Abstract
Tonoplast-enriched vesicles isolated from maize (Zea mays L.) coleoptiles and seeds synthesize ATP from ADP and inorganic phosphate (Pi) and inorganic pyrophosphate from Pi. The synthesis is consistent with reversal of the catalytic cycle of the H+-ATPase and H+-pyrophosphatase (PPase) vacuolar membrane-bound enzymes. This was monitored by measuring the exchange reaction that leads to 32Pi incorporation into ATP or inorganic pyrophosphate. The reversal reactions of these enzymes were dependent on the proton gradient formed across the vesicle membrane and were susceptible to the uncoupler carbonyl cyanide p(trifluoromethoxy)-phenylhydrazone and the detergent Triton X-100. Comparison of the two H+ pumps showed that the H+-ATPase was more active than H+-PPase in coleoptile tonoplast vesicles, whereas in seed vesicles H+-PPase activity was clearly dominant. These findings may reflect the physiological significance of these enzymes in different tissues at different stages of development and/or differentiation.
Two distinct proton pumps are found in the vacuolar membrane of plants, a V-ATPase (EC 3.6.1.3) and a membrane-bound H+-PPase (EC 3.6.1.1). Each of these enzymes can generate a transmembrane electrochemical H+ gradient using the energy derived from hydrolysis of its substrate (ATP or PPi, respectively). The H+ gradient is then used to energize the secondary transport of the different substances needed for the plant's development (Rea and Sanders, 1987). The physiological role of the membrane-bound PPase is not clear at present. It is currently accepted that the V-ATPase plays a predominant role in the maintenance of a transmembrane electrochemical H+ gradient, whereas the H+-PPase seems to serve as an ancillary backup system for the pumping of protons (Rea et al., 1992; Taiz, 1992; Baykov et al., 1993). Recently, it was found that H+-PPase is overexpressed in response to energetic stresses such as chilling and anoxia (Carystinos et al., 1995; Darley et al., 1995). The cellular PPi content, unlike that of ATP, remains stable during marked changes in respiratory state (Weiner et al., 1987; Dancer and ap Rees, 1989). These observations suggest that the H+-PPase may exert a key role in the survival strategies of plants under conditions of limited ATP supply (Macrì et al., 1995, and refs. therein).
The coexistence of two different enzymatic systems playing the same role in the same membrane is apparently paradoxical. Based on thermodynamic considerations, Rea and Sanders (1987) and Schmidt and Briskin (1993a) raised the hypothesis that, instead of both enzymes always operating in parallel to pump protons into the vacuole, the gradient generated by one of the proton pumps might drive the reversal reaction of the other, which therefore behaves as a synthase. The catalytic cycles of both enzymes can be reversed using the energy derived from the H+ gradient. Dupaix et al. (1989) and Schmidt and Briskin (1993b) demonstrated the synthesis of ATP from ADP and Pi and showed that synthesis was driven by the H+ gradient formed by PPi hydrolysis in tonoplast vesicles. Based on 18O-exchange measurements at the PPi-binding site, Baykov et al. (1994) concluded that PPi formation had occurred in Vigna radiata tonoplast H+-PPase during PPi hydrolysis.
The present study shows that the H+ gradient generated across the vacuolar membrane by the hydrolysis of either PPi or ATP may drive both PPi and ATP synthesis (as indicated by isotope exchange rather than net synthesis), which is consistent with the reversal of the tonoplast H+-PPase and H+-ATPase. Furthermore, there is a significant difference between the activities of tonoplast-enriched vesicles from maize coleoptiles and seeds, suggesting a differential expression or regulation of these enzymes depending on plant cell development and/or differentiation.
MATERIALS AND METHODS
Seeds of maize (Zea mays L.) were soaked in water for 24 h. Afterward, some of the seeds were used for isolation of tonoplast vesicles, and the remainder were sown on wet filter paper and germinated in the dark at 28°C. Coleoptiles of 5-d-old seedlings were harvested for preparation of vesicles. The maize seeds were provided by Sementes Agroceres S.A. (São Paulo, Brazil).
Tonoplast-Enriched Vesicles
Vacuolar membrane (tonoplast) vesicles were isolated from whole seeds or etiolated coleoptiles using differential centrifugation as described by Giannini and Briskin (1987), with minor modifications. About 50 g of coleoptiles or 150 g of seeds was homogenized using either a mortar and pestle or a domestic food liquidizer in 2 mL/g (fresh weight) of ice-cold buffer containing 10% (v/v) glycerol, 0.5% (v/v) PVP (PVP-40, 40 kD), 5 mm EDTA, 0.13% (w/v) BSA, and 0.1 m Tris-HCl buffer, pH 8.0. Just prior to use, 150 mm KCl, 3.3 mm DTT, and 1 mm PMSF were added to the buffer. The homogenate was strained through four layers of cheesecloth and centrifuged at 8,000g for 10 min. The supernatant was centrifuged once more at 8,000g for 10 min and then at 100,000g for 40 min. The pellet was resuspended in a small volume of ice-cold buffer containing 10 mm Tris-HCl, pH 7.6, 10% (v/v) glycerol, 1 mm DTT, and 1 mm EDTA. The suspension containing the coleoptile vesicles was layered over a 10/25/46% (w/w) discontinuous Suc gradient that contained, in addition to Suc, 10 mm Tris-HCl buffer, pH 7.6, 1 mm DTT, and 1 mm EDTA.
For vesicles from seeds a better yield was obtained using a 10/30/46% (w/w) gradient, in agreement with a previous study (Hoh et al., 1995) showing that during the subcellular fractionation of pea cotyledons at early developmental stages, a peak of V-ATPase activity was found in the fractions between 30 and 32% (w/w) on a Suc gradient. After centrifugation at 100,000g for 3 h in a swinging bucket, the vesicles that sedimented at the interface between 10 and 25 or 30% Suc were collected, diluted with 3 volumes of ice-cold water, and centrifuged at 100,000g for 40 min. Bafilomycin A1 or NO3−-inhibited H+-ATPase and K+-dependent H+-PPase activities were used as marker enzymes for the tonoplast membrane (Sze, 1985). The pellet was resuspended in a medium containing 10 mm Tris-HCl, pH 7.6, 10% (v/v) glycerol, 1 mm DTT, and 1 mm EDTA. The vesicles were either used immediately or frozen under liquid N2 and stored at −70°C until use. Protein concentrations were determined by the method of Lowry et al. (1951).
ATPase and PPase Activity
ATPase activity was determined by measuring the release of Pi, either colorimetrically (Fiske and Subbarow, 1925) or using [γ-32P]ATP, as previously described (de Meis, 1988). Between 85 and 100% of the vesicle ATPase activity measured at pH 7.0 was inhibited by either 50 mm KNO3 or 10 nm Bafilomycin A1, two specific inhibitors of the V-type H+-ATPase (Bowman et al., 1988; White, 1994). In all experiments the ATPase activity was measured with and without NO3− or Bafilomycin A1, and the difference between these two activities was attributed to the vacuolar H+-ATPase. KF, an inhibitor of PPase (Maeshima and Yoshida, 1989), completely inhibited PPase activity. ATPase and PPase activities of tonoplast preparations were unaffected by either vanadate (0.1 mm), an inhibitor of plasma membrane ATPase, or oligomicin (10 nm), an inhibitor of mitochondrial ATPases.
Electrochemical Gradient of Protons
The accumulation of H+ by the vesicles was determined by measuring the fluorescence quenching of ACMA using a fluorimeter (model F-3010, Hitachi, Tokyo). The excitation wavelength was set at 415 nm and the emission wavelength was set at 485 nm. The reaction medium contained 10 mm Mops-Tris, pH 7.0, 2 μm ACMA, 5 mm MgCl2, and 100 mm KCl. In different vesicle preparations tested, the inclusion of 50 mm KNO3 or 50 nm Bafilomycin A1 in the assay medium promoted more than 85% inhibition of the fluorescence quenching measured after the addition of ATP. Both substances are specific V-type ATPase inhibitors and, when added after the H+ gradient was formed, promoted a passive backflow of protons (data not shown). The addition of either 3 μm FCCP, a proton ionophore, or 0.02% of the detergent Triton X-100 abolished the H+ gradient formed by either ATP or PPi hydrolysis. There was no formation of an H+ gradient when PPi was used as the substrate if the vesicles were previously treated with 10 mm KF or when KCl was not included in the assay medium (data not shown). These data are consistent with an ATP- and PPi-dependent H+ transport mediated by the tonoplast H+-ATPase and by the K+-dependent H+-PPase (Griffith et al., 1986; White, 1994).
Pi ↔ PPi and Pi ↔ ATP Exchange
The synthesis of PPi and ATP by tonoplast vesicles was assayed by measuring the amount of [32P]PPi and [γ-32P]ATP formed during the cleavage of nonradioactive ATP or PPi (de Meis, 1984; Behrens and de Meis, 1985; de Meis et al., 1985). The assay medium contained 50 mm Mops-Tris buffer, pH 7.0, 5 mm MgCl2, 5 mm [32Pi]Pi (20,000 cpm/nmol Pi), 100 mm KCl, and tonoplast vesicles to a final concentration of 0.05 mg/mL protein. The reaction was started by the addition of either 1 mm PPi or 1 mm ATP. After the reaction was completed, a sample of the reaction medium was quenched with ice-cold TCA and used to determine the total amount of Pi esterified as either ATP or PPi. The rest of the assay medium was filtered (0.45-μm filters, Millipore) to remove the tonoplast vesicles, and the filtrate was used to distinguish between the fraction of Pi used to synthesize ATP and that used to form PPi. To part of the sample, 0.1 mm ATP, 0.15 mm CaCl2, and 50 μg/mL Ca2+-dependent ATPase (EC 3.6.1.38), prepared as describe by Eletr and Inesi (1972), were added. To the second sample, 0.1 mm PPi and 10 μg/mL yeast PPase (EC 3.6.1.1) were added. The addition of 0.1 mm ATP or PPi was required to optimize the hydrolysis activity (data not shown).
These two samples were incubated for 30 min at 35°C and then quenched with TCA. The Ca2+-dependent ATPase preparation used was able to catalyze the hydrolysis of the γ-Pi of ATP at the rate of 3.0 μmol Pi mg−1 min−1. In agreement with previous reports (de Meis, 1984, 1988; de Meis et al., 1985, 1986), we did not detect any measurable cleavage of PPi with the Ca2+-dependent ATPase. The yeast PPase used was able to catalyze the hydrolysis of PPi only at the rate of 4.0 μmol mg−1 min−1. The 32Pi was extracted from the different TCA-quenched samples as phosphomolybdate using isobutyl alcohol-benzene, as previously described (de Meis, 1984; de Meis et al., 1985).
The small amount of radioactivity found in controls (not incubated with the tonoplast vesicles) was subtracted from that found in the experiments with vesicles. The radioactivity remaining in the aqueous phase after extraction of the free 32Pi with isobutyl alcohol-benzene (representing total 32Pi esterified) was subtracted from that in the sample treated with Ca2+-ATPase or yeast PPase; the difference was attributed to either ATP or PPi synthesized by the tonoplast enzymes. Concentrations of radioactive PPi synthesized were calculated on the basis of two 32Pi incorporated into each PPi. In earlier reports it was shown that this method makes it possible to quantify the amount of radioactive ATP and PPi formed by solubilized enzyme and by chromatophores of Rhodospirillum rubrum. In these reports, the radioactive ATP and PPi remaining in the aqueous phase after extraction were copurified with nonradioactive ATP and PPi, respectively, and characterized by TLC and autoradiography (de Meis, 1984; Behrens and de Meis, 1985; de Meis et al., 1985).
Materials
Bafilomycin A1, A23157, PPase purified from yeast, FCCP, ADP, and ATP were purchased from Sigma. All other reagents used were analytical grade. A 500 mm Pi-Tris stock solution adjusted to pH 7.0 was prepared by mixing aqueous solutions of phosphoric acid and Tris base. One-millimolar stock solutions of FCCP or ACMA in ethanol were used. The final concentration of ethanol in the assay medium never exceeded 0.03%.
RESULTS
ATPase and PPase Activity in Tonoplast Vesicles from Coleoptiles and Seeds
A single, central vacuole is typical of later stages of cell development in most vegetative tissues. However, two functionally distinct kinds of vacuoles have been found in plant cells at early stages of development and/or differentiation (Hoh et al., 1995; Paris et al., 1996). In this work we compared the activity of proton pumps in fractions of tonoplast vesicles derived from 5-d-old coleoptiles, representative of mature vegetative tissues, with that from hydrating seeds, representative of tissues in development.
Tonoplast vesicles catalyzed the hydrolysis of both ATP and PPi, regardless of whether they were derived from coleoptiles or from seeds. The hydrolysis of both substrates was coupled with proton translocation. However, the activities of the two enzymes varied depending on the origin of the vesicles. For the coleoptile vesicles the velocities of ATP hydrolysis and H+ accumulation were faster than the rate of H+ transport supported by PPi hydrolysis (Table I). In contrast, for the seed vesicles the initial velocities of ATP hydrolysis and H+ transport were several times slower than those of PPi hydrolysis and H+ transport (Table I). The H+ gradient formed by PPi hydrolysis in seed vesicles was always steeper than that formed in coleoptile vesicles (Table I). These data suggest that there is either a differential expression or a differential activation of H+-PPase and H+-ATPase in coleoptile and seed vacuolar membranes.
Table I.
The difference in H+-ATPase and H+-PPase activities between tonoplast vesicles derived from coleoptiles and seeds
| Parameter | Enzyme
Activity
|
|||
|---|---|---|---|---|
| Coleoptiles
|
Seeds
|
|||
| H+-ATPase | H+-PPase | H+-ATPase | H+-PPase | |
| H+ Gradient | ||||
| % | ||||
| Steady state (ΔF) | 53 ± 6 (n = 6) | 46 ± 3 (n = 6) | 25 ± 2 (n = 5) | 63 ± 7 (n = 5) |
| F min−1 | ||||
| Initial velocity | 286 ± 10 (n = 6) | 35 ± 3.8 (n = 6) | 14 ± 1 (n = 6) | 128 ± 32 (n = 5) |
| Hydrolysis | ||||
| nmol mg−1min−1 | ||||
| Initial velocity | 140 ± 30 (n = 3) | 57 ± 7 (n = 3) | 28 ± 8 (n = 3) | 131 ± 31 (n = 3) |
The initial rates of ATP and PPi hydrolysis and the formation of an H+ gradient were calculated from experiments like those shown in Figure 1 (hydrolysis). Initial velocities of H+-gradient formation are given in arbitrary units. Values represent the means ± se of n independent experiments.
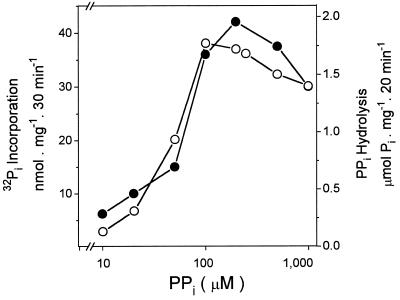
In the presence of 5 mm MgCl2, the concentration of PPi needed for maximal rates of substrate hydrolysis (Fig. 1) was found to vary between 0.1 and 0.2 mm. In agreement with previous reports (White et al., 1990; Leigh et al., 1992), a decrease of the PPase was observed in the presence of an excess of substrate (Fig. 1). The half-maximal ATPase activity was reached at about 0.2 to 0.4 mm ATP (data not shown), a Km similar to that found for tonoplast H+-ATPase from oat roots.
Figure 1.
H+-PPase of seed vesicles: substrate dependence for PPi hydrolysis and 32Pi incorporation. The assay medium composition was 50 mm Mops-Tris buffer, pH 7.0, 0.1 mm ADP, 100 mm KCl, 5 mm 32Pi, 5 mm MgCl2, and 0.05 mg/mL tonoplast vesicles, at 35°C. ○, PPi hydrolysis; •, 32Pi incorporation.
PPi and ATP Synthesis in Tonoplasts from Coleoptiles and Seeds
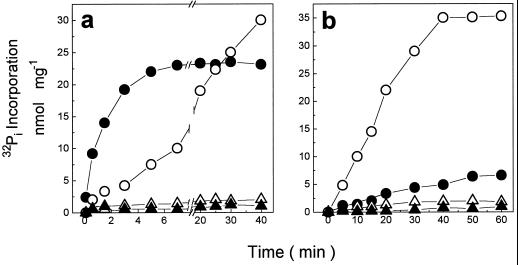
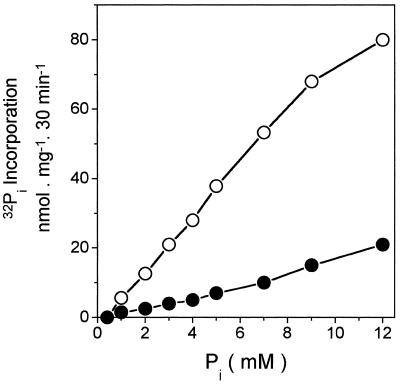
The proton gradient generated by the hydrolysis of either PPi or ATP can be used to promote reversal of the catalytic cycle of tonoplast-bound H+-PPase and H+-ATPase as measured by 32Pi incorporation. When the reaction was triggered by ATP in coleoptile vesicles, the rate was faster than that observed with PPi-dependent 32Pi incorporation (Fig. 2a). In contrast, in seed tonoplast vesicles, PPi-dependent 32Pi incorporation was much faster than ATP-dependent 32Pi incorporation (Fig. 2b). Figure 2 shows that the incorporation of 32Pi stopped after 6 min (Fig. 2a) and after 40 min (Fig. 2b). At present, we do not know why the 32Pi incorporation did not continue as in the case of ATP and PPi hydrolysis. The presence of uncoupler agents such as Triton X-100 (Fig. 2) and FCCP (data not shown) blocked the [32P]phosphate-exchange reactions, indicating that reversal of the catalytic cycle of tonoplast H+-PPase and H+-ATPase requires the H+ gradient. The substrate dependence for the Pi-exchange reaction was almost the same as that for the substrate hydrolysis (Fig. 1). The rate of exchange was found to vary with the Pi concentration in the medium (Fig. 3). The affinity of both the H+-PPase and the H+-ATPase for Pi seems to be low. Saturation was not reached even in the presence of 12 mm Pi. Thus, we were not able to measure the apparent Km for Pi.
Figure 2.
Time course of 32Pi incorporation by tonoplast vesicles from coleoptiles (a) and seeds (b). The assay medium composition was 50 mm Mops-Tris buffer, pH 7.0, 0.1 mm ADP, 100 mm KCl, 5 mm 32Pi, 5 mm MgCl2, and 0.05 mg/mL tonoplast vesicles at 35°C. Triangles show when 0.02% Triton X-100 was present in the assay medium. The reaction was started by the addition of either 1 mm ATP (•, ▴) or 1 mm PPi (○, ▵) and was arrested by the addition of TCA. Essentially the same results were obtained in five experiments using different vesicle preparations.
Figure 3.
PPi dependence of 32Pi incorporation by tonoplast vesicles from seeds. The assay medium composition was 50 mm Mops-Tris buffer, pH 7.0, 0.1 mm ADP, 100 mm KCl, 5 mm MgCl2, and 0.05 mg/mL tonoplast vesicles at 35°C, using as the substrate either 1 mm ATP (•) or 1 mm PPi (○). The reaction was carried out in the presence of increasing Pi concentrations, maintaining the radioactive 32Pi: nonradioactive Pi proportions constant at 20,000 cpm/nmol of nonradioactive Pi. Essentially the same results were obtained in four independent experiments.
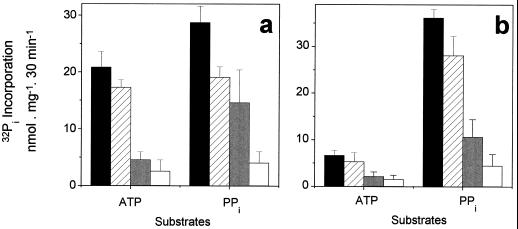
The [32P]phosphate-exchange assay adopted in this study does not allow us to identify directly which of the two compounds (ATP or PPi) has been synthesized. To this end, the reaction medium containing the Pi esterified during the exchange assay was subjected to enzymatic hydrolysis by PPase purified from yeast or by sarcoplasmic reticulum Ca2+-ATPase isolated from rabbit skeletal muscle. When the substrate was ATP, most of the 32Pi incorporated into products during the exchange reaction disappeared after treatment with PPase from yeast, indicating that a large fraction of PPi had been formed (Fig. 4). On the other hand, when the substrate was PPi, the 32Pi incorporation was significantly affected by the Ca2+-ATPase as well as by the PPase (Fig. 4). Since two molecules of Pi were incorporated for each PPi molecule synthesized, similar amounts of PPi and ATP were synthesized under these conditions (Table II). The same proportions were observed with tonoplast vesicles derived from coleoptiles as with vesicles from seeds.
Figure 4.
Identification of the products synthesized by coleoptiles (a) and seeds (b) using the sarcoplasmic reticulum Ca2+-ATPase and the PPase from yeast. The assay medium composition was 50 mm Mops-Tris buffer, pH 7.0, 100 mm KCl, 0.1 mm ADP, 5 mm MgCl2, 5 mm 32Pi, and 0.05 mg/mL tonoplast protein. The reactions were started by adding either 1 mm ATP or 1 mm PPi. After 30 min the reactions were stopped by filtration to remove vesicles, and the filtrates were divided into four aliquots. Three of them were incubated with 0.05 mg/mL Ca2+-ATPase from skeletal muscle sarcoplasmic reticulum (hatched bars), 0.01 mg/mL PPase from yeast (gray bars), or both (white bars); one aliquot (control; black bars) was not incubated with either enzyme. The Ca2+-ATPase reaction medium was supplemented with 0.15 mm CaCl2, 2 μm A23187, and 0.1 mm ATP when ATP was not already present. The PPase reaction medium was supplemented with 0.1 mm PPi when PPi was not already present. The reaction was stopped after 30 min by the addition of TCA (10%, w/v). The medium was subjected to phosphomolybdate extraction, and the radioactivity remaining in the aqueous phase was counted. Values represent the means + se of six independent experiments.
Table II.
Identification of products synthesized using specific inhibitors of tonoplast proton pumps
| Additions | Products Synthesized
|
|||
|---|---|---|---|---|
| Coleoptiles
|
Seeds
|
|||
| ATP | PPi | ATP | PPi | |
| nmol mg−1 30 min−1 | ||||
| ATP | 3.5 ± 0.3 (n = 6) | 8.2 ± 2.5 (n = 6) | 1.3 ± 0.5 (n = 6) | 2.3 ± 1.1 (n = 6) |
| ATP + KF | 3.0 ± 1.6 (n = 3) | 0 | 1.6 ± 1.1 (n = 3) | 0 |
| PPi | 9.6 ± 0.9 (n = 6) | 7.1 ± 2.8 (n = 6) | 8.0 ± 1.2 (n = 6) | 12.8 ± 2.3 (n = 6) |
| PPi + Bafilomycin A1 | 0 | 12.5 ± 1.6 (n = 3) | 0 | 11.3 ± 2.4 (n = 3) |
The enzymatic identification of products synthesized (Fig. 4) is compared with experiments of phosphate exchange performed either in the presence of 10 nm Bafilomicin A1, a specific inhibitor of V-ATPase, or with vesicles preincubated with 10 mm KF, an inhibitor of H+-PPase. The specific activity of products was calculated from radioactivity remaining after phosphomolybdate extraction. The total 32Pi incorporated in the presence of each substrate and in the absence of inhibitors (data not shown) are in the range presented as “Control” in Figure 4. The reaction medium contained 50 mm MOPS-Tris, pH 7.0, 5 mm MgCl2, 5 mm 32Pi, 100 mm KCl, 0.1 mm ADP, and either 1 mm ATP or 1 mm PPi. The reaction was started by the addition of 0.05 mg/mL protein. Values represent the means ± se of n independent experiments. PPi values were corrected for two Pi incorporated into each PPi.
Additional evidence for the identification of the products synthesized in these reactions was obtained using inhibitors of vacuolar ATPase and PPase. Bafilomycin A1 was able to inhibit the ATP synthesis promoted by PPi hydrolysis, as reported previously by Schmidt and Briskin (1993b). Preincubation of vesicles with fluoride blocked the PPi synthesis dependent on ATP hydrolysis. Regardless of the origin of vesicles (coleoptiles or seeds), the amount of 32Pi incorporation recovered after treatment with fluoride was essentially identical to the 32Pi incorporation recovered after treatment with PPase from yeast (Table II). Likewise, when Bafilomycin A1 was used, seed vesicles showed the same 32Pi incorporation as that recovered after treatment with sarcoplasmic reticulum Ca2+-ATPase. However, in coleoptile vesicles the amount of 32Pi incorporation found in the presence of Bafilomycin A1 was higher than that recovered after treatment with Ca2+-ATPase (Table II). It may be that eliminating the synthesis of ATP driven by PPi hydrolysis increases the H+ gradient available for reversal of the reaction catalyzed by the H+-PPase, thus increasing PPi synthesis.
ADP and KCl Dependence of the Reversal Reaction of Tonoplast ATPase and PPase from Coleoptiles and Seeds
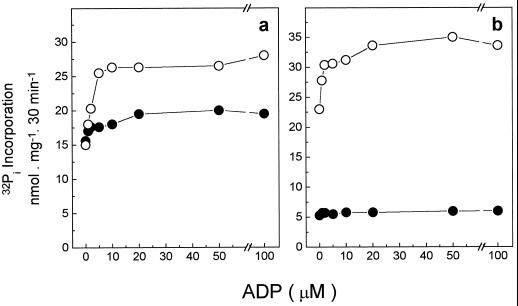
The 32Pi incorporation with PPi was enhanced when ADP was added to the medium (Fig. 5). Maximal increase was obtained with 5 μm ADP, a concentration in the range of that required for mitochondrial ATP synthase (Catterall and Pedersen, 1972). The incorporation obtained in the absence of ADP probably reflects the synthesis of PPi. Two molecules of Pi are needed for the synthesis of each PPi molecule and, therefore, the value measured must be divided by 2 (i.e. the 15 nmol 32Pi incorporated in the absence of ADP shown in Fig. 5a represented the synthesis of only 7.5 nmol [32P]PPi). On the other hand, one molecule of 32Pi was needed for the synthesis of each ATP molecule, and the 3 to 14 nmol 32Pi incorporated after the addition of ADP reflects the synthesis of 3 to 14 nmol ATP (Fig. 5a). The data in Figure 5 and Table II show that the amount of radioactive PPi synthesized in coleoptiles was the same regardless of whether ATP or PPi was used as the substrate for the transport of H+. In this organ, what varied was the amount of ATP formed during the exchange reaction, more [γ-32P] ATP being synthesized when PPi was cleaved than when nonradioactive ATP was hydrolyzed. A different profile was observed with seeds. In this case, a significant incorporation of 32Pi was observed only when PPi was cleaved.
Figure 5.
ADP dependence for 32Pi incorporation in tonoplast vesicles from coleoptiles (a) and seeds (b). The assay medium composition was 50 mm Mops-Tris buffer, pH 7.0, 100 mm KCl, 5 mm MgCl2, 5 mm 32Pi, 0.05 mg/mL tonoplast protein, and the concentration of ADP is shown on the abscissa. The reactions were started by either 1 mm ATP (•) or 1 mm PPi (○). Essentially the same results were obtained in three experiments using different vesicle preparations.
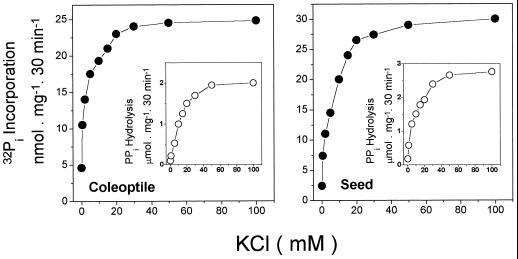
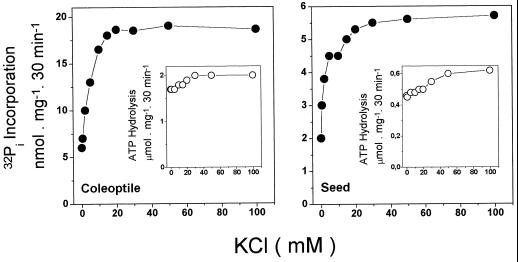
Figures 6 and 7 show the K+ dependence of the forward and reverse reactions in tonoplast vesicles derived from coleoptiles and from seeds. With PPi as the substrate (Fig. 6) both reactions were strongly stimulated by K+. This indicates that if the PPase is not activated by K+; there is no H+ gradient formation and the medium 32Pi cannot be used to synthesize either PPi or ATP. By contrast, with ATP as the substrate (Fig. 7), the forward reaction (Fig. 7, insets) exhibited only a small stimulation by KCl, whereas most of the 32Pi incorporation showed a K+ dependence. In this case, the gradient is formed by the ATPase, but the energy derived from the gradient can be used only to synthesize PPi if the PPase is activated by K+.
Figure 6.
KCl dependence for PPi hydrolysis and Pi-exchange reactions catalyzed by tonoplast vesicles from coleoptiles and seeds. The assay medium composition was 50 mm Mops-Tris buffer, pH 7.0, 5 mm MgCl2, 5 mm 32Pi, 0.1 mm ADP, and 0.05 mg/mL vacuolar membrane protein. •, 32Pi incorporation when 1 mm PPi was used as the substrate. In the case of the Pi-exchange assay the medium also contained 0.1 mm ADP. ○, Hydrolysis activities. Essentially the same results were obtained in four experiments using different vesicle preparations.
Figure 7.
KCl dependence for ATP hydrolysis and Pi-exchange reactions catalyzed by tonoplast vesicles from coleoptiles and seeds. The assay medium composition was 50 mm Mops-Tris buffer, pH 7.0, 5 mm MgCl2, 5 mm 32Pi, 0.1 mm ADP, and 0.05 mg/mL vacuolar membrane protein. •, 32Pi incorporation when 1 mm ATP was used as the substrate. In the case of the Pi-exchange assay the medium also contained 0.1 mm ADP. ○, Hydrolysis activities. Essentially the same results were obtained in four experiments using different vesicle preparations.
DISCUSSION
In the present work we examined the activities of tonoplast H+ pumps from coleoptiles and seeds that represent different stages of plant development. Mature coleoptiles manifest a premature senescence, since their function is linked to the early stages of growth of the seedlings. The coleoptile protects the leaf primordia and has been shown to serve as a gravi-guiding system for shoots of germinating seedlings (Edelmann, 1996). The balance of activity between the two proton pumps in coleoptile tonoplast vesicles is consistent with the pattern established for most vegetative tissue preparations, in which the tonoplast H+-ATPase usually can generate a pH gradient across the vacuolar membrane of similar or greater magnitude than the H+-PPase (Chanson et al., 1985; Giannini and Briskin, 1987; Rea and Sanders, 1987). The remarkable preponderance of H+-PPase activity in preparations from whole seeds contrasts with what has been found in coleoptiles and in most vegetative tissues.
In the early stages of seed hydration, the mitochondria are functionally deficient and the adenylate energy of the cell is low (Ivanov and Khavkin, 1976; Bewley and Black, 1985, and refs. therein). The prevalence of H+-PPase in developing seeds supports the idea that the physiological significance of this enzyme would be to maintain the proton gradient under conditions of limited ATP supply (Carystinos et al., 1995; Darley et al., 1995; Macrì et al., 1995). Suzuki and Kasamo (1993) showed that H+-PPase activities are about 3-fold higher than the V-ATPase activities in 7-d-old pumpkin cotyledons. However, this situation changes over the next few days, as the V-ATPase assumes the major role in proton pumping.
Maeshima et al. (1994) studied the induction of H+-PPase and H+-ATPase in germinating pumpkin seeds. Their data showed an abundance of the H+-PPase in 2-d-old pumpkin cotyledons as well as the remarkable activation of the H+-PPase compared with the H+-ATPase activity in tonoplasts from 2- to 6-d-old pumpkin cotyledons. These findings, as well as ours with maize seeds, support the hypothesis that PPi may serve as the key energy source during seed germination and/or early developmental stages in plant cells.
In hydrating seeds another source of energy might be derived from the presence of another pathway for the production of ATP. Here we have extended previous studies of the reversal reaction of both tonoplast H+ pumps (Dupaix et al., 1989; Schmidt and Briskin 1993b; Baykov et al., 1994). We show that the proton gradient generated by hydrolysis of either PPi or ATP can be used to promote reversal of the catalytic cycle of both H+-PPase and H+-ATPase. In accordance with predictions based on thermodynamic calculations (Roberts, 1990, and refs. therein; Schmidt and Briskin, 1993a), our assessment of the products synthesized shows that reversal of H+-PPase is favored over reversal of H+-ATPase (Table II). Although the analysis of mass-action ratios and equilibrium constants for tonoplast H+-PPase activities by these authors indicates that in vivo H+-PPase may be operating near equilibrium, this is the first time to our knowledge that PPi synthesis has been verified experimentally in tonoplasts.
ATP synthesis coupled to an electrochemical gradient generated by H+-PPase has been described in red beet tonoplasts (Schmidt and Briskin, 1993b). However, these authors concluded that under most physiological conditions reversal of the V-ATPase would be unlikely because of the difference in the free energy values for ATP synthesis and PPi hydrolysis. They suggested that high PPi and low Pi concentrations would be required to form a gradient of the necessary magnitude. On the contrary, under our conditions ATP synthesis was driven by PPi hydrolysis in the presence of a Pi concentration 5 times higher than that of PPi. These results may reflect a difference in the conditions required for isotope exchange compared with those required for net synthesis.
The role of the gradient in both the ATP ↔ Pi exchange and the PPi ↔ Pi exchange is still not clear. In earlier reports it was assumed that a transport ATPase could catalyze only an exchange reaction when an ionic gradient was formed across the vesicle membrane. The notion was that the energy derived from the gradient represented an absolute energetic requirement for the exchange reaction. Later experimentation revealed that during the exchange reaction the catalytic cycle of some, but not all, transport enzymes can be reversed in the absence of a gradient. An example is the membrane-bound Ca2+-ATPase found in different animal tissues. This enzyme can catalyze an ATP ↔ Pi exchange in both the presence and absence of a transmembrane Ca2+ gradient (de Meis and Carvalho, 1974; de Meis and Vianna, l979; Plank et al., 1979; de Meis et al., 1986).
For other enzymes, such as the ATP synthase of mitochondria, an ATP ↔ Pi exchange can be measured only in the presence of an H+ gradient. For most transport enzymes so far studied, the gradient seems to be needed to increase the affinity of the enzyme for Pi and to improve the ratio between the rates of hydrolysis and the synthesis of ATP (for reviews, see de Meis, 1993; Boyer, 1997; Weber and Senior, 1997). During the exchange reaction some of the energy derived from the cleavage is retained by the enzyme for the synthesis of a new ATP or PPi molecule. This is observed in enzymes that undergo a conformational change during the catalytic cycle. In these enzymes the products of the hydrolysis are released from the enzyme surface before returning to the conformation that allows the beginning of a new catalytic cycle. At this stage of the cycle the enzymes re-bind the products, reverse the catalytic cycle, and synthesize a new ATP or PPi molecule (de Meis, 1981, 1989; Sakamoto and Tonomura, 1983; de Meis et al., 1986).
In aqueous solutions under conditions close to those found in vivo, the hydrolysis of both ATP and PPi is accompanied by a large change in free energy (George et al., 1970; Hayes et al., 1978). During the catalytic cycle of several enzymes involved in energy transduction, there are steps in which the hydrolysis of these compounds is accompanied by only a small energy change (George et al., 1970; Hayes et al., 1978; de Meis, 1984). It has been proposed that the large change in energy of hydrolysis of the Pi compounds is promoted by a small change in water structure in the microenvironment on the enzyme (de Meis, 1984; de Meis et al., 1985, and refs. therein). These studies have important implications for the equilibrium of enzyme reaction, especially for seeds in which water concentration is low. The present data provide further evidence for the reversibility of the reaction mechanisms of the tonoplast ATP- and PPi-dependent proton pumps. Given the appropriate thermodynamic conditions in vivo, these pumps may operate as systems of energy conservation, with a role in maintaining the cytosolic ATP and PPi levels.
This notion is consistent with structural studies showing that the vacuolar H+-PPase and the R. rubrum H+-PPase (Nore et al., 1991) have evolved from a common ancestral gene, just as V- and F-type ATPases seem to have evolved from a single enzyme present in a common ancestor (Kibak et al., 1992). The coupling observed between the proton gradient and the Pi exchange suggests that the tonoplast H+-PPase and H+-ATPase share functional as well as structural similarities with the H+-PPase of R. rubrum and the F-type ATP synthases, both of which can couple the synthesis of their own products to the electrochemical H+ gradient generated in the membranes in which they are embedded.
ACKNOWLEDGMENT
The authors are grateful to Dr. Martha Sorenson for the helpful discussion of the manuscript.
Abbreviations:
- ACMA
9-amino-6-chloro-2-methoxyacridine
- FCCP
carbonyl cyanide p(trifluoromethoxy)-phenylhydrazone
- PPase
pyrophosphatase
- V-ATPase
vacuolar ATPase
Footnotes
This research was supported by grants from Programa de Apois ao Desenvolvimento Cientifico e Tecnologico-Conselho Nacional de Desenvolvimento Científico e Tecnológico, Financiadora de Estudos e Projetos, and Fundação de Amparo á Pesquisa do Estado do Rio de Janeiro. A.R.F. is a recipient of a fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico.
LITERATURE CITED
- Baykov AA, Bakuleva NP, Rea PA. Steady-state kinetics of substrate hydrolysis by vacuolar H+-pyrophosphatase. A simple three-state model. Eur J Biochem. 1993;217:755–762. doi: 10.1111/j.1432-1033.1993.tb18303.x. [DOI] [PubMed] [Google Scholar]
- Baykov AA, Kasho VN, Bakuleva NP, Rea PA. Oxygen exchange reactions catalyzed by vacuolar H+-translocating pyrophosphatase. FEBS Lett. 1994;350:323–327. doi: 10.1016/0014-5793(94)00800-0. [DOI] [PubMed] [Google Scholar]
- Behrens MI, de Meis L. Synthesis of pyrophosphate by chromatophores of Rhodospirillum rubrum in the light and by soluble yeast inorganic pyrophosphatase in water-organic solvent mixtures. Eur J Biochem. 1985;152:221–227. doi: 10.1111/j.1432-1033.1985.tb09187.x. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M (1985) Seeds—Physiology of Development and Germination. Plenum Press, New York, pp 139–152
- Bowman EJ, Siebers A, Altendorf K. Bafilomicins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer PD. The ATP-synthase, a splendid molecular machine. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- Carystinos GD, MacDonald HR, Monroy AF, Dhindsa RS, Poole RJ. Vacuolar H+-translocating pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiol. 1995;108:641–649. doi: 10.1104/pp.108.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Pedersen PL. Adenosine triphosphatase from rat liver mitochondria II. Interaction with adenosine diphosphate. J Biol Chem. 1972;247:7969–7976. [PubMed] [Google Scholar]
- Chanson A, Fichmann J, Spear D, Taiz L. Pyrophosphate-driven proton transport by microsomal membranes of corn coleoptiles. Plant Physiol. 1985;79:159–164. doi: 10.1104/pp.79.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer J, ap Rees T. The effects of 2,4-dinitrophenol and anoxia on the inorganic pyrophosphate content of spadix of Arum maculatum and root apices of Pisum sativum. Planta. 1989;178:421–424. doi: 10.1007/BF00391871. [DOI] [PubMed] [Google Scholar]
- Darley CP, Davies JM, Sanders D. Chill-induced changes in the activity and abundance of the vacuolar proton-pumping pyrophosphatase from mung bean hypocotyls. Plant Physiol. 1995;109:659–665. doi: 10.1104/pp.109.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meis L (1981) The sarcoplasmic reticulum. Transport and energy transduction. In EE Bittar, ed, Wiley Series on Transport in the Life Sciences, Vol 2. John Wiley, New York, pp 1–163
- de Meis L. Pyrophosphate of high and low energy: contributions of pH, Ca2+, Mg2+ and water to free energy of hydrolysis. J Biol Chem. 1984;259:6090–6097. [PubMed] [Google Scholar]
- de Meis L. Approaches to the study of mechanisms of ATP synthesis in sarcoplasmic reticulum. Methods Enzymol. 1988;157:190–206. doi: 10.1016/0076-6879(88)57075-1. [DOI] [PubMed] [Google Scholar]
- de Meis L. Role of water in the energy of hydrolysis of phosphate compounds—energy transduction in biological membranes. Biochim Biophys Acta. 1989;973:333–349. doi: 10.1016/s0005-2728(89)80440-2. [DOI] [PubMed] [Google Scholar]
- de Meis L. The concept of energy-rich phosphate compounds: water, transport ATPases and entropic energy. Arch Biochem Biophys. 1993;973:333–349. doi: 10.1006/abbi.1993.1514. [DOI] [PubMed] [Google Scholar]
- de Meis L, Behrens MI, Celis H, Romero I, Puyou MTG, Puyou AG. Orthophosphate-pyrophosphate exchange catalyzed by soluble and membrane-bound inorganic pyrophosphatase. Eur J Biochem. 1986;158:149–157. doi: 10.1111/j.1432-1033.1986.tb09732.x. [DOI] [PubMed] [Google Scholar]
- de Meis L, Behrens MI, Petretski JH, Politi MJ. Contribution of water to free energy of hydrolysis of pyrophosphate. Biochemistry. 1985;24:7783–7789. doi: 10.1021/bi00347a042. [DOI] [PubMed] [Google Scholar]
- de Meis L, Carvalho MGC. Role of the Ca2+ concentration gradient in the adenosine 5′triphosphate. Inorganic phosphate exchange catalyzed by sarcoplasmic reticulum. Biochemistry. 1974;13:5032–5038. doi: 10.1021/bi00721a026. [DOI] [PubMed] [Google Scholar]
- de Meis L, Vianna AL. Energy interconversion by the Ca2+-transport ATPase of sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]
- Dupaix A, Johannin G, Arrio B. ATP synthesis and pyrophosphate-driven proton transport in tonoplast-enriched vesicles isolated from Catharanthus roseus. FEBS Lett. 1989;249:13–16. [Google Scholar]
- Edelmann HG. Coleoptiles are gravi-guiding systems vital for gravi-insensitive shoots of germinating grass seedlings. Planta. 1996;200:281–282. doi: 10.1007/BF00208320. [DOI] [PubMed] [Google Scholar]
- Eletr S, Inesi G. Phospholipid orientation in sarcoplasmic reticulum membranes: spin label ESR and proton NMR studies. Biochim Biophys Acta. 1972;282:174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- Fiske CF, Subbarow Y. The colorometric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- George P, Witonsky RJ, Trachtman M, Wu C, Dorwart W, Richman L, Richman W, Shurayh F, Lentz B. “Squiggle-H2O”: an enquiry into the importance of solvation effects in phosphate ester and anhydride reactions. Biochim Biophys Acta. 1970;223:1–15. doi: 10.1016/0005-2728(70)90126-x. [DOI] [PubMed] [Google Scholar]
- Giannini JL, Briskin DP. Proton transport in plasma membrane and tonoplast vesicles from red beet (Beta vulgaris L.) storage tissue. Plant Physiol. 1987;84:613–618. doi: 10.1104/pp.84.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith CJ, Rea PA, Blumwald E, Poole RJ. Mechanism of stimulation and inhibition of tonoplast H+-ATPase of Beta vulgaris by chloride and nitrate. Plant Physiol. 1986;81:120–125. doi: 10.1104/pp.81.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DM, Kenyon GL, Kollman PA. Theoretical calculations of the hydrolysis energy of some “high-energy” molecules. 2. A survey of some biologically important hydrolytic reactions. J Am Chem Soc. 1978;106:4331–4340. [Google Scholar]
- Hoh B, Hinz G, Jeong B-K, Robinson DG. Protein storage vacuoles form de novo during pea cotyledon development. J Cell Sci. 1995;108:299–310. doi: 10.1242/jcs.108.1.299. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Khavkin EE. Protein patterns of developing mitochondria at the onset of germination in maize (Zea mays L.) FEBS Lett. 1976;65:383–385. doi: 10.1016/0014-5793(76)80152-4. [DOI] [PubMed] [Google Scholar]
- Kibak H, Taiz L, Starke T, Bernasconi P, Gogarten JP. Evolution of structure and function of V-ATPases. J Bioenerg Biomembr. 1992;24:415–424. doi: 10.1007/BF00762534. [DOI] [PubMed] [Google Scholar]
- Leigh RA, Pope AJ, Jennings IR, Sanders D. Kinetics of the vacuolar H+-pyrophosphatase. The roles of magnesium, pyrophosphate, and their complexes as substrates, activators, and inhibitors. Plant Physiol. 1992;100:1698–1705. doi: 10.1104/pp.100.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Macrì F, Zancani M, Petrussa E, Dell'Antone C, Vianello A. Pyrophosphate and H+-pyrophophatase maintain the vacuolar proton gradient in metabolic inhibitor-treated Acer pseudoplatanus cells. Biochim Biophys Acta. 1995;1229:323–328. [Google Scholar]
- Maeshima M, Hara-Nishimura I, Takeuchi Y, Nishimura M. Accumulation of vacuolar H+-pyrophosphatase and H+-ATPase during reformation of the central vacuole in germinating pumpkin seeds. Plant Physiol. 1994;106:61–69. doi: 10.1104/pp.106.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima M, Yoshida S. Purification and properties of vacuolar membrane proton-translocating inorganic pyrophosphatase from mung bean. J Biol Chem. 1989;264:20068–20073. [PubMed] [Google Scholar]
- Nore BF, Sakai-Nore Y, Maeshima M, Baltscheffsky M, Nyren P. Immunological cross-reactivity between proton-pumping inorganic pyrophosphatase of widely phylogenic separated species. Biochem Biophys Res Commun. 1991;181:962–967. doi: 10.1016/0006-291x(91)92030-n. [DOI] [PubMed] [Google Scholar]
- Paris N, Stanley CM, Jones RL, Rogers JC. Plant cells contain two functionally distinct vacuolar compartments. Cell. 1996;85:563–572. doi: 10.1016/s0092-8674(00)81256-8. [DOI] [PubMed] [Google Scholar]
- Plank B, Hellmann G, Punzengruber C, Suko J. ATP-Pi and ITP-Pi exchange by cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1979;550:259. doi: 10.1016/0005-2736(79)90212-8. [DOI] [PubMed] [Google Scholar]
- Rea PA, Kim Y, Sarafian V, Poole RJ, Davies JM, Sanders D. Vacuolar H+-translocating pyrophosphatases: a new category of ion translocase. Trends Biochem Sci. 1992;17:348–353. doi: 10.1016/0968-0004(92)90313-x. [DOI] [PubMed] [Google Scholar]
- Rea PA, Sanders D. Tonoplast energization: two H+ pumps, one membrane. Physiol Plant. 1987;71:131–141. [Google Scholar]
- Roberts JKM. Observation of uridine triphosphate: glucose-1-phosphate uridyltransferase activity in maize root tips by saturation transfer 31P-NMR. Estimation of cytoplasmic PPi. Biochim Biophys Acta. 1990;1051:29–36. doi: 10.1016/0167-4889(90)90170-i. [DOI] [PubMed] [Google Scholar]
- Sakamoto J, Tonomura Y. Synthesis of enzyme-bound ATP by mitochondrial soluble F1-ATPase in the presence of dimethyl sulfoxide. J Biochem Tokyo. 1983;93:1601–1614. doi: 10.1093/oxfordjournals.jbchem.a134299. [DOI] [PubMed] [Google Scholar]
- Schmidt AL, Briskin DP. Energy transduction in tonoplast vesicles from red beet (Beta vulgaris L.) storage tissue: H+/substrate stoichiometries for the H+-ATPase and H+-PPase. Arch Biochem Biophys. 1993a;301:165–173. doi: 10.1006/abbi.1993.1129. [DOI] [PubMed] [Google Scholar]
- Schmidt AL, Briskin DP. Reversal of the red beet tonoplast H+-ATPase by a pyrophosphate-generated proton electrochemical gradient. Arch Biochem Biophys. 1993b;306:407–414. doi: 10.1006/abbi.1993.1530. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kasamo K. Effects of aging on the ATP- and pyrophosphate-dependent pumping of protons across the tonoplast isolated from pumpkin cotyledons. Plant Cell Physiol. 1993;34:613–619. [Google Scholar]
- Sze H. H+-translocating ATPases: advances using membrane vesicles. Annu Rev Plant Physiol. 1985;36:175–208. [Google Scholar]
- Taiz L. The plant vacuole. J Exp Biol. 1992;172:113–122. doi: 10.1242/jeb.172.1.113. [DOI] [PubMed] [Google Scholar]
- Weber J, Senior AE. Catalytic mechanism of F1-ATPase. Biochim Biophys Acta. 1997;1319:19–58. doi: 10.1016/s0005-2728(96)00121-1. [DOI] [PubMed] [Google Scholar]
- Weiner H, Stitt M, Heldt HW. Subcellular compartmentation of pyrophosphate and alkaline phosphatase in leaves. Biochim Biophys Acta. 1987;893:13–21. [Google Scholar]
- White PJ. Bafilomycin A1 is a non-competitive inhibitor of the tonoplast H+-ATPase of maize coleoptiles. J Exp Bot. 1994;45:1397–1402. [Google Scholar]
- White PJ, Marshall J, Smith JAC. Substrate kinetics of the tonoplast H+-translocating inorganic pyrophosphatase and its activation by free Mg2+ Plant Physiol. 1990;93:1063–1070. doi: 10.1104/pp.93.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]