Abstract
We examined performance and fMRI activity in participants (n=235) aged 17-81yrs on a non-verbal recognition memory task, figural memory. Reaction time, error rate and response bias measures indicated that the youngest and oldest participants were faster, made fewer errors and showed a more conservative response bias than participants in the median age ranges. Encoding and Recognition phases activated a distributed bilateral network encompassing prefrontal, subcortical, lateral and medial temporal and occipital regions. Activation during Encoding phase did not correlate with age. During Recognition, task-related activation for correctly identified targets (Hit-Targets) correlated linearly positively with age; non-task related activity correlated negative quadratically with age. During correctly identified distractors (Hit-Distractors) activity in task-related regions correlated positive linearly with age, non-task activity showed positive and negative quadratic relationships with age. Missed-Targets activity did not correlate with age. We concluded that figural memory performance and fMRI activity during Recognition but not Encoding was affected both by continued maturation of the brain in the early 20s and compensatory recruitment of additional brain regions during recognition memory in oldage.
Keywords: healthy aging, fMRI, non-verbal memory, figural memory
1. Introduction
Normal aging is accompanied by changes in the structure and function of the brain, and these changes underlie the alterations in cognition and memory seen in old age. As the life expectancy of the population increases, so too does the burden of impairments associated with both normal and abnormal cognitive aging. One of the most commonly investigated effects in the cognitive aging literature is the reduction in memory function in older adults. Memory decline may occur as early as the 50s in otherwise healthy individuals, and it is thought to be due to problems with encoding and retrieval of new information (Beason-Held et al., 2005; Cabeza, et al., 1997; Daselaar et al., 2003).
Typically, older adults perform worse than younger ones on tests of recognition memory (Grady, 2008; Rajah & D’Esposito, 2005; Salthouse, 2003; 2011). This decreased performance is often associated with changes in fMRI activity, both in regions activated by young participants, and in additional regions not activated by them (e.g., Cabeza, 2002; Daselaar et al., 2006; Grady et al., 2008). Over-recruitment of the latter regions has been attributed to either compensatory processes or dedifferentiation of function (Rajah & D’Esposito, 2005). According to the compensation view, age-related increases or decreases in activation in task-related regions represent functional deficits and concomitant activation increases in non-task-related regions represent an attempt to compensate for this deficiency. The strongest evidence for this hypothesis is when increased activity in non-task-related regions is accompanied by non-significant performance differences between young and old adults. Note however activation increases in non-task regions without concomitant performance improvement have also been interpreted as unsuccessful (or partially successful) compensatory attempts. Conversely, according to the dedifferentiation view, age-related activity changes reflect reductions in regional localization specificity. Activity spreads and neural regions become less functionally specialized due to changes in the specificity of neurotransmission. The dedifferentiation model posits that this spreading of activation may be beneficial or detrimental to performance – in other words, the model does not deny that some of this activation spread may benefit performance and therefore can be considered compensatory under certain circumstances. In their literature review, Rajah and D’Esposito (2005) concluded that different regions within the prefrontal cortex (PFC) may show compensation or dedifferentiation under different task conditions.
In the general population, memory for pictures tends to be better than that for words – the ‘picture superiority effect’ (e.g. Paivio, 1971; Neslon et al., 1976; Sternberg, 2006), a process that continues into very old age (> 90yrs; Cherry et al., 2008; Ally et al., 2008). The mechanism of the picture superiority effect remains a matter of debate, but most major hypotheses posit that it relies on the ability to encode the picture both verbally and visually, whereas words are primarily encoded verbally. Recent research from Resnick and colleagues (Golski et al., 1998; Beason-Held et al., 2005; see also Beason-Held et al., 2008a; 2008b; Maki et al., 2011) has focused on the development of the figural memory task, a visual recognition memory task that employs picture stimuli that are resistant to verbal encoding (Figure 1). In a sample of elderly (63-82yrs) participants, Beason-Held et al. (2005) demonstrated increases in regional cerebral blood flow (rCBF) using positron emission tomography (PET)2 in prefrontal cortex, anterior cingulate, lateral and medial temporal and occipital regions during encoding of verbal and figural stimuli relative to baseline. Medial temporal regions exhibited greater rCBF during encoding of figural than verbal stimuli, suggesting that older adults use more resources to perform the figural compared to the verbal memory task. Since this study included only older adults with no younger comparison group, it is difficult to determine how these results fit with the dedifferentiation vs. compensation hypotheses. The figural memory task has been specifically developed to measure changes in visual recognition with age, however to date there has been no systematic study of changes in performance or brain activation on the task associated with healthy aging.
Figure 1.
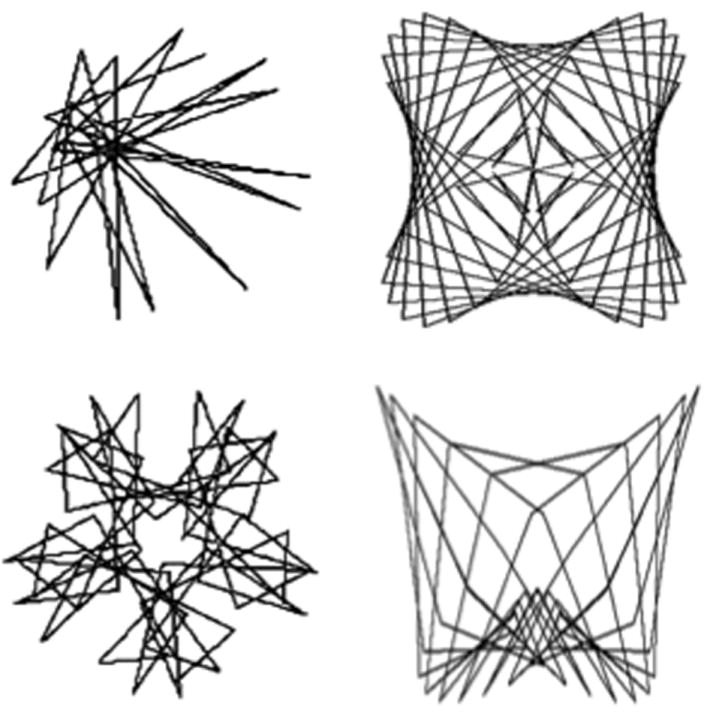
Two examples of task-stimuli for the Figural Memory task. Top: examples of targets; Bottom: examples of distractors.
In general, the vast majority of studies of cognitive aging compare young (~20-30yrs) and old (~60-70yrs) participant groups, or ‘young-old’ (~60-70yrs) and ‘old-old’ (~>75yrs) groups to examine the effects of age on memory. This artificial categorizing of age differs substantially between studies and vitiates the continuous nature of age as a variable. Implicitly, these studies assume that the performance of the young group represents an optimal baseline, and therefore changes relative to the young group represent age-related decline (Whitson et al., in press); also that there is some discrete step from ‘intact’ or ‘optimized’ memory function to ‘impaired’ or ‘deficient’ memory function occurring somewhere in middle age. In a recent review of longitudinal and cross-sectional studies of memory and cognition across the adult lifespan, Salthouse (2011) showed that memory and cognition show both linear and quadratic relationships with age and concluded there is no evidence of a discrete step between a period of stability and a period of negative change. While the use of extreme age groups in the study of aging effects on memory and cognition can be more efficient for detecting age differences than can a continuous sample, it also inflates estimates of age relations, because variance associated with middle-aged adults is ignored and can potentially miss non-linear relationships between age and memory.
In this study, we investigate changes in figural memory performance and associated neural activity across the adult age span. In a large (n=235) sample with a wide age range (17-81 years), we examined behavioral performance and fMRI signal during the Figural Memory task. These sample characteristics allowed us to examine age as a continuous variable across the adult lifespan, thereby increasing our sensitivity to detect when in the lifespan changes in figural memory occur. We examine fMRI activity related to Encoding and Recognition task phases, including to Hit Targets (true positive responses), Missed Targets (false negatives) and Hit Distractors (true negatives), and examine linear and non-linear age relationships. Furthermore, since evidence suggests that older adults may recruit additional brain regions to perform memory tasks we examined relationships between fMRI activity and age in both task and non-task-related regions. Last, given the well-documented reductions in whole brain and gray matter volume with age (see Fjell & Walhovd, 2010; Glorioso & Sabille, 2011 for reviews), we assessed how changes in gray matter volume are related to fMRI activity and its relationship with age.
2. Methods
2.1 Participants
Participants were 235 individuals aged 17-81 years (mean 34.86 years; 127 female; 144 Caucasian, 13 Hispanic, 12 African American, 6 Other and 60 Unreported race) who volunteered for research at the Olin Neuropsychiatry Research Center. As seen in Figure 2A, ages 18-20 were oversampled, with older age ranges sampled with a frequency of around 25 per 10-year age bin. Age was not associated with sex. Participants in the age ranges >25 years were derived from a representative community sample of normal aging in the Hartford CT metropolitan area, recruited by random digit dialing to residential addresses in a manner proportional to age and ethnicity distribution in each catchment area in the prior national census. Participants in the age ranges <25 years were derived from ongoing studies of cognition in college-aged students in Connecticut. Participants were excluded for significant histories of major central neurological illness, past/present/family history of Axis-1 psychiatric disorder as indexed by SCID-IV (First, 2002), head injury leading to unconsciousness >10 minutes, mild cognitive impairment or signs of Alzheimer’s disease (see Schretlen et al., 2003), pregnancy or positive urine toxicology screens for abused substances on the day of testing. All participants were screened for visual acuity by ensuring they could correctly identify standard test stimuli before entry into the scanner. Participants needing visual correction were provided with MR-compatible lenses. Note that there were no specific exclusion criteria for older adults other than overt brain pathology (e.g. stroke, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, etc) non-correctable visual acuity, or substance abuse so this group should not be considered as ‘super-healthy aging’3. Participants gave written informed consent using procedures approved by Yale University and Hartford Hospital institutional review boards.
Figure 2.
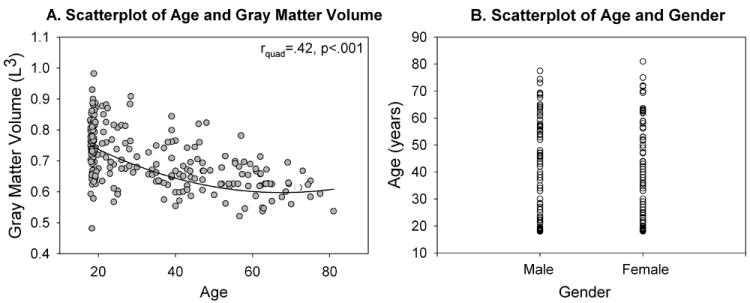
Demographics. (A) Gray matter volume showed a significant quadratic relationship with age, (B) Sex was evenly distributed across the age range
2.2 Task
Participants completed the figural memory task in the fMRI scanner. The task stimuli (20 targets, 20 distractors, counterbalanced between participants) were black line drawings presented against a white background that were matched across uniqueness and resistant to verbal encoding (Figure 1; Beason-Held et al., 2005; Golski et al., 1998). The task consisted of two phases, an encoding and a recognition phase. During encoding, 20 target stimuli were presented (duration 3-sec, inter-stimulus interval (ISI) 4-sec) and participants instructed to silently examine each item and try and remember it for later. Following each stimulus, participants pressed a response button with their dominant index finger to confirm that they saw the stimulus. After a 5-min delay (with no other cognitive task presented), the recognition phase followed the encoding phase, where 20 target and 20 distractor stimuli were presented (duration 3-sec, ISI 4-sec) in a fixed pseudo-random sequence. Participants indicated whether the item had been seen previously with their dominant index (‘yes’) and middle (‘no’) fingers; accuracy was emphasized over speed.
2.3 MRI Acquisition
Magnetic resonance images were acquired using a Siemens Allegra 3T dedicated head scanner equipped with 40mT/m gradients and a standard quadrature head coil. Functional images were acquired using a T2*-weighted gradient-echo echo-planar imaging (EPI) protocol (ascending axial acquisition, 405 volumes, TR=1.86s, TE=27ms, FOV=220mm, matrix=64×64, flip angle=70°, voxel size=3.4×3.4×4mm, gap=1mm, 36slices). Anatomical images were collected using a T1-weighted 3D MPRAGE protocol (TR=2500ms, TE=2.74ms, flip angle=8°, 176×256matrix, FOV=176×256mm2, voxel size=1×1×1mm, 176slices). Functional images acquired during the 5 minute delay period were discarded.
2.4 Data Analysis
2.4.1 Behavioral
Trials during the encoding phase were classified as successfully encoded if the target was correctly identified in the subsequent recognition phase (Encoded). Targets that were subsequently forgotten in recognition phase were classified as Encoded-Forgotten. Trials during the recognition phase were classified as Hit Targets (true positives), Missed Targets (false negatives), Hit Distractors (true negatives) and Missed Distractors (false positives). All participants correctly identified targets on at least 50% of trials. Reaction time was calculated for Hit Targets, Missed Targets, Hit Distractors and Missed Distractors and was analyzed with a 2 stimulus type (target, distractor) × 2 accuracy (hit, miss) repeated measures ANOVA. Error rate was calculated as the number of Missed Target/Distractors divided by the total number of Target/Distractors (20), respectively. Error rate was analyzed with a 2 stimulus type (target, distractor) repeated-measures ANOVA. Effect sizes are reported as r values. For RT and error rate, non-linear relationships with age were calculated using the curve estimation procedure in SPSS v17. Note that no non-linear relationships of a higher order than quadratic reached significance. Signal detection theory analyses are shown in Supplementary Analyses.
2.4.2 Structural MRI
Given the well-documented reduction in whole brain volume and gray matter volume with age (see Fjell & Walhovd, 2010 for a review), we examined changes in fMRI activity related to both age alone and age when accounting for changes in gray matter volume. Gray matter was extracted using the unified segmentation routine in SPM5 (Wellcome Department of Imaging Neuroscience, UK). Segmented images were modulated as part of the algorithm to represent volumetric data. Resulting segmented data were lightly cleaned to improve tissue class accuracy, and then spatially smoothed with a 9mm full width half maximum Gaussian kernel. Gray matter volume (L3) was then extracted from the segmented images. For participants where the segmentation routine failed due to image quality issues (11%), the gray matter volume was estimated as the average gray matter volume for all other participants within that participant’s age range. Total gray matter volume showed a significant quadratic relationship with age (rquad=.42, p<.001; Figure 2A) a pattern consistent with previous reports (e.g. Jerningan & Gamst, 2005; Peelle et al., 2012).
2.4.3 Functional MRI
Functional images were preprocessed using SPM5. The first 6 images were discarded to allow for T1 saturation effects. Slice acquisition timing differences were corrected using the central slice as reference. Motion was corrected using INRI-align (Freire et al., 2002) and images spatially normalized into Montreal Neurological Institute (MNI) space. Normalised EPIs were smoothed with a 9mm FWHM Gaussian kernel. The threshold for allowable movement was 1 voxel (3.4mm).
Events in the encoding phase were classified as Encoded or Encoded-Forgotten, and events in the recognition phase were characterized as Hit Targets, Missed Targets, Hit Distractors and Missed Distractors. The Encoding phase included a maximum of 73 images per subject and the Recognition phase included a maximum of 149 images (dependent on accuracy). On average, the number of Encoding images were 54 per subject, and Recognition images was 111. The duration of each event was determined by RT; since the BOLD response increases linearly with the duration of processing (Poldrack et al., 2011) any changes in BOLD activation between conditions or participants could be attributable merely to changes in RT. Thus, this approach allows us to attribute changes in BOLD activation between conditions and across the age range to the task, unconfounded by changes in RT.
For first level analysis, a canonical haemodynamic response function was fitted to the onset of each event. Realignment parameters were included in the model as covariates of no interest. The following contrasts were constructed at the first-level: Encoded > baseline, Hit Targets > baseline, Hit Distractors > baseline, Missed Target > baseline and Miss Distractors >. baseline. In this context, ‘baseline’ refers to implicit baseline – i.e. all unmodelled variance (error term of the GLM) in the data. Second-level analyses were conducted on these contrast images and thresholded at p<.01 FDR corrected, minimum cluster size k=5 voxels. Note that Miss Distractors > baseline results did not survive correction for multiple comparisons and so is not discussed further. To determine regions that were activated by the task, the above contrasts were first submitted to separate one-sample t-tests. Three separate analyses were used to examine the effects of age. In the first, we examined task-related regions that were correlated with age by using multiple regression as implemented in SPM5 with age as a covariate and mask of task-related activity identified in the one-sample t-tests. In the second, we examined task-related regions that were correlated with age with the effect of gray matter volume covaried out by using the above analysis with an additional covariate of gray matter volume. In the third, as discussed in the Introduction current evidence suggests that older adults may recruit additional brain regions to perform memory tasks relative to younger adults (Grady, 2008; Rajah & D’Esposito, 2005), therefore we re-ran the above multiple regressions, this time with a mask of non-task activity entered in the analysis. Note that negative correlations with age were not observed in any analysis, regardless of controlling for gray matter volume, or searching in task- or non-task-related regions.
Lastly, since behavior showed a number of significant non-linear relationships with age, we examined whether the regions identified in the above analyses also showed non-linear relationships with age4. We therefore constructed ROIs (10mm spheres) around each region showing a significant correlation with age identified in the SPM analysis, extracted contrast values using MarsBar (Brett, 2002), and entered them into the curve estimation algorithm in SPSS.
3. Results
3.1 Behavioral Results
Behavioral results are summarized in Figure 3. The significant main effect of accuracy confirmed that Hit trials were performed faster than Missed trials (F(1,217)=190.84, p<.001,r=.68). The significant accuracy × stimulus interaction (F(1,217)=15.45, p<.001,r=.26) confirmed that the increase in RT for Misses was larger for Targets than for Distractors.
Figure 3.
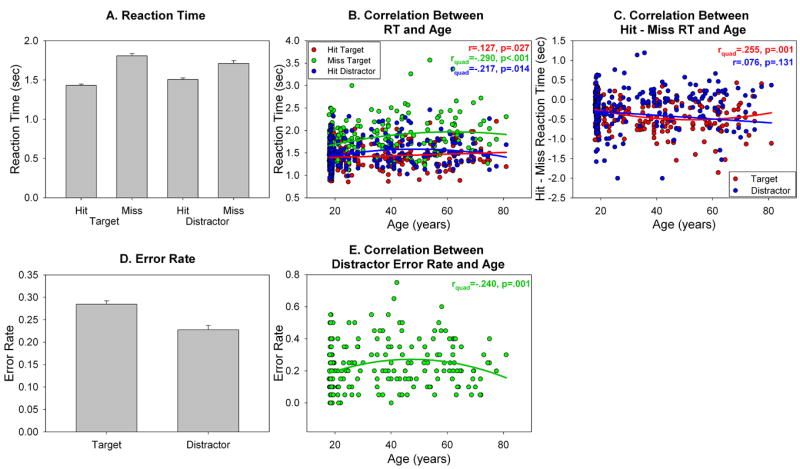
Behavioral results. (A) Reaction time (sec) for Hit Targets, Missed Targets, Hit Distractors and Missed Distractors. (B). Relationship between RT and age for Hit Targets, Missed Targets and Hit Distractors. Missed Target and Hit Distractor RT showed a non-linear relationship with age; Hit Target RT showed a linear relationship with age. (C) Relationship between Hit-Missed RT and age, to visualize the accuracy × stimulus × age 3-way interaction. Hit-Missed RT for Targets was non-linearly related to age; Hit-Missed RT for Distractors was linearly related to age. (D) Error rate for Targets and Distractors. (E) Non-linear relationship between Distractor error rate and age.
To test the hypothesis that figural memory performance would change across the age range, we examined the data for linear and non-linear relationships between age and RT. Age showed a significant linear relationship with Hit Target RT (r=.127, p=.027) such that RT linearly increased as age increased (Figure 3B, red line). Age also showed a significant quadratic relationship with Missed Target RT (rquad=-.290, p<.001) and Hit Distractor RT (rquad=-.217, p=.014) such that RT increased until around 30 years of age, plateaued, and then decreased around 60 years of age (Figure 3B, green & blue lines). The 2 stimulus type (target, distractor) × 2 accuracy (hit, missed) × age repeated measures ANCOVA showed a significant three way interaction between accuracy, stimulus and age (F(1,216)=7.69, p=.006,r=.19). Examination of the scatterplot and the quadratic relationship between age and Hit-Missed RT suggested that the difference between Hit and Missed RT started to decrease around > 60 years of age (Figure 3C, red line); the effect for Distractors was minimal (Figure 3C). Thus age had a disproportionate effect on Missed Target RT across the age distribution.
For error rate (Figure 3D), the main effect of stimulus (F(1,231)=20.56, p<.001,r=.28) confirmed that error rate for Targets was higher than error rate for Distractors. Age showed a quadratic relationship with Distractor error rate (rquad=.240, p=.001) such that Distractor error rate increased until around 30 years, and decreased around 60 years (Figure 3E). No such effect was shown in the Target stimulus. Consistent with this, the 2 stimulus type (Target, Distractor) × age repeated measures ANCOVA showed a significant interaction between stimulus and age (F(1,230)=4.48, p=.042).
3.2 fMRI Results
During Encoding, participants activated a distributed bilateral network that encompassed frontal, parietal, temporal, occipital, hippocampus, parahippocampal gyrus, amygdala and subcortical regions (Figure 4A, Table 1). To test the hypothesis that activation during Encoding would differ across the age range, we examined relationships between task- and non-task related fMRI activity and age. To test the hypothesis that functional changes with age may be in response to structural changes in aging, we also examine relationships between task- and non-task related regions while controlling for gray matter volume. No task- or non-task-related region was correlated with age before or after controlling for gray matter volume.
Figure 4.
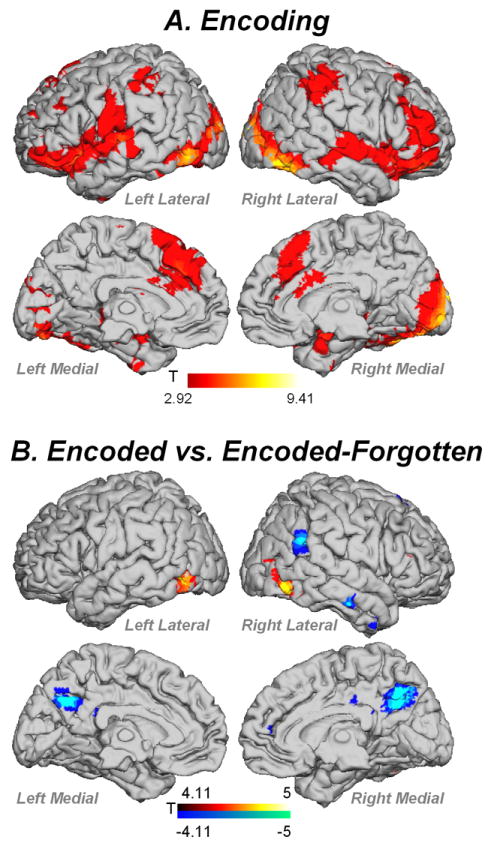
(A). fMRI activity for Encoding > Baseline contrast. (B) Comparison of encoding for trials subsequently remembered ‘Encoded’ vs. subsequently forgotten ‘Endoded-Forgotten’ targets. Red = Encoded > Encoded-Forgotten; Blue = Encoded-Forgotten > Encoded. Contrasts thresholded at p<.01 FDR corrected, k=5 voxels.
Table 1.
MNI coordinates and T values for regions activated in Encoding > Baseline. Contrast thresholded at p<.01 FDR corrected k=5 voxels
| Region (BA) | Left Hemisphere | T | Right Hemisphere | T |
|---|---|---|---|---|
| Superior Orbitofrontal (11) | -24 48 -9 | 3.65 | 24 36 -15 | 4.12 |
| Middle Oribitofrontal (11) | -27 42 -12 | 4.02 | 33 42 -12 | 4.21 |
| Inferior Orbitofrontal (11) | -45 39 -15 | 5.65 | 48 39 -15 | 5.74 |
| Superior Medial Frontal (9/6) | -3 33 36 | 5.81 | 3 30 42 | 4.72 |
| Superior Frontal (8) | -15 27 57 | 4.50 | 21 27 54 | 3.47 |
| Middle Frontal (9/46) | -45 30 33 | 3.48 | 42 42 12 | 5.88 |
| Inferior Frontal Triangularis | -45 18 3 | 4.06 | 57 21 9 | 3.12 |
| Inferior Frontal Operculum (47) | -48 15 -6 | 6.81 | 48 15 0 | 5.05 |
| Supplementary Motor Area (6) | -6 18 63 | 4.34 | 6 24 51 | 4.04 |
| Mid Cingulate (32) | -3 15 39 | 3.31 | 9 33 33 | 4.33 |
| Anterior Cingulate (24) | 0 9 30 | 4.22 | 15 30 21 | 3.58 |
| Insula | -36 -9 15 | 4.09 | 45 18 -9 | 6.56 |
| Precentral (6) | -48 -6 24 | 4.76 | 51 -6 24 | 3.15 |
| Postcentral (4) | -54 -15 27 | 4.68 | 51 -33 57 | 4.77 |
| Caudate | 21 0 15 | 3.93 | ||
| Putamen | -33 -6 -6 | 3.75 | 33 -15 -6 | 3.21 |
| Thalamus | -18 -30 0 | 3.38 | 21 -27 -3 | 4.34 |
| Amygdala | -37 -3 -15 | 3.68 | 21 -3 -18 | 6.13 |
| Parahippocampal | -18 -18 -18 | 3.73 | 21 -3 -18 | 4.80 |
| Hippocampus | -27 -27 -9 | 3.75 | 21 -30 -6 | 5.30 |
| Heschl’s (22) | -48 -15 6 | 4.86 | 51 -15 3 | 3.99 |
| Superior Temporal Pole (22) | -48 12 -6 | 6.58 | 48 18 -15 | 5.64 |
| Superior Temporal (22) | -60 -18 3 | 6.61 | 63 -18 0 | 5.26 |
| Middle Temporal (37/21) | -54 -24 -3 | 3.48 | 66 -27 -3 | 3.49 |
| Inferior Temporal (19) | -45 -54 -18 | 3.49 | 51 -60 -6 | 3.95 |
| Inferior Parietal (40) | -51 -36 45 | 4.06 | 48 -39 45 | 5.22 |
| Cuneus (18) | -6 -96 18 | 4.50 | 15 -96 12 | 6.05 |
| Superior Occipital (18) | -18 -96 16 | 5.67 | 18 -102 12 | 8.98 |
| Middle Occipital (19) | -24 -93 9 | 5.46 | 42 -87 0 | 6.89 |
| Inferior Occipital | -45 -75 -9 | 7.28 | 45 -84 -3 | 6.90 |
| Fusiform (20) | -36 -84 -18 | 4.61 | 30 -3 -42 | 3.56 |
| Lingual (19) | -12 -90 -9 | 5.80 | 9 -84 -6 | 4.92 |
| Cerebellum 6 | -36 -69 -24 | 7.50 | 39 -69 -24 | 9.22 |
| Cerebellum Crus 1 | -24 -81 -21 | 7.40 | 42 -57 -27 | 5.82 |
During the Recognition phase, a distributed network was activated for Hit Targets encompassing prefrontal, motor, subcortical, hippocampal, temporal, parietal and occipital regions (Figure 5A, Table 2). Task-related activity in supplementary motor area, precentral gyrus, cingulate and parahippocampal gyrus was positively correlated with age (Figure 5B). When controlling for gray matter volume, task-related activity in the supplementary motor area, cingulate and parahippocampal gyrus was correlated with age (Figure 5C). For non-task regions, fMRI activity in prefrontal, premotor, parietal, medial and lateral temporal and occipital regions showed significant correlation with age before and after controlling for gray matter volume (Figure 5D, E; Table 2).
Figure 5.
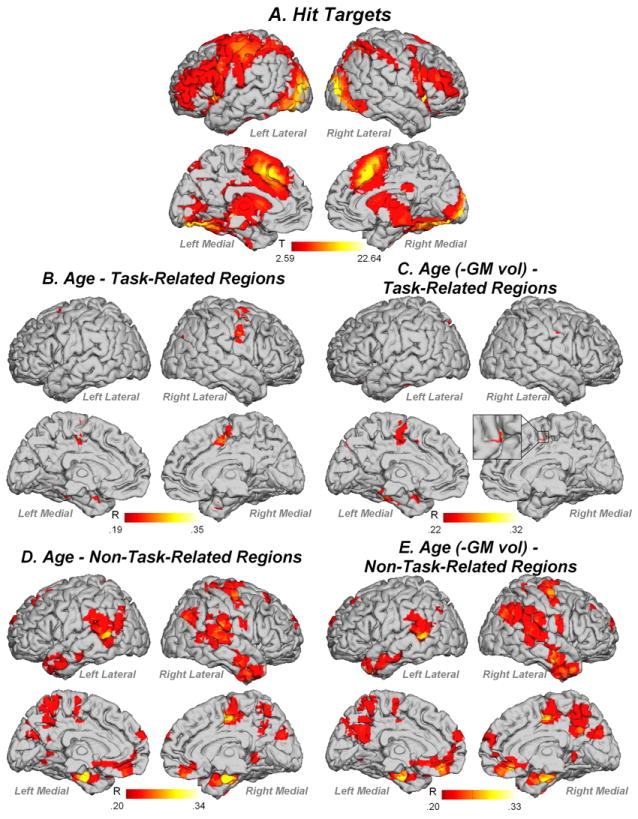
fMRI activity for (A) Hit Targets, (B) task-related regions correlated with age, not controlling for gray matter volume, (C) task-related regions correlated with age when controlling for gray matter volume, (D) non-task-related regions correlated with age, not controlling for gray matter volume, (E) non-task-related regions correlated with age, controlling for gray matter volume. Contrast thresholded at p<.01 FDR corrected, k=5 voxels
Table 2.
MNI coordinates and T values for regions activated by Hit Target > Baseline. Contrast thresholded at p<.01 FDR corrected k=5 voxels.
| Region (BA) | Left Hemisphere | T | Age r | Age r [-GM vol] | Right Hemisphere | T | Age r | Age r [-GM vol] |
|---|---|---|---|---|---|---|---|---|
|
TASK-RELATED REGIONS
| ||||||||
| Superior Orbitofrontal (11) | -24 54 -6 | 4.84 | 21 39 -15 | 6.57 | ||||
| Superior Frontal (6) | -24 -6 63 | 4.95 | 24 0 57 | 2.85 | ||||
| Superior Medial Frontal (6) | -6 30 39 | 15.87 | 6 30 42 | 17.70 | ||||
| Anterior Cingulate (32) | -3 30 27 | 9.74 | 9 33 24 | 12.41 | ||||
| Middle Frontal (46) | -39 12 36 | 8.45 | 45 42 21 | 9.69 | ||||
| Middle Orbitofrontal (11) | -39 48 -6 | 5.91 | 36 51 -6 | 4.84 | ||||
| Inferior Orbitofrontal (11) | -36 27 -6 | 13.42 | 36 30 -9 | 12.92 | ||||
| Inferior Frontal Triangularis (46) | -39 24 27 | 11.84 | 39 24 12 | 11.00 | ||||
| Inferior Frontal Operculum (44) | -48 6 21 | 11.65 | 48 15 30 | 14.43 | ||||
| Supplementary Motor Area (8) | -3 18 48 | 21.65 | .24 | 9 21 48 | 19.50 | .35 | .32 | |
| Mid Cingulate (32) | -3 18 36 | 11.49 | .27 | 12 24 33 | 15.68 | .22 | ||
| Insula | -36 15 -3 | 18.22 | 39 21 0 | 19.09 | ||||
| Rolandic Operculum | -45 -3 12 | 10.10 | 48 3 12 | 5.52 | ||||
| Precentral (6) | -51 3 33 | 14.32 | 39 0 45 | 6.67 | .27 | |||
| Postcentral (40) | -39 -30 48 | 16.11 | 48 -27 45 | 10.93 | ||||
| Caudate | -9 12 6 | 7.62 | 12 9 12 | 10.37 | ||||
| Putamen | -24 3 6 | 12.34 | 24 3 6 | 11.08 | ||||
| Thalamus | -12 -15 6 | 16.63 | 12 -15 6 | 13.68 | ||||
| Subthalamic Nucleus | -9 -15 -9 | 12.15 | 9 -15 -9 | 12.59 | ||||
| Posterior Cingulate (23) | -6 -39 24 | 4.13 | 6 -39 24 | 4.71 | ||||
| Superior Parietal (7) | -24 -57 48 | 14.87 | 24 -69 54 | 15.30 | ||||
| Precuneus (7) | -6 -75 54 | 3.28 | 12 -66 48 | 6.14 | ||||
| Inferior Parietal (40) | -33 -45 48 | 14.54 | 39 -48 42 | 12.58 | ||||
| Hippocampus | -21 -27 -3 | 13.05 | 24 -27 -3 | 10.58 | ||||
| Parahippocampus | -18 -39 -6 | 4.36 | 30 -36 -12 | 5.86 | .28 | .30 | ||
| Amygdala | -15 -6 -15 | 4.46 | 21 3 -15 | 3.66 | ||||
| Superior Temporal (41) | -51 -24 9 | 4.35 | 51 12 -12 | 5.67 | ||||
| Superior Temporal Pole (38) | -51 12 -9 | 5.91 | 39 12 -27 | 3.29 | ||||
| Middle Temporal (37) | -51 -66 0 | 5.37 | 48 -69 0 | 8.40 | ||||
| Inferior Temporal (37) | -48 -51 -15 | 12.61 | 48 -57 -9 | 10.94 | ||||
| Heschl’s (22) | -33 -30 12 | 4.99 | ||||||
| Cuneus (18) | -12 -84 15 | 6.21 | 15 -72 36 | 3.53 | ||||
| Superior Occipital (18) | -24 -90 24 | 14.08 | 24 -96 18 | 17.88 | ||||
| Middle Occipital (19) | -30 -72 33 | 1.317 | 36 -84 21 | 17.80 | ||||
| Inferior Occipital (18) | -30 -84 -9 | 17.36 | 36 -84 -9 | 18.85 | ||||
| Lingual (18) | -15 -87 -12 | 15.43 | 21 -87 -9 | 19.29 | ||||
| Fusiform (37) | -36 -57 -12 | 15.39 | .28 | 36 -54 -15 | 17.55 | |||
| Cerebellum 1 | -36 -60 -33 | 8.13 | -30 -72 -30 | 6.05 | ||||
| Cerebellum 4/5 | -21 -48 -21 | 7.24 | .22 | 21 -48 -21 | 12.06 | |||
| Cerebellum 6 | -33 -57 -24 | 11.44 | .27 | 30 -48 -30 | 15.33 | |||
|
| ||||||||
|
NON-TASK-RELATED REGIONSa
| ||||||||
| Medial Orbitofrontal (11) | 6 36 -18 | 4.34 | .27 | .30 | ||||
| Anterior Cingulate (32) | -6 51 0 | 3.46 | .22 | .24 | ||||
| Superior Medial Frontal (10) | -9 63 30 | 3.92 | .25 | .21 | 9 60 36 | 4.04 | .26(.22) | |
| Superior Frontal (6) | -24 33 48 | 3.56 | .23 | .21 | 24 -9 72 | 3.93 | .25 | .24 |
| Middle Frontal (8) | -27 15 60 | 3.09 | .20 | 33 42 39 | 3.35 | .22 | ||
| Supplementary Motor Area (6) | 9 -12 51 | 5.52 | .34 | .31 | ||||
| Mid Cingulate (24) | 0 -24 48 | 3.68 | .23 | .21 | 9 -12 45 | 4.14 | .26 | .25 |
| Precentral (6) | 63 -6 27 | 3.75 | .24 | .24 | ||||
| Caudate | -3 9 -9 | 3.18 | .22 | .19 | ||||
| Rolandic Operculum | 54 -30 21 | 4.44 | .28 (-.28) | .25 | ||||
| Precuneus (7) | -9 -57 24 | 3.17 | .20 | .22 | 6 -63 60 | 4.02 | .25 | .23 |
| Superior Parietal (7) | 24 -45 69 | 4.32 | .27 (.26) | .23 | ||||
| Inferior Parietal (40) | -60 -36 30 | 4.10 | .26 (-.26) | .20 | 60 -30 30 | 4.00 | .25 (-.26) | .25 |
| Amygdala | -27 0 -27 | 3.84 | .24 | 30 0 -24 | 3.15 | .20 | .21 | |
| Hippocampus | -24 -12 -21 | 3.90 | .25 | .22 | 24 -15 -18 | 3.10 | .20 | .20 |
| Parahippocampal | -21 -15 -27 | 5.41 | .33 (.26) | .31 | 24 -12 -27 | 5.68 | .35 | .33 |
| Superior Temporal (42) | -60 -45 18 | 4.14 | .26 | .26 | 60 -33 15 | 4.91 | .31 (-.32) | .26 |
| Middle Temporal (20/21/38) | -63 -57 6 | 5.34 | .33 | .29 | 60 -9 -18 | 4.53 | .28 (-.24) | .29 |
| Middle Temporal Pole (38) | -42 12 -42 | 3.70 | .24 | .27 | 42 6 -45 | 4.11 | .27 | |
| Inferior Temporal (20) | -45 -24 -18 | 3.69 | .23 | .21 | 51 -3 -33 | 3.86 | .25 (-.26) | .23 |
| Lingual (19) | 12 -48 0 | 3.79 | .24 | .21 | ||||
| Middle Occipital (19) | -42 -72 21 | 3.80 | .24 (.25) | .20 | 51 -69 24 | 3.34 | .21 | .21 |
| Cuneus (19) | 6 -81 27 | 4.13 | .26 | .25 | ||||
| Cerebellum 6 | -12 -66 -12 | 3.88 | .25 | .20 | ||||
Notes:
Values in brackets represent the quadratic r value. –GM vol: correlation when controlling for gray matter volume
Hit Distractors activated a bilateral distributed network (Figure 6A, Table 3). Age was positively correlated with task-related activity in prefrontal regions, premotor cortex, putamen, parahippocampal gyrus, superior and inferior parietal lobules, and temporal cortex (Figure 6B). When controlling for gray matter volume, age correlated with task-related activity in prefrontal cortex, premotor cortex, superior and inferior parietal lobi and lingual gyrus (Figure 6C). For non-task-related regions, fMRI activity in prefrontal, premotor, lateral and medial temporal and occipital regions showed significant correlation with age, before and after controlling for gray matter volume (Figure 6D, E; Table 3).
Figure 6.
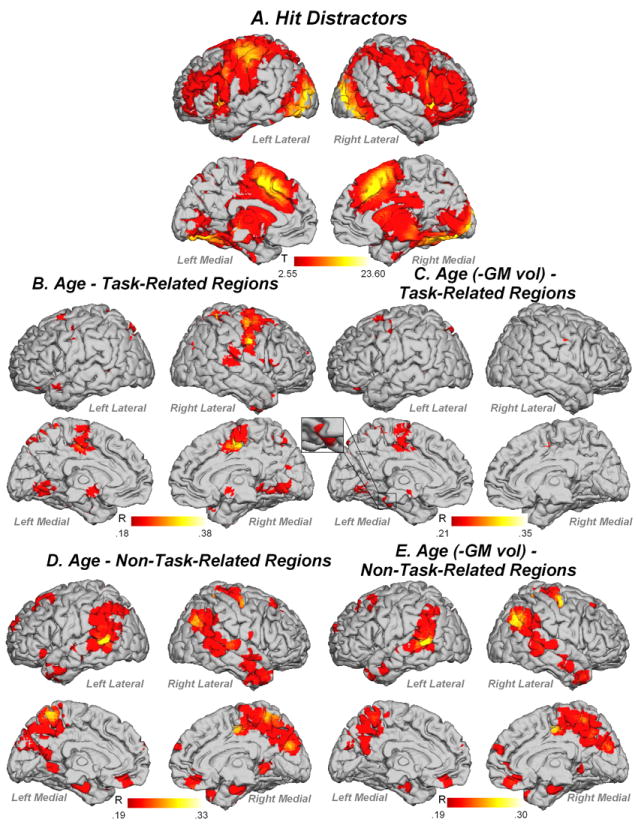
fMRI activity for (A) Hit Distractors, (B) task-related regions correlated with age, (C) task-related regions correlated with age when controlling for gray matter volume, (D) non-task-related regions correlated with age, (E) non-task related regions correlated with age when controlling for gray matter volume. Contrast thresholded at p<.01 FDR corrected k=5 voxels.
Table 3.
MNI coordinates and T values for Hit Distractor > Baseline contrast. Contrast thresholded at p<.01 FDR corrected k=5 voxels
| Region (BA) | Left Hemisphere | T | Age r | Age r [-GM vol] | Right Hemisphere | T | Age r | Age r [-GM vol] |
|---|---|---|---|---|---|---|---|---|
|
TASK-RELATED REGIONS
| ||||||||
| Superior Orbitofrontal (11) | -24 51 -9 | 4.07 | 24 36 -15 | 5.88 | ||||
| Middle Orbitofrontal (11) | -33 51 -6 | 2.62 | 27 51 -6 | 7.02 | ||||
| Inferior Orbitofrontal (11) | -33 24 -9 | 15.31 | 33 24 -9 | 19.46 | ||||
| Superior Frontal (6) | -12 18 45 | 13.00 | .28 | 18 6 60 | 5.72 | .28 | .28 | |
| Middle Frontal (10/9/8) | -33 48 18 | 8.01 | .18 | .24 | 36 48 3 | 7.41 | .26 | .25 |
| Inferior Frontal Triangularis (47) | -42 21 27 | 7.20 | 42 24 3 | 17.28 | .16 | |||
| Inferior Frontal Operculum (47) | -42 6 24 | 10.45 | 45 18 0 | 17.20 | .27 | |||
| Anterior Cingulate (24/32) | -6 24 27 | 12.08 | 12 27 24 | 12.99 | ||||
| Supplementary Motor Area (32/6) | -3 9 54 | 21.29 | .38 | .24 | 6 21 45 | 22.46 | .35 | |
| Mid Cingulate (32/24) | -6 15 33 | 12.51 | .22 | .21 | 6 21 36 | 18.08 | .33 | .24 |
| Insula | -30 24 0 | 20.94 | 33 24 0 | 23.60 | .24 | .23 | ||
| Rolandic Operculum (13) | -51 -21 18 | 13.51 | 45 6 12 | 9.79 | .20 | |||
| Caudate | -15 12 6 | 10.26 | 21 24 0 | 4.44 | ||||
| Putamen | -24 3 3 | 15.59 | 18 9 33 | 5.08 | .19 | |||
| Thalamus | -12 -18 6 | 18.00 | 12 -12 3 | 14.91 | ||||
| Substantia Nigra | -12 -21 -9 | 12.38 | 12 -21 -9 | 11.99 | ||||
| Amygdala | -24 0 -15 | 7.03 | 33 -6 -12 | 5.59 | ||||
| Hippocampus | -24 -30 -6 | 8.08 | 24 -27 -3 | 10.57 | ||||
| Parahippocampal | -18 -36 -6 | 5.37 | .22 | 33 -33 -15 | 7.14 | .20 | ||
| Precentral (4/6) | -39 -15 60 | 19.11 | .21 | 45 3 30 | 12.12 | .34 | .32 | |
| Postcentral (40) | -42 -30 48 | 20.09 | .29 | .25 | 51 -27 45 | 12.75 | .23 | |
| Posterior Cingulate (23) | -3 -30 27 | 9.38 | 3 -30 27 | 9.92 | ||||
| Superior Parietal (7) | -27 -60 51 | 14.10 | .26 | .25 | 27 -63 51 | 14.21 | .29 | |
| Precuneus (7) | -12 -66 48 | 5.75 | .24 | .21 | ||||
| Inferior Parietal (40) | -30 -48 48 | 14.52 | .21 | 27 -63 45 | 16.48 | .23 | .24 | |
| Heschl’s (13) | -36 -24 12 | 6.60 | ||||||
| Superior Temporal Pole (38) | -51 6 0 | 8.67 | .18 | 45 15 -21 | 16.58 | .17 | .25 | |
| Superior Temporal (41) | -51 -21 12 | 10.90 | 66 -33 18 | 3.28 | .33 | .30 | ||
| Middle Temporal (37) | -48 -63 3 | 4.28 | .28 | 42 -66 3 | 6.90 | |||
| Inferior Temporal (37) | -33 -9 -33 | 3.83 | 48 -54 -18 | 14.37 | .29 | |||
| Fusiform (18) | -33 -69 -9 | 16.81 | .24 | .22 | 33 -54 -18 | 20.67 | ||
| Cuneus (18) | -15 -75 33 | 3.84 | .21 | 21 -93 12 | 16.51 | |||
| Lingual (17) | -18 -87 -12 | 17.63 | .32 | .26 | 15 -87 -6 | 15.04 | .28 | .25 |
| Superior Occipital (18) | -27 -93 24 | 15.63 | .23 | 24 -93 12 | 18.30 | .20 | ||
| Middle Occipital (19) | -30 -93 15 | 20.47 | 33 -90 9 | 22.48 | .26 | |||
| Inferior Occipital (18) | -33 -81 -12 | 20.21 | 42 -66 -15 | 17.50 | ||||
| Cerebellum 6 | -27 -51 -21 | 12.61 | .31 | .25 | 33 -54 -24 | 18.95 | .23 | |
| Cerebellum 1 | -36 -78 -21 | 13.18 | 33 -57 -33 | 10.69 | ||||
| Cerebellum 4/5 | -21 -48 -18 | 7.24 | .26 | .23 | 15 -54 -24 | 12.59 | .23 | .20 |
| Cerebellum 2 | -6 -72 -33 | 4.94 | 6 -72 -33 | 6.80 | ||||
|
| ||||||||
|
NON-TASK-RELATED REGIONSa
| ||||||||
| Medial Orbitofrontal (11) | -2 42 -21 | 3.30 | .26 | .26 | 3 42 -21 | 3.58 | .27 | .25 |
| Anterior Cingulate (32) | 3 33 -6 | 4.02 | .26 | |||||
| Superior Frontal (10) | -21 63 24 | 3.64 | .20 | .19 | 27 24 51 | 3.42 | .19 | |
| Superior Medial Frontal (10) | 0 63 24 | 3.19 | .23 | .21 | 3 63 27 | 3.27 | .23 | .19 |
| Supplementary Motor Area (6) | 12 -12 51 | 5.14 | .31 | .30 | ||||
| Middle Frontal Gyrus (8) | -27 15 60 | 4.17 | .20 | 33 42 39 | 3.53 | .22 | ||
| Precentral (6) | 48 -15 57 | 4.75 | .27 | .27 | ||||
| Mid Cingulate (31) | -3 -18 45 | 3.62 | .23 | 12 -36 57 | 4.39 | .24(.24) | .23 | |
| Rolandic Operculum | 45 -12 21 | 3.38 | .20 | .22 | ||||
| Superior Temporal (22/39) | -60 -45 18 | 3.78 | .26 | .25 | 57 -39 15 | 4.56 | .26 (-.26) | .25 |
| Superior Temporal Pole (22) | 39 18 -36 | 3.32 | .26 (-.25) | |||||
| Middle Temporal (21/22) | -63 -57 6 | 5.05 | .33 | .29 | 54 -9 -15 | 3.49 | .24 | .24 |
| Middle Temporal Pole (38) | -42 12 -33 | 3.19 | .20 | .21 | 42 9 -45 | 3.80 | .25(-.24) | .27 |
| Inferior Temporal (21) | -42 12 -42 | 3.50 | .24 | .27 | 54 -15 -21 | 3.10 | .23 | .28 |
| Parahippocampal | -18 -9 -30 | 3.21 | .33 | .30 | 27 -12 -27 | 3.36 | .33 | .30 |
| Hippocampus | -21 -15 -21 | 3.03 | .28 | .24 | 30 -9 -27 | 2.95 | .27 | .23 |
| Precuneus (7) | -9 -54 66 | 4.02 | .26 (.25) | .24 | 6 -54 66 | 3.74 | .19 | .21 |
| Inferior Parietal (39/40) | -60 -48 24 | 3.50 | .23 | .21 | 45 -72 33 | 4.94 | .28 (.26) | .27 |
| Lingual (18) | -9 -51 -3 | 3.73 | .19 | 12 -48 -3 | 2.98 | .24 | .21 | |
| Superior Occipital (19) | -12 -87 39 | 3.00 | .19 | .22 | ||||
| Middle Occipital (19) | -39 -72 36 | 3.01 | .23 | .20 | 48 -69 24 | 3.41 | .21 | .20 |
| Cuneus (19) | -12 -84 36 | 3.85 | .21 | .23 | 6 -81 27 | 4.44 | .26 | .24 |
Notes:
Values in brackets represent the quadratic r value. –GM vol: correlation when controlling for gray matter volume
Missed Targets activated a distributed network similar to that obtained in Hit Targets (Figure 7, Table 4). Age did not correlate with any task-or non-task-related region before or after controlling for gray matter volume. In order to determine if the reason for Missed Targets was due to an issue with encoding or retrieval, we conducted an additional analysis comparing encoding of subsequently remembered stimuli vs. encoding of subsequently forgotten stimuli (Encoded > Encoded-Forgotten; Figure 4B; Table 5). This comparison showed that Encoded > Encoded-Forgotten trials showed increased activity in bilateral middle occipital gyrus and right anterior cingulate (BA 24), and Encoded-Forgotten > Encoded showed increased activity in precuneus, right inferior parietal lobule and right inferior temporal gyrus (BA 20). This activity was not significantly related to age regardless of controlling for gray matter volume.
Figure 7.
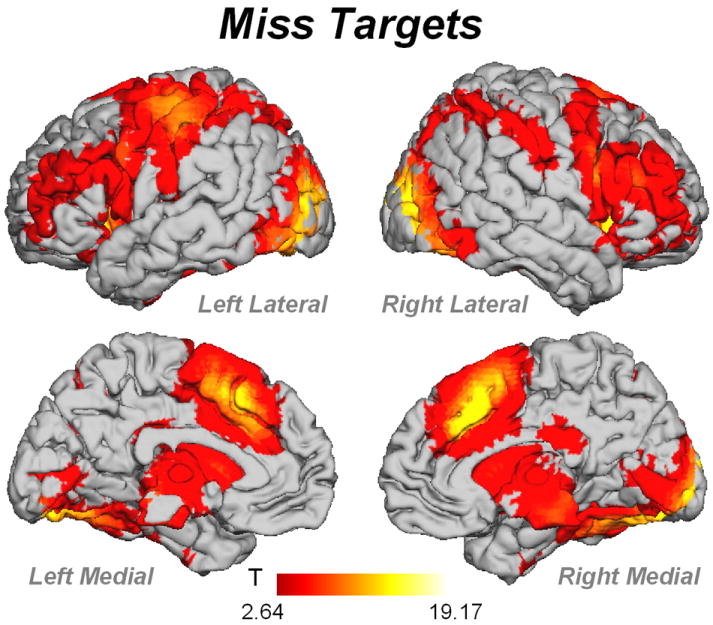
fMRI activity for Missed Targets. Contrast thresholded at p<.01 FDR corrected k=5 voxels
Table 4.
MNI coordinates and T values for Missed Target > Baseline contrast. Contrast thresholded at p<.01 FDR corrected k=5 voxels.
| Region (BA) | Left Hemisphere | T | Right Hemisphere | T |
|---|---|---|---|---|
| Superior Orbitofrontal (10) | 24 57 -6 | 4.60 | ||
| Middle Orbitofrontal (11) | -30 51 -12 | 3.17 | ||
| Inferior Orbitofrontal (47/11) | -33 27 -6 | 12.84 | 36 24 -9 | 16.60 |
| Superior Frontal (6) | -24 6 63 | 3.23 | 15 18 48 | 7.76 |
| Superior Medial Frontal (6) | -3 27 39 | 14.06 | 9 27 42 | 15.60 |
| Middle Frontal (46) | -36 54 18 | 6.53 | 48 33 21 | 11.21 |
| Inferior Frontal Triangularis (46) | -42 24 24 | 8.94 | 36 27 9 | 11.54 |
| Inferior Frontal Operculum(9) | -45 6 24 | 9.67 | 48 9 27 | 13.53 |
| Anterior Cingulate (32/33) | 0 27 30 | 10.11 | 9 33 24 | 10.70 |
| Mid Cingulate (32) | -6 24 33 | 12.79 | 9 21 36 | 16.41 |
| Supplementary Motor Area (6/8) | -6 18 51 | 15.37 | 6 18 51 | 17.74 |
| Insula | -33 21 0 | 17.60 | 33 24 0 | 19.73 |
| Rolandic Operculum | -51 0 12 | 4.08 | 48 3 12 | 4.61 |
| Precentral (6) | -48 6 30 | 11.83 | 48 9 30 | 13.33 |
| Postcentral (40) | -42 -30 45 | 16.90 | 45 -33 54 | 5.54 |
| Caudate | -15 6 15 | 7.19 | 15 12 3 | 10.07 |
| Putamen | -24 3 3 | 11.80 | 18 9 3 | 11.57 |
| Substantia Nigra | -6 -12 -12 | 8.60 | 6 -12 -12 | 9.39 |
| Subthalamic Nucleus | -9 -15 -6 | 11.09 | 9 -15 -3 | 10.97 |
| Thalamus | -12 -18 6 | 13.55 | 12 -12 -3 | 11.71 |
| Superior Parietal (7) | -27 -60 51 | 11.17 | 27 -66 51 | 11.66 |
| Precuneus (7) | -12 -69 51 | 5.49 | 18 -72 45 | 6.41 |
| Inferior Parietal (40) | -30 -48 48 | 13.06 | 42 -36 45 | 9.79 |
| Posterior Cingulate (23) | 3 -30 27 | 9.45 | ||
| Hippocampus | -24 -30 -3 | 5.25 | 21 -30 -3 | 6.55 |
| Parahippocampal | 24 -39 -6 | 2.77 | ||
| Heschl’s (41) | -36 -27 12 | 4.22 | ||
| Superior Temporal (41) | -48 -30 15 | 3.47 | ||
| Superior Temporal Pole (38) | -48 12 -3 | 7.84 | 48 12 -15 | 5.08 |
| Middle Temporal (37) | -45 -60 -3 | 6.06 | 48 -69 0 | 6.16 |
| Inferior Temporal (37) | -42 -63 -9 | 10.87 | 42 -63 -6 | 7.88 |
| Fusiform (18) | -24 -81 -6 | 12.45 | 24 -81 -12 | 16.31 |
| Superior Occipital (19) | -27 -87 24 | 13.47 | 27 -87 27 | 10.60 |
| Middle Occipital (19) | -30 -90 18 | 16.65 | 33 -90 9 | 17.72 |
| Inferior Occipital (18) | -30 -81 -12 | 16.08 | 39 -69 -12 | 13.77 |
| Cuneus (18) | -12 -84 15 | 4.74 | 18 -69 36 | 5.51 |
| Lingual (18) | -18 -87 -12 | 15.82 | 18 -87 -9 | 15.79 |
| Cerebellum 4/5 | -30 -39 -30 | 3.74 | 18 -51 -24 | 10.40 |
| Cerebellum 6 | -27 -51 -24 | 7.48 | 27 -48 -24 | 13.16 |
| Cerebellum 1 | -24 -84 -21 | 10.10 | 36 -51 -36 | 8.28 |
| Cerebellum 2 | -6 -75 -30 | 4.42 | 6 -75 -30 | 4.61 |
Table 5.
MNI coordinates and T values for Encoded vs. Encoded-Forgotten contrast. Contrast thresholded at p<.01 FDR corrected, k=5 voxels.
| Region (BA) | Left Hemisphere | T | Right Hemisphere | T |
|---|---|---|---|---|
|
Encoded > Encoded-Forgotten
| ||||
| Anterior Cingulate (24) | 15 15 30 | 4.63 | ||
| Middle Occipital Gyrus (18/37) | -48 -69 -6 | 4.91 | 51 -66 -9 | 4.68 |
|
| ||||
|
Encoded-Forgotten > Encoded
| ||||
| Precuneus/Posterior Cingulate (7/31) | 3 -63 39 | 5.21 | ||
| Inferior Parietal Lobule (40) | 51 -54 30 | 5.05 | ||
| Inferior Temporal Gyrus (20) | 63 -15 -21 | 4.89 | ||
When examining fMRI data for non-linear relationships with age, we found that no task-related region in any analysis showed significant non-linear relationship with age after correcting for multiple comparisons. In contrast, a number of non-task-related regions showed quadratic relationships with age. For Hit Targets, right temporal and bilateral inferior parietal regions showed a negative quadratic relationship with age and left parahippocampal gyrus, left occipital and right medial frontal regions showed a positive quadratic relationship with age (Figure 8, Table 2). For Hit Distractors, right superior temporal gyrus, and right superior and middle temporal poles showed negative quadratic relationships with age, and right mid cingulate, left precuneus and right inferior parietal lobule showed positive quadratic relationships with age (Figure 9).
Figure 8.
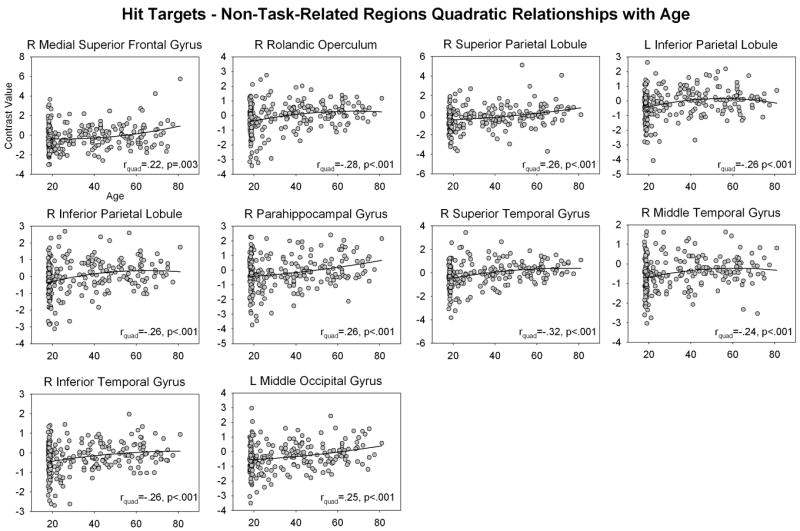
Non-linear relationships between Hit Target non-task-related activity and age. Contrast values were extracted from ROIs constructed around non-task-related activity identified in Hit Targets > Baseline contrast (see text). Abbreviations: L = left, R = right
Figure 9.
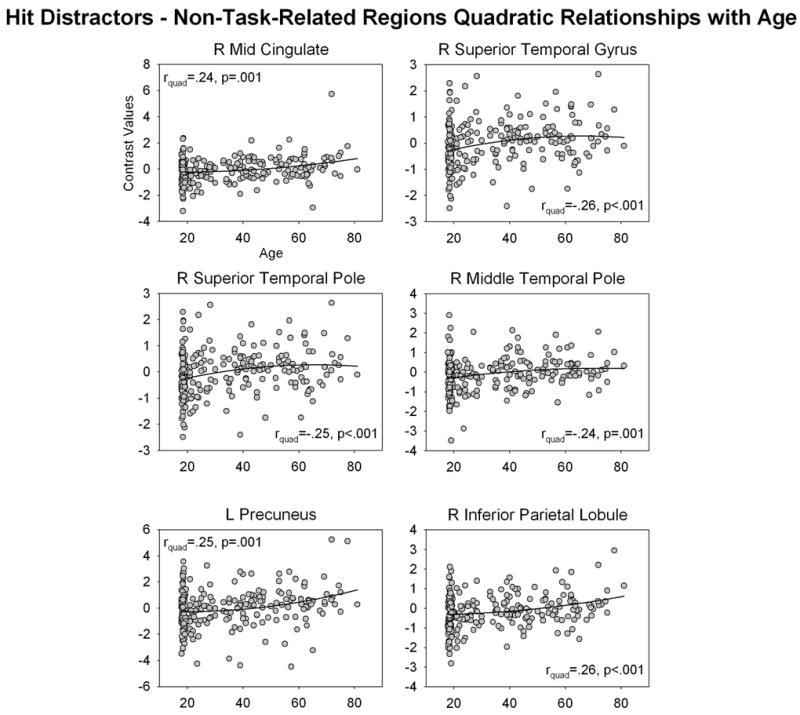
Non-linear relationships between Hit-Distractor non-task-related activity and age. Contrast values were extracted from ROIs constructed around non-task-related activity identified in Hit Distractor > Baseline contrast (see text). Abbreviations: L = left, R = right
4. Discussion
In this study, we present the first investigation of performance and fMRI activity during a figural memory task across the adult age span. We found that task performance and fMRI activity showed both linear and quadratic relationships with age. As this is the first systematic fMRI investigation of this task in healthy participants (but see Maki et al., 2011 who investigated figural memory using fMRI in women receiving hormone therapy), and the first to investigate performance in a non-elderly population, we present a detailed discussion of the results averaged across age in Supplementary Material.
4.1 Changes in figural memory performance across the adult age range
We demonstrated several linear and non-linear effects of age on performance: Hit Target RT showed a linear increase with age, and Missed Target, Hit Distractor RT and Distractor error rate showed a quadratic relationship with age, such that RT and error rate increased until 30-years of age, plateaued and then decreased around 60-years of age. Signal detection theory analyses (Supplementary Analysis 1) showed that younger and older participants were more likely to adopt a conservative response criterion when uncertain.
It is likely that our quadratic effects of age on behaviour represent two developmental processes. The changes in performance in the youngest (~17-30yrs) age ranges is likely to reflect continued maturation of the brain that is known to continue into the late 20s / early 30s (Lebel et al., 2008; Ostby et al., 2009; Stiles & Jernigan, 2010). This is consistent with the reduction in gray matter volume seen in the youngest age ranges in our sample (Figure 2A). It seems that as this maturation continues, participants slow down, become less accurate, and adopt a more liberal response criterion. At the other end of the age spectrum, we see that as age increases past ~60-yrs of age, participants again speed up, become more accurate and adopt a more conservative response criterion. The change in response criterion is consistent with previous reports of response conservativeness during recognition memory tasks in healthy aging adults (e.g. Hirschman, 1995). Also note that this pattern of results is in contrast to that seen in older adults with Alzheimer’s disease (Beth et al., 2009; Snodgrass & Corwin, 1988; Budson et al., 2006) who tend to adopt a less conservative response threshold. Thus, these results are consistent with our excluding mild cognitive impairment/early Alzheimer’s in our sample. In contrast to these expected results, the finding that older adults became faster and more accurate as age increased was not expected and is in contrast to that seen in other studies of recognition memory performance in older adults. Previous studies have shown performance decrements during recognition memory performance, albeit smaller than that seen in tests of recall (Dobbs & Rule, 1989; Kemps et al., 2006). As we discuss below, it is likely that this better performance in the oldest age ranges is due to successful compensation.
When examining the quadratic relationships between age and performance in Figure 3, it is interesting to note the end-points of the fitted lines: the lines start and end at roughly the same level of RT/error rate/bias. This suggests that if we had taken the most common approach in cognitive aging studies and compared the youngest (say 20-30 years) to the oldest (say >65years) groups, we would have found little evidence of an effect of age on performance. By extension, the study of Golski et al. (1998) who compared old (60-69yrs) and older (70-85yrs) participants would have caught only the tail-end of the changes in figural memory performance across the age distribution. It is likely that this is why they did not find age differences on RT or bias in their study. These findings highlight the advantage of treating age as the continuous variable that it is, rather than creating then comparing artificial age categories.
4.2 Changes in figural memory fMRI activity across the adult age range
Intriguingly, we found no significant effects of age during the encoding phase, regardless of whether we controlled for changes in gray matter volume, or if we examined task- or non-task-related regions. This finding is compatible with the finding that stimulus discriminability (Pr) did not differ with age (Supplementary Analysis 1). This clearly suggests that changes in performance related to age were not related to difficulties in encoding the stimuli, rather they were due to changes in the ability to recognize the stimuli during the recognition phase. This result is consistent with previous reports that older adults can properly encode information, but struggle when required to retrieve the information (Drag & Bieliauskas, 2009; Kemps et al., 2006; Nyberg et al., 2003).
For correctly recognized Targets, task-related activity increased linearly in supplementary motor area, precentral gyrus, cingulate and parahippocampal gyrus with increasing age. The increase in task-related activity in the parahippocampal gyrus was related to changes in gray matter volume, as this relationship disappeared once gray matter volume was controlled, and may therefore represent an attempt to compensate for structural changes in the brain that occur with age (Figure 2A; Stiles & Jernigan, 2010). Increases in activation in frontal areas with age have been widely reported (see Grady et al., 2008 for a review), and it is currently a matter of debate whether these increases in activity represent compensation or neural inefficiency. Since the increase in activation in these regions was accompanied by a linear increase in Hit Target RT with age, it appears that the increase in prefrontal activation during Hit Targets represents an attempt to compensate for cognitive decline that occurs in older age. Since this over-recruitment of task-related regions was associated with better performance, it appears that this compensation was successful.
These linear increases with age in Hit Target task-related regions were accompanied by non-linear increases in activity in non-task-related regions with age. Although the pattern of results in these scatterplots is complex, they can be broadly described as showing early changes in activity with age in the youngest age range (<25-30-yrs), followed by accelerated changes approximately > 60yrs. Right medial superior frontal gyrus, superior parietal lobule, parahippocampal gyrus, and left middle occipital gyrus show a slight increase in activity after 60-yrs; the right rolandic operculum, superior, middle and inferior temporal gyri and bilateral inferior parietal lobule showed a slight decrease in activity after 60-yrs. Thus, there was substantial variability in the way that older participants recruited non-task-related regions to Hit Targets. The finding that older adults differentially recruit a broader range of neural regions to perform tasks of memory and cognition than younger adults is consistent with a large number of previous studies (see Grady, 2008; Rajah & D’Esposito, 2005 for reviews); it is also consistent with our conclusion that older participants adopted a compensatory strategy to achieve successful performance on the task.
The pattern of results for Hit Distractors is consistent with this conclusion. For Hit Distractors, task-related activity increased linearly with age in a network of regions including bilateral prefrontal cortex, motor areas, parietal cortex, temporal cortex and occipital cortex. Many of these regions, including bilateral superior/middle frontal gyri, motor areas, superior parietal cortex, right temporal cortex and bilateral occipital cortex, remained associated with age even when accounting for changes in gray matter volume, suggesting that these changes with age are specifically due to changes in function during task performance, and are not an attempt to compensate or overcome gray matter atrophy. In contrast, in some regions activity was related to changes in both age and gray matter volume, including right inferior frontal gyrus, putamen, bilateral parahippocampal gyrus, left inferior parietal lobule, superior temporal pole and bilateral temporal cortex. Thus changes in activity in these regions with age are likely to reflect changes related to overcoming gray matter changes in the brain that occur with age. In addition, like Hit Targets, Hit Distractor non-task-related activity showed a complex pattern of non-linear relationships with age, with activity in right mid cingulate and bilateral parietal cortex showing a positive quadratic relationship with age, and activity in the right temporal lobe showing a negative quadratic relationship with age. This is consistent with our interpretation that older adults differentially recruit a range of additional regions to achieve successful recognition memory performance. Together, these results are consistent with our conclusion that older adults over-recruit task-related regions and also recruit additional non-task-related regions in order to achieve successful performance on the figural memory task.
We saw no significant effects of age on fMRI activity for Missed Targets, or for Encoded vs. Encoded-Forgotten trials. This suggests that changes related to compensation were restricted to correct recognition of Targets and Distractors, which is consistent with our earlier interpretation. The finding that activity for Encoded vs. Encoded-Forgotten trials did not differ with age is in contrast to previous results (Duverne et al., 2009). Duverne et al. (2009) showed that the medial parietal/right inferior parietal activation seen in Encoded-Forgotten > Encoded trials occurred in young (18-29 yrs) but not older (63-76-yrs). It is unclear why we did not see this effect. Duverne et al. (2009) used a different analysis strategy, and used a less conservative threshold than used here. However, even when reducing our threshold to p<.001 uncorrected, we do not see this effect. In Duverne et al., the older participants showed a decrement in performance relative to the younger subjects. Given that in our sample, the older adults actually performed better than the participants in the median age ranges, it is possible that our older adults did not show the same level of impairment during encoding than the Duverne et al. sample.
Our findings add to the growing body of research on age-related memory decline. The finding of over-recruitment of brain regions during memory tasks with increasing age is consistent with previous reports (Grady et al., 2008; Duverne et al., 2009; Morcom et al., 2007; Cabeza et al., 2002; Daselaar et al., 2006). However as noted previously, the finding of better performance with increasing age is somewhat unexpected. Typically, older adults show memory decline across multiple domains of memory, including immediate and delayed recall for stories, arbitrary pairs of words, faces, and lists of unrelated words (see Salthouse, 2003 for a review). Previous studies using picture stimuli that can be verbalized (e.g. pictures of nouns, nameable objects) have shown reduced recognition accuracy and patterns of neural over-recruitment for old vs. young groups (Duverne et al., 2009; Morcom et al., 2007). Interestingly, Ally et al. (2008) showed that older adults showed an intact picture superiority effect for noun pictures vs. noun words. Thus, while older adults continue to show better memory for pictures than words, even though overall, memory recognition is worse for older vs. younger adults. Since our stimuli could be encoded visually but not verbally, our results suggest that this intact picture superiority effect might not be related to the fact that verbalizable pictures can be encoded both verbally and visually, rather that older adults benefit more from the visual encoding of stimuli. In addition, we argue that the over-recruitment seen in the fMRI data suggests that older adults successfully employed compensatory strategies to achieve improved memory performance, consistent with previous reports (Duverne et al., 2009; Morcom et al., 2007). Interestingly, Wang et al. (2009) demonstrated the ‘old-old’ adults (84-96 years) showed no additional over-recruitment relative to ‘young-old’ (64-77 years) participants, suggesting that if we increased the age range of our sample, we would see a plateauing of this over-recruitment. Indeed, some plateauing is already evident in Figures 8 & 9.
4.3 Strengths and limitations of the current study and future directions
There were a number of significant strengths of our study. Firstly, we treated age as the continuous variable that it is, rather than comparing artificial age categories, as in the vast majority of studies of cognitive aging. As noted previously, examination of the scatterplots suggests that had we compared only the youngest and oldest adults in our samples, the effects of age on performance would likely have been minimized. Our results are compatible with Salthouse’s (2011) conclusions that normal aging shows linear and non-linear effects on performance and fMRI activity, and that there is no evidence of a discrete step from ‘intact’ to ‘impaired’ performance. In the Introduction, we noted that studies that artificially categorize participant age implicitly assume that such a discrete step must occur somewhere in middle age. Examining the scatterplots of performance and fMRI activity presented here, it is clear that changes occur somewhere around the beginning of the 6th decade. Note however that our results point to a gradual, not discrete, change with increasing age. A second strength of our study is that we used a large sample with relatively consistent sampling frequency across the age ranges of 21-81. This allowed us to take the approach of treating age as a continuous variable. Note that Salthouse (2011) illustrated that age-related changes in cognition with increasing age appear to be due to changes in mean performance, rather than increases in variability with age. Examining the scatterplots of performance and fMRI activity with age in the current study is consistent with this conclusion: the spread of points within the plots is relatively uniform across the age ranges. A third strength of our study is that we examined effects of age on both task- and non-task-related fMRI activity. This allowed us to determine whether older adults recruited additional neural regions to achieve superior figural memory performance. A fourth strength of our study is that we distinguished between regions showing effects of age related to presence or absence of changes related to gray matter volume. It is well-established that changes in brain volume are associated with age and can impact on fMRI activity and task performance (see Fjell & Walhovd, 2010 for a review). Lastly, a fifth strength of our study is that we examined the data for presence or absence of non-linear effects of age on performance and fMRI activity. This was possible because of our large sample size, however studies that simply look for linear effects of age may well miss effects that are present in the data. For example, in this study, the increase in performance at the older age ranges would have been missed had we examined the data for linear effects only.
Given these strengths, it is also important to note the limitations of our study. Firstly, our use of gray matter volumes extracted from segmented images is a rather gross estimate of changes of brain volume with increasing age. We chose to use gray matter volume rather than total brain volume (gray + white + cerebrospinal fluid volume) due to issues with T1 image quality (indeed, for a small proportion of participants, the gray matter volume could not be reliably estimated from the images5). Furthermore, these analyses were designed to merely shed light on how changes in fMRI activity with increasing age could be attributable to structural brain changes. Future studies should endeavor to quantify how changes in figural memory fMRI activity is related to changes in volume of specific brain structures (e.g. hippocampus) and changes in white matter volume, cortical thickness, etc. Secondly, our study did not include a verbal memory task as a comparison condition for the figural memory task, as such we cannot conclude that our findings are specific to a change in figural memory that is not also seen in verbal memory. Thirdly, the distribution of ages in the sample is skewed towards younger participants. It is possible that this may have affected the results, and future studies should endeavor to explore this possibility. Lastly, despite the strengths of our sample, the upper age range of the sample falls within the ‘young-old’ age range used in previous studies. It is possible that the pattern of results will change in ‘old-old’ adults. For example, Wang et al. (2010) showed that subjects aged 84-96 yrs showed a different pattern of performance and fMRI activity during encoding of visual (but verbalizable) stimuli compared to subjects aged 67-77 yrs. They noted that changes in compensation may occur somewhere between these age ranges, such that it is no longer successful, or shows reduced success. Future studies should examine older age ranges to determine if this is also the case in the figural memory task.
4.4 Conclusions
In this study we presented the first analysis of changes in figural memory performance across the healthy adult age spectrum. We found that performance actually improved in older age ranges, and this was accompanied by linear increases in task-related activity, and linear and non-linear changes in activity in non-task-related regions. We attributed this pattern of results to successful compensation for cognitive decline that occurs in older age. As noted by Rugg and colleagues (Duverne et al., 2009; Wang et al., 2010), this generalized cognitive decline could be attributable to neurodegeneration, a reduction in neural efficiency, or as a consequence of normal aging. Note that previous studies of cognitive aging have argued that changes in fMRI activity with age could attributable to compensation or to dedifferentiation of functional localization (Grady, 2008; Rajah & D’Esposito, 2005). However we note that both Grady and Rajah and D’Esposito argue that changes in fMRI activity together with intact performance are taken as the strongest evidence for compensation in the literature. Since in our study, changes in fMRI activity were associated with better, not just intact, performance, we strongly prefer the compensation interpretation over dedifferentiation.
Supplementary Material
Footnotes
PET provides a more direct measure of brain metabolism than BOLD fMRI, however it also has some limitations. In addition to the issues with radiation exposure, PET also has a relatively limited spatial resolution and substantially poorer temporal resolution relative to BOLD fMRI (Huettel et al., 2004).
Also note that we do not have complete data for blood pressure across the sample, which may impact the BOLD response
Although it is possible to find significant non-linear relationships in areas not showing linear relationships, for brevity we chose to focus only on regions previously identified in the linear correlation analysis.
Note that we re-ran the analyses with only participants who had useable T1 scans, and the results were stable.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beason-Held LL, Golski S, Kraut MA, Esposito G, Resnick SM. Brain activation during encoding and recognition of verbal and figural information in older adults. Neurobiol Aging. 2005;26:237–250. doi: 10.1016/j.neurobiolaging.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Kraut MA, Resnick SM. I. Longitudinal changes in aging brain function. Neurobiol Aging. 2008a;29:483–496. doi: 10.1016/j.neurobiolaging.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason-Held LL, Kraut MA, Resnick SM. II. Temporal patterns of longitudinal change in aging brain function. Neurobiol Aging. 2008b;29:497–513. doi: 10.1016/j.neurobiolaging.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beth EH, Budson AE, Waring JD, Ally BA. Response bias for picture recognition in patients with Alzheimer’s Disease. Cogn Behav Neurol. 2009;22:229–235. doi: 10.1097/WNN.0b013e3181b7f3b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using the MarsBar toolbox for SPM99. Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover JE, Gabrieli JDE. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer’s Disease: separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Functional neuroanatomy of recall and recognition: a PET study of episodic memory. J Cogn Neurosci. 1997;9:254–265. doi: 10.1162/jocn.1997.9.2.254. [DOI] [PubMed] [Google Scholar]
- Cherry KE, Hawley KS, Jackson EM, Volaufova J, Su LJ, Jazwinski M. Pictorial superiority effects in oldest-old people. Memory. 2008;16:728–741. doi: 10.1080/09658210802215534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SMm, Veltman DJ, Rombouts SARB, Raajimakers JGW, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Dobbs A, Rule B. Adult age differences in working memory. Psychol Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. J Geriatr Psychiatry Neurol. 2010;23:75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance and the neural correlates of successful memory encoding. Cereb Cortex. 2009;19:733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? I E E E Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Glorioso C, Sibille E. Betweeen destiny and disease: genetics and molecular pathways of human central nervous system aging. Prog Neurobiol. 2011;93:165–181. doi: 10.1016/j.pneurobio.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JDE. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124:1841–1854. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- Golski S, Zonderman AB, Malamut BL, Resnick SM. Verbal and figural recognition memory: task development and age associations. Exp Aging Res. 1998;24:359–385. doi: 10.1080/036107398244193. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Hirschman E. Decision processes in recognition memory: criterion shifts and the list-strength paradigm. J Exp Psychol Learn Mem Cogn. 1995;21:302–313. doi: 10.1037//0278-7393.21.2.302. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age – consistency and interpretation of observed effects. Neurobiol Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Kemps E, Newson R. Comparison of adult age differences in verbal and visuospatial memory: the importance of ‘pure’, parallel, and validated measures. J Clin Exp Neuropsychol. 2006;28:341–356. doi: 10.1080/13803390490918228. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Maki PM, Dennerstein L, Clark M, Guthrie J, LaMontagne P, et al. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–243. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, Reed VS, Walling JR. Pictorial superiority effect. J Exp Psychol Hum Learn. 1976;2:523–528. [PubMed] [Google Scholar]
- Nyberg L, Maitland S, Ronnlund S. Selective adult age differences in an age-invariant multifactor model of declarative memory. Psychol Aging. 2003;18:149–160. doi: 10.1037/0882-7974.18.1.149. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11:1150–1160. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Paivio A. Imagery and verbal processes. New York: Rinehart & Winston; 1971. [Google Scholar]
- Petrides M. Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 9. Amsterdam: Elsevier; 1994. pp. 59–82. [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Proc R Soc Lond B Biol Sci. 1996;351:1455–1492. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Proc R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragananth C. Working memory for visual objects: complementary roles of inferior temporal, medial temporal and prefrontal cortex. Neurosci. 2006;139:277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Roland PE, Gulyas B. Visual memory, visual imagery, and visual recognition of large filed patterns by the human brain: functional anatomy by positron emission tomography. Cereb Cortex. 1995;1:79–93. doi: 10.1093/cercor/5.1.79. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Memory aging from 18 to 80. Alzheimer Dis Assoc Disord. 2003;17:162–167. doi: 10.1097/00002093-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Ann Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Sternberg G. Conceptual and perceptual factors in the picture superiority effect. Eur J Cogn Psychol. 2006;18:813–847. [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JDE. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Y, Metzak P, He Y, Woodward TS. Age-related changes in topological patterns of large-scale brain functional networks during memory encoding and recognition. Neuroimage. 2010;50:862–872. doi: 10.1016/j.neuroimage.2010.01.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


