Abstract
Objective
To better understand the associations between certificate of need regulations and intensity-modulated radiotherapy (IMRT) dissemination.
Methods
Using Surveillance, Epidemiology, and End Results (SEER)-Medicare data, we identified men (66 years or older) treated with radiotherapy for prostate cancer diagnosed between 2001 and 2007. Based on data from the American Health Planning Association, we sorted Health Service Areas (HSAs) according to the stringency of certificate of need regulations (low vs. high) in that market. We assessed our outcomes (i.e., the probability of IMRT adoption and IMRT utilization in HSAs) using Cox proportional-hazards and Poisson regression models, respectively.
Results
Low and high stringency markets were similar in terms of racial composition (80% vs. 85% white, p=0.08), population density (1,085 vs. 558 people/square mile, p=0.08), and income (median: $38,683 vs. 40,309, p=0.44), but low stringency markets had more patients with stage T1 disease (45% vs. 36%, p<0.01). The probability of IMRT adoption across the two groups of HSAs was similar (p=0.65). However, among adopting HSAs, those with high stringency consistently had greater use of IMRT (p<0.01).
Conclusions
Certificate of need regulations fail to create significant barriers to entry for IMRT. Among HSAs that acquire IMRT, high stringency markets demonstrate a greater propensity for using IMRT. These findings raise questions regarding the ability of certificate of need regulations to control technology dissemination.
Keywords: intensity-modulated radiotherapy, certificate of need, adoption, utilization, health service area
INTRODUCTION
The implementation of new expensive technology is a leading contributor to the rapid growth in healthcare spending.1 For a variety of reasons, costly innovations can disseminate broadly before their effectiveness is well established.2 One regulatory mechanism that may help curb unfettered adoption of expensive technology is state-based certificate of need laws. The rationale of certificate of need legislation is to limit healthcare costs by controlling unnecessary expansion of health facilities and services.3 Intensity-modulated radiotherapy (IMRT) is an example of a promising yet expensive technology for the treatment of prostate cancer that diffused rapidly over the past decade. Compared with its immediate predecessor, 3-dimensional conformal therapy, IMRT typically delivers higher doses of radiation to the prostate, which may lead to improved cancer control4 and lower toxicity.5 However, it is considerably more expensive, both in terms of start-up costs, which are well over a million dollars,6 and in terms of per episode costs, which exceed $30,000.7
Due to the significant capital outlay required to purchase the infrastructure to deliver IMRT, it is a natural target of certificate of need laws. The effects of these regulations on the adoption and implementation of IMRT, however, are poorly understood. On the one hand, application fees may discourage hesitant investors from purchasing IMRT equipment,3 which may constrain its diffusion. Moreover, state agencies may prevent overuse by approving only a limited number of IMRT machines in their jurisdiction. On the other hand, states have little incentive to limit the approval of applications within their markets because they do not benefit directly from limiting healthcare costs.8 For instance, since Medicare is a major payer, taxpayers across the nation bear much of the financial burden of providing new services.8 Further, politically powerful hospitals or physician groups may be able to convince state agencies to approve their proposals.3 For these reasons, certificate of need laws may not always limit the dissemination of IMRT.
Understanding how these regulations impact technology dissemination has real-world implications for policymakers, particularly given the growth of costly prostate cancer technologies.
MATERIAL and METHODS
Data Sources and Study Population
Using Surveillance, Epidemiology, and End Results (SEER)-Medicare data, we identified men aged 66 years or older diagnosed with prostate cancer between 2001 and 2007. SEER is a nationally representative population-based registry that comprises approximately 26% of the United States’ population.9 Next, we identified men undergoing radiation therapy (i.e., all forms of external beam radiotherapy, brachytherapy) within the first 12 months of diagnosis using Healthcare Common Procedure Coding System (HCPCS) codes in the outpatient and carrier files.7 Only fee-for-service beneficiaries eligible for both Medicare Parts A and B from 12 months prior to diagnosis until 12 months after diagnosis were included in the study. Men aged 65 years were excluded to ensure accurate comorbidity estimation using Medicare claims for the 12-month period prior to diagnosis.10 Using these criteria, our study population consisted of 55,162 patients treated with radiotherapy for localized prostate cancer.
Identifying Health Care Markets
We divided the SEER registries into healthcare markets using 164 Health Service Area (HSA) boundaries specified by the Area Resource File. Briefly, HSAs were originally defined by the National Center for Health Statistics as a single county or cluster of contiguous counties that are relatively self-contained with respect to hospital care.11 We chose HSAs as our unit of exposure because radiation treatment for prostate cancer is elective, discretionary, requires daily visits, and hence, is generally delivered locally. One HSA was excluded because it lacked patients undergoing radiation therapy for prostate cancer during the study period.
For each HSA, we characterized its level of certificate of need regulation using data from the American Health Planning Association’s National Directory of Health Planning, Policy, and Regulatory Agencies.12 The American Health Planning Association surveys state regulatory agencies to obtain information on certificate of need programs. Based on previous studies,13 we sorted HSAs into two groups according to the stringency of certificate of need regulations (low vs. high) presiding over that market. Low stringency represents markets with either a state-defined equipment expenditure threshold over $1.5 million or no certificate of need regulations. We chose a threshold of $1.5 million due to the approximate cost of IMRT equipment.6 A market with a threshold over $1.5 million is considered low stringency because IMRT equipment generally costs less than this threshold, and thus, investors would circumvent the review process. We grouped markets with no certificate of need regulations with low stringency markets because certificate of need laws will presumably have the same regulatory effects, or lack thereof, in both types of markets. In both cases, purchasers of IMRT equipment will avoid the review process established by certificate of need agencies. Conversely, high stringency markets reflect those with an equipment expenditure threshold less than or equal to $1.5 million. Purchasing equipment that costs more than a given expenditure threshold requires prior approval. In some circumstances, changes in equipment expenditure thresholds occurred over time. However, these modifications never resulted in a market crossing stringency categories. After contacting certificate of need agencies to confirm cost thresholds for review, we performed a sensitivity analysis for a range of threshold values from $1.0 million to $1.7 million and found no changes in our results.
Outcomes
To assess the effects of certificate of need regulations on IMRT dissemination, we first characterized the probability of adopting IMRT (i.e., a healthcare market that acquires the ability to deliver IMRT). To reduce measurement error, a market was considered an adopter if it contained 5 or more patients treated with IMRT within a 12-month period. The time of adoption was backdated to the first claim for IMRT. Because certificate of need regulations may influence the diffusion of IMRT within an HSA even after it acquires the capability, we next measured utilization among adopting HSAs (n=128). For this ratio, the numerator was the number of men treated with IMRT and the denominator was the number of men treated with radiation.
Statistical Analysis
We contrasted aggregate patient and HSA population characteristics according to level of certificate of need stringency using chi-square and Student t tests for categorical and continuous variables, respectively. Next, we fit a Cox proportional-hazards model to assess the probability of IMRT adoption across the certificate of need stringency exposure. This model met the proportional-hazards assumptions. Time-to-event was calculated from the beginning of the study (January 1, 2001) until the first date of an IMRT claim or the end of the observation window (December 31, 2008).
Among HSAs that adopted IMRT during the study period, we fit a Poisson regression model to assess a market’s utilization of IMRT in the first 12 months after adoption. This model was back-transformed to generate the predicted probability of IMRT utilization according to certificate of need stringency. For both outcomes (i.e., probability of IMRT adoption and probability of IMRT use among adopters), models were adjusted for patient (age, tumor grade and stage, comorbidity) and HSA population (racial composition, population density, education, income, percent speaking English as a secondary language) characteristics. Comorbidity was measured using a well-established modification of the Charlson index.12 All analyses were performed using SAS v9.2 (Cary, NC). The probability of a type I error was set at 0.05 and all testing was two-sided. The Institutional Review Board of the University of Michigan approved the study protocol.
RESULTS
The population characteristics of HSAs are shown in Table 1. Of the 163 HSAs, 114 (70%) comprised low stringency markets, whereas 49 (30%) consisted of high stringency markets. Although patient comorbidity differed statistically, this difference was small and unlikely of clinical significance. Patients treated in low stringency markets more often had lower stage disease (45% T1 vs. 36%, p<0.01). Low and high stringency markets were similar in terms of patient age (73 vs. 73 years, p=0.50), racial composition (80% vs. 85% white, p=0.08), population density (1,085 vs. 558 people/square mile, p=0.08), education (20% vs. 19% with at least a college education, p=0.54), income (median: $38,683 vs. 40,309, p=0.44), and tumor grade (54% vs. 57% well/moderately differentiated, p=0.15). Markets with low stringency had a greater percentage of residents speaking English as a secondary language (3% vs. 1%, p<0.01).
Table 1.
Population characteristics of Health Service Areas according to level of certificate of need stringency
| Health Service Area | Certificate of need regulation | p value | |
|---|---|---|---|
| Low stringency |
High stringency |
||
| No. Health Service Areas | 114 | 49 | -- |
| No. patients treated with radiation | 39,654 | 15,508 | -- |
| Mean patient age, years | 73 | 73 | 0.50 |
| White population, % | 80 | 85 | 0.08 |
| Population density, (people per square mile) | 1,085 | 558 | 0.08 |
| At least college education, % | 20 | 19 | 0.54 |
| Median income, $ | 38,683 | 40,309 | 0.44 |
| English secondary language, % | 3 | 1 | <0.01 |
| Well/moderately differentiated tumor grade, % | 54 | 57 | 0.15 |
| Tumor stage T1, % | 45 | 36 | <0.01 |
| Charlson Score 2 or higher, % | 12 | 10 | 0.04 |
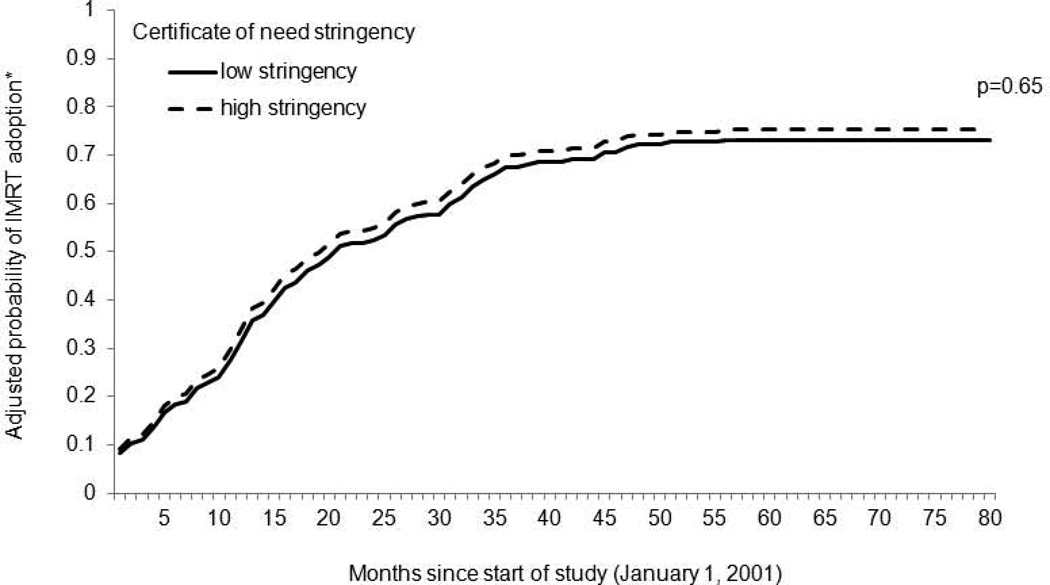
After adjusting for patient- and market-level characteristics, the probability of IMRT adoption across the two groups of HSAs was very similar (p=0.65) (Figure 1). The likelihood of adoption increased in both types of markets over time. By 2007, 73% in low stringency and 75% in high stringency markets had adopted IMRT.
Figure 1. Probability of IMRT adoption according to certificate of need stringency.
The probability of adoption across the two groups of HSAs was very similar (p=0.65). The likelihood of IMRT adoption increased in both types of markets over time.
HSA, health service area; IMRT, intensity-modulated radiotherapy
*Adjusted for patient-level (i.e., age, tumor grade and stage, comorbidity) and market-level (i.e., racial composition, population density, education, income, English as a secondary language) characteristics.
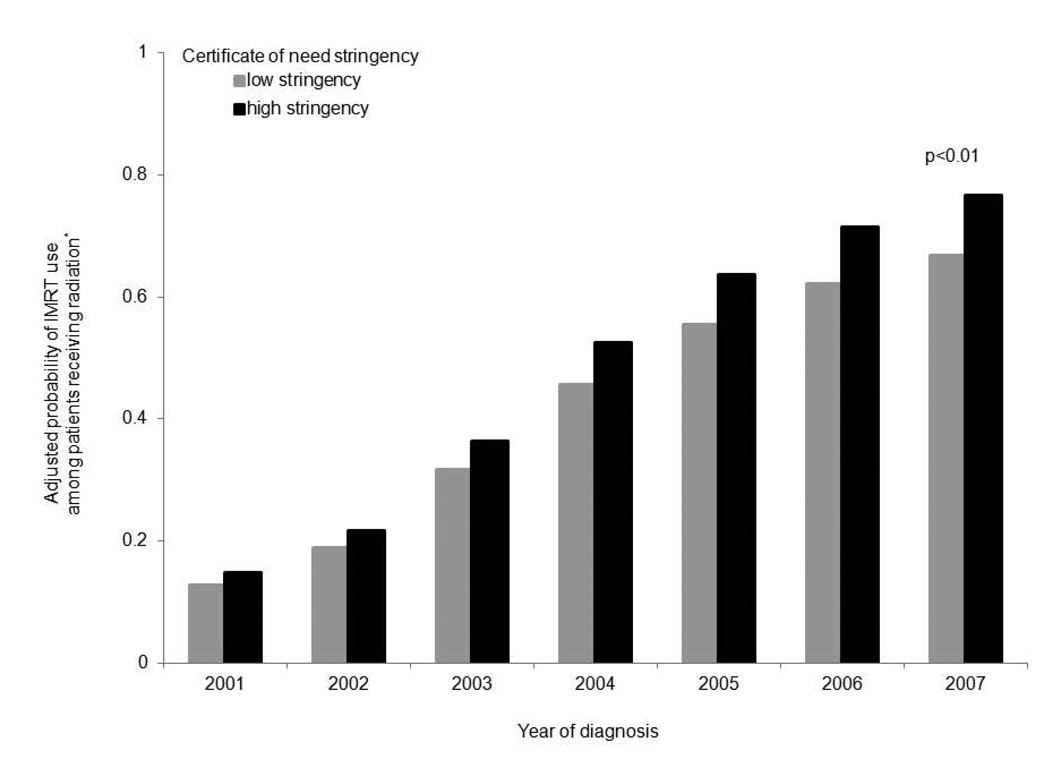
The population characteristics of HSAs that adopted IMRT are shown in Table 2. With the exception of tumor stage, both categories of markets had statistical differences in patient- and market-level characteristics that were small and clinically insignificant. Again, 9% more patients in low stringency markets had clinical T1 disease (46% vs. 37%, p<0.01). After adjusting for patient- and market-level factors, the probability of IMRT utilization among patients treated with radiation is illustrated in Figure 2. Independent of certificate of need stringency, markets that adopted IMRT demonstrated rapid growth in utilization; both categories of HSAs had a greater than 50% increase in IMRT utilization over time. However, HSAs with high stringency consistently had a greater propensity for IMRT utilization compared to HSAs with low stringency (p<0.01 for the overall effect of certificate of need stringency on utilization).
Table 2.
Population characteristics among Health Service Areas that have adopted intensity-modulated radiotherapy according to level of certificate of need stringency
| Health Service Area | Certificate of need regulation | p value | |
|---|---|---|---|
| Low stringency |
High stringency |
||
| No. Health Service Areas | 92 | 36 | -- |
| No. patients treated with radiation | 39,153 | 15,180 | -- |
| Mean patient age, years | 74 | 73 | <0.01 |
| White population, % | 79 | 83 | <0.01 |
| Population density, (people per square mile) | 1,530 | 860 | <0.01 |
| At least college education, % | 22 | 22 | 0.59 |
| Median income, $ | 41,884 | 43,357 | 0.21 |
| English secondary language, % | 3 | 2 | <0.01 |
| Well/moderately differentiated tumor grade, % | 56 | 58 | <0.01 |
| Tumor stage T1, % | 46 | 37 | <0.01 |
| Charlson Score 2 or higher, % | 11 | 10 | <0.01 |
Figure 2. The probability of IMRT utilization among men treated with radiation in markets that have adopted IMRT, according to certificate of need stringency.
Independent of certificate of need stringency, markets that adopted IMRT demonstrated rapid growth in utilization over time. However, HSAs with high certificate of need stringency consistently had a greater propensity for IMRT utilization compared to HSAs with low stringency (p<0.01 for the overall effect of certificate of need stringency on utilization).
HSA, health service area; IMRT, intensity-modulated radiotherapy
*Adjusted for patient-level (i.e., age, tumor grade and stage, comorbidity) and market-level (i.e., racial composition, population density, education, income, English as a secondary language) characteristics.
COMMENT
In 1974, the federal government mandated certificate of need regulations in all states in an attempt to control healthcare costs.14,15 This was largely in response to the growing belief that the increasing availability of health insurance contributed to Roemer’s law, that is a bed created is a bed used.14 However, by the early 1980’s, evidence accumulated suggesting that certificate of need laws were ineffective at controlling costs.15 As such, in 1986, the federal government allowed states to decide whether to uphold these regulations.16 By 2001, 14 states had dropped certificate of need laws altogether, while an additional 12 states dropped them for radiation therapy, specifically. Indeed, the effects of certificate of need laws on the diffusion of other technologies have been mixed.17,18 Nonetheless, proponents of certificate of need regulations argue that state-run programs had considerable heterogeneity wherein states with ineffective programs abandoned these regulations while states with successful programs maintained them.16 A variety of factors could contribute to this heterogeneity, including local political considerations, rigors of the review process, and the availability of agency resources, which may influence the effectiveness of certificate of need regulations.16,19 As a result, healthcare markets across the country face varying levels of certificate of need regulations. However, how these disparate markets affect radiation delivery remains largely unknown.
We found that markets with different certificate of need stringencies had similar rates of adoption. That is, certificate of need approval had no bearing on whether a market adopted IMRT. Further, markets with high stringency that acquired IMRT had a higher propensity for implementing the new technology. Collectively, these findings highlight that certificate of need regulations fail to create significant barriers for adopting IMRT.
There are at least three potential reasons why certificate of need regulations do not curb the acquisition of IMRT technology. First, critics of certificate of need laws contend that states have little incentive to limit the approval of applications within their markets because they do not benefit directly from containing costs; they argue that since Medicare is a major payer, taxpayers across the nation bear much of the financial burden of providing new services in a market.8 By approving the use of new services, states may enjoy the benefit of providing new technologies, while having some of the costs absorbed by national payers.8 Second, powerful hospitals or physician groups may be able to convince state agencies to approve their proposals.3 Critics worry that industrial lobbying may fuel a process whereby certificate of need agencies serve the welfare of investors instead of the public interest.3,20 For instance, an understaffed and underfinanced certificate of need agency may approve a proposal from a financially and politically strong organization instead of incurring the costs needed to defend the application's denial.3 Third, although certificate of need agencies implement application fees, these costs are typically a few thousand dollars.12 With equipment outlays over $1,500,0006 and annual profits as much as $400,000 per investor,21 these financial considerations are orders of magnitude higher than the application fees, which are thus unlikely to deter investors from purchasing IMRT.
The inability of certificate of need regulations to impede adoption is compounded by the greater utilization of IMRT in markets with high stringency. The implications of this are twofold. First, markets with stringent certificate of need regulations may inadvertently protect institutions that have adopted IMRT from competition,8,22 thereby providing unwarranted economic advantages to those institutions approved to provide services.23 For example, established providers may lobby against competitors who seek approval in their market.24 By fostering anticompetitive markets, certificate of need regulations may actually increase healthcare costs.24,25 Second, whether or not this increased utilization improves quality of care is unknown. On the one hand, limiting the delivery of IMRT to fewer centers may improve care by cultivating higher-volume practices.26 On the other hand, by thwarting competition, institutions in high stringency markets may overtreat patients, wherein IMRT is prescribed to the marginal patient to recoup sunk costs. Indeed, evidence from prior studies shows that certificate of need regulations are unlikely to affect quality of care.15,27
Regardless, the findings of unrestricted adoption and propagated utilization in markets with stringent certificate of need regulations have real-world policy implications. Insofar as certificate of need laws are intended to contain costs by controlling the dissemination of new, expensive technologies,8 these regulations appear to be largely ineffective. Throughout the period of study, highly stringent markets had similar likelihoods of IMRT adoption as less stringent markets, suggesting that certificate of need regulations generate ineffective barriers to entry. Although we did not examine cost specifically, the similar rates of adoption among HSAs support the notion that capital expenditures were similar across markets. To heighten entry barriers, certificate of need agencies could leverage the application fee to increase costs to potential investors. One caveat, however, is that a more expensive application fee may favor larger, wealthier institutions,3 while further stifling competition. Moreover, agencies could limit their consideration to applications that demonstrate extreme and urgent need.3 However assessing need may be difficult.
Whether certificate of need regulations prevent the overuse of services remains unclear. After accounting for the greater proportion of lower-staged patients in high stringency markets as well as other clinical and market characteristics, HSAs with stringent regulations were more likely to use IMRT in lieu of other types of radiation. However, the market-level framework of this analysis prevented us from addressing whether the increased utilization was attributed to the overtreatment of patients. For these same reasons, we were unable to measure patient-level outcomes. Further examination of both these endpoints is warranted.
The decreased utilization in less stringent markets suggests that competing mechanisms may influence the utilization of technology. For example, managed care organizations, which employ cost-controlling strategies, may have a stronger foothold in markets less regulated by certificate of need laws. Insofar as healthcare policy desires to play a role in overseeing the dissemination of technology, it is imperative to understand the interplay among these various mechanisms.
In addition, policymakers must grapple with how much evidence is sufficient to approve new technology. Given prostate cancer’s protracted clinical course, a robust trial involving IMRT would take more than 10 years to complete. To completely deny access to new technology, such as IMRT, until definitive evidence arises would, in many ways, impede advances in medicine because of the amount of time that would elapse. However, IMRT has now been available for a decade, and we still lack definitive evidence supporting its relative effectiveness. One concept that may help amass evidence involving new technologies is coverage with evidence development. This policy would grant Medicare coverage for designated new treatments provided that patients participate in research, such as a clinical trial or disease registry.28 If patients treated with IMRT had participated in such a trial, there would be prospectively collected data on thousands of patients to help determine IMRT’s relative health benefits.
Our findings should be interpreted in the context of several limitations. First, our outcomes are based on patterns of radiation treatment for Medicare beneficiaries. Although approximately one-third of patients with prostate cancer are less than 65 years old,29 those treated with radiation tend to be older than those undergoing alternative therapies (median age 69 years),30 making our findings generalizable to the vast majority of men undergoing radiation for prostate cancer. Second, as with all observational data, our inference may be biased by unmeasured differences between markets. For instance, states without certificate of need regulations may have other types of health care regulatory mechanisms that were not accounted for, such as licensure or limits on capital diffusion.23 Accordingly, we adjusted for several measured market factors to minimize confounding. Third, certificate of need programs are heterogeneous with respect to several characteristics, such as expenditure thresholds, rigors of the approval process, and political considerations.16 Although details about each agency’s review process were unknown, we accounted for differences in expenditure thresholds by stratifying certificate of need programs based on whether they were above or below the estimated cost of acquiring IMRT technology.
Despite these limitations, this study elucidates two key findings. First, markets with stringent certificate of need regulations have a similar propensity to acquire IMRT technology. To the extent that IMRT likely represents an expensive technology with small incremental benefits, this finding highlights the ineffectiveness of these regulations. IMRT equipment and support for ancillary staff cost millions of dollars,6 and thus, effectively regulating the number of facilities may help slow the rapid growth in healthcare costs. This will become even more relevant as more expensive prostate cancer treatments, such as proton beam therapy, come down the pike. Second, markets with stringent certificate of need regulations tend to utilize more IMRT. Going forward, a greater understanding is needed to decipher whether this increased utilization among highly stringent markets signifies an improvement in quality of care or rather represents overuse among institutions protected by certificate of need regulations.
ACKNOWLEDGMENT
SUPPORT
Bruce Jacobs is supported in part by the American Cancer Society Postdoctoral Fellowship Grant (121805-PF-12-008-01-CPHPS). Bruce Jacobs and Florian Schroeck are supported in part by the National Institutes of Health Training Grant NIH 5 T32 DK007782-12.
Brent Hollenbeck is supported in part by the American Cancer Society Pennsylvania Division—Dr. William and Rita Conrady Mentored Research Scholar Grant (MSRG-07-006-01-CPHPS), the American Urological Association Foundation, and Astellas Pharma US, Inc.
The views expressed in this article do not reflect the views of the federal government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
Bruce L. Jacobs: none
Yun Zhang: none
Ted A. Skolarus: none
John T. Wei: none
James E. Montie: none
Florian R. Schroeck: none
Brent K. Hollenbeck: none
Contributor Information
Bruce L. Jacobs, Email: brucejac@med.umich.edu.
Yun Zhang, Email: seanyz@med.umich.edu.
Ted A. Skolarus, Email: tskolar@med.umich.edu.
John T. Wei, Email: jtwei@med.umich.edu.
James E. Montie, Email: jmontie@med.umich.edu.
Florian R. Schroeck, Email: fschroec@med.umich.edu.
Brent K. Hollenbeck, Email: bhollen@med.umich.edu.
REFERENCES
- 1.Newhouse JP. Medical care costs: how much welfare loss? J Econ Perspect. 1992;6:3–21. doi: 10.1257/jep.6.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Barbash GI, Glied SA. New technology and health care costs - the case of robot-assisted surgery. N Engl J Med. 2010;363:701–704. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 3.Salkever DS, Bice TW. The impact of certificate-of need controls on hospital investment. Milbank Mem Fund Q Health Soc. 1976;54:185–214. [PubMed] [Google Scholar]
- 4.Vora SA, Wong WW, Schild SE, et al. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1053–1058. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Carreyrou J, M T. A Device to Kill Cancer, Lift Revenue. Wall Street Journal. 2010 Dec 7; [Google Scholar]
- 7.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellinger FJ. The effect of certificate-of-need laws on hospital beds and healthcare expenditures: an empirical analysis. Am J Manag Care. 2009;15:737–744. [PubMed] [Google Scholar]
- 9.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; 2010. [Google Scholar]
- 10.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. [accessed January 3, 2012]; Available from URL: http://www.cdc.gov/nchs/
- 12.Falls Church, VA: American Health Planning Association; 2007. National Directory of Health Planning, Policy, and Regulatory Agencies (2001,2003,2007) [Google Scholar]
- 13.Popescu I, Vaughan-Sarrazin MS, Rosenthal GE. Certificate of need regulations and use of coronary revascularization after acute myocardial infarction. JAMA. 2006;295:2141–2147. doi: 10.1001/jama.295.18.2141. [DOI] [PubMed] [Google Scholar]
- 14.Melhado EM. Health planning in the United States and the decline of public-interest policymaking. Milbank Q. 2006;84:359–440. doi: 10.1111/j.1468-0009.2006.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conover CJ, Sloan FA. Does removing certificate-of-need regulations lead to a surge in health care spending? J Health Polit Policy Law. 1998;23:455–481. doi: 10.1215/03616878-23-3-455. [DOI] [PubMed] [Google Scholar]
- 16.Conover CJ, Sloan FA. Evaluation of certificate of need in Michigan. Volume 2. Durham, NC: Center for Health Policy, Law, Management, Terry Sanford Institute of Public Policy, Duke University; 2003. [Google Scholar]
- 17.Lewin-ICF and Alpha Center. Evaluation of the Ohio Certificate-of-Need Program. Washington, DC: Lewin-ICF; 1991. [Google Scholar]
- 18.Teplensky JD, Pauly MV, Kimberly JR, et al. Hospital adoption of medical technology: an empirical test of alternative models. Health Serv Res. 1995;30:437–465. [PMC free article] [PubMed] [Google Scholar]
- 19.Salkever DS. Regulation of prices and investment in hospitals in the United States. In: Culyer AJ, Newhouse JP, editors. Handbook of Health Economics, edition 1, volume 1. chapter 28. Amsterdam: Elsevier; 2000. pp. 1489–1535. [Google Scholar]
- 20.Havighurst CC. Regulation of health facilities and services by certificate-of-need. Virginia Law Review. 1973;599:1143–1232. [Google Scholar]
- 21.FAQ'S. [accessed January 27, 2012]; Available from URL: http://online.wsj.com/public/resources/documents/urorad-whois12082010.pdf. [Google Scholar]
- 22.Burda D. CONspiracies to crush competition. Hospitals using CON laws to thwart rival's projects. Mod Healthc. 1991;21:28–30. 32-24, 36. [PubMed] [Google Scholar]
- 23.Vaughan-Sarrazin MS, Hannan EL, Gormley CJ, et al. Mortality in Medicare beneficiaries following coronary artery bypass graft surgery in states with and without certificate of need regulation. JAMA. 2002;288:1859–1866. doi: 10.1001/jama.288.15.1859. [DOI] [PubMed] [Google Scholar]
- 24.Improving health care: A dose of competition, Federal Trade Commission and the Department of Justice. 2004 [Google Scholar]
- 25.Rivers PA, Fottler MD, Frimpong JA. The effects of certificate of need regulation on hospital costs. Journal of Health Care Finance. 2010;36:1–16. [Google Scholar]
- 26.Short MN, Aloia TA, Ho V. Certificate of need regulations and the availability and use of cancer resections. Ann Surg Oncol. 2008;15:1837–1845. doi: 10.1245/s10434-008-9914-1. [DOI] [PubMed] [Google Scholar]
- 27.DiSesa VJ, O'Brien SM, Welke KF, et al. Contemporary impact of state certificate-of-need regulations for cardiac surgery: an analysis using the Society of Thoracic Surgeons' National Cardiac Surgery Database. Circulation. 2006;114:2122–2129. doi: 10.1161/CIRCULATIONAHA.105.591214. [DOI] [PubMed] [Google Scholar]
- 28.Miller FG, Pearson SD. Coverage with evidence development: ethical issues and policy implications. Med Care. 2008;46:746–751. doi: 10.1097/MLR.0b013e3181789453. [DOI] [PubMed] [Google Scholar]
- 29.Ries LAG, Krapcho M, Stinchcomb DG, et al., editors. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; 2008. [Google Scholar]
- 30.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]