Abstract
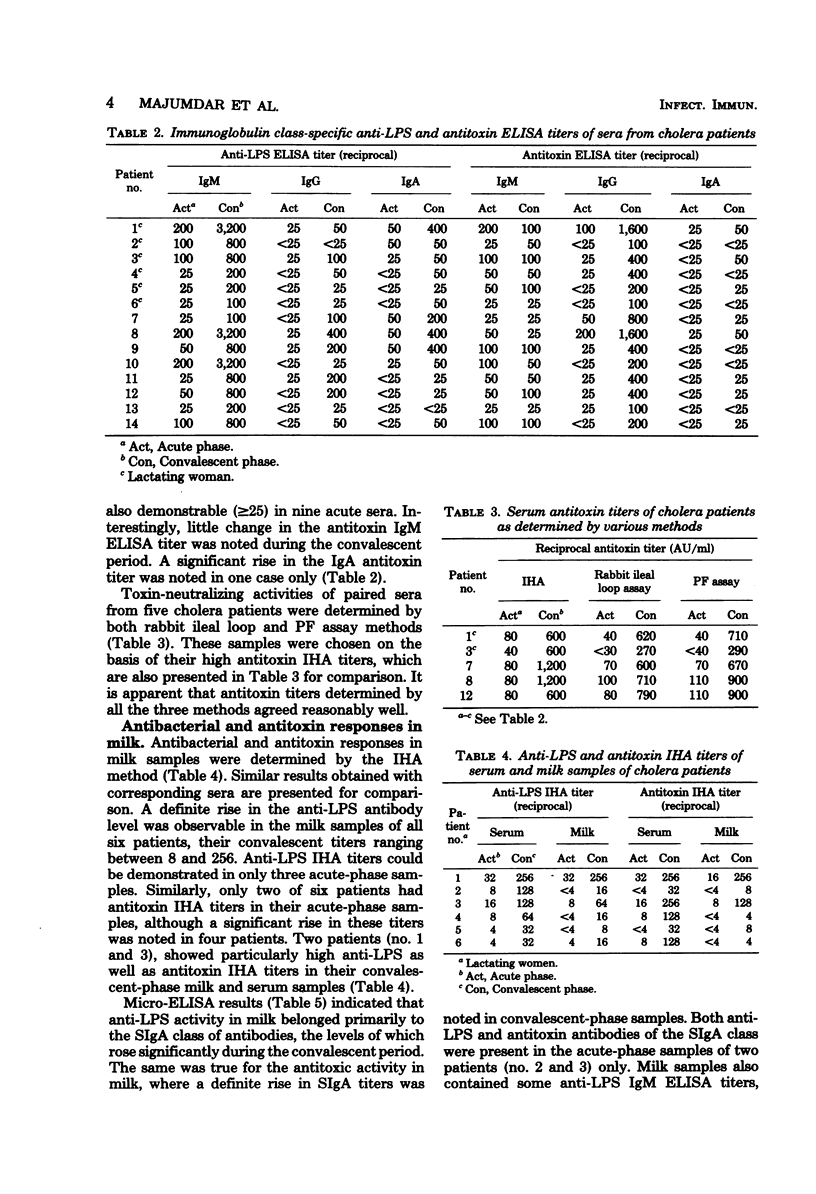
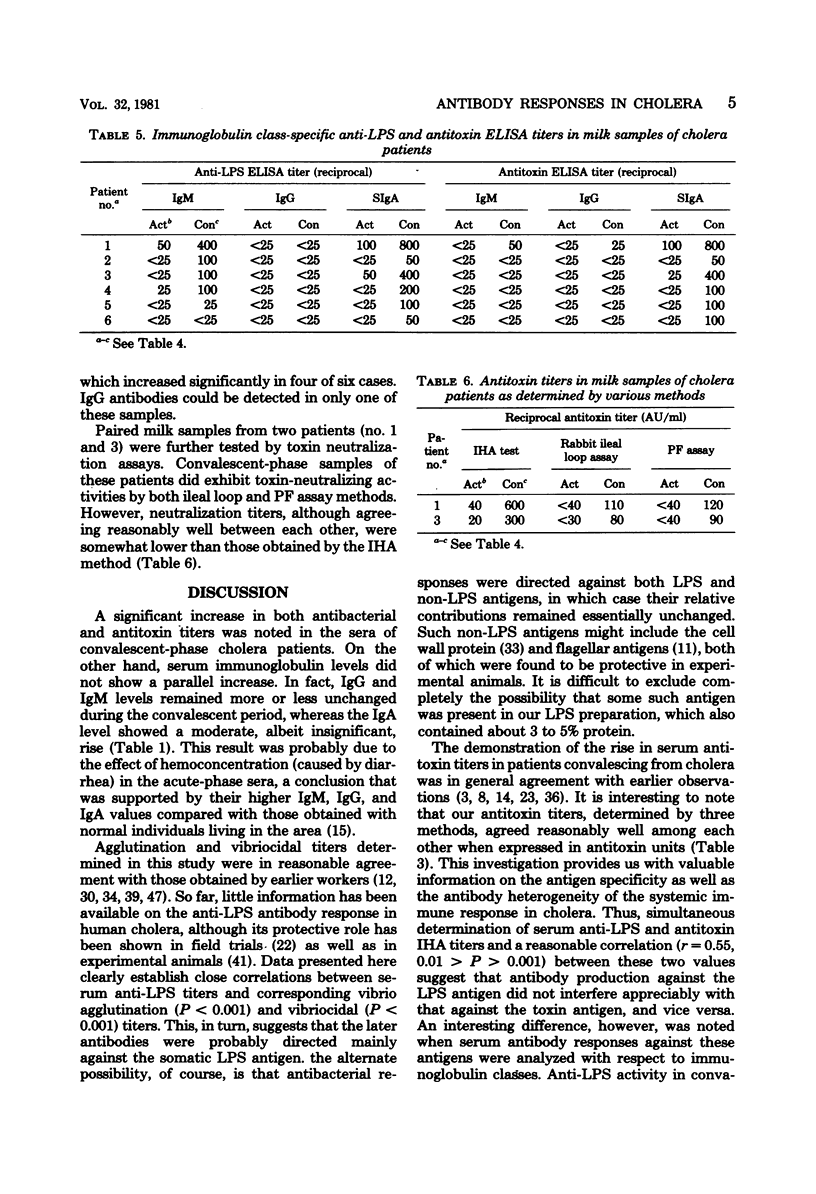
Antibacterial and antitoxin responses in the acute and convalescent (7 to 10 days) sera of 14 cholera patients were determined by various serological techniques. Similar studies were also carried out with corresponding milk samples of six of these patients who were lactating women. A significant rise in antibacterial titers was observed in all convalescent serum and milk samples. A similar rise in antitoxin titers was observable in all serum and four milk samples. Specificity of the antibacterial titers was further evaluated by the indirect hemagglutination test using lipopolysaccharide antigen, and close correlations were noted between these titers and vibrio agglutination (P<0.001) and vibriocidal (P<0.001) titers of sera. Serum and milk convalescent cholera patients could effectively neutralize cholera toxin action in vivo, although the neutralizing activity of serum was higher than that of milk. Determination of antibody titers by the enzyme-linked immunosorbent assay demonstrated that anti-lipopolysaccharide activity in sera belonged predominantly to immunoglobulin M (IgM) and, to a lesser extent, to IgG and IgA, whereas such activity in milk was mostly contributed by secretory IgA, although some IgM antibodies also could be detected. On the other hand, antitoxic activity in convalescent sera primarily belonged to IgG, whereas such activity in milk was almost exclusively contributed by secretory IgA. These results demonstrate that an antibody response in the mammary gland was stimulated due to the antigen exposure in the gut and are consistent with the idea of a common homing pattern of immunocytes within the secretory immune system. Moreover, some differences in the antibody production mechanism between the systemic and secretory immune systems are indicated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardyce R. A., Shearman D. J., McClelland D. B., Marwick K., Simpson A. J., Laidlaw R. B. Appearance of specific colostrum antibodies after clinical infection with Salmonella typhimurium. Br Med J. 1974 Aug 3;3(5926):307–309. doi: 10.1136/bmj.3.5926.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benenson A. S., Saad A., Mosley W. H., Ahmed A. Serological studies in cholera. 3. Serum toxin neutralization--rise in titre in response to infection with Vibrio cholerae, and the level in the "normal" population of East Pakistan. Bull World Health Organ. 1968;38(2):287–295. [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., McDermott M., Befus D., O'Neill M. A common mucosal immunologic system involving the bronchus, breast and bowel. Adv Exp Med Biol. 1978;107:53–59. doi: 10.1007/978-1-4684-3369-2_7. [DOI] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- DE S. N. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959 May 30;183(4674):1533–1534. doi: 10.1038/1831533a0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Eubanks E. R., Guentzel M. N., Berry L. J. Evaluation of surface components of Vibrio cholerae as protective immunogens. Infect Immun. 1977 Feb;15(2):533–538. doi: 10.1128/iai.15.2.533-538.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Peterson J. W. In vitro detection of antibody to cholera enterotoxin in cholera patients and laboratory animals. Infect Immun. 1970 Jan;1(1):21–29. doi: 10.1128/iai.1.1.21-29.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose A. C., Haldar J. P., Pal S. C., Mishra B. P., Mishra K. K. Serological investigations on Indian kala-azar. Clin Exp Immunol. 1980 May;40(2):318–326. [PMC free article] [PubMed] [Google Scholar]
- Goldblum R. M., Ahlstedt S., Carlsson B., Hanson L. A., Jodal U., Lidin-Janson G., Sohl-Akerlund A. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975 Oct 30;257(5529):797–798. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- Hanson L. A., Ahlstedt S., Carlsson B., Kaijser B., Larsson P., Baltzer I. M., Akerlund A. S., Edén C. S., Svennerholm A. M. Secretory IgA antibodies to enterobacterial virulence antigens: their induction and possible relevance. Adv Exp Med Biol. 1978;107:165–176. doi: 10.1007/978-1-4684-3369-2_20. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Hanson L. A., Carlson B., Lindblad B. S., Rahimtoola J. Neutralizing antibodies against Escherichia coli and Vibrio cholerae enterotoxins in human milk from a developing country. Scand J Immunol. 1976;5(6-7):867–871. doi: 10.1111/j.1365-3083.1976.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Mechanisms of disease and immunity in cholera: a review. J Infect Dis. 1977 Aug;136 (Suppl):S105–S112. doi: 10.1093/infdis/136.supplement.s105. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H., Batty I. International reference preparation for human serum IgG, IgA, IgM. Immunochemistry. 1974 Nov;11(11):759–759. doi: 10.1016/0019-2791(74)90277-8. [DOI] [PubMed] [Google Scholar]
- Kasai G. J., Burrows W. The titration of cholera toxin and antitoxin in the rabbit ileal loop. J Infect Dis. 1966 Dec;116(5):606–614. doi: 10.1093/infdis/116.5.606. [DOI] [PubMed] [Google Scholar]
- Kately J. R., Patel C. B., Friedman H. Involvement of T- and B-lymphocytes in the immune response to the protein exotoxin and the lipopolysaccharide antigens of Vibrio cholerae. Ann N Y Acad Sci. 1975 Feb 28;249:404–412. doi: 10.1111/j.1749-6632.1975.tb29089.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamm M. E., Weisz-Carrington P., Roux M. E., McWilliams M., Phillips-Quagliata J. M. Development of the IgA system in the mammary gland. Adv Exp Med Biol. 1978;107:35–42. doi: 10.1007/978-1-4684-3369-2_5. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Arnold R. R., Michalek S. M., Prince S. J., Babb J. L. Induction of secretory antibodies in humans following ingestion of Streptococcus mutans. Adv Exp Med Biol. 1978;107:177–184. doi: 10.1007/978-1-4684-3369-2_21. [DOI] [PubMed] [Google Scholar]
- Merson M. H., Black R. E., Sack D. A., Svennerholm A. M., Holmgren J. Maternal cholera immunisation and scecretory IgA in breast milk. Lancet. 1980 Apr 26;1(8174):931–932. doi: 10.1016/s0140-6736(80)90860-0. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Connelly K. M., Cohn J., Skandera C. A. Remote-site stimulation of secretory IgA antibodies following bronchial and gastric stimulation. Adv Exp Med Biol. 1978;107:113–122. doi: 10.1007/978-1-4684-3369-2_14. [DOI] [PubMed] [Google Scholar]
- Neoh S. H., Rowley D. Protection of infant mice against cholera by antibodies to three antigens of Vibrio cholerae. J Infect Dis. 1972 Jul;126(1):41–47. doi: 10.1093/infdis/126.1.41. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. Intestinal antibodies. J Infect Dis. 1978 May;137(5):661–662. doi: 10.1093/infdis/137.5.661. [DOI] [PubMed] [Google Scholar]
- Rudbach J. A. Molecular immunogenicity of bacterial lipopolysaccharide antigens: establishing a quantitative system. J Immunol. 1971 Apr;106(4):993–1001. [PubMed] [Google Scholar]
- Sack R. B., Barua D., Saxena R., Carpenter C. C. Vibriocidal and agglutinating antibody patterns in cholera patients. J Infect Dis. 1966 Dec;116(5):630–640. doi: 10.1093/infdis/116.5.630. [DOI] [PubMed] [Google Scholar]
- Stoliar O. A., Pelley R. P., Kaniecki-Green E., Kkaus M. H., Carpenter C. C. Secretory IgA against enterotoxins in breast-milk. Lancet. 1976 Jun 12;1(7972):1258–1261. doi: 10.1016/s0140-6736(76)91735-9. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M. Experimental studies on cholera immunization. 4. The antibody response to formalinized Vibrio cholerae and purified endotoxin with special reference to protective capacity. Int Arch Allergy Appl Immunol. 1975;49(4):434–452. [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J., Hanson L. A., Lindblad B. S., Quereshi F., Rahimtoola R. J. Boosting of secretory IgA antibody responses in man by parenteral cholera vaccination. Scand J Immunol. 1977;6(12):1345–1349. doi: 10.1111/j.1365-3083.1977.tb00376.x. [DOI] [PubMed] [Google Scholar]
- Van Weemen B. K., Schuurs A. H.W.M. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971 Jun 24;15(3):232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- Voller A., Bartlett A., Bidwell D. E. Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol. 1978 Jun;31(6):507–520. doi: 10.1136/jcp.31.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward W. E. Cholera reinfection in man. J Infect Dis. 1971 Jan;123(1):61–66. doi: 10.1093/infdis/123.1.61. [DOI] [PubMed] [Google Scholar]



