328(85)90062-2. [DOI] [PubMed] [Google Scholar]
- 5.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. J Neurophysiol. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- 6.Nishijo H, Ono T, Nishino H. J Neurosci. 1988;8:3570–3583. doi: 10.1523/JNEUROSCI.08-10-03570.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paton JJ, Belova MA, Morrison SE, Salzman CD. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreiman G, Koch C, Fried I. Nat Neurosci. 2000;3:946–953. doi: 10.1038/78868. [DOI] [PubMed] [Google Scholar]
- 9.Whalen P, Phelps EA. The Human Amygdala. Oxford University Press; New York: 2009. [Google Scholar]
- 10.Mormann F, et al. J Neurosci. 2008;28:8865–8872. doi: 10.1523/JNEUROSCI.1640-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kriegeskorte N, et al. Neuron. 2008;60:1126–1141. doi: 10.1016/j.neuron.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pessoa L, Adolphs R. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar M, Neta M. Neuropsychologia. 2007;45:2191–2200. doi: 10.1016/j.neuropsychologia.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.New J, Cosmides L, Tooby J. Proc Natl Acad Sci USA. 2007;104:16598–16603. doi: 10.1073/pnas.0703913104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallortigara G, Rogers LJ. Behav Brain Sci. 2005;28:575–589. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.