Abstract
Cardiomyocytes from common experimental animals rapidly exit the cell cycle upon isolation, impeding studies of basic cell biology and applications such as myocardial repair. Here we examined proliferation of cardiomyocytes derived from human and mouse embryonic stem (ES) cells. While mouse ES cell-derived cardiomyocytes showed little proliferation, human cardiomyocytes were highly proliferative under serum-free conditions (15–25% BrdU+/sarcomeric actin+). The cells exhibited only a small serum dose-response, and proliferation gradually slowed with increasing differentiation of the cells. Neither cell density nor different matrix attachment factors affected cardiomyocyte proliferation. Blockade of phosphatidylinositol 3-kinase (PI 3-kinase) and Akt significantly reduced cardiomyocyte proliferation, whereas MEK inhibition had no effect. Antibody blocking of the insulin-like growth factor-1 (IGF-1) receptor significantly inhibited cardiomyocyte proliferation, while addition of IGF-1 or IGF-2 stimulated cardiomyocyte proliferation in a dose-dependent manner. Thus, cardiomyocytes derived from human ES cells proliferate extensively in vitro, and their proliferation appears to be mediated primarily via the PI 3-kinase/Akt signaling pathway, using the IGF-1 receptor as one upstream activator. This system should permit identification of regulatory pathways for human cardiomyocyte proliferation and may facilitate expansion of cardiomyocytes from human ES cells for therapeutic purposes.
Keywords: Human embryonic stem cells, Cardiomyocyte proliferation, Insulin-like growth factor (IGF), Phosphatidylinositol 3-kinase (PI 3-kinase), Akt
1. Introduction
The characterization of mammalian cardiomyocyte proliferation has been based primarily upon developmental studies of rodent species. While cardiomyocytes exhibit significant proliferative potential during organogenesis, they permanently withdraw from the cell cycle soon after birth. This relatively brief period of cardiomyocyte proliferation (~2 weeks for rodent species) and the tendency for isolated fetal cardiomyocytes from most species to cease proliferating in vitro have made the mechanisms governing cardiomyocyte growth difficult to study. Most insights into potential mechanisms regulating cardiomyocyte proliferation have come from transgenic or knock-out mouse models that exhibit a cardiac hypoor hyperplasia phenotype. A comprehensive discussion of the specific outcomes of various mouse models has recently been reviewed [1]. Attempts to create permanent lines of proliferating cells that retain the proper cardiomyocyte phenotype have met with limited success [2–5]. Although various mitogens have been purported to stimulate proliferation of cardiomyocytes in different model systems (see [6] and [1] for thorough reviews), the limited availability of human cardiomyocytes has restricted study to relatively few investigations in vivo [7–9] or in vitro [10–13], and such studies were dependent on the availability of human fetal tissue sources.
Human embryonic stem (ES) cell lines have recently been established that are capable of differentiating into cell types from all three germ layers, thus facilitating the development of new cell-based therapies for human tissue repair [14]. Human ES cells spontaneously differentiate into cardiomyocytes with normal transcription factor and structural protein expression profiles, as well as calcium handling and electrophysiological properties [15,16]. Additional work has characterized the methods to efficiently cultivate human ES-derived cardiomyocytes, and protocols have been developed to enrich for fractions of cells containing cardiomyocytes [16]. An interesting preliminary observation of cardiomyocytes derived from human ES cells was the relatively high percentage of cells capable of proliferating in vitro, even after several weeks of differentiation. A subsequent study also reported a progressive decrease in the percentage of proliferating cardiomyocytes derived from human ES cells as they matured over time during differentiation [17].
Based on the preliminary reports regarding human ES cell-derived cardiomyocyte proliferation, we sought to examine in vitro conditions that might influence the proliferation of enriched cardiomyocyte cultures and investigate signaling pathways that might significantly affect cardiomyocyte proliferation. We report here that the high proliferative ability of early human cardiomyocytes appears to be independent of typical exogenous signals such as serum, attachment factors or plating density. This high level of human ES cell-derived cardiomyocyte proliferation appears to be driven largely by a cell-autonomous pathway involving phosphatidylinositol 3-kinase (PI 3-kinase) and Akt, and utilizing insulin-like growth factor (IGF) receptor as one of the upstream activators.
2. Materials and methods
2.1. ES cell culture and Percoll enrichment
Mouse and human ES cells were cultured and Percoll enrichment of differentiating human ES cells was performed similar to previously established techniques [16,18–20] (see Section 2 in the Online Data Supplement for details of culture and enrichment methods).
2.2. Proliferation assay
Human embryoid body (EB) outgrowths were dissociated after 10–47 days of differentiation and following Percoll separation, fraction IV cells (the densest of the cell fractions containing the highest percentage of cardiomyocytes) were typically re-plated at a density of ~3.5–5.0 × 104 cells per cm2 in two-well plastic chamber slides (Nunc) pre-adsorbed with gelatin. The cells were plated overnight in differentiation media containing 20% FBS to allow the cells to attach before the samples were switched the following day to the appropriate treatment conditions. The cardiomyocytes were cultured for 3 days after Percoll separation prior to the addition of BrdU (10 µmol/l), and then cultured an additional 24 hours (for a total of 4 days post-Percoll). The samples were fixed with ice-cold methanol for 10 min and immunostained (as described below). Each experimental condition was assayed in triplicate, and a minimum of 500 cardiomyocyte nuclei was counted per well. Only flattened, adherent cardiomyocytes (sarcomeric actin+) with clearly discernible nuclei were included in cell counts, and small round cells that sometimes attached on top of cardiomyocyte clusters were omitted to ensure confidence in the accuracy of the counts. The results are reported as the mean percentage of BrdU+ cardiomyocyte nuclei ± the standard deviation, and statistically significant differences were detected between the treatment groups by performing a Student’s t-test (P < 0.05). Results are reported in terms of cell nuclei, as opposed to cell number, due to the fact that the cardiomyocytes primarily attached and grew in colonies, making it difficult to clearly delineate the edges of individual cell boundaries by immunostaining. The majority of the experiments described herein were conducted with the H7 human ES cell line, although a limited number of comparable experiments were performed in parallel with the H1 line to corroborate significant experimental findings.
2.3. Attachment factors
For some experiments, two-well chamber slides were coated with fibronectin, laminin or Matrigel (Becton Dickinson) by incubating them overnight at 4 °C at a concentration of 5, 10 or 100 µg/ml in serum-free DMEM media, respectively. Alternatively, substrates were briefly coated with 0.67% gelatin (Sigma) at room temperature, the protein solution was aspirated and the chamber slides were air-dried before use. Unless otherwise stated, the majority of the experiments were performed with gelatin-coated substrates.
2.4. Inhibition assays
The PI 3-kinase inhibitors LY294002 (10 µmol/l) and wortmannin (500 nmol/l), the MEK (MAP kinase-kinase) inhibitor PD98059 (40 µmol/l), and the Akt inhibitor (catalog # 124005, 10 µmol/l) were obtained from CalBiochem. Control samples were treated with an equal volume of the vehicle (DMSO, Sigma) alone. Blocking antibodies for erythropoietin (EPO), the EPO receptor and appropriate isotype controls (10 µg/ml) were obtained from R&D Systems, and the blocking antibody for the IGF-1 receptor (1 µg/ml) was obtained from Oncogene Research Products. Each of the candidate molecules was added individually to the enriched cultures of cardiomyocytes in serum-free conditions at a concentration approximately equal to 10 times the normal effective dose for 50% inhibition (ED50), in order to ensure near-maximal inhibition. The estimated ED50 values for each molecule were based upon reported values from the commercial vendor as well as published reports. The blocking antibodies were diluted in serum-free media containing 0.1% BSA (Sigma). The blocking reagents were added to the enriched cardiomyocyte cultures 48 hours after re-plating (24 hours before the BrdU pulse).
2.5. Stimulation assays
Recombinant human IGF-1 and IGF-2 were obtained from R&D Systems and diluted in serum-free media containing 0.1% BSA. The enriched cultures were treated with 0.1, 1, or 10 ng/ml of IGF-1 (reported ED50 ~ 1 ng/ml) or 1, 10 or 100 ng/ml of IGF-2 (reported ED50 ~ 10 ng/ml) during the final 48 hours of culture.
2.6. Immunostaining
Chamber slide samples were blocked with a 1.5% solution of goat serum in PBS. The samples were incubated overnight at 4 °C with a primary antibody against sarcomeric actin (5C5, 1:5000, Sigma), followed by a secondary biotinylated-goat anti-mouse antibody at room temperature for 1 hour (1:400, IgM, Vector) and an avidin/biotinylated alkaline phosphatase complex for 30 min at room temperature (ABC-AP, Vector). The enzymatic reaction was developed with the addition of the alkaline phosphatase substrate Vector Red (Vector). Antigen retrieval for BrdU was performed by incubating with 1.5 N HCl at 37 °C for 15 min and rinsing twice with 0.1 mol/l Borax buffer (pH 8.5) for 5 min. The samples were incubated with a peroxidase-conjugated anti-BrdU antibody (1:50, Roche) overnight at 4 °C prior to development with 3,3′-diamino benzidine tetrahydrochloride (DAB). The cells were counterstained with hematoxylin, rinsed with acid alcohol briefly, incubated in Scott’s blue and mounted with Aqua-mount mounting media before coverslipping. In parallel studies, fractionated cells were immunostained with an antibody for fast skeletal myosin heavy chain (MY32) in order to specifically detect the presence of any mature skeletal muscle cells. In agreement with previously published reports, the cultures were consistently negative for skeletal muscle (see Supplementary Information Fig. 1), indicating the 5C5 antibody for sarcomeric actin can be used confidently as a reliable means of identifying cardiomyocytes.
Pelleted EB sections were immunostained similarly to the chamber slides, with the following exceptions. In some instances, an antibody for sarcomeric myosin heavy chain (MF20, ATCC) was used in lieu of the sarcomeric actin antibody. A secondary biotinylated-horse anti-mouse antibody was incubated for 1 hour at room temperature (1:400, Vector), followed by an avidin/biotinylated horseradish peroxidase complex for 30 min at room temperature (ABC, Vector) and developed with DAB. BrdU staining was developed with VIP (Vector) and nuclei were counterstained with methyl green.
3. Results
3.1. Comparison of mouse and human ES cell-derived cardiomyocyte proliferation
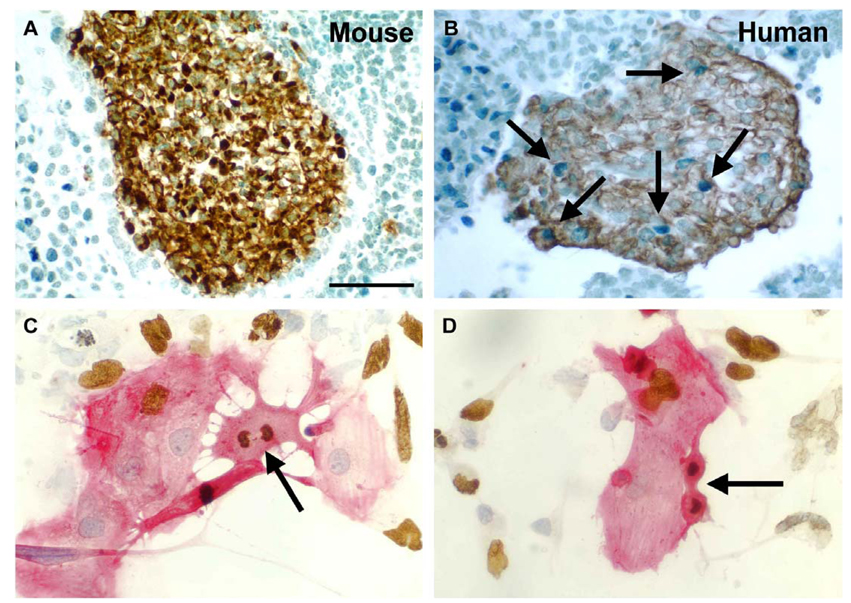
Mouse EB outgrowths containing beating cell foci (indicative of cardiomyogenesis) were observed typically between 9 and 12 days of differentiation and analyzed immunohistochemically at various time points during the course of differentiation (4–17 days). Clusters of cardiomyocytes were clearly identified by sarcomeric myosin heavy chain or sarcomeric actin staining, and the number and size of cardiomyocyte colonies appeared to increase with time. However, very few of the cardiomyocytes (< 1%) at any stage of differentiation incorporated the BrdU label, indicating that proliferation rates were very low (Fig. 1A). This suggested that committed cardiomyocytes differentiated from mouse ES cells and readily identifiable by immunohistochemical labeling, possessed a limited capacity to proliferate. These results are similar to findings that mouse cardiomyocytes differentiated from ES cells rapidly exit the cell cycle upon differentiation [21]. In contrast, a readily identifiable number of cardiomyocytes from human EB outgrowths were clearly BrdU+ (Fig. 1B). Thus, future studies focused on characterizing the proliferation of cardiomyocytes derived from human ES cells.
Fig. 1.
A and B. Comparison of mouse and human ES-cell derived cardiomyocyte proliferation. Mouse ES cells were differentiated for 12 days (A) and human ES cells were differentiated for 14 days (B). The cultures were pulsed with BrdU and sections from cell pellets were immunostained for myosin heavy chain (mouse) or sarcomeric actin (human) to detect cardiomyocytes; cell nuclei were counterstained with methyl green. BrdU+ cardiomyocytes (brown cytoplasm, blue nuclei) were rarely seen in differentiating mouse ES cultures, whereas numerous BrdU+ cardiomyocytes were detected in differentiating human ES cultures, as noted by the arrows. Scale bar = 50 µm. C and D. Mitotic figures and BrdU incorporation in human ES cell-derived cardiomyocytes. H7 human ES cells were differentiated for 19 days before Percoll separation and fraction IV cells were cultured for an additional 4 days with 20% FBS. Cells were stained for sarcomeric actin (red) and BrdU (brown), and nuclei were counterstained with hematoxylin (blue). Multiple BrdU+ cardiomyocytes were evident; mitotic cardiomyocytes in metaphase (C) and telophase (D) are shown by the arrows.
3.2 Serum titration
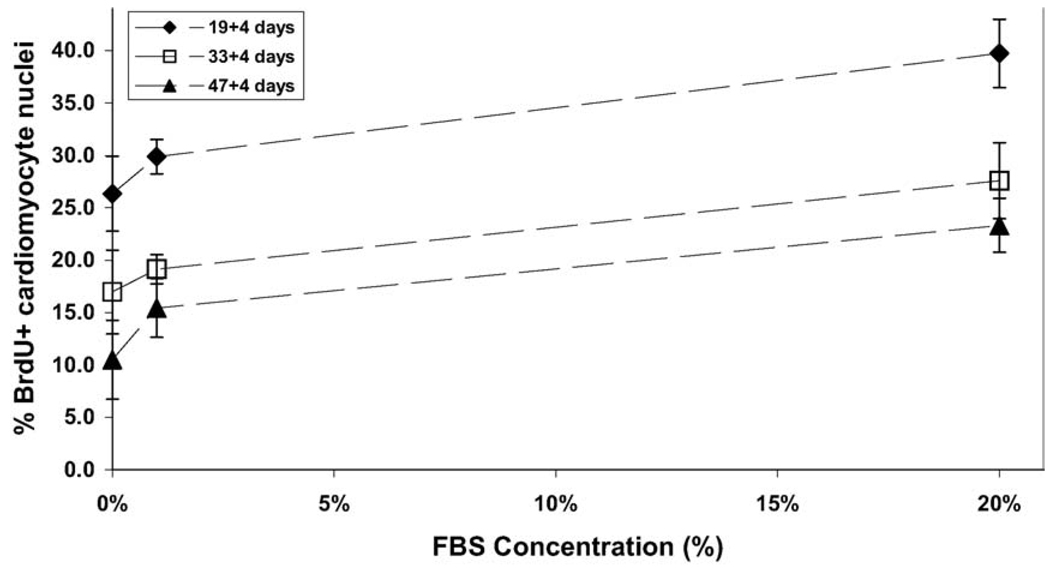
Human cardiomyocytes exhibited high levels of proliferation under standard culture conditions, typically averaging 20–40% BrdU+ cells after a 24-hour labeling period. Dose-response studies showed only a modest dependency on serum, such as the reduction from 39.7 ± 3.3% BrdU+ cardiomyocytes with 20% FBS to 26.3 ± 3.6% in serum-free media, after 3 weeks of differentiation (Fig. 2). This modest serum-dependence was consistently observed for the enriched cultures of cardiomyocytes at various stages of differentiation. A progressive decrease in the overall level of cardiomyocyte proliferation was observed with increasing maturity of the differentiating cells (Fig. 2). This suggested that the enriched cultures of cardiomyocytes were proliferating in an autonomous fashion that decreased over time with increasing differentiation of the cells. Mitotic figures within BrdU+ cardiomyocytes were readily evident throughout the cultures (Fig. 1C, D). Few, if any, bi-nucleated cardiomyocytes were apparent at any time, suggesting that BrdU incorporation accurately reflects cytokinesis and not simply DNA synthesis in the absence of cell division.
Fig. 2.
Serum-dependence of human ES cell-derived cardiomyocyte proliferation. H7 human ES cells were differentiated for varying times (19, 33, or 47 days) before Percoll separation, and fraction IV cells were cultured for an additional 4 days at various FBS concentrations (0%, 1% or 20%). The percentage of BrdU+ cardiomyocytes was moderately sensitive to the concentration of FBS and decreased as the cells were differentiated for longer periods of time.
In some instances, there was considerable variability in the baseline of cardiomyocyte proliferation between separate experiments; however, there was no apparent correlation between the purity of the cardiomyocyte cultures in independent experiments and the baseline levels of proliferation (i.e. proliferation scaling directly or inversely with purity). In general, it appeared that cardiomyocytes derived from lower passage ES cells exhibited higher levels of proliferation than cardiomyocytes differentiated from older passage ES cells. Due to the inherent variability between separate preparations of ES cell-derived cardiomyocytes, it was often difficult to make comparisons between distinct experiments. Thus, all of the subsequent studies analyzing factors that influenced proliferation were internally controlled by studying single batches of Percoll-enriched cells and then repeated to ensure the validity of the findings. Based on the higher levels of proliferation observed for the less mature cardiomyocytes, all subsequent experiments were performed with cultures of cardiomyocytes enriched by Percoll centrifugation after 17–21 days of differentiation.
3.3. Attachment factors
Cardiomyocytes were plated onto substrates pre-coated with different attachment factors to examine the potential role of the extracellular matrix in influencing proliferation. Although small reproducible trends were noted in independent experiments, no statistically significant differences were observed in cardiomyocyte proliferation based upon the nature of the adhesive molecules pre-adsorbed to the culture surfaces. In general, the highest rates of proliferation were observed on gelatin-coated substrates followed by laminin- and fibronectin- and then Matrigel-coated wells (see Supplementary Information, Fig. 2). All subsequent experiments were performed with gelatin-coated substrates.
3.4. Cell density
The enriched fraction of cardiomyocytes was plated at varying densities to assess whether a paracrine factor produced within the cultures potentially stimulated proliferation. The percentage of BrdU+ cardiomyocytes remained constant over a 10-fold range of plating density, suggesting paracrine stimulation was not responsible (see Supplementary Information, Fig. 3).
3.5. Inhibition studies
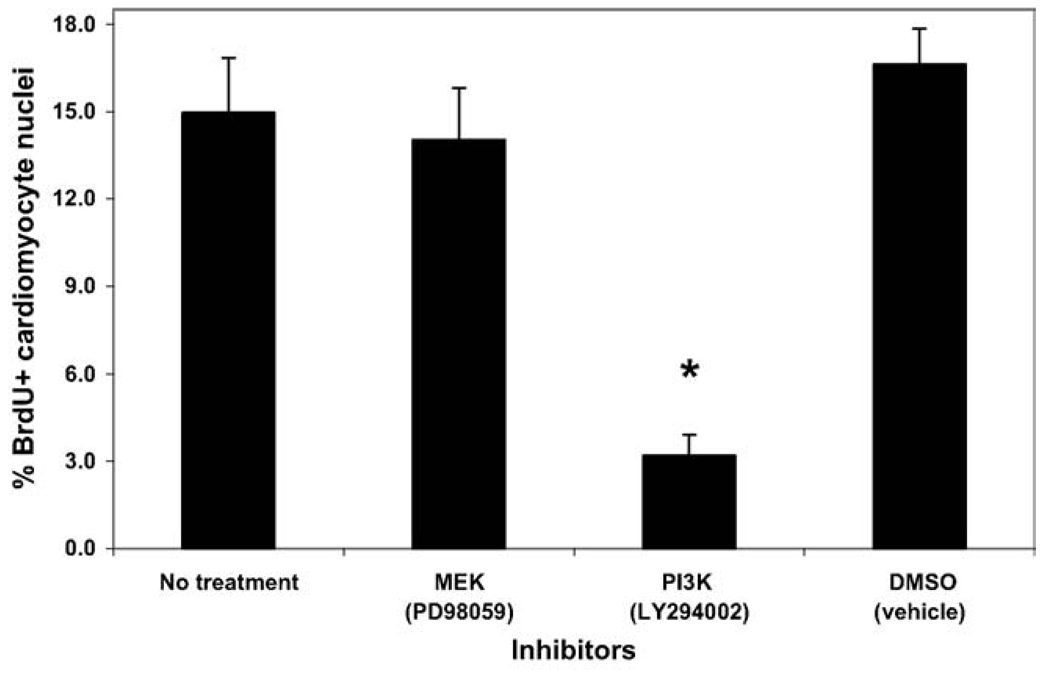
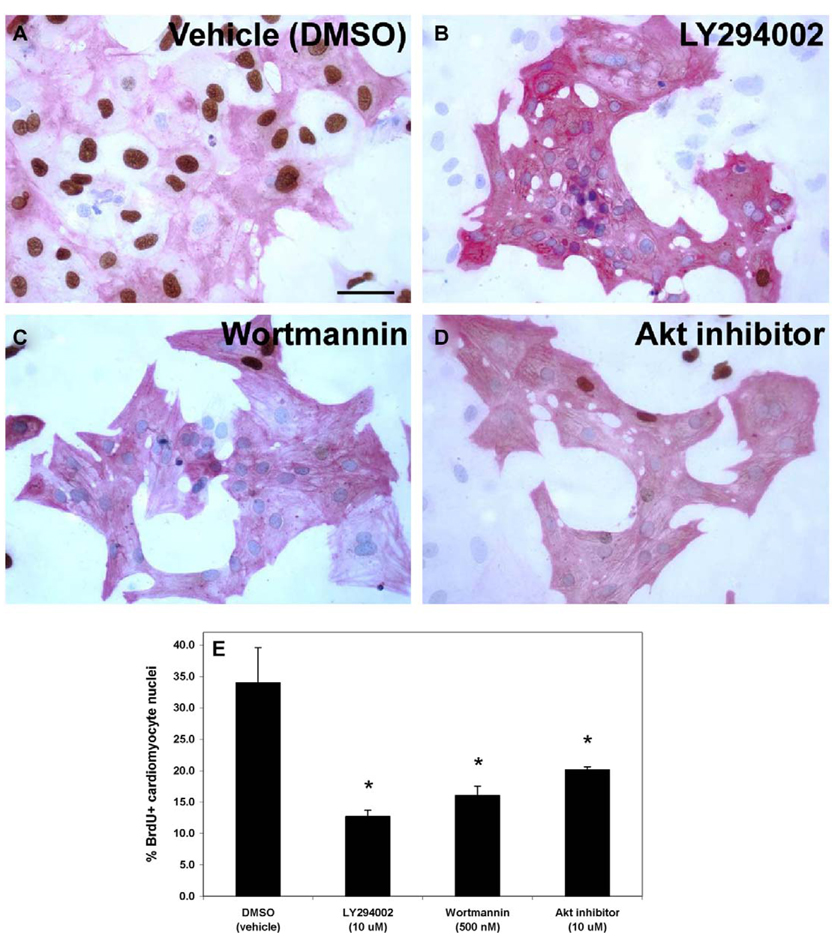
Based on the persistently high proliferation of human cardiomyocytes under serum-free conditions, a series of inhibition experiments was conducted to decipher important signaling pathways and potential growth factor receptors that significantly influenced the cardiomyocyte proliferation. The PI 3-kinase inhibitor LY294002 significantly decreased the number of BrdU+ cardiomyocytes from 15.0 ± 1.9% (DMSO vehicle alone) to 3.2 ± 0.7%, whereas the MEK inhibitor PD98059 did not affect the percentage of proliferating cardiomyocytes (Fig. 3). Subsequent studies with an additional inhibitor of PI 3-kinase (wortmannin) and an inhibitor of Akt (a downstream target of PI 3-kinase) also significantly decreased the proliferation of the human cardiomyocytes to a similar extent (Fig. 4). No significant cell death was observed in the cultures treated with the inhibitory compounds at the concentrations used.
Fig. 3.
Signaling pathway inhibition of human ES cell-derived cardiomyocyte proliferation. H7 human ES cells were differentiated for 19 days before Percoll separation, and fraction IV cells were cultured for an additional 4 days in serum-free media with various signaling inhibitors. The inhibitors were added for the final 48 hours of culture prior to fixation. Only the PI 3-kinase inhibitor (LY294002) significantly inhibited human ES cell-derived cardiomyocyte proliferation (* = P < 0.05).
Fig. 4.
PI 3-kinase inhibition of human ES cell-derived cardiomyocyte proliferation. H7 human ES cells were differentiated for 18 days before Percoll separation, and fraction IV cells were cultured for an additional 4 days in serum-free media with various signaling inhibitors. The inhibitors were added for the final 48 hours of culture prior to fixation. Cells were stained for sarcomeric actin (red) and BrdU (brown), and nuclei were counterstained with hematoxylin (blue). A–D) Significantly fewer BrdU+ cardiomyocyte nuclei were apparent in the treated cultures (panels B–D) as opposed to the control (DMSO vehicle alone; panel A). Scale bar = 50 µm. E) Quantification of the data indicated that the PI 3-kinase inhibitors, LY294002 and wortmannin, and the Akt inhibitor significantly inhibited human ES cell-derived cardiomyocyte proliferation compared to the control (* = P < 0.05).
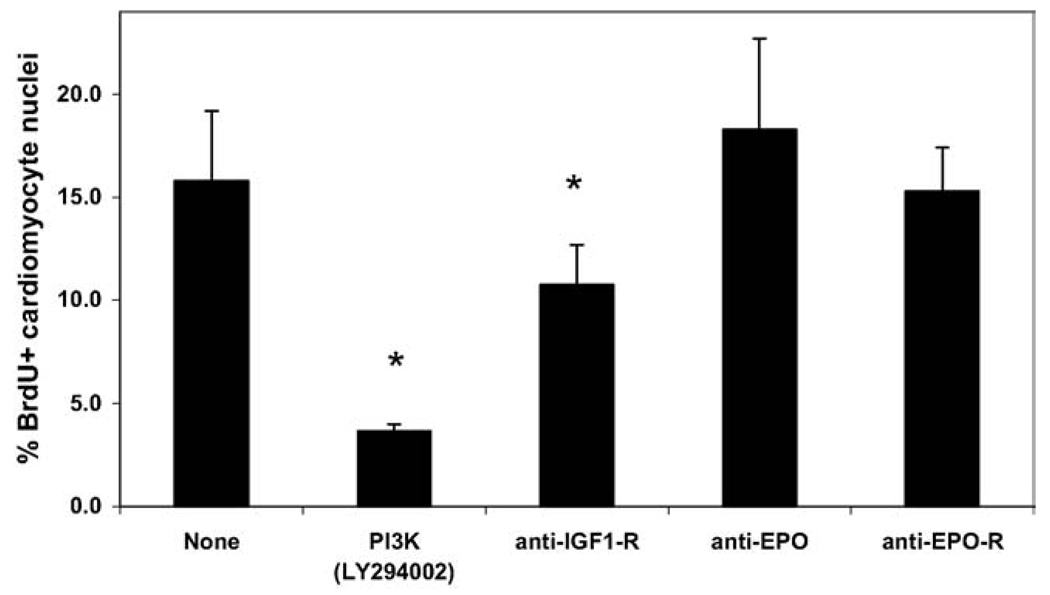
Based upon the results of the PI 3-kinase inhibition experiments, we attempted to inhibit growth factors and growth factor receptors that are reported to signal through the PI3 kinase pathway in order to determine which, if any, might significantly influence cardiomyocyte proliferation. Initial experiments blocking either the PDGF or EGF receptors with small molecule inhibitors (AG1296 and AG1478, respectively) did not decrease the percentage of cardiomyocyte proliferation (see Supplementary Information, Fig. 4). Similarly, the addition of heparin to the serum-free culture media to inhibit FGF signaling did not decrease proliferation. In subsequent experiments, enriched cultures of cardiomyocytes were treated with blocking antibodies to EPO, the EPO receptor or the IGF-1 receptor. Only treatment with the IGF-1 receptor-blocking antibody significantly inhibited cardiomyocyte proliferation, although the levels of inhibition achieved were not as extensive as with the PI 3-kinase inhibition (Fig. 5).
Fig. 5.
Antibody blocking of growth factor receptors. H7 human ES cells were differentiated for 20 days before Percoll separation, and fraction IV cells were cultured for an additional 4 days in serum-free media with various signaling inhibitors. The inhibitors were added for the final 48 hours of culture prior to fixation. Only the PI 3-kinase inhibitor (LY294002) and the IGF-1 receptor blocking antibody significantly inhibited human ES cell-derived cardiomyocyte proliferation (* = P < 0.05), whereas antibodies to the EPO ligand or receptor had no effect.
3.6. Stimulation studies
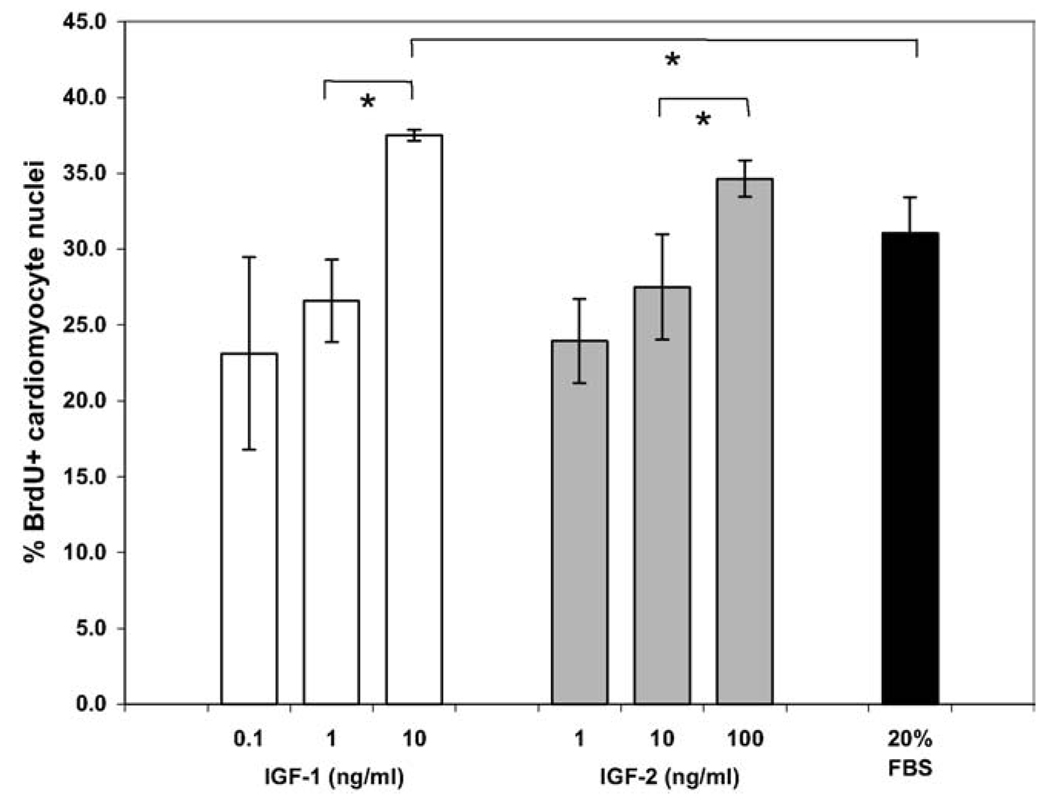
Based upon the results of the blocking studies with the IGF-1 receptor antibody, recombinant human IGF-1 or IGF-2 were added to serum-free cultures of hESC-derived cardiomyocytes to determine whether proliferation could be further stimulated. Addition of either IGF-1 or IGF-2 (Fig. 6) increased the percentage of proliferating cardiomyocytes in a dose-dependent manner from 23 ± 6.4% to 37.5 ± 0.4% or 24.0 ± 2.8% to 34.7 ± 1.2%, respectively; statistically significantly increases were observed at concentrations roughly equal to 10 times the reported ED50 value for both molecules. IGF-1 at a concentration of 10 ng/ml increased cardiomyocyte proliferation (37.5 ± 0.4% BrdU+) to an even greater level than 20% FBS treatment (31.0 ± 2.4%).
Fig. 6.
Stimulation of human ES cell-derived cardiomyocyte proliferation. H7 human ES cells were differentiated for 17 days before Percoll separation, and fraction IV cells were cultured for an additional 4 days in serum-free media. Various concentrations of recombinant human IGF-1 or IGF-2 were added for the final 48 hours of culture prior to fixation. IGFs increased cardiomyocyte proliferation in a dose-dependent manner to levels greater than treatment with 20% FBS (* = P < 0.05).
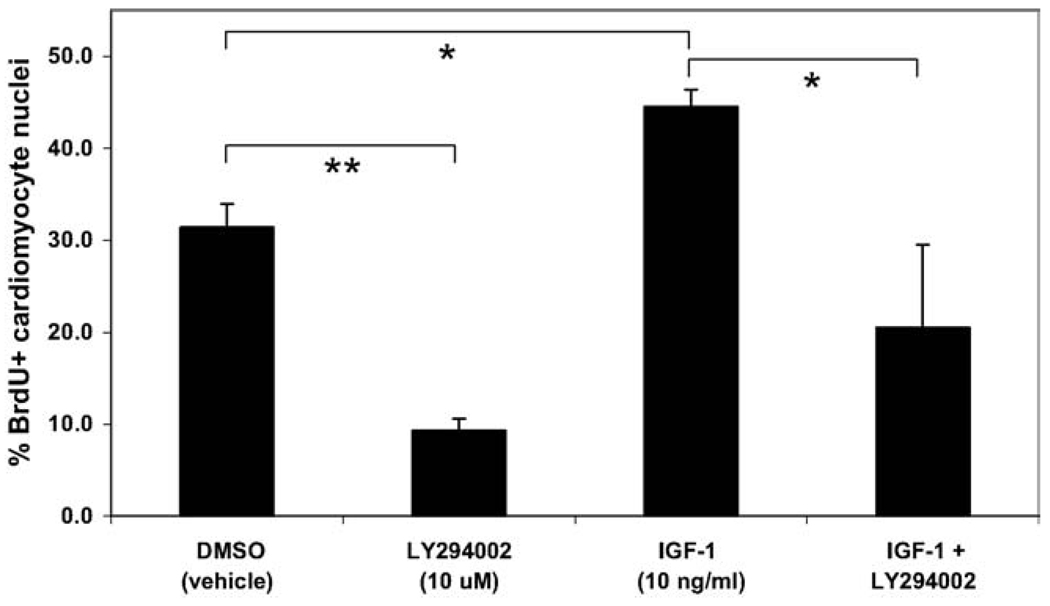
In order to directly demonstrate that IGF stimulation of proliferation was mediated via the PI 3-kinase pathway, IGF-1 and LY294002 were added independently and in combination to enriched cardiomyocyte cultures. Similar to previous results, treatment with LY294002 inhibited cardiomyocyte proliferation, whereas addition of IGF-1 stimulated BrdU incorporation. When IGF-1 and LY294002 were added together, a significant decrease in proliferation was attained compared to IGF-1 treatment alone (from 44.6 ± 1.9% to 20.5 ± 9.0%), indicating that IGF-stimulated proliferation was mediated via the PI 3-kinase pathway (Fig. 7).
Fig. 7.
IGF stimulation of human ES cell-derived cardiomyocyte proliferation is mediated via PI 3-kinase. H7 human ES cells were differentiated for 21 days before Percoll separation, and fraction IV cells were cultured for an additional 4 days in serum-free media with various treatments for the final 48 hours of culture prior to fixation. The PI 3-kinase inhibitor LY294002 significantly inhibited and IGF-1 significantly stimulated hESC-derived cardiomyocyte proliferation (** = P < 0.01 and * = P < 0.05, respectively), similar to previous results. Dual treatment with IGF-1 and LY294002 significantly inhibited proliferation (P < 0.05) compared to treatment with IGF-1 alone, suggesting that IGF-1 stimulated proliferation is mediated via the PI 3-kinase pathway.
4. Discussion
These results represent the first report of the molecular mechanisms governing the proliferation of cardiomyocytes derived from human ES cells. Similar to other recent reports, we found that cardiomyocytes differentiating from human ES cells exhibit a high level of proliferation, which progressively decreases as the cells mature in culture [16,17]. In stark contrast, cardiomyocytes derived from mouse ES cells appeared non-proliferative using similar experimental techniques. Human cardiomyocyte proliferation was largely independent of the concentration of serum in the culture media, attachment to various extracellular matrix factors and the cell density at which they were plated. These findings suggest the cultures may produce an autocrine/paracrine factor(s) that stimulates cell growth. Lastly, based upon inhibition and stimulation studies, signaling via the IGF/PI 3-kinase/Akt pathway appears to mediate the proliferation of cardiomyocytes derived from human ES cells, and addition of IGF-1 or IGF-2 can stimulate cardiomyocyte proliferation in a dose-dependent manner.
Previous studies in mice and rats have clearly established the role of IGF, PI 3-kinase and Akt signaling in cardiomyocyte hyperplasia, hypertrophy and survival. IGF signaling stimulates cardiomyocyte DNA synthesis in vitro [22] and the decrease in cardiomyocyte proliferation during postnatal development is coupled with decreased levels of the IGF ligands and receptor [23]. Cardiac-specific overexpression of IGF-1 leads to an increased number of myocytes in the heart [24] and in a transgenic model of cardiomyopathy, prevents dilation and stimulates cardiomyocyte proliferation [25]. Transgenic mice with cardiac-restricted expression of constitutively active PI 3-kinase or Akt exhibit increased heart size, whereas dominant-negative forms of PI 3-kinase or kinase-deficient Akt reduce heart size and weight [26,27]. These consequences were attributed to cardiomyocyte hypertrophy, rather than hyperplasia, presumably due to the choice of promoter (α myosin heavy chain), which may not be active during the stage of development in which hyperplastic cardiomyocyte growth occurs. Subsequent studies from the same group have demonstrated that IGF and PI 3-kinase activity are also involved in physiological hypertrophy of cardiomyocytes [28,29]. Another mouse model of cardiac IGF-1 overexpression has demonstrated that, while initially stimulating physiological cardiac hypertrophy, prolonged expression of IGF-1 in the heart progressively leads to a pathological hypertrophic condition [30]. IGF-1 and PI 3-kinase activation can protect primary cardiomyocytes from apoptosis [31,32], and IGF-1 signaling via PI 3-kinase and Akt has been shown to reduce cardiomyocyte apoptosis in vitro [33]. Adenoviral gene delivery of constitutively active PI 3-kinase or Akt inhibits apoptosis of primary cardiomyocytes in vitro [33,34] and constitutively active Akt can protect cardiomyocytes from apoptosis after ischemia reperfusion injury in vivo in mice [34] or rats [35].
PI 3-kinase signaling has previously been reported to be essential for differentiation of cardiomyocytes from mouse ES cells [36,37]. Treatment of differentiating mouse ES cell cultures with the PI 3-kinase inhibitor LY294002 reduced the number of alpha-actinin positive cardiomyocytes and the number of embryoid bodies containing beating foci. Recent work also suggests that cardiotrophin-1 stimulated proliferation of mouse ES cell-derived cardiomyocytes is mediated by PI 3-kinase [38]. However, cardiomyocytes derived from mouse ES cells exhibit a relatively low level of proliferation which ceases entirely by 21 days of differentiation [21]. This short period of proliferative activity during murine cardiomyogenesis makes the mouse system more challenging to interrogate molecular mechanisms governing cardiomyocyte proliferation than working with human ES cells. The disparate behavior between the two species could be attributed to differences in the concentrations of IGF, the isoforms of IGF expressed, or to differences in their downstream signal transduction pathways. Such possibilities are the focus of ongoing studies. This example highlights just one of the many differences that may exist between murine and human ES cells and the behavior of their differentiated progeny.
It was recently reported that fetal sheep cardiomyocytes demonstrated an increase in BrdU+ myocytes upon treatment with IGF-1. Stimulation by IGF-1 could be blocked by PI 3-kinase inhibition using the LY294002 compound [39]. However, the maximum amount of stimulation achievable was only about 5% of the cells, which is much lower than the levels observed in the current study with human ES-derived cardiomyocytes. In contrast to human ES-derived cardiomyocytes, fetal sheep cardiomyocyte proliferation could also be inhibited with a MEK inhibitor (ERK pathway), although the same exact inhibitor was not examined in the current study.
The apparent conservation of the IGF/PI 3-kinase/Akt pathway in cardiomyocyte proliferation and hypertrophy suggests that these molecules act consistently to promote cardiomyocyte growth. Future studies should focus on elucidating the molecular mechanisms whereby signaling via these growth pathways transitions from a hyperplastic to a hypertrophic phenotype. One hypothesis is that cardiomyocytes are pre-programmed for a particular number of cell divisions from the onset of differentiation. A second notion is that intrinsic molecular mechanisms to inhibit proliferation are activated at a specific stage of cardiomyocyte differentiation. Determination of why and how cardiomyocytes lose their proliferative capacity, and if the process is reversible, are remaining challenges that need to be addressed in order to allow these cells to be responsive to proliferative signals for expansion purposes.
One caveat to this experiment is the fact that our cell preparation, though enriched, does not consist purely of cardiomyocytes. Previous reports have demonstrated that retinoic acid and EPO can stimulate cardiomyocyte proliferation in a non-cell autonomous manner by acting upon epicardial cells [40,41]. In order to resolve whether cardiomyocyte proliferation is truly cell-autonomous, highly pure cultures of human ES-derived cardiomyocytes would need to be examined by a similar series of experiments. Thus, the technical challenge of improving the purity of cardiomyocyte cultures derived from human ES cells is the focus of ongoing studies in the laboratory.
In addition, although IGF promoted cardiomyocyte proliferation, it is not necessarily the only mitogen capable of stimulating cardiomyocyte proliferation via the PI 3-kinase/Akt pathway. This is suggested by the fact that inhibition of IGF signaling with a blocking antibody did not reduce the levels of proliferation as much as blockade of downstream PI 3-kinase did with the LY294002 compound. Thus future studies to define additional growth factors or combinations of cytokines capable of stimulating human ES-derived cardiomyocyte proliferation are underway.
In conclusion, these results suggest that molecular-based approaches to stimulate the IGF/PI 3-kinase/Akt signaling pathways may be used in conjunction with enhanced purification techniques to augment the production of cardiomyocytes from differentiating human ES cells for therapeutic and diagnostic purposes. In addition to providing the first molecular insights into human cardiomyocyte proliferation, this work also supports the notion that targeting of the IGF signaling, PI 3-kinase and/or Akt pathways could be used to stimulate cardiomyocyte proliferation for cardiac repair applications.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Guenter Daum and Dr. Bardia Askari for helpful discussions about signaling inhibitors and kindly providing some of the reagents. We also are grateful to Dr. Joseph Gold and Dr. Chunhui Xu for sharing their expertise in the culture, differentiation and enrichment methods of the human ES cells. We also thank Qiang Fu and Veronica Poppa for technical assistance with cell culture and immunostaining. This work was supported in part by NIH grants HL64387, HL61553, HL3174 and GM69983. TM was supported in part by a training grant fellowship through grant HL07403-25.
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at 10.1016/j.yjmcc.2005.09.007.
Footnotes
Conflict-of-interest disclosure
Dr Murry’s laboratory receives sponsorship from Geron Corporation for cardiac repair studies involving human ES cells. The current study however was not sponsored by Geron, but instead was paid for by grants from the National Institutes of Health.
References
- 1.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 2.Steinhelper ME, Lanson NA, Jr, Dresdner KP, Delcarpio JB, Wit AL, Claycomb WC, et al. Proliferation in vivo and in culture of differentiated adult atrial cardiomyocytes from transgenic mice. Am J Physiol. 1990;259:H1826–H1834. doi: 10.1152/ajpheart.1990.259.6.H1826. [DOI] [PubMed] [Google Scholar]
- 3.Katz EB, Steinhelper ME, Delcarpio JB, Daud AI, Claycomb WC, Field LJ. Cardiomyocyte proliferation in mice expressing alpha-cardiac myosin heavy chain-SV40 T-antigen transgenes. Am J Physiol. 1992;262:H1867–H1876. doi: 10.1152/ajpheart.1992.262.6.H1867. [DOI] [PubMed] [Google Scholar]
- 4.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rybkin II, Markham DW, Yan Z, Bassel-Duby R, Williams RS, Olson EN. Conditional expression of SV40 T-antigen in mouse cardiomyocytes facilitates an inducible switch from proliferation to differentiation. J Biol Chem. 2003;278:15927–15934. doi: 10.1074/jbc.M213102200. [DOI] [PubMed] [Google Scholar]
- 6.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 7.Xavier-Vidal R, Mandarim-de-Lacerda CA. Cardiomyocyte proliferation and hypertrophy in the human fetus: quantitative study of the myocyte nuclei. Bull Assoc Anat (Nancy) 1995;79:27–31. [PubMed] [Google Scholar]
- 8.Mandarim-de-Lacerda CA, dos Santos MB, Pessanha MG. Quantitative study of the myocardium in human embryos. Anat Anz. 1995;177:179–184. doi: 10.1016/s0940-9602(11)80071-3. [DOI] [PubMed] [Google Scholar]
- 9.Huttenbach Y, Ostrowski ML, Thaller D, Kim HS. Cell proliferation in the growing human heart: MIB-1 immunostaining in preterm and term infants at autopsy. Cardiovasc Pathol. 2001;10:119–123. doi: 10.1016/s1054-8807(01)00065-5. [DOI] [PubMed] [Google Scholar]
- 10.Claycomb WC, Delcarpio JB, Guice SE, Moses RL. Culture and characterization of fetal human atrial and ventricular cardiac muscle cells. In Vitro Cell Dev Biol. 1989;25:1114–1120. doi: 10.1007/BF02621262. [DOI] [PubMed] [Google Scholar]
- 11.Goldman BI, Wurzel J. Effects of subcultivation and culture medium on differentiation of human fetal cardiac myocytes. In Vitro Cell Dev Biol. 1992;28A:109–119. doi: 10.1007/BF02631014. [DOI] [PubMed] [Google Scholar]
- 12.Goldman B, Mach A, Wurzel J. Epidermal growth factor promotes a cardiomyoblastic phenotype in human fetal cardiac myocytes. Exp Cell Res. 1996;228:237–245. doi: 10.1006/excr.1996.0322. [DOI] [PubMed] [Google Scholar]
- 13.Hornberger LK, Singhroy S, Cavalle-Garrido T, Tsang W, Keeley F, Rabinovitch M. Synthesis of extracellular matrix and adhesion through beta(1) integrins are critical for fetal ventricular myocyte proliferation. Circ Res. 2000;87:508–515. doi: 10.1161/01.res.87.6.508. [DOI] [PubMed] [Google Scholar]
- 14.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 15.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 17.Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, et al. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–H2363. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 18.Wobus AM. Potential of embryonic stem cells. Mol Aspects Med. 2001;22:149–164. doi: 10.1016/s0098-2997(01)00006-1. [DOI] [PubMed] [Google Scholar]
- 19.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 20.Doevendans PA, Kubalak SW, An RH, Becker DK, Chien KR, Kass RS. Differentiation of cardiomyocytes in floating embryoid bodies is comparable to fetal cardiomyocytes. J Mol Cell Cardiol. 2000;32:839–851. doi: 10.1006/jmcc.2000.1128. [DOI] [PubMed] [Google Scholar]
- 21.Klug MG, Soonpaa MH, Field LJ. DNA synthesis and multinucleation in embryonic stem cell-derived cardiomyocytes. Am J Physiol. 1995;269:H1913–H1921. doi: 10.1152/ajpheart.1995.269.6.H1913. [DOI] [PubMed] [Google Scholar]
- 22.Kajstura J, Cheng W, Reiss K, Anversa P. The IGF-1-IGF-1 receptor system modulates myocyte proliferation but not myocyte cellular hypertrophy in vitro. Exp Cell Res. 1994;215:273–283. doi: 10.1006/excr.1994.1343. [DOI] [PubMed] [Google Scholar]
- 23.Cheng W, Reiss K, Kajstura J, Kowal K, Quaini F, Anversa P. Downregulation of the IGF-1 system parallels the attenuation in the proliferative capacity of rat ventricular myocytes during postnatal development. Lab Invest. 1995;72:646–655. [PubMed] [Google Scholar]
- 24.Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, et al. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welch S, Plank D, Witt S, Glascock B, Schaefer E, Chimenti S, et al. Cardiac-specific IGF-1 expression attenuates dilated cardiomyopathy in tropomodulin-overexpressing transgenic mice. Circ Res. 2002;90:641–648. doi: 10.1161/01.res.0000013780.77774.75. [DOI] [PubMed] [Google Scholar]
- 26.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, et al. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, et al. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 30.Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 1999;13:1923–1929. doi: 10.1096/fasebj.13.14.1923. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Ma W, Markovich R, Chen JW, Wang PH. Regulation of cardiomyocyte apoptotic signaling by insulin-like growth factor I. Circ Res. 1998;83:516–522. doi: 10.1161/01.res.83.5.516. [DOI] [PubMed] [Google Scholar]
- 32.Wu W, Lee WL, Wu YY, Chen D, Liu TJ, Jang A, et al. Expression of constitutively active phosphatidylinositol 3-kinase inhibits activation of caspase 3 and apoptosis of cardiac muscle cells. J Biol Chem. 2000;275:40113–40119. doi: 10.1074/jbc.M004108200. [DOI] [PubMed] [Google Scholar]
- 33.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, et al. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 34.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 36.Klinz F, Bloch W, Addicks K, Hescheler J. Inhibition of phosphatidylinositol-3-kinase blocks development of functional embryonic cardiomyocytes. Exp Cell Res. 1999;247:79–83. doi: 10.1006/excr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 37.Sauer H, Rahimi G, Hescheler J, Wartenberg M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 2000;476:218–223. doi: 10.1016/s0014-5793(00)01747-6. [DOI] [PubMed] [Google Scholar]
- 38.Sauer H, Neukirchen W, Rahimi G, Grunheck F, Hescheler J, Wartenberg M. Involvement of reactive oxygen species in cardiotrophin-1-induced proliferation of cardiomyocytes differentiated from murine embryonic stem cells. Exp Cell Res. 2004;294:313–324. doi: 10.1016/j.yexcr.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 39.Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1481–R1489. doi: 10.1152/ajpregu.00232.2003. [DOI] [PubMed] [Google Scholar]
- 40.Chen TH, Chang TC, Kang JO, Choudhary B, Makita T, Tran CM, et al. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev Biol. 2002;250:198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- 41.Stuckmann I, Evans S, Lassar AB. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev Biol. 2003;255:334–349. doi: 10.1016/s0012-1606(02)00078-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.