Abstract
The current study examined whether healthy older adults (OA) and individuals at the earliest stages of dementia of the Alzheimer’s type (DAT) differ from younger adults (YA) and from each other on a simple, extended continuous tapping task using intervals (500 ms, 1000 ms, and 1500 ms) thought to differentially engage attention control systems. OA groups sped up their tapping at the slowest target rate compared to the YA; this pattern was magnified in the early stage DAT groups. Performance variability appeared especially sensitive to DAT-related changes, as reliable differences between healthy OA and very mild DAT individuals emerged for multiple tap rates. These differences are proposed to result from breakdowns in attentional control that disrupt error-correction processes and the ability to resolve discrepancies between internally-generated temporal expectancies and the external temporal demands of the repetitive timing task.
Keywords: Aging, Attention, Alzheimer type dementia, Dementia, Time perception, Sensory motor performance
An important goal in the cognitive neuropsychology of aging is to better understand how normal age-related declines differ from those associated with Alzheimer’s disease (AD). AD-related neuropathological changes occur years prior to apparent clinical symptoms (Bennett et al., 2006; Morris et al., 1996; Price & Morris, 1999). Recent research has sought to identify pre-clinical markers which distinguish healthy older adults (OA) from those with prodromal AD. Though episodic memory impairments are considered the hallmark of AD and discriminate healthy OA from those with mild dementia of the Alzheimer’s type (DAT: Albert, Moss, Blacker, Tanzi, & McArdle, 2007; Albert, Moss, Tanzi & Jones, 2001; Storandt & Hill, 1989; Storandt, Grant, Miller & Morris, 2006), declines in attention have recently been shown to also be powerful markers (e.g., Hutchison, Balota, & Duchek, 2010).
Declines in Attention with Aging and DAT
Declines in attentional control are found in healthy OA and individuals with early stage DAT (see Balota & Faust, 2001 and Perry & Hodges, 1999 for relevant reviews); selective and divided attention are especially sensitive to early stage DAT-related breakdowns (see Faust & Balota, 2007 for a review). Namely, DAT individuals have trouble maintaining a representation of task demands and inhibiting the intrusion of inappropriate information; this may contribute to episodic memory impairments through continued activation of irrelevant information that disrupts encoding and retrieval processes (Balota et al. 1999; Castel, Balota, & McCabe, 2009; Craik & Lockhart, 1972; Jacoby, 1999). Vigilance is resistant to normal age-related declines (Tucker, Stern, Basner & Rakitin, 2011) but may show DAT-related deficits under increased task difficulty (Berardi, Parasuraman & Haxby, 2005).
Attention and Timing
Attention is critical in temporal perception and performance, and so investigations of simple timing tasks may afford a useful paradigm for examining age and AD related changes. One popular information-processing conception of timing posits a pacemaker-accumulator device with an attention-mediated gate (Gibbon, Church, & Meck, 1984; Lejeune, 1998; Rousseau, Picard, & Pitre, 1984; Zakay & Block, 1997). The degree of attention to time influences the opening and closing of the gate and, subsequently, the number of pulses that pass to the accumulator and represent the target interval. The impact of attentional manipulations on perceived interval length depends on whether attention is disrupted during encoding or response (Brown, 1997; Fortin, Rousseau, Bourque, & Kirouac, 1993; Macar, Grondin, & Casini, 1994; Zakay, 1998). Though the impact on timing variability is less clear (Brown, 1997; Perbal, Droit-Volet, Isingrini, & Pouthas, 2002), Rakitin (2005) examined this relationship using choice time production with 3 and 5 second target durations. Under conditions requiring greater attentional control (stimulus-response incompatibility), the coefficient of variation (COV) increased, especially for the shortest interval. Presumably, non-scalar variability linked to opening and closing of the attentional gate contributed more to the shorter interval’s total variability.
Most studies manipulating attention and timing have involved durations beyond the upper range (3 – 5 sec) of the “psychological present” (see Pöppel, 2004 for a review) where people have difficulty perceiving two stimuli as part of a unified event, causing timing to rely more on executive control. However, engagement of executive functions may also occur at shorter durations. For example, Michon (1985) argued that 500 ms delineates automatic versus cognitively-mediated temporal processes. Likewise, there is differential engagement of brain networks during timing of durations shorter (sensorimotor regions) versus longer (right prefrontal and parietal regions) than 1 second (see Koch, Oliveri & Caltagirone, 2009 and Lewis & Miall, 2006 for relevant reviews). The latter brain areas are associated with attention and executive functions (Lewis & Miall, 2003). In the context of a continuous tapping paradigm, like that used in the current study, slower tapping rates and longer tapping bouts lead to drift, with breaks in patterns of drift occurring at about 1000 and 1300 ms (Collier & Ogden, 2004; Madison, 2001). Increased drift is linked to increased response dispersion and breaks implicate possible shifts in processes used for timing.
Timing, Aging, and AD
A meta-analysis (Block, Zakay & Hancock, 1998) of the aging and time literature found more variable estimates and shorter productions with age when demands on controlled attention were greater at encoding than at test. Of the few studies that have explored the impact of DAT on timing, most have utilized temporal discrimination with supra-second durations. Although the results are somewhat mixed, DAT appears linked to more variable estimates (Nichelli et al., 1993; Papagno, Allegra, & Cardaci, 2004; Rueda & Schmitter-Edgcombe, 2009).
Simple continuation tapping involves the continuation of a tapping pulse to an external signal after that signal has been removed. This task allows partitioning of timing variability into a component attributable to a putative internal clock and one associated with peripheral motor processes (Wing & Kristofferson, 1973). This paradigm has revealed declines in special populations, such as individuals with prodromal Huntington’s disease, using sub-second intervals (Rowe et al., 2010). Similarly, differences were found between non-demented Parkinson’s patients on medication and normal, healthy controls on durations of 300 and 600 ms (Harrington, Haaland & Hermanowicz, 1998). Although Duchek, Balota and Ferraro (1994) did not replicate this finding with Parkinson’s patients using a 550 ms interval, they did find increased clock variability in mild DAT. Neither very mild DAT nor normal aging led to deficiencies in clock or motor variability. Others, however, have found a positive relationship between clock variability and age in this task (Woodruff-Pak & Jaeger, 1998).
Stronger age- and DAT-related differences may emerge in this paradigm at slower tapping rates. When tapping at rates ranging from 150 to 1709 ms, OA speed up at the longest interval (McAuley, Jones, Holub, Johnston & Miller, 2006). Likewise, Krampe, Doumas, Lavrysen and Rapp (2010) found that during tapping at fast (550 ms) and slow (2100 ms) rates, dual tasking led both older and younger adults (YA) to speed up their tapping at the slow rate, while OA also sped up during the fast rate. Dual tasking also led to increased variability for both groups; importantly, this was magnified at the slow rate for OA. Thus, even with simple repetitive tapping, attention plays a critical role.
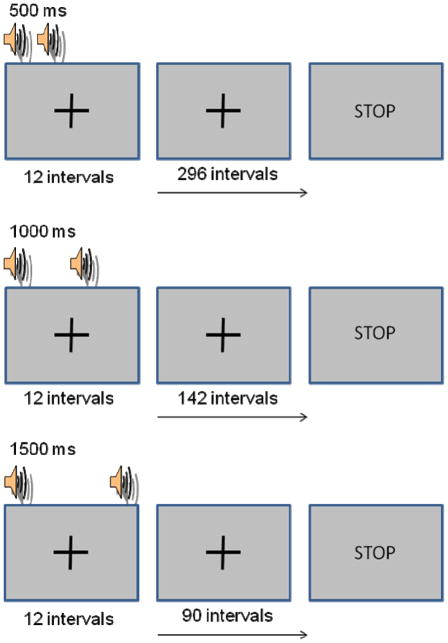
The current study expands upon work by Duchek and colleagues (1994) to examine whether normal aging and very mild DAT lead to breakdowns on a simple synchronization-continuation tapping task (see Figure 1) when slower tap rates, intended to tax attention, are included. Its apparent simplicity in terms of performance demands and instructions make it well-suited for individuals with cognitive declines. We utilize intervals (500, 1000, and 1500 ms) well within the “psychological present” to ensure each inter-tap interval (ITI) is perceived as a unified event. Each tap rate condition lasts approximately three minutes. This is longer than the typical bout required for this procedure and should engage attentional mechanisms to maintain an accurate representation of the rate over time. While this differs from traditional attention/vigilance tasks, participants must monitor their internal environment to detect a generated expectancy indicating when to execute a tap.
Figure 1.
Schematic of the synchronization-continuation timing task. Participants produced 12 intervals in sync with an auditory pacer, then the tones disappeared and tapping continued while participants tried to maintain the target tapping rate for the rest of the trial. Time on task was roughly equated for the 3 tapping rate conditions.
Slower tapping rates should be more likely to engage attentional mechanisms. To the degree that dual-task interference serves as a valid model of aging and DAT-related decline, we expect these groups to show increased variability and speed of tapping, especially at the slowest rates, compared to YA (Krampe et al., 2010; McAuley et al. 2006). These changes may be magnified even in the very earliest stages of the disease, i.e., levels of DAT that are comparable to the cognitive decline seen in MCI (see Storandt et al., 2006). Given recent evidence that variability measures are sensitive to early stage AD (e.g., Duchek et al., 2009; Hultsch et al., 2000; Tse et al., 2010), we are especially interested in how intra-individual variability in timing differs for the healthy older and very mild DAT groups. Although we report results from a small sample of individuals with mild DAT (Clinical Dementia Rating, CDR, of 1), we are most interested in individuals with very mild DAT (CDR of .5), because they are at the earliest detectable transition from healthy aging to early stage AD (see Storandt, et al., 2006). The mild DAT group was examined to insure that increases in dementia severity do not produce unexpected patterns of results.
Methods
Participants
A total of 282 individuals completed this study, approved by the Institutional Review Board at Washington University; participants gave informed consent prior to participating. Sixty-six college-aged YA (mean age = 20.27, SD = 1.56) recruited from the Washington University Psychology department undergraduate pool participated for course credit. The remaining 216 older adults were recruited from the Washington University Alzheimer’s Disease Research Center (ADRC) and were screened for depression, untreated hypertension, reversible dementias, and other disorders associated with cognitive impairment. Presence and severity of dementia was assessed using the Washington University Clinical Dementia Rating (CDR) scale (Morris, 1993; Morris & Fulling, 1988). CDR scale values and their matching dementia status are: 0 = no dementia, .5 = very mild dementia, 1 = mild dementia, 2 = moderate dementia, and 3 = severe dementia.1 Note that individuals classified as CDR .5 in our study scored, on average, 27 on the MMSE, suggesting they are at the very earliest detectable stages of dementia. Out of the OA, 133 were classified as healthy CDR 0, 58 as very mild dementia, or CDR .5, and 25 as mild dementia, or CDR 1 (see Table 1).
Table 1.
Psychometric Means (SD) as a Function of Group
| Healthy Old | CDR .5 | CDR 1 | |
|---|---|---|---|
| N | 119 | 46 | 21 |
| Gender (# males) | 43 | 28 | 9 |
| Handedness (# right) | 114 | 44 | 21 |
| Age | 74.62 (7.42) | 75.76 (7.54) | 79.76 (5.97) *+ |
| Education | 15.59 (2.83) | 15.07 (2.60) | 13.50 (3.57)* |
| MMSE | 29.01 (1.27) | 27.00 (3.16)* | 23.59 (4.36)*+ |
| Logical Memory | 13.51 (4.33) | 10.27 (4.33)* | 5.19 (4.63)*+ |
| Forward Digit Span | 6.77 (1.05) | 6.64 (.97) | 6.38 (1.36) |
| Backward Digit Span | 4.95 (1.32) | 4.45 (1.30)* | 4.24 (1.37)* |
| WMS Associate Recall | 14.33 (3.71) | 11.02 (3.99)* | 8.19 (2.91)*+ |
| Word Fluency S-P | 32.67 (11.72) | 26.93 (9.11)* | 17.05 (6.52)*+ |
| SRT Free | 29.62 (6.59) | 22.06 (8.06)* | 11.50 (10.00)*+ |
| Trailmaking A | 37.40 (13.96) | 44.39 (24.19)* | 80.48 (50.18)*+ |
| Trailmaking B | 92.53 (35.44) | 113.36 (45.35)* | 160.29 (31.46)*+ |
| Boston Naming | 53.91 (6.75) | 53.14 (5.66) | 41.33 (12.33)*+ |
| Animal Fluency | 19.70 (5.58) | 17.05 (5.28)* | 11.48 (4.08)*+ |
| WAIS Digit Symbol | 47.94 (10.72) | 37.84 (11.70)* | 19.76 (14.56)*+ |
Note.
p < .05 indicates a significant difference when compared against healthy OA.
p < .05 indicates a significant difference when compared against very mildly demented (CDR .5) individuals. Tests of memory included the forward and backward digit span, the logical and associate memory components from the Wechsler Memory Scale (WMS; Wechsler & Stone, 1973), as well as free recall from the selective reminding test (SRT Free; Grober, Buschke, Crystal, Bang & Dresner, 1988). Psychomotor processing speed was assessed with the Digit Symbol subtest of the Wechsler Adult Intelligences Scale (WAIS) (Wechsler, 1955). Visual perceptual-motor performance was examined with parts A and B of the Trail Making test (Armitage, 1945). The Word Fluency Test S-P (Thurstone & Thurstone, 1949), the Animal Fluency Test (Goodglass & Kaplan, 1983b) and The Boston Naming Test (Goodglass & Kaplan, 1983a) evaluated semantic/lexical retrieval. Statistical analysis of differences in education and psychometric performance controlled for age. The number of male participants and the number of right-hand dominant individuals are reported for gender and handedness.
Participants completed simple synchronization-continuation tapping. Some were excluded from further analysis due to: 1) difficulty understanding instructions, 2) finger pain, wrist pain or numbness, or 3) changing the response finger during tapping. This eliminated 2 YA, 14 healthy OA, 12 CDR .5, and 3 CDR 1 individuals, with the primary causes due to pain or motor issues; difficulty with instructions eliminated only 1 YA, 3 OA, 2 CDR .5, and 1 CDR 1 adult(s). Of the remaining participants, the OA and CDR .5 groups did not differ in age, p = .380. However, age differences emerged between the CDR .5 and CDR 1 groups, t(65) = −2.14, p = .036, and between the OA and CDR 1 groups, t(138) = −3.00, p = .003, hence age was controlled in the analyses comparing these groups.
Apparatus
An IBM-compatible computer running E-prime software (Schneider, Eschman, & Zuccolotto, 2002) was used to control stimulus presentation and collect data. Stimuli were displayed on a 15 inch monitor.
Psychometric Testing
All OA were administered a separate 2-hour standard neuropsychological battery with an experimenter blind to their CDR score. The psychometric tests included in this battery are reported in Table 1.
Continuous Tapping Task
Participants repetitively tapped at three rates (500, 1000, and 1500 ms). At the start of a trial they heard a 50 ms 1000 Hz repeating tone and were asked to synchronize their taps with these tones until they disappeared (after 12 ITIs). Participants continued tapping at the same rate until STOP appeared on the computer screen. Taps were made using the index finger of the dominant hand on the space bar of the keyboard.2 Participants completed 3 short practice trials at a rate of 1250 ms. The total number of unpaced ITIs differed for each target rate (500 ms: 296 ITIs, 1000 ms: 142 ITIs, 1500 ms: 90 ITIs) to roughly equate time on task (~ 3 minutes). No explicit performance feedback was given.
An exit questionnaire assessed whether participants had problems completing the task. If they did, as noted earlier, they were eliminated from further analyses. Slightly less than half of all the participants (128 individuals) received the same presentation order of tapping rates (1000 ms followed by 500 ms then 1500 ms) in an effort to examine individual differences. However, we also report data from a counterbalanced set of participants. Thus, in all analyses, we control for tap rate order.3
Data Analysis
Psychometrics
Table 1 provides information about the gender, dominant handedness, and psychometric performance of our OA groups. We performed univariate comparisons, controlling for age, to evaluate group differences, which are reported in the table.
Continuous Tapping
Several performance measures of unpaced tapping were assessed across the complete trial (time on task was equated) and across the first 90 ITIs for each tap rate (hence, equating the number of tap events). The former set of data may be more sensitive to breakdowns in vigilance linked to time on task. Accuracy measures included 1) the accuracy index (AI, Baudouin, Vanneste, Pouthas & Isingrini, 2006), a relative timing measure calculated by dividing the mean tapping rate by the target rate (values less than 1 indicate faster tapping than the target rate and values greater than 1 indicate slower tapping), and 2) absolute error (AE), calculated by averaging the absolute difference between each produced and target ITI. The AI is useful for comparing accuracy across rates, and has been proposed to index the integrity of the decision rule used to compare different temporal representations (Gallistel & Gibbon, 2000; Gibbon & Fairhurst, 1994; Malapani & Fairhurst, 2002). We also included a measure of drift, or people’s ability to maintain the representation of the target interval over time; larger negative slopes indicated a faster loss of the representation. This performance change across the tapping series was calculated by estimating the slope of a linear regression across each individual’s trimmed tapping intervals for a particular rate and dividing it by the target rate. Resultant values were multiplied by 100 for ease of interpretation. Variability measures included 1) the standard deviation (SD) and 2) coefficient of variation (COV), which involves dividing the SD by mean tapping rate. The COV is often considered a measure of timing sensitivity and allows for comparisons of variability to be made across tap rates.4
For all dependent measures, we conducted mixed-factor ANOVAs, controlling for tap rate order, with group as the between-participants and tap rate as the within-participants factor. A set of orthogonal planned contrasts evaluating expected group differences and interactions, using one-tailed tests were performed. These explored simple effects comparing pairs of groups. The first (AGE) contrast (1, −1, 0, 0) compared the YA and healthy OA groups, with the expectation that YA would show better timing performance. The second (DAT) contrast (0 1 −1 0) was a focused analyses of the OA and very mild CDR .5 groups to determine if very mild DAT led to reliably worse performance. When significant effects involving this second contrast emerged we conducted Bonferroni-corrected post-hoc univariate tests (critical p = .017) comparing these two groups at each tap rate to explore the specific conditions impacted by the earliest detectable stages of Alzheimer’s disease. The final (SEVERITY) contrast (0 0 1 −1) compared the very mild and mild DAT groups to determine how disease severity impacts performance. Of critical interest were the first two contrasts for revealing normal age-related and DAT-specific declines in simple repetitive tapping.
Where sphericity was violated in omnibus tests we applied the Huynh-Feldt correction. Effect sizes are reported as partial eta-squared.5 Outliers were identified within each duration on each timing measure by standardizing the values within each group and searching for scores beyond ±3 standard deviations from the mean. Analyses of each performance measure excluded the relevant outliers.6, 7, 8
Results
Timing Measures: Omnibus Comparisons
Results from the omnibus analyses comparing all participant groups across the entire tapping trial are reported in Table 2. Since the analyses across the first 90 ITIs showed the same pattern of significance they are not included in Table 2. Likewise, for all analyses below, results across the first 90 ITIs are only reported when they differ from those across the full trial. For all timing measures, we found significant main effects of tap rate and group (all p < .01). All interactions were also significant (all p < .01), except for the standard deviation measure (p = .303). Figures 2 – 6 show the timing performance means for each group across the first 90 ITIs (Panel A) and the entire tapping trial (Panel B). Significant differences between the healthy OA and CDR .5 groups are indicated by asterisks.
Table 2.
Continuous Tapping - ANOVA table of omnibus results for each dependent measure (columns) across the full trial
| AI
|
AE
|
Slope
|
SD
|
CV | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | MS | np2 | df | F | MS | np2 | df | F | MS | np2 | df | F | MS | np2 | df | F | MS | np2 | |
| Source – BS | ||||||||||||||||||||
| G | 3 | 9.98*** | .325 | .11 | 3 | 25.59*** | 509535.92 | .25 | 3 | 9.42*** | .128 | .11 | 3 | 7.27*** | 8983.19 | .09 | 3 | 17.76*** | .033 | .19 |
| Error (G) | 235 | .033 | 234 | 19911.82 | 232 | .014 | 230 | 1236.49 | 231 | .002 | ||||||||||
| Source – WS | ||||||||||||||||||||
| D | 2 | 51.84*** | .681 | .18 | 2 | 150.77*** | 1912209.61 | .39 | 2 | 24.36*** | .222 | .10 | 2 | 178.90*** | 123699.93 | .44 | 2 | 23.99*** | .014 | .09 |
| Error (D) | 455 | .013 | 373 | 12683.19 | 355 | .009 | 360 | 691.44 | 456 | .001 | ||||||||||
| D × G | 6 | 13.48*** | .177 | .15 | 5 | 17.06*** | 216429.05 | .18 | 5 | 4.71** | .043 | .06 | 5 | 1.21 | 839.00 | .02 | 6 | 5.09*** | .017 | .06 |
| Error (D × G) | 455 | .013 | 373 | 12683.19 | 355 | .009 | 360 | 691.44 | 456 | .001 | ||||||||||
Note. MS is the mean squared for the indicated effect or error. Degrees of freedom (df) are reported using the Huynh-Feldt correction for violations of sphericity and rounded to the nearest whole number.
p< .001,
p < .01,
p < .05, one-tailed.
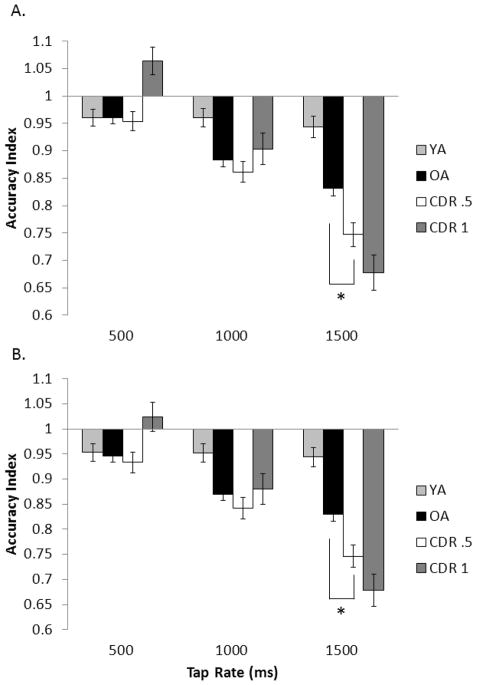
Figure 2.
Mean tapping accuracy index for all four participant groups across the unpaced intervals A) for the first 90 ITIs and B) for the full trial for each tapping rate. Error bars are ± 1 standard error. The asterisk (*) indicates that in the comparison between just the healthy older adult group and the CDR .5 group, the groups differed at the indicated tap rate.
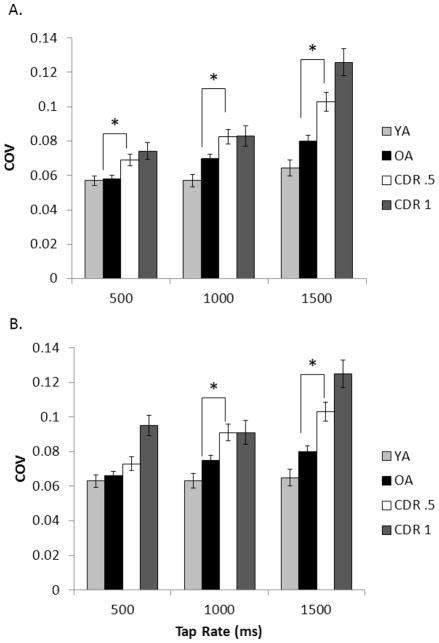
Figure 6.
COV from the timing task for all four participant groups across the unpaced intervals A) for the first 90 ITIs and B) for the full trial at each tapping rate. Error bars are ± 1 standard error. The asterisk (*) indicates that in the comparison between just the healthy older adult group and the CDR .5 group, the groups differed at the indicated tap rate.
Timing Measures: Planned Contrasts
Accuracy Index
Figure 2 displays the AI. AI was fairly stable across all tapping rates for YA, while aging was linked to an increased rate of tapping as target rate decreased. These observations were supported by a significant AGE contrast effect, F(1, 235) = 15.35, p < .001, ηp2 = .061, and an AGE × tap rate interaction, F(2, 234) = 7.05, p < .001, ηp2 = .057. Importantly, the DAT contrast analysis revealed a significant DAT × tap rate interaction, F(2, 234) = 3.09, p = .024, ηp2 = .026, and a DAT contrast effect, F(1, 235) = 5.07, p =.013, ηp2 = .021, indicating that DAT led to faster tapping above and beyond that seen with normal aging. Post hoc tests confirmed that the only reliable group difference occurred at the 1500 ms rate (p = .002). For the SEVERITY contrast, we found a significant contrast × tap rate interaction, F(2, 234) = 5.77, p =.002, ηp2 = .047, likely driven by opposite patterns in tapping performance at 500 ms. However, the overall contrast effect was not significant (p =.236).
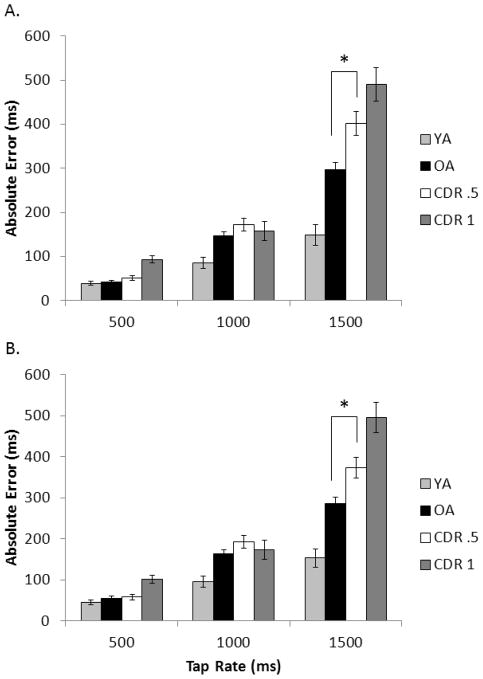
Absolute error
As demonstrated in Figure 3, OA typically showed greater AE than YA, especially at slower tapping rates. This was supported by a significant AGE contrast × tap rate interaction, F(2, 233) = 12.77, p < .001, ηp2 = .099, and a significant AGE effect, F(1, 234) = 26.85, p < .001, ηp2 = .103. Importantly, the DAT contrast analysis revealed a greater increase in AE as tap rate slowed for the CDR .5 group compared to healthy OA, supported by a DAT × tap rate interaction, F(2, 233) = 4.69 p = .005, ηp2 = .039. The DAT contrast effect also reached significance, F(1, 234) = 7.53, p = .004, ηp2 = .031, with a reliable effect only at the 1500 ms rate (p = .005). The SEVERITY contrast × tap rate interaction reached significance, F(2, 233) = 6.31, p = .001, ηp2 = .051 as did the SEVERITY contrast, F(1, 234) = 4.79, p = .015, ηp2 = .020, indicating an increase in AE with increased DAT severity.
Figure 3.
Mean absolute error for all four participant groups across the unpaced intervals A) for the first 90 ITIs and B) for the full trial for each tapping rate. Error bars are ± 1 standard error. The asterisk (*) indicates that in the comparison between just the healthy older adult group and the CDR .5 group, the groups differed at the indicated tap rate
Slope
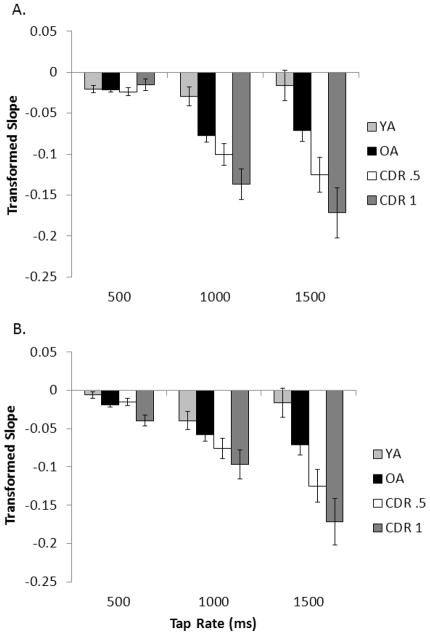
Figure 4 illustrates that while YA showed little performance change across trials, OA showed increasingly negative slopes as tap rate slowed, supported by a significant AGE contrast × tap rate interaction across the first 90 ITIs, F(2, 231) = 3.08, p = .024, ηp2 = .026, but not across the full trial (p = .090). The AGE contrast effect did emerge, F(1, 232) = 6.42, p = .006, ηp2 = .027. The DAT contrast was significant, F(1, 232) = 3.56, p = .031, ηp2 = .015, due to the CDR .5 group being more prone to drift than the healthy OA group. The DAT × tap rate interaction reached significance when evaluated across the full trial (with reliance on vigilance at a premium), F(2, 231) = 3.07, p = .024, ηp2 = .026, but not across the first 90 ITIs, (p = .166). There were no reliable group differences at any tap rate. There was no interaction of the SEVERITY contrast × tap rate interaction (p = .388) but the contrast effect did emerge across the full trial F(1, 232) = 2.81, p = .048, ηp2 = .012, but not the first 90 ITIs (p = .153). Namely, mild DAT tended to show greater drift than very mild DAT individuals.
Figure 4.
Transformed slope across the unpaced timing intervals A) for the first 90 ITIs and B) for the full trial for each tapping rate in all four participant groups. Error bars are ± 1 standard error. The asterisk (*) indicates that in the comparison between just the healthy older adult group and the CDR .5 group, the groups differed at the indicated tap rate.
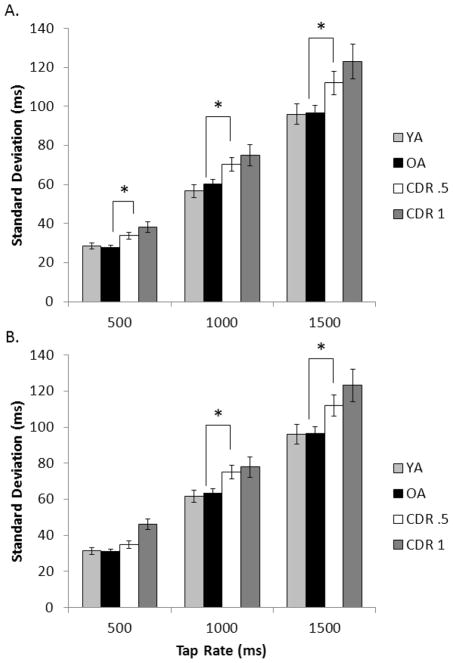
Standard deviation
Figure 5 shows the SD results. We found neither an AGE contrast (p = .425), nor an AGE × tap rate interaction (p = .442); normal aging was not associated with increased SD. There was a DAT contrast × tap rate interaction across the full trials, F(2, 229) = 2.59 p = .039, ηp2 = .022, but not the first 90 ITIs (p = .170). The DAT contrast was significant, F(1, 230) = 8.18, p = .003, ηp2 = .034. The CDR .5 group produced larger SD than the OA group at the 1000 ms (p = .005) and 1500 ms rates (p = .015) and at the 500 ms tap rate when examined across the first 90 ITIs (p = .003). Neither the SEVERITY × tap rate interaction (p = .178) nor the SEVERITY contrast (p = .070) reached significance, indicating fairly stable variability when moving from very mild to mild DAT.
Figure 5.
Standard deviation from the timing task for all four participant groups across the unpaced intervals A) for the first 90 ITIs and B) for the full trial at each tapping rate. Error bars are ± 1 standard error. The asterisk (*) indicates that in the comparison between just the healthy older adult group and the CDR .5 group, the groups differed at the indicated tap rate.
Coefficient of variation
YA showed similar COVs while OA showed increasing COVs as tap rate decreased, as revealed in Figure 6. This was supported by a significant AGE contrast × tap rate interaction, F(2, 230) = 2.95, p = .028, ηp2 = .025, as well as a significant AGE contrast effect, F(1, 231) = 5.90, p = .008, ηp2 = .025. The DAT contrast revealed a significant effect, F(1, 231) = 11.79, p = .001, ηp2 = .049, because the CDR .5 group consistently showed higher COVs than healthy OA. While COV increased for both groups at slower tap rates, the magnitude of this change was larger for the CDR .5 group across the full trial, F(2, 230) = 3.42, p = .018, ηp2 = .029, but not across the first 90 ITIs (p = .079). Post-hoc tests confirmed reliable differences at 1000 ms, (p = .006), and 1500 ms, (p < .001), and at 500 ms determined across the first 90 ITIs (p = .008). There was a SEVERITY contrast × tap rate interaction, F(2, 230) = 4.13, p = .009, ηp2 = .035, and a significant SEVERITY contrast across the full trial, F(1, 231) = 4.59, p = .017, ηp2 = .019, that was simply a trend across the first 90 ITIs (p = .060). Therefore, COV appears sensitive to increasing DAT severity.
Discussion
The purpose of the current study was to examine whether aging and the earliest detectable stages of Alzheimer’s disease lead to declines on a simple repetitive tapping task, particularly at slower rates where extant literature suggests increasing reliance on attention. We also lengthened time on task to increase likelihood of performance drift and tap into vigilance processes. Our major focus, however, was to determine what conditions lead to DAT-specific declines above and beyond those associated with normal aging.
Age-related Differences in Timing
As expected, YA were more accurate and resistant to drift across all tap rates than OA. All groups were fairly accurate at the fastest rate even when evaluated across the full trial (the condition which required production of the most ITIs). Thus, peripheral (motor) systems do not appear to be major contributors to accuracy declines. In general, OA showed an expected increase in tapping speed, especially at the 1500 ms rate. Surprisingly, aging did not lead to increases in variability as measured by SD. However, increases in COV did emerge. These patterns suggest that while healthy OAs’ overall level of variability was equivalent to YA, it increased disproportionately with their observed decreasing tap rate. Thus, aging is associated with reduced timing sensitivity at slower tap rates.
While OA’s faster tapping could signify an increase in speed of an internal pacemaker when there is no external pacing signal, this seems unlikely, given that OA typically show a slower preferred tapping tempo compared to YA (McAuley et al., 2006; Vanneste, Pouthas & Wearden, 2001). Instead, as McAuley and colleagues (2006) argue, aging may be associated with narrowing of the interval range to which individuals can successfully entrain their motor responses. When repetitively reproducing an interval outside this preferred range, OA experience difficulty sustaining the rate and, therefore, adjust it toward their preferred tempo, thought to harbor around 650 to 750 ms (McCauley et al., 2006; Vanneste, Pouthas & Wearden, 2001; though see Baudouin, Vanneste, & Isingrini, 2004). Another possibility is that poorer online error correction processes contribute to difficulties sustaining an accurate representation of the target rate, thus increasing drift (Krampe et al., 2010) and leading to tempo adjustments. Indeed, we found that OA had generally larger negative slopes than YA across a tapping trial.
Interestingly, we found similar age-related changes in timing performance for the full trial and the first 90 ITIs. Therefore, we have expanded upon work by Duchek and colleagues (1994) to show that simply lengthening the target tap rate to tax controlled attention is sufficient to elicit normal age-related changes in timing performance, while extending the tapping bout may be less critical. This is consistent with work showing that vigilance appears stable with normal aging (Tucker et al., 2011). However, the role of bout length in eliciting age-related changes merits additional study, because while Krampe et al. (2010) did not find these changes during tapping at a supra-second rate under single task conditions, we did. In their study a single bout at the slowest rate lasted less than a minute while ours required tapping for approximately 3 minutes.
Differences Between Healthy Aging and CDR .5
We focus now on the behavioral timing differences specifically linked to the earliest stages of DAT. In general the CDR .5 group showed faster rates of tapping, increased variability, and larger negative slopes than healthy OA, indicating they had more difficulty maintaining an accurate representation of the target interval across the tapping trial, even when evaluated only across 90 ITIs. These patterns were similar and, in some cases, more pronounced at increased disease severity as shown by the CDR 1 group. Measures of accuracy for the CDR .5 group only reliably differed from healthy OA at the 1500 ms tap rate, suggesting that this condition was most sensitive to DAT-specific accuracy declines. Differences in SD and COV, however, emerged across even faster tapping rates, supporting the idea that increases in intra-individual variability, even in timing, may be a particularly sensitive marker of DAT (Duchek et al., 2009; Hultsch et al., 2000). The emergence of significant variability differences between the OA and very mild DAT group at all tap rates across just the first 90 ITIs suggests that there may be an ideal task length over which healthy OA maintain stability in performance, but individuals with the earliest signs of Alzheimer’s disease (CDR.5 individuals) do not. Note that even 90 ITIs produced at the fastest rate in our study constitute a bout nearly 3 times longer than that typically used for sub-second tap rates in this paradigm (Duchek et al., 1994; Ivry & Hazeltine, 1995).
Much extant work on timing in DAT has investigated temporal discrimination rather than response timing. The most consistent result from this earlier work points to increased performance variability at early stages of DAT, similar to the current findings (Caselli, Iaboli & Nichelli, 2009; Nichelli et al., 1993; Papagno, Allegra, & Cardaci, 2004; Rueda & Schmitter-Edgcombe, 2009). We have now demonstrated that DAT-related breakdowns emerge even for a simple repetitive tapping task when target tap rates are lengthened. In general, it appears that DAT-related changes in the current paradigm are similar to those found under conditions of divided attention with longer intervals (Krampe et al., 2010). McAuley and colleagues (2006) argue that task event structure strongly influences the degree to which attentional resources are coordinated to support performance. With continuous tapping, rate can influence one’s ability to focus attentional resources at the right moments in time to facilitate accurate performance. This stems from the dynamic attending approach which argues that attention involves both internal temporal expectancies and rhythms created by external events (e.g. a pacing signal) (Large & Jones, 1999). The internal rhythm is consistent with McAuley’s view that ideal tempo drives predictions about where and when to focus attention. Successful “attending” to external events occurs when synchrony is achieved via entrainment of internal to external rhythms. In the current task, achieving synchrony after disappearance of the pacing signal requires that individuals generate expectancies about when they should press the button for each subsequent reproduction, engaging attentional selection, inhibition of competing internal expectancies, and error correction processes. Since individuals at the early stages of DAT experience breakdowns in attentional control systems that support these processes as well as the maintenance of current task goals (Balota & Faust, 2001), they experience more difficulty resolving these competing influences. This leads to poorer error-correction processes, regressions towards their ideal tempo, and increased drift and variability, especially at the slower target rates where there are larger deviations between the target ITI and internal expectancies.
We note that the age- and DAT-related changes in our study appear at first glance to be inconsistent with the predictions of the pacemaker-accumulator account of timing involving an attentional gate (Rakitin, 2005; Zakay & Block, 1997). Namely, full attention to timing during paced ITIs followed by OAs’ difficulty attending to time during the continuation phase should have led them to tap more slowly and show increased variability that was most marked at the fastest tap rate. However, results consistent with this model are typically obtained with the use of discrete, as opposed to repetitive timing tasks. As mentioned earlier, studies using a repetitive tapping paradigm obtained results similar to those found in the present study, even for YA under divided attention (Krampe et al., 2010; McAuley et al., 2006). This implies that continuous repetitive tapping engages additional online processes that are not employed during discrete timing tasks.
Conclusion
In sum, both DAT and healthy aging are linked to poorer accuracy and sensitivity at the slowest tap rate. Poorer variability in early stage DAT also consistently emerged for the 1000 and 1500 ms rates, and in some cases, for the 500 ms rate. Thus, mechanisms that contribute to timing variability may suffer greater impacts at early stages of DAT than mechanisms associated with overall accuracy. Explorations of these mechanisms and identification of tasks which are sensitive to these increases in intra-individual variability may be advantageous for developing useful tools for discriminating healthy aging from DAT. As shown here, a timing paradigm involving the continuous marking of events in the supra-seconds range over an extended period could prove helpful. Benefits of the current paradigm include its simplicity and the fact that the slowest tapping condition takes less than 5 minutes. Further work examining performance at longer intervals with even higher disagreement between internal and external temporal expectancies may be useful for identifying the conditions most sensitive to these early declines.
Acknowledgments
This work was supported by grants NIA T32 AG000030-32, NIA PO1 AGO3991, and NIA PO1 AGO26276. We would like to thank Christopher Grant, Amy Heidebreder, Rebecca Howard, and Jeanne Mishkin for their help with data collection, Jeremy Missuk for his invaluable assistance with coding and analyzing data, John Morris and the Clinical Core for their careful description of the participants, Martha Storandt for the psychometric data, and Jan Duchek for her help in coordination of the data analyses.
Footnotes
The criteria to determine inclusion as DAT are consistent with those of the National Institute of Neurological and Communication Disorders and Stroke – Alzheimer’s disease and Related Disorders Association which indicate probable AD (Mckhann et al., 1984). Recruitment, screening and evaluations methods used at the ADRC enable a diagnosis of DAT in individuals characterized as MCI in other studies (see Berg et al., 1998; Morris et al., 2001 for details and see http://alzheimer.wustl.edu/cdr/PDFs/CDR_OverviewTranscript-Revised.pdf for a summary of CDR diagnostic procedures). CDR scale diagnoses have high reliability (Burke et al., 1988) and validity (based on subsequent autopsy findings), with 93% accuracy (Berg et al., 1998; Storandt et al., 2006).
The Psychology Software Tools (PST) response box was not used because of greater force required to depress the buttons than on a computer keyboard. We were concerned that older adults would get unduly fatigued using the PST box for an extended period of time.
The percentage of participants given the default tap rate order is shown below, as are the percentages given counterbalanced orders which included the 1500 ms condition in the first, middle, and last positions.
| OA | CDR .5 | CDR1 | YA | |
|---|---|---|---|---|
|
|
||||
| Default order (1000, 500, 1500) | 38% | 33% | 77% | 80% |
| 1500 First | 25% | 28% | 14% | 8% |
| 1500 Middle | 25% | 26% | 5% | 9% |
| 1500 Last (including default order) | 50% | 46% | 82% | 83% |
A series of mixed-factor ANOVAs were run within the healthy OA and CDR 5 groups to assess whether an order effect related to the time of presentation of the 1500 ms condition influenced tapping performance. The position of the 1500 ms tap rate (first, middle, or last) served as the between subjects variable. For the healthy OA group, there was a significant main effect of order on SD, F(2, 110) = 3.34, MSe = 1112.73, p = .039, ηp2 = .06, with individuals who had orders placing the 1500 ms tap rate last showing more variable performance at nearly all tap rate conditions. There were no other main effects of order or interactions between order and tap rate for OA, (all p’s > .08, ηp2 < .05). No main effects of order or interactions between order and tap rate reached significance for the CDR .5 group (all p’s > .32, ηp2 < .06). Thus, it does not appear that order of presentation of the 1500 ms condition led to specific fatigue effects for the CDR .5 group.
Though we applied the Wing-Kristofferson model to the de-trended ITI data, we do not report these results, because we found broad violations of the model for all age groups across all of the target tapping intervals. Namely, negative estimates of motor variability were found across the board. It is possible that this model may not apply to versions of synchronization-continuation tapping which are extended in time.
The partial eta-squared measure indicates the degree of variance accounted for by an independent variable excluding variance contributed by other sources. It is useful for comparing an effect in the current study with one in a different study involving additional independent variables (Pierce, Block & Aguinis, 2004).
When outliers were included in the AGE contrast, the AGE contrast became non-significant for COV across both the full trial (p = .141) and the first 90 ITIs. For the slope across the full trial the interaction of AGE × tap rate became significant (p = .045).
When outliers were included in DAT contrast, the DAT × tap rate interaction across the full trial became non-significant for AI (p = .073), Slope (p = .075), SD (p = .292) and COV (p = .121).
When outliers were included in SEVERITY contrast, the SEVERITY effect became significant across the first 90 ITIs for AI (p = .041), SD (p = .004), and COV (p = .047), but non-significant for slope (p = .298). The SEVERITY × tap rate interaction for the first 90 ITIs became significant for the slope, (p = .049) but non-significant for AE (p = .127).
The authors report no conflicts of interest.
References
- Albert M, Moss MB, Blacker D, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Albert M, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7:631–639. doi: 10.1017/S1355617701755105. [DOI] [PubMed] [Google Scholar]
- Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychological Monographs. 1945;60(1 Whole No 177):1–48. [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, III, McDermott KB, Yerys BE. Veridical and false memories in healthy older adults and in dementia of the Alzheimer’s type. Cognitive Neuropsychology. 1999;16:361–384. doi: 10.1080/026432999380834. [DOI] [Google Scholar]
- Balota DA, Faust ME. Attention in dementia of the Alzheimer’s type. In: Bolla F, Cappa S, editors. Handbook of neuropsychology: Vol. 6. Aging and dementia. 2. New York: Elsevier Science; 2001. pp. 51–80. [Google Scholar]
- Baudouin A, Vanneste S, Isingrini M. Age-Related Cognitive Slowing: The Role of Spontaneous Tempo and Processing Speed. Experimental Aging Research. 2004;30(3):225–239. doi: 10.1080/03610730490447831. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Vanneste S, Pouthas V, Isingrini M. Age-related changes in duration reproduction: Involvement of working memory processes. Brain and Cognition. 2006;62(1):17–23. doi: 10.1016/j.bandc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Berardi A, Parasuraman R, Haxby JV. Sustained Attention in Mild Alzheimer’s Disease. Developmental Neuropsychology. 2005;28(1):507–537. doi: 10.1207/s15326942dn2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Miller JP, Storandt M, Rubin EH, Morris JC, Saunders AM. Clinicopathologic Studies in Cognitively Healthy Aging and Alzheimer Disease: Relation of Histologic Markers to Dementia Severity, Age, Sex, and Apolipoprotein E Genotype. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Block RA, Zakay D, Hancock PA. Human aging and duration judgments: A meta-analytic review. Psychology and Aging. 1999;13(4):584–596. doi: 10.1037/0882-7974.13.4.584. [DOI] [PubMed] [Google Scholar]
- Brown SW. Attentional resources in timing: Interference effects in concurrent temporal and nontemporal working memory tasks. Perception & Psychophysics. 1997;59(7):1118–1140. doi: 10.3758/bf03205526. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek JM, Berg L. The reliability of the Washington University Clinical Dementia Rating. Archives of Neurology. 1988;45:31–32. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- Caselli L, Iaboli L, Nichelli P. Time estimation in mild Alzheimer’s disease patients. Behavioral and Brain Functions. 2009;5 doi: 10.1186/1744-9081-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Balota DA, McCabe DP. Memory efficiency and the strategic control of attention at encoding: Impairments of value-directed remembering in Alzheimer’s disease. Neuropsychology. 2009;23(3):297–306. doi: 10.1037/a0014888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier GL, Ogden RT. Adding drift to the decomposition of simple isochronous tapping: An extension of the Wing-Kristofferson model. Journal of Experimental Psychology: Human Perception and Performance. 2004;30(5):853–872. doi: 10.1037/0096-1523.30.5.853. [DOI] [PubMed] [Google Scholar]
- Craik FI, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning & Verbal Behavior. 1972;11(6):671–684. [Google Scholar]
- Duchek JM, Balota DA, Ferraro FR. Component analysis of a rhythmic finger tapping task in individuals with senile dementia of the Alzheimer type and in individuals with Parkinson’s disease. Neuropsychology. 1994;8:218–226. doi: 10.1037/0894-4105.8.2.218. [DOI] [Google Scholar]
- Duchek JM, Balota DA, Tse C, Holtzman DM, Goate AM. The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer’s disease. Neuropsychology. 2009;23(6):746–758. doi: 10.1037/a0016583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust ME, Balota DA. Inhibition, facilitation, and attentional control in dementia of the Alzheimer’s type: The role of unifying principles in cognitive theory development. In: Gorfein DS, MacLeod CM, editors. Inhibition in cognition. Washington, DC: American Psychological Association; 2007. pp. 213–238. [Google Scholar]
- Fortin C, Rousseau R, Bourque PE, Kirouac E. Time estimation and concurrent nontemporal processing: Specific interference from short-term-memory demands. Perception & Psychophysics. 1993;53(5):536–548. doi: 10.3758/bf03205202. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Gibbon J. Time, rate, and conditioning. Psychological Review. 2000;107 (2):289–344. doi: 10.1037/0033-295X.107.2.289. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Fairhurst S. Ratio versus difference comparators in choice. Journal of the Experimental Analysis of Behavior. 1994;62(3):409–434. doi: 10.1901/jeab.1994.62–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 2. Philadelphia: Lea & Febiger; 1983a. [Google Scholar]
- Goodglass H, Kaplan E. J Animal Naming (Fluency in Controlled Association) Philadelphia: Lea & Febiger; 1983b. Boston Diagnostic Aphasia Examination Booklet, III, ORAL EXPRESSION. [Google Scholar]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;3:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Hermanowitz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12(1):3–12. doi: 10.1037/0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS, Hunter MA, Levy-Bencheton J, Strauss E. Intraindividual variability in cognitive performance in older adults: comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology. 2000;14(4):588–598. doi: 10.1037/0894-4105.14.4.588. [DOI] [PubMed] [Google Scholar]
- Hutchison KA, Balota DA, Duchek JM. The utility of Stroop task switching as a marker for early-stage Alzheimer’s disease. Psychology and Aging. 2010;25(3):545–559. doi: 10.1037/a0018498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Hazeltine RE. Perception and production of temporal intervals across a range of durations: Evidence for a common timing mechanism. Journal of Experimental Psychology: Human Perception and Performance. 1995;21(1):3–18. doi: 10.1037/0096-1523.21.1.3. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. Deceiving the elderly: Effects of accessibility bias in cued-recall performance. Cognitive Neuropsychology. 1999;16:417–436. doi: 10.1080/026432999380861. [DOI] [Google Scholar]
- Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philosophical Transactions from the Royal Society B. 2009;364:1907–1918. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampe RT, Doumas M, Lavrysen A, Rapp M. The costs of taking it slowly: Fast and slow movement timing in older age. Psychology and Aging. 2010;25(4):980–990. doi: 10.1037/a0020090. [DOI] [PubMed] [Google Scholar]
- Large EW, Jones MR. The dynamics of attending: How people track time-varying events. Psychological Review. 1999;106 (1):119–159. doi: 10.1037/0033-295X.106.1.119. [DOI] [Google Scholar]
- Lejeune H. Switching or gating? The attentional challenge in cognitive models of psychological time. Behavioural Processes. 1998;44(2):127–145. doi: 10.1016/S0376-6357(98)00045-X. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: Evidence from neuroimaging. Current Opinion in Neurobiology. 2003;13(2):250–255. doi: 10.1016/S0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Remembering the time: A continuous clock. Trends in Cognitive Sciences. 2006;10(9):401–406. doi: 10.1016/j.tics.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Macar F, Grondin S, Casini L. Controlled attention sharing influences time estimation. Memory & Cognition. 1994;22(6):673–686. doi: 10.3758/bf03209252. [DOI] [PubMed] [Google Scholar]
- Madison G. Variability in isochronous tapping: Higher order dependencies as a function of intertap interval. Journal of Experimental Psychology: Human Perception and Performance. 2001;27(2):411–422. doi: 10.1037/0096-1523.27.2.411. [DOI] [PubMed] [Google Scholar]
- Malapani C, Fairhurst S. Scalar timing in animals and humans. Learning and Motivation. 2002;33:156–176. doi: 10.1006/lmot.2001.1105. [DOI] [Google Scholar]
- McAuley JD, Jones MR, Holub S, Johnston HM, Miller NS. The time of our lives: Life span development of timing and event tracking. Journal of Experimental Psychology: General. 2006;135(3):348–367. doi: 10.1037/0096-3445.135.3.348. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Michon JA. The compleat time experiencer. In: Jackson JAMJL, editor. Time, mind, and behavior. Berlin: Springer Verlag; 1985. pp. 20–54. [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Fulling K. Early Alzheimer’s disease: Diagnostic considerations. Archives of Neurology. 1988;45(3):345–349. doi: 10.1001/archneur.1988.00520270127033. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel DW, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46(3):707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Archives of Neurology. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Vernneri A, Molinari M, Tavani F, Grafmna J. Precision and accuracy of subjective time estimation in different memory disorders. Cognitive Brain Research. 1993;1(2):87–93. doi: 10.1016/0926-6410(93)90014-V. [DOI] [PubMed] [Google Scholar]
- Papagno C, Allegra A, Cardaci M. Time estimation in Alzheimer’s disease and the role of the central executive. Brain and Cognition. 2004;54(1):18–23. doi: 10.1016/S0278-2626(03)00237-9. [DOI] [PubMed] [Google Scholar]
- Perbal S, Droit-Volet S, Isingrini M, Pouthas V. Relationships between age-related changes in time estimation and age-related changes in processing speed, attention and memory. Neuropsychology, and Cognition. 2002;9(3):201–216. doi: 10.1076/anec.9.3.201.9609. [DOI] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain: A Journal of Neurology. 1999;122(3):383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Pierce CA, Block RA, Aguinis H. Cautionary note on reporting eta-squared values from multifactor ANOVA designs. Educational and Psychological Measurement. 2004;64 (6):916–924. doi: 10.1177/0013164404264848. [DOI] [Google Scholar]
- Pöppel E. Lost in time: A historical frame, elementary processing units and the 3-second window. Acta Neurobiologiae Experimentalis. 2004;64:295–301. doi: 10.55782/ane-2004-1514. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and ‘preclinical’ Alzheimer’s disease. Annals of Neurology. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::AID-ANA12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Rakitin BC. The effects of spatial stimulus-response compatibility on choice time production accuracy and variability. Journal of Experimental Psychology: Human Perception and Performance. 2005;31(4):685–702. doi: 10.1037/0096-1523.31.4.685. [DOI] [PubMed] [Google Scholar]
- Rousseau R, Picard D, Pitre E. An adaptive counter model for time estimation. Annals of the New York Academy of Sciences. 1984;423:639–642. doi: 10.1111/j.1749-6632.1984.tb23480.x. [DOI] [Google Scholar]
- Rowe KC, Paulsen JS, Langbehn DR, Duff K, Beglinger LJ, Wang C, Moser DJ. Self-paced timing detects and tracks change in prodromal Huntington disease. Neuropsychology. 2010;24(4):435–442. doi: 10.1037/a0018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda AD, Schmitter-Edgecombe M. Time estimation abilities in mild cognitive impairment and Alzheimer’s disease. Neuropsychology. 2009;23(2):178–188. doi: 10.1037/a0014289. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschmann A, Zuccolotto A. E-Prime user’s guide. Pittsburgh, PA: Psychology Software Tools, Inc; 2002. [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JA. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Storandt M, Hill RD. Very mild senile dementia of the Alzheimer type: II. Psychometric test performance. Archives of Neurology. 1989;46(4):383–386. doi: 10.1001/archneur.1989.00520400037017. [DOI] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone LG. Examiner manual for the SRA Primary Mental abilities Test. Chicago: Science Research Associates; 1949. [Google Scholar]
- Tse CS, Balota DA, Yap MJ, Duchek JM, McCabe DP. Effects of healthy aging and early stage dementia of the Alzheimer’s type on components of response time distributions in three attentional tasks. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AM, Stern Y, Basner RC, Rakitin BC. The prefrontal model revisited: Double dissociations between young sleep deprived and elderly subjects on cognitive components of performance. Sleep. 2011;34(8):1039–1050. doi: 10.5665/sleep.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Pouthas V, Wearden JH. Temporal control of rhythmic performance: A comparison between young and old adults. Experimental Aging Research. 2001;27(1):83–102. doi: 10.1080/036107301750046151. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale (Manual) San Antonio, TX: Psychological corp; 1955. [Google Scholar]
- Wechsler D, Stone CP. Manual: Wechsler Memory Scale. New York: Psychological Corporation; 1973. [Google Scholar]
- Wing AM, Kristofferson AB. The timing of interresponse intervals. Perception & Psychophysics. 1973;13(3):455–460. [Google Scholar]
- Woodruff-Pak DS, Jaeger ME. Predictors of eyeblink classical conditioning over the adult age span. Psychology and Aging. 1998;13(2):193–205. doi: 10.1037/0882-7974.13.2.193. [DOI] [PubMed] [Google Scholar]
- Zakay D, Block RA. Temporal cognition. Current Directions in Psychological Science. 1997;6(1):12–16. doi: 10.1111/1467-8721.ep11512604. [DOI] [Google Scholar]
- Zakay D. Attention allocation policy influences prospective timing. Psychonomic Bulletin & Review. 1998;5(1):114–118. [Google Scholar]