Abstract
OBJECTIVES
To determine (i) the presence of fatty acid amide hydrolase (FAAH) in the urinary bladder; (ii) whether or not endogenous fatty acid ethanolamides are synthesized by the bladder; (iii) the effects of FAAH inhibition on referred hyperalgesia associated with acute bladder inflammation in rats.
MATERIALS AND METHODS
Immunohistochemistry and immunoblotting were performed to detect FAAH in the bladder. Acrolein (1 mM, 400 µL) was instilled into bladders of female Wistar rats to induce cystitis. Referred mechanical hyperalgesia was assessed by application of Von Frey monofilaments to the hind paws. Animals were killed 4, 24, 48 and 72 h after acrolein instillation, and the fatty acid ethanolamide content of bladders was measured using isotope-dilution liquid chromatography/mass spectrometry. Other rats were treated with the FAAH inhibitor URB597 (0.3 mg/kg, i.p.) after the induction of cystitis, and the mechanical sensitivity of the hind paws was determined.
RESULTS
Immunohistochemistry and immunoblotting showed the presence of FAAH in the bladder, with greatest abundance in the urothelium. Acrolein-induced cystitis increased fatty acid ethanolamide content (including anandamide) in the bladder in a time-dependent manner. Inhibition of FAAH diminished referred hyperalgesia associated with acute bladder inflammation.
CONCLUSIONS
The results obtained in the present study indicate that (i) FAAH is present in the urinary bladder; (ii) fatty acid ethanolamides are increased during bladder inflammation; (iii) inhibition of FAAH could be an effective therapeutic approach for the treatment of bladder pain. These results raise the possibility that inhibitors of enzymes responsible for metabolism of fatty acid ethanolamides could inhibit pain associated with bladder inflammation.
Keywords: cystitis, bladder, fatty acid amide hydrolase, fatty acid ethanolamide, endocannabinoid
INTRODUCTION
Visceral pain accompanying bladder disorders is often difficult to control, and the pathogenesis underlying visceral pain is not fully understood [1]. Increasing evidence suggests that inflammatory mediators released from inflamed tissues sensitize bladder afferent nerves, causing persistent visceral pain [2,3]. Fatty acid ethanolamides, such as anandamide (AEA) and palmitoylethanolamide (PEA), are increased in inflamed tissues [4,5], although there is only one report describing increased AEA in inflamed bladder tissues [6]. AEA is an endocannabinoid that activates both cannabinoid receptors 1 and 2 [7,8], and the analgesic effects of AEA have been reported in animal models of inflammatory pain [9–11]. The effects of PEA appear to be mediated by binding to the peroxisome proliferators-activated α receptor [12,13]. Systemic administration of AEA or PEA prevented referred hyperalgesia associated with turpentine-induced bladder inflammation in rats [9,10].
Fatty acid amide hydrolase (FAAH) is primarily responsible for the degradation of AEA, although it also has the capacity to hydrolyze other fatty acid ethanolamides, including oleoylethanolamide (OEA) and PEA [14,15]. FAAH is abundantly expressed in various tissues [12]. To date, the expression of FAAH in the bladder has not been described. Inhibition of FAAH is a promising therapeutic approach for the treatment of inflammatory pain by increasing the abundance of fatty acid ethanolamides, and FAAH inhibition may exert the analgesic effect without evoking cannabinoid-like side-effects [16].
Acrolein, a metabolite of cyclophosphamide, induces cystitis that is accompanied by referred hyperalgesia of somatic structures [17]. Referred hyperalgesia also accompanies bladder disorders in humans [18]. In the present study, the presence of FAAH within the rat bladder wall is shown and time-dependent changes in the abundance of fatty acid ethanolamides in the bladder during inflammation are investigated. The inhibition of FAAH on referred hyperalgesia that accompanied bladder inflammation is also evaluated.
MATERIALS AND METHODS
ANIMALS
Ten- to 14-week-old female Wistar rats (200–300 g) were used. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin, Madison.
BLADDER INFLAMMATION
Rats were anaesthetized by inhalation of isoflurane (2–5%) in oxygen. Bladders were catheterized with lubricated PE 10 tubing (Intramedic, Sparks, MD, USA), emptied by light abdominal compression, and acrolein (1 mM, 400 µL; Ultra Scientific, Kingstown, RI, USA) or saline (0.9%, 400 µL) was instilled. Rats remained anaesthetized, and the catheter was left in place for 30 min after instillation of acrolein or saline.
TREATMENT WITH FAAH INHIBITOR
FAAH inhibitor URB597 was prepared in 20% dimethyl sulphoxide and 80% saline. Rats were treated with URB597 (0.3 mg/kg, i.p.), 1 h before evaluating sensitivity to peripheral application of mechanical stimuli. Peripheral application of mechanical stimuli was carried out 4 and 24 h after instillation of acrolein or saline. The dose and time of administration were based on previous studies showing that brain concentrations of AEA reached a peak 1 h after i.p. administration of URB597 in rats [15]. Control rats received the vehicle.
EVALUATION OF REFERRED HYPERALGESIA
Referred hyperalgesia was assessed by response to peripheral application of mechanical stimuli, using Von Frey monofilaments and the up-down method [17,19]. Rats were placed in individual chambers with a wire mesh floor. Once the animal was acclimated to the enclosure, five Von Frey monofilaments with forces of 2, 4, 6, 8 and 15 g were applied perpendicular to the plantar surface of the hind paw with sufficient force to cause the monofilament to bend slightly. A positive response was recorded when the animal withdrew or licked the paw, and the 50% probability withdrawal threshold was determined [17,19]. Mechanical sensitivity was determined before and 4 and 24 h after intravesical administration of acrolein or saline.
HISTOLOGY
Rats were euthanized after the last mechanical sensitivity measurement. Bladders were collected and weighed. Bladders were fixed in 10% formalin. Tissue sections were stained with haematoxylin and eosin. Morphological evidence of inflammation was assessed by comparison of bladder weights and by histological evaluation of tissues stained with haematoxylin and eosin [20].
IMMUNOBLOTTING
Rats were killed 48 h after intravesical instillation of acrolein or saline. The bladder mucosa (urothelium and lamina propria) was separated from the detrusor, homogenized using protein extraction reagent (T-PER; Pierce Biotechnology, Rockford, IL, USA) containing protease inhibitors (Roche, Indianapolis, IN, USA). Homogenates were centrifuged, the supernatants were collected, and protein concentrations were measured. Protein samples were resolved with SDS polyacrylamide gel (10%) and transferred to nitrocellulose membranes. Membranes were washed and incubated overnight at 4 °C with an antibody against FAAH (dilution 1 : 3000; Cayman, Ann Arbor, MI, USA). Membranes were washed and incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (dilution 1 : 10 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and signal was shown using a chemiluminescent substrate (Pierce Biotechnology). Membranes were stripped and re-blotted with α-tubulin antibody (dilution 1 : 10 000; Santa Cruz Biotechnology) as loading control. Protein abundance was estimated by optical density measurement with Image J software (NIH, Bethesda, MD, USA).
MEASUREMENT OF FATTY ACID ETHANOLAMIDE CONTENT
Rats were killed 4, 24, 48 and 72 h after intravesical instillation of acrolein or saline. Bladders were rapidly frozen in liquid nitrogen and stored at −80 °C until analysis. The frozen-pulverized bladders were mixed with acetonitrile containing 8.4 pmol of [2H8]AEA, sonicated in a cooled bath for 30 min and incubated overnight at −20 °C to precipitate proteins. Extracted lipids were resuspended in methanol. AEA, PEA and OEA were quantified using isotope dilution, atmospheric pressure, chemical ionization and liquid chromatography/mass spectrometry, as described previously [21,22].
IMMUNOHISTOCHEMISTRY
Tissue sections were rinsed in PBS and blocked with 10% normal goat serum. Sections were incubated overnight at 4 °C with antibody against FAAH (dilution 1 : 500; Cayman). Staining was sown with goat anti-rabbit fluorophore-conjugated secondary antibody (dilution 1 : 500; Invitrogen, Carlsbad, CA, USA) for 90 min. Sections were examined with a Nikon E600 microscope (Nikon, Tokyo, Japan), and digital images were captured using a Spot digital camera. Control tissue sections, using normal rabbit IgG instead of specific antibody, were processed under identical conditions.
STATISTICAL ANALYSIS
All data are expressed as the mean ± SEM. Individual values obtained at specific intervals were compared to control values using a two-tailed Student’s t-test. Significance levels were set at 95% confidence limits.
RESULTS
FAAH IN THE BLADDER
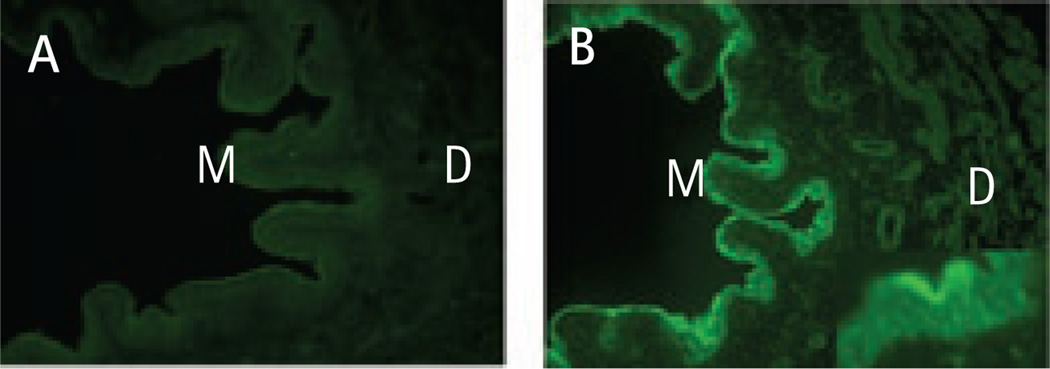
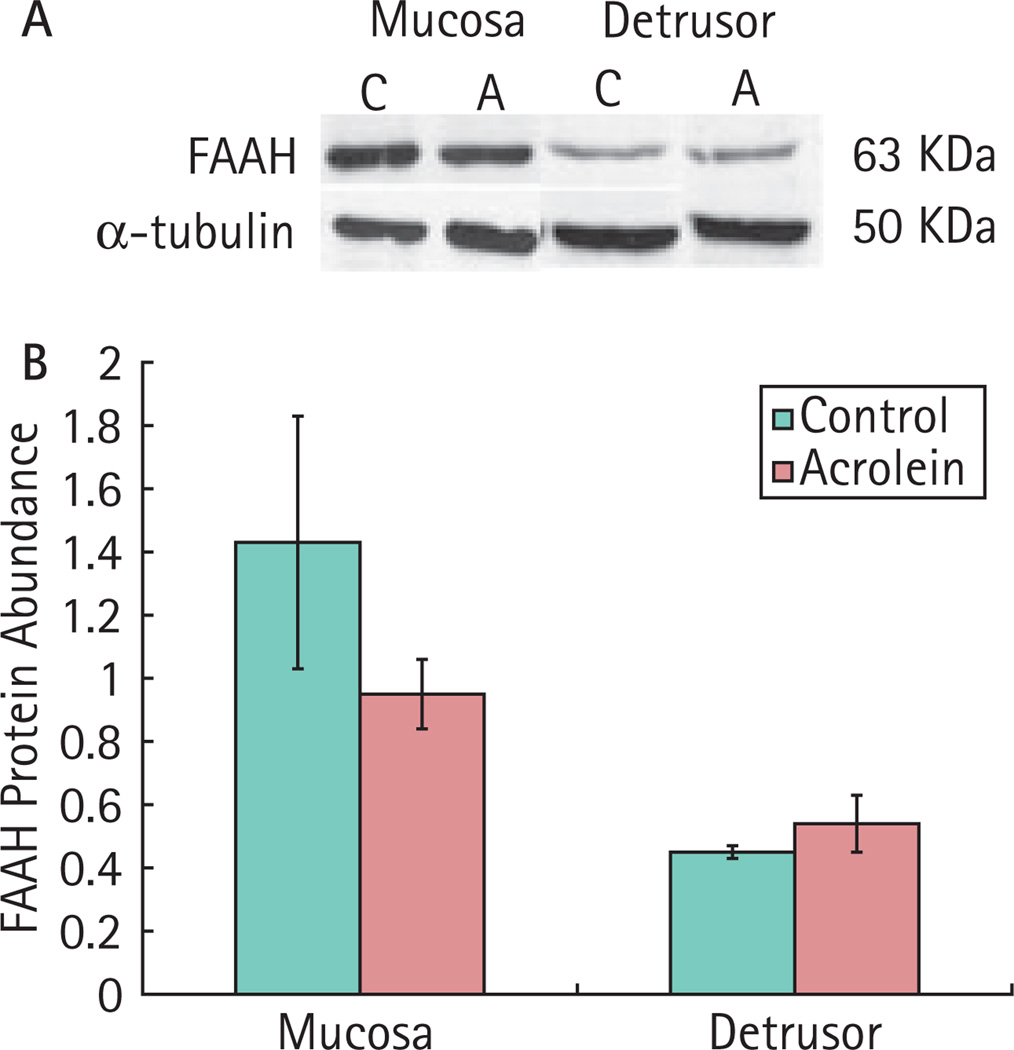
Immunohistochemistry showed FAAH-like immunoreactivity in the mucosa, submucosa and detrusor of rat bladder. FAAH-like immunoreactivity appeared more intense in the urothelium than in detrusor (Fig. 1). Similarly, immunoblotting showed that FAAH was more abundant in mucosa than in detrusor. FAAH abundance in the inflamed bladder was not significantly different from that in control animals (Fig. 2).
FIG. 1.
Presence of fatty acid amide hydrolase (FAAH) immunoreactivity in normal rat bladder. A, No staining was observed using normal rabbit IgG. B, Representative immunofluorescence images of normal rat bladder probed with a selective FAAH antibody. Note the intense FAAH immunoreactivity in urothelium. Images are shown at ×100 magnification, except the inset in B, which is a ×400 image of mucosa. M, mucosa; D, detrusor.
FIG. 2.
Immunoblotting for fatty acid amide hydrolase (FAAH) in bladders of rats with and without bladder inflammation. Intensity of FAAH was normalized to that of α-tubulin in the same sample. A, Representative blots of FAAH expression. B, Bar graph summarizing the data (mean ± SEM). C, control, n = 4; A, acrolein-treated, n = 7, P > 0.05.
INFLAMMATION INCREASED THE ABUNDANCE OF BLADDER FATTY ACID ETHANOLAMIDES
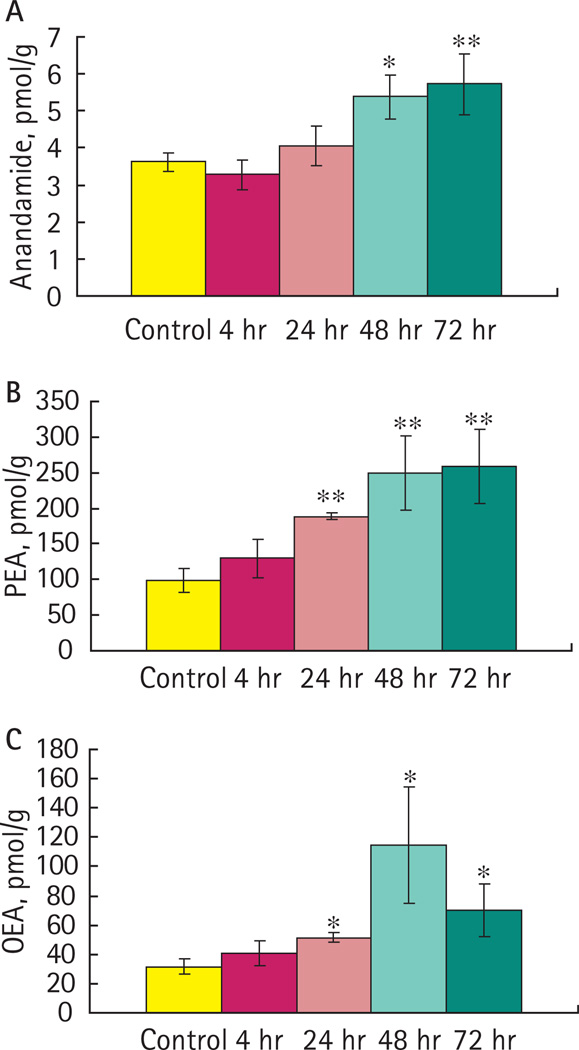
Inflammation induced a time-dependent increase in abundance of AEA, PEA and OEA. The AEA content of bladders was significantly increased after 48 and 72 h compared to control animals (Fig. 3A). The contents of PEA (Fig. 3B) and OEA (Fig. 3C) in the bladder were significantly increased 24, 48 and 72 h after instillation of acrolein.
FIG. 3.
Concentrations of fatty acid ethanolamides in bladder in the presence or absence of inflammation. A, Concentrations of anandamide (AEA) were significantly increased 48 and 72 h after bladder inflammation. B, Concentrations of palmitoylethanolamide (PEA) were significantly increased 24, 48 and 72 h after bladder inflammation. C, Concentrations of oleoylethanolamide (OEA) were significantly increased 24, 48 and 72 h. Data are the mean ± SEM. *P < 0.05; **P < 0.01; control, n = 8; acrolein-treated, n = 4.
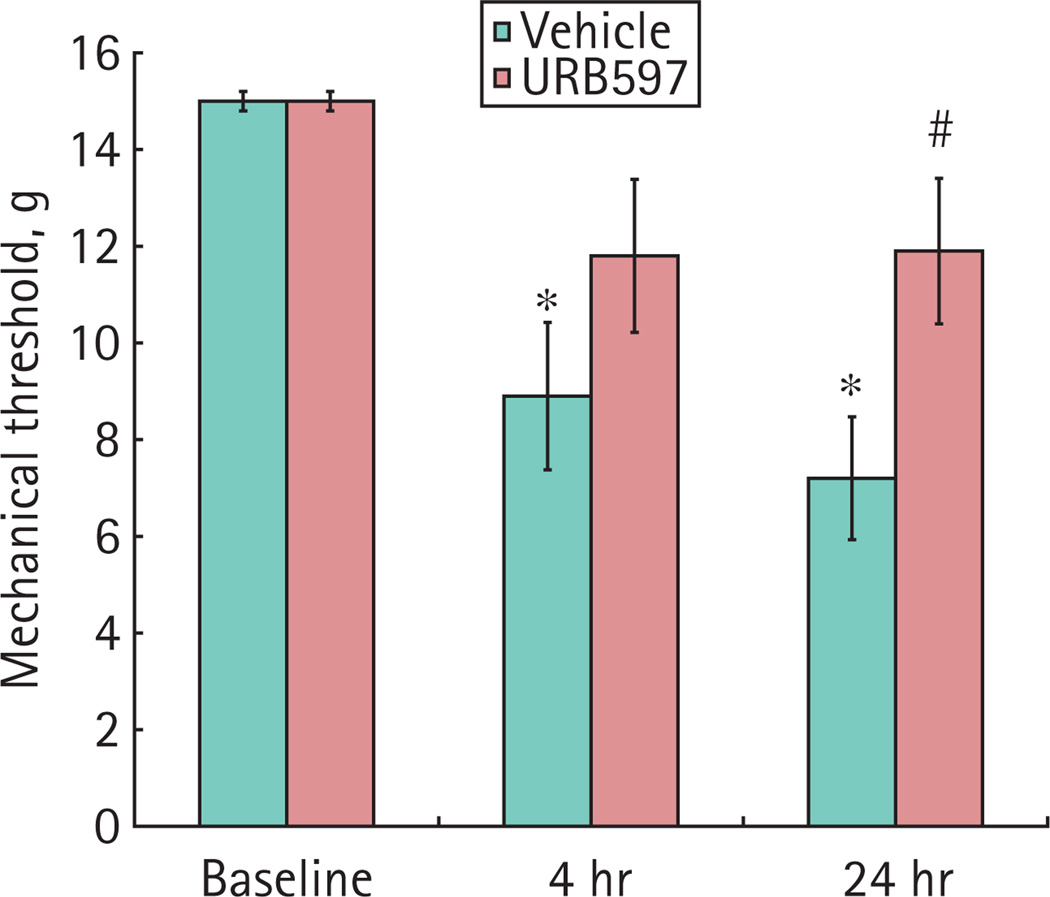
FAAH INHIBITOR URB597 ATTENUATED REFERRED HYPERALGESIA
The baseline mechanical sensitivity was similar in all animals. The mechanical sensitivity was significantly increased 4 and 24 h after intravesical acrolein in rats that received vehicle. URB597 attenuated increased mechanical sensitivity that accompanied cystitis (Fig. 4). URB597 did not affect the mechanical sensitivity in rats that received intravesical saline.
FIG. 4.
Referred hyperalgesia in the presence of bladder inflammation. Mechanical sensitivity was measured in the hind paws of rats. Treatment with URB597 prevented a increased mechanical sensitivity in rats with bladder inflammation. Data are the mean ± SEM. *P < 0.05; acrolein-treated, n = 5; acrolein-treated plus URB597, n = 6.
BLADDER INFLAMMATION
In control, saline-treated rats, the bladder weight (normalized to body weight, mg/g) was 0.47 ± 0.03 (n = 10). Bladder weight was increased 24 h after instillation of acrolein to 0.84 ± 0.04 (n = 6). In URB597-treated rats, the bladder weight was 0.72 ± 0.04 (n = 6). Bladders from acrolein-infused, URB597-treated rats weighed 16% less than bladders from acrolein-infused rats treated with vehicle, although this reduction in bladder weight was not statistically significant (P = 0.0755). Histological evaluation of the bladders showed that acrolein consistently induced oedema and leukocytic infiltration of the bladder wall, which was unaffected by treatment with the FAAH inhibitor.
DISCUSSION
The present study shows, for the first time, that FAAH is expressed in the rat bladder, primarily in the mucosa. Immunohistochemical analysis showed that FAAH protein expression was unchanged 48 h after bladder inflammation. FAAH activity in the bladder was not determined, nor were other time points examined, so it remains possible that both the abundance and activity of FAAH in bladder vary over the course of inflammation. However, the data from the present study suggest that the expression of FAAH remains relatively stable during bladder inflammation.
Visceral pain associated with bladder inflammation is difficult to assess directly, and referred hyperalgesia of the hind paws or ventral abdominal wall has been used as a surrogate metric for the assessment of visceral pain [3]. Systemic inhibition of FAAH decreased referred hyperalgesia, although this effect could be the result of the inhibition of FAAH within the bladder, afferent innervation, spinal cord or brain. Future studies will be designed to further localize the site of action of FAAH inhibition on referred hyperalgesia and visceral pain. URB597 is a potent, selective and irreversible FAAH inhibitor that has been shown to increase concentrations of AEA and PEA in various tissues [15]. Transgenic mice lacking FAAH (FAAH−/− mice) showed increased withdrawal latency in the tail immersion test (water bath at 56 °C) and this effect was reversed by the cannabinoid receptor 1 antagonist SR141716A [23]. Therefore, URB597 probably exerts analgesic effects by increasing concentrations of AEA or other fatty acid ethanolamides [15,16]. In the present study, URB597 given at this dosage and equivalent times did not appear to significantly affect bladder inflammation, suggesting that the effect of URB597 is not dependent on reduced inflammation. However, the present study was not designed to determine the effects of FAAH inhibition on inflammation, and no firm conclusions can therefore be made regarding whether or not FAAH inhibition can ameliorate the severity of bladder inflammation.
Hansen et al. [5] showed that concentrations of AEA were increased by neurodegeneration in neonatal rat cortex. Another study showed that bacterial lipopolysaccharide induced synthesis of AEA in macrophages [13]. Capsaicin and KCl stimulated AEA production and release in cultured primary sensory neurones [24]. Intraplantar injection of formalin increased the abundance of PEA in rat paw skin [25]. The results obtained in the present study provide the first evidence that bladder inflammation increases the abundance of not only AEA, but also other fatty acid ethanolamides, including PEA and OEA. In the present study, AEA and PEA concentrations were significantly increased 48 and 24 h, respectively, and remained increased for up to 72 h after bladder inflammation. The concentration of AEA has been reported to be increased in rat bladders 72 h after systemic treatment with cyclophosphamide [6]. In previous studies, it was reported that mechanical sensitivity induced by bladder inflammation occurred within 4 h of instillation of acrolein and that the sensitivity returned to basal level by 72 h after the initiation of bladder inflammation [17]. The time course of increased production of AEA and PEA observed in inflamed bladders coincides with the time course of regression of referred hyperalgesia. This suggests that referred hyperalgesia induced by bladder inflammation regresses as endogenous production of AEA and PEA increases, leading to the hypothesis that enhanced AEA in the bladder wall suppresses the increased sensitivity of afferent nerve fibres that is associated with inflammation. However, these data do not confirm a cause-effect relationship between increased substrates of FAAH activity and reduced referred hyperalgesia.
PEA may also contribute to analgesia induced by FAAH treatment by the activation of peroxisome proliferator-activated α receptors [26,27]. It was also observed that cystitis increases OEA abundance in bladder. Little is known about the functions of OEA. Systemic administration of URB597 increases the concentration of OEA in rat brain [15]. Increased concentrations of OEA in the bladder could contribute to the analgesic effect of AEA because OEA has been reported to inhibit the catalytic effects of FAAH on AEA in cell culture [28].
In conclusion, the results obtained in the present study indicate that the endocannabinoid signaling system, including FAAH, is present in rat bladder and that inflammation induces a time-dependent increase in fatty acid ethanolamides. Furthermore, the inhibition of FAAH attenuates peripheral hyperalgesia associated with bladder inflammation. These results suggest that FAAH inhibitors or endogenous fatty acid ethanolamides themselves could be effective therapeutic agents for the treatment of bladder pain associated with cystitis.
ACKNOWLEDGEMENTS
The current research was supported by NIH award R01DK066349 (D.E.B.) and R01DA09155 (C.J.H.)
Abbreviations
- AEA
anandamide
- FAAH
fatty acid amide hydrolase
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamide
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Phatak S, Foster HE., Jr The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45–53. doi: 10.1038/ncpuro0385. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002;59:43–50. doi: 10.1016/s0090-4295(01)01637-5. [DOI] [PubMed] [Google Scholar]
- 3.Cervero F, Laird JM. Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol. 2004;61:45–54. doi: 10.1002/neu.20084. [DOI] [PubMed] [Google Scholar]
- 4.Darmani NA, Izzo AA, Degenhardt B, et al. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available preclinical data, and first human studies. Neuropharmacology. 2005;48:1154–1163. doi: 10.1016/j.neuropharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Hansen HH, Schmid PC, Bittigau P, et al. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J Neurochem. 2001;78:1415–1427. doi: 10.1046/j.1471-4159.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- 6.Dinis P, Charrua A, Avelino A, et al. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci. 2004;24:11253–11263. doi: 10.1523/JNEUROSCI.2657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayn MH, Ballesteros I, de Miguel F, et al. Functional and immunohistochemical characterization of CB1 and CB2 receptors in rat bladder. Urology. 2008;72:1174–1178. doi: 10.1016/j.urology.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi V, Philips BJ, Su R, et al. Differential expression of functional cannabinoid receptors in human bladder detrusor and urothelium. J Urol. 2009;181:1932–1938. doi: 10.1016/j.juro.2008.11.078. [DOI] [PubMed] [Google Scholar]
- 9.Farquhar-Smith WP, Rice AS. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology. 2001;94:507–513. doi: 10.1097/00000542-200103000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 11.Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- 12.Lever IJ, Robinson M, Cibelli M, et al. Localization of the endocannabinoiddegrading enzyme fatty acid amide hydrolase in rat dorsal root ganglion cells and its regulation after peripheral nerve injury. J Neurosci. 2009;29:3766–3780. doi: 10.1523/JNEUROSCI.4071-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Batkai S, Pacher P, et al. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- 14.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 15.Fegley D, Gaetani S, Duranti A, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- 16.Piomelli D, Tarzia G, Duranti A, et al. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R111–R122. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol. 2005;173:1983–1987. doi: 10.1097/01.ju.0000158452.15915.e2. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139:158–167. doi: 10.1016/j.pain.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Bjorling DE, Elkahwaji JE, Bushman W, et al. Acute acrolein-induced cystitis in mice. BJU Int. 2007;99:1523–1529. doi: 10.1111/j.1464-410X.2007.06773.x. [DOI] [PubMed] [Google Scholar]
- 21.Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signaling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- 22.Patel S, Carrier EJ, Ho WS, et al. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Cravatt BF, Demarest K, Patricelli MP, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahluwalia J, Yaqoob M, Urban L, Bevan S, Nagy I. Activation of capsaicinsensitive primary sensory neurones induces anandamide production and release. J Neurochem. 2003;84:585–591. doi: 10.1046/j.1471-4159.2003.01550.x. [DOI] [PubMed] [Google Scholar]
- 25.Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 26.LoVerme J, Fu J, Astarita G, et al. The nuclear receptor peroxisome proliferator-actived receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 27.LoVerme J, Russo R, La Rana G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson KO, Vandevoorde S, Lambert DM, Tiger G, Fowler CJ. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br J Pharmacol. 2001;133:1263–1275. doi: 10.1038/sj.bjp.0704199. [DOI] [PMC free article] [PubMed] [Google Scholar]