Abstract
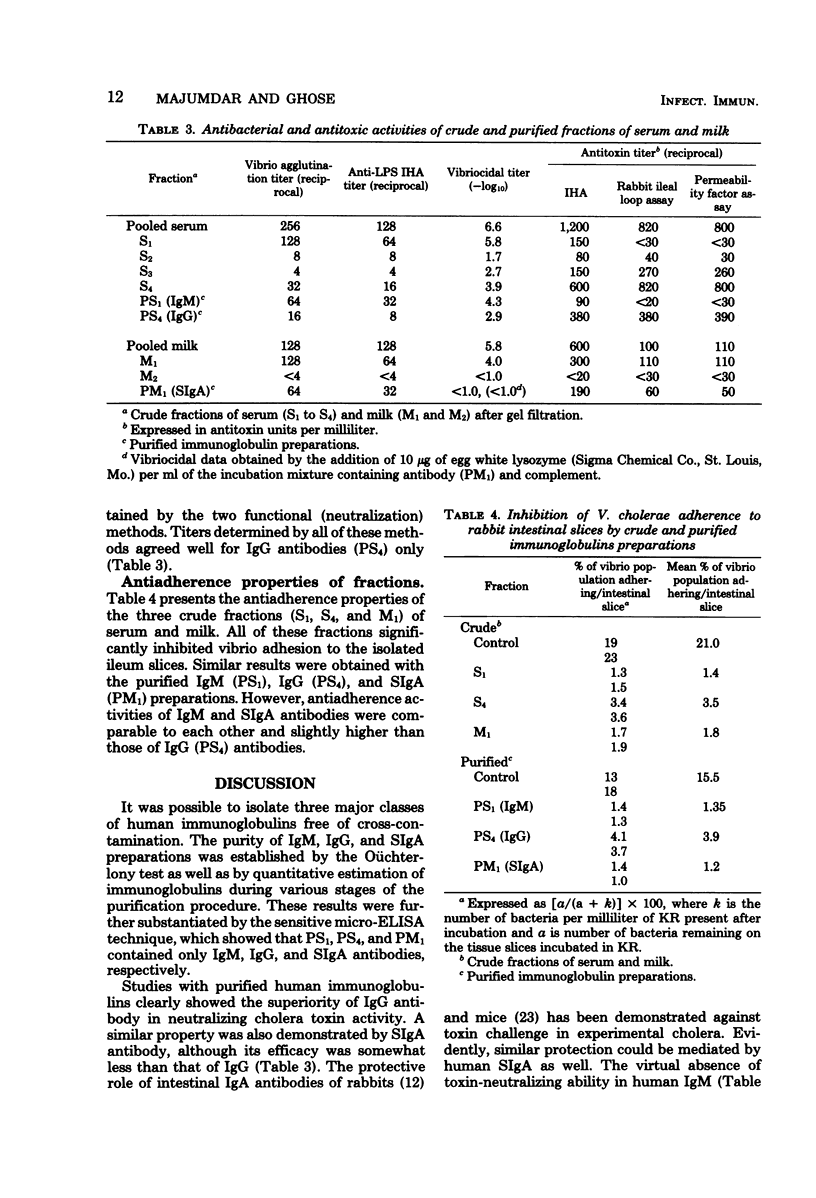
Different classes of immunoglobulins (immunoglobulin M [IgM], IgG, and secretory IgA) were purified from pooled serum and milk samples of convalescent cholera patients by gel filtration and immunoadsorbent techniques. The purity of these preparations was established by immunodiffusion and the enzyme-linked immunosorbent assay, using class-specific antisera. The biological properties of antibodies present in these crude and purified immunoglobulin preparations were evaluated by tests related to cholera. Purified human IgM and IgG exhibited both agglutinating and vibriocidal properties. On the other hand, human secretory IgA was not vibriocidal (even in the presence of lysozyme), although it showed agglutinating properties. Both IgG and secretory IgA could effectively neutralize cholera toxin action in vivo, whereas such activity was virtually absent in IgM. The toxin-neutralizing capacity of IgG was, however, higher than that of secretory IgA. All three classes of human antibodies could significantly inhibit the adherence of Vibrio cholerae to intestinal slices in vitro. These results are discussed in relation to the protective immune mechanism during cholera infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHASKARAN K. SEGREGATION OF GENETIC FACTORS DURING RECOMBINATION IN VIBRIO CHOLERAE, STRAIN 162. Bull World Health Organ. 1964;30:845–853. [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Curlin G. T., Craig J. P., Subong A., Carpenter C. C. Antitoxic immunity in experimental canine cholera. J Infect Dis. 1970 May;121(5):463–470. doi: 10.1093/infdis/121.5.463. [DOI] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Heddle R. J., Knop J., Steele E. J., Rowley D. The effect of lysozyme on the complement-dependent bactericidal action of different antibody classes. Immunology. 1975 Jun;28(6):1061–1066. [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Enzyme-linked immunosorbent assays for cholera serology. Infect Immun. 1973 May;7(5):759–763. doi: 10.1128/iai.7.5.759-763.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Mechanisms of disease and immunity in cholera: a review. J Infect Dis. 1977 Aug;136 (Suppl):S105–S112. doi: 10.1093/infdis/136.supplement.s105. [DOI] [PubMed] [Google Scholar]
- Kaur J., McGhee, Burrows W. Immunity to cholera: the occurrence and nature of antibody-active immunoglobulins in the lower ileum of the rabbit. J Immunol. 1972 Feb;108(2):387–395. [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Craig J. P., Hoover D., Bergquist E. J., Waterman D., Holley H. P., Hornick R. B., Pierce N. P., Libonati J. P. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73(1):3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Majumdar A. S., Dutta P., Dutta D., Ghose A. C. Antibacterial and antitoxin responses in the serum and milk of cholera patients. Infect Immun. 1981 Apr;32(1):1–8. doi: 10.1128/iai.32.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- O'Daly J. A., Cebra J. J. Rabbit secretory IgA. I. Isolation of secretory component after selective dissociation of the immunoglobulin. J Immunol. 1971 Aug;107(2):436–448. [PubMed] [Google Scholar]
- Parikh I., March S., Cuatercasas P. Topics in the methodology of substitution reactions with agarose. Methods Enzymol. 1974;34:77–102. doi: 10.1016/s0076-6879(74)34009-8. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Reynolds H. Y. Immunity to experimental cholera. I. Protective effect of humoral IgG antitoxin demonstrated by passive immunization. J Immunol. 1974 Sep;113(3):1017–1023. [PubMed] [Google Scholar]
- Steele E. J., Chaicumpa W., Rowley D. Isolation and biological properties of three classes of rabbit antibody to Vibrio cholerae. J Infect Dis. 1974 Aug;130(2):93–103. doi: 10.1093/infdis/130.2.93. [DOI] [PubMed] [Google Scholar]
- Svennerholm A., Lange S., Holmgren J. Correlation between intestinal synthesis of specific immunoglobulin A and protection against experimental cholera in mice. Infect Immun. 1978 Jul;21(1):1–6. doi: 10.1128/iai.21.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Willis D. L., Berry L. J. Flagella-induced immunity against experimental cholera in adult rabbits. Infect Immun. 1979 Jul;25(1):220–228. doi: 10.1128/iai.25.1.220-228.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]