Abstract
Loss of STAT5 from liver tissue results in hepatosteatosis and enhanced cell proliferation. This study now demonstrates that liver-specific Stat5-null mice develop severe hepatosteatosis as well as hepatocellular carcinomas at 17 months of age even in the absence of chemical insults. To understand STAT5’s role as tumor suppressor we have identified and investigated new STAT5 target genes. Expression of Nox4, the gene encoding the Reactive Oxygen Species (ROS) generating enzyme NOX4, was induced by growth hormone through STAT5. In addition, the genes encoding the pro-apoptotic proteins PUMA and BIM were induced by growth hormone through STAT5, which bound to GAS motifs in the promoter regions of all three genes. We further show that STAT5-induced activation of Puma and Bim was dependent on NOX4. Treatment of mice with TGF-β, an inducer of apoptosis, resulted in cleaved caspase 3 in control but not in liver-specific Stat5-null mice. This study for the first time demonstrates that cytokines through STAT5 regulate the expression of the ROS generating enzyme NOX4 and key pro-apoptotic proteins. We propose that STAT5 harnesses several distinct signaling pathways in liver and thereby functions as a tumor suppressor. Besides suppressing the activation of STAT3, STAT5 induces the expression of pro-apoptotic genes and the production of ROS.
Keywords: STAT5, NOX4, PUMA, BIM, HCC
Introduction
Signal Transducers and Activators of Transcription (STAT) 5A and 5B are latent transcription factors that are induced by a plethora of cytokines, including growth hormone, prolactin and several interleukins.1 Recently, context-specific tumor suppressor functions have been associated with STAT5, such as inhibiting expression of NPM1-ALK2 and suppressing STAT3 and TGF-β activity in liver.3 Although active STAT5 has been detected in many human tumors, in normal cells constitutively active STAT5A induces senescence4. In particular, SOCS1 expression induced by aberrant STAT5 signaling can facilitate the process of cellular senescence, which is an important tumor suppressor mechanism5.
Mice from which the Stat5a/b locus has been deleted specifically in liver tissue displayed altered metabolic pathways and developed fatty liver (nonalcoholic steatohepatitis).6, 7 Treatment of these mice with CCl4 led to liver fibrosis and hepatocellular carcinoma (HCC) suggesting that STAT5 is a tumor suppressor.3 Aberrant activation of the TGF-β and STAT3 pathways in these mice appears to contribute to the CCl4-induced fibrosis and HCC.3
Defects in apoptosis can be pivotal contributors to the development of cancer and the impaired response of tumor cells to therapy.8 It is not clear to what extent STAT5 regulates apoptotic mechanism in liver tissue. The proapoptotic BH3-only proteins PUMA, BIM, and BID are essential for the activation of BAX- and BAK-dependent cell death programs.9 PUMA expression is reduced in melanoma tumor tissue,10 and loss of PUMA dramatically accelerated myc-induced lymphomagenesis in vivo.11 Concomitant loss of PUMA and BIM in respective knock-out mice exacerbated hyperplasia of lymphatic organs, and promoted spontaneous malignancies.12 Loss of PUMA- and BAX/BAK-dependent apoptosis also enhanced tumorigenesis in a hypoxia-induced tumor model.13 In liver, JNK1-dependent PUMA expression induced hepatocyte lipoapoptosis.14 Moreover, BIM and PUMA induction, and BAX activation by palmitate induced apoptosis in hepatocytes.15 BIM and BID are critical contributors in hepatocyte apotosis caused by TNFα in vivo.16 TNFα can cooperate with FasL to induce hepatocyte apoptosis by activating BIM and BID.17 These results demonstrate that PUMA and BIM can function as tumor suppressors in mice.
Recent studies have demonstrated that NOX4 as a source of oxidative stress promotes apoptosis in vascular endothelial cell18 and hepatocyte,19 mitochondrial dysfunction in cardiac myocytes,20,21 and cellular senescence in hepatocytes.22
In a quest to further understand STAT5’s role as a liver-specific tumor suppressor we have identified novel STAT5 target genes in liver and mouse embryonic fibroblasts. This study for the first time explores the link between STAT5 and NOX4 and the apoptotic proteins PUMA and BIM.
Materials and Methods
Mice breeding
Stat5f/f;Alb-Cre mice were generated by breeding Stat5f/f mice with Alb-Cre transgenic mice.23 Stat5f/f and Alb-Cre transgenic mice were on a mixed background. Only 8- to 68-week-old male mice were used in the experiments unless otherwise indicated. Animals were treated humanely and experiments and procedures were performed according to the protocol approved by the Animal Use and Care Committee at the National Institute of Diabetes and Digestive and Kidney Diseases.
Liver induced by CCl4 or GH
Hepatic fibrosis in mice was induced by intraperitoneal (i.p.) injection with 2 ml/kg body weight of 10% CCl4 (Sigma, St. Louis, MO) dissolved in olive oil (Sigma, St. Louis, MO), 3 times per week for 12 weeks. For growth hormone (GH) stimulation, mice were injected i.p. with GH (2µg/g body weight) (mGH, NHPP, NIDDK). Four hours after injection mice were euthanized and livers were harvested for analyses.
Cell Culture
Mouse hepatocyte AML12 cells were obtained from ATCC (Manassas, VA) and cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12 medium supplemented with 10% fetal bovine serum (FBS), 5 µg/mL insulin, 5 µg/mL transferrin, 5 ng/mL selenium, and 40 ng/mL dexamethasone at 37°C with 5% CO2.
Antibodies, immunoblotting and immunostaining
In brief, liver tissue was lysed by adding NuPAGE LDS Sample buffer (Invitrogen, Carlsbad, CA). Western blotting was performed according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). The rabbit polyclonal anti-STAT5 (C-17), anti-STAT3 (C-20), anti-β-actin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-STAT5, anti-phospho-STAT3 (Cell Signaling Technology, Beverly, MA), anti-NOX4 (Novus Biologicals, Littleton, CO), anti-PUMA (Abcam, Cambridge, MA) and anti-BIM (Cell Signaling Technology, Beverly, MA) were used for probing western blots. Immunohistochemistry was performed using standard procedures. In short, liver tissues were removed and fixed in 10% neutral buffered formalin and embedded in paraffin wax. Five µm sections were prepared for hematoxylin and eosin (H&E) staining and immunofluorescence analyses. After deparaffinization, antigen unmasking was performed in a Decloaking chamber (Biocare Medical, San Diego, CA) using BORG Decloaker Solution (Biocare Medical, San Diego, CA) for 5 min at 125°C. The sections were blocked for 30 min in TBS-T containing 3% goat serum. Primary antibodies used in this study included rabbit anti-phospho-STAT5 (Tyr694), anti-cleaved Caspase-3 (Cell Signaling Technology, Beverly, MA), rabbit anti-NOX4 (Novus Biologicals, Littleton, CO), rabbit anti-PUMA (Abcam, Cambridge, MA), anti-BIM (Cell Signaling Technology, Beverly, MA), anti-phospho-histone H3 (Upstate Biotechnology, Lake Placid, NY) and anti-Ki 67 (Santa Cruz Biotechnology, Santa Cruz, CA) in addition to mouse anti-β-catenin (BD Transduction Laboratories, San Jose, CA). For double-labeling immunofluorescence analyses, sections exposed to a pair of primary antibodies were incubated in a 1:400 dilution of goat anti-rabbit IgG conjugated with a red fluorophore (Alexa Fluor 594; Molecular Probes, Eugene, OR) and goat anti-mouse IgG conjugated with a green fluorophore (Alexa Fluor 488; Molecular Probes, Eugene, OR) for 30 min at room temperature. Images were obtained with a Retiga Exi camera on a Olympus BX51 microscope (Olympus America, Center Valley, PA) using Image-Pro 5.1 software.
Chromatin immunoprecipitation assay
For growth hormone (GH) stimulation, mice were injected with 2 µg/g body weight of GH by i.p. They were sacrificed 45 minutes after injection and liver tissue was harvested. Non-injected mice were used as controls. Liver tissue was cross-linked in 1.5% formaldehyde for 15 min at 37°C and sonicated using the Misonix Sonicator 3000 (Misonix, Farmingdale, NY, USA). Immunoprecipitation was carried out in TE buffer containing protease inhibitors (Sigma, St. Louis, MO). Chromatin was incubated with protein A Dynabeads (Invitrogen, Carlsbad, CA), which were pre-incubated with STAT5A or IgG antibody (R&D Systems, Minneapolis, MN, USA). Immunoprecipitated DNA was eluted and amplified by real-time PCR using a 7900 HT fast real-time PCR system (Applied Biosystems, Foster City, CA) and analyzed using SDS2.3 Software (Applied Biosystems, Foster City, CA). Sequence-specific primers used for amplification of the putative STAT5 binding sites (GAS sites) within the Socs2, Nox4, Puma and Bim genes were as following: For the Socs2 GAS sequence, forward primer 5’-GGAGGGCGGAGTCGCAGGC-3’, reverse primer 5’-GACTTGGCAAGAGTTAACCGTC-3’; the primer sets for Nox4 gene were: GAS1, forward 5’-AGGCTACTTCCGGCTCAAAT-3’, reverse 5’-GCGCATACACCCTACTTCCT-3’; GAS2, forward 5’-CCCAATCAGGGCATACATTT-3’, reverse 5’-TTTCCCATTCCTAGCACAGC-3’; the primer sets for Puma gene were: GAS1, forward 5’-AGCAGGAACCTGTCTCAGGA-3’, reverse 5’-TAAAGGCTGACCCCTTCTCA-3’; the primer sets for Bim gene were: GAS1, forward 5’-GAAGAGGGGTGAGCATCTTG-3’, reverse 5’-CAGTTGGAAGCCTCAGAAGG-3’; GAS2, forward 5’-GGGTCGGTACTGGCATCTAA-3’, reverse 5’-GCTCGGCGTTAATCACTTTC-3’.
RNA isolation and quantitative real-time PCR analysis
Total RNA was isolated from liver tissue of Stat5f/f, Stat5f/f;Alb-Cre mice and hepatocytes using RNeasy mini kit (Qiagen, Valencia, CA) and one µg of RNA was reverse transcribed (cDNA reverse transcription kit; Applied Biosystems, Foster City, CA). Real-time quantification of mRNA transcript levels was performed using the TaqMan gene Expression Master Mix (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Real-time PCR was carried out using an ABI Prism 7900HT (Applied Biosystems, Foster City, CA). TaqMan probes for Nox4 (Mm00479246_m1), Socs2 (Mm00850544_g1), Puma (Mm00519268_m1), Bim (Mm00437795_m1) and beta-actin (4352341E) were used (Applied Biosystems, Foster City, CA) for Real-time PCR. The SYBR primer were Cdkn2b, forward 5’-CCCTGCCACCCTTACCAGA-3’, reverse 5’-CAGATACCTCGCAATGTCACG-3’; GAPDH, forward 5’-AACGACCCCTTCATTGAC-3’, reverse 5’-TCCACGACATACTCAGCAC-3’.
Statistics
All statistical analyses were performed using the Student’s t test (2 tailed, unpaired). A P value of 0.05 or less was considered significant.
Results
STAT5-dependent regulation of Nox4, Puma and Bim in liver tissue
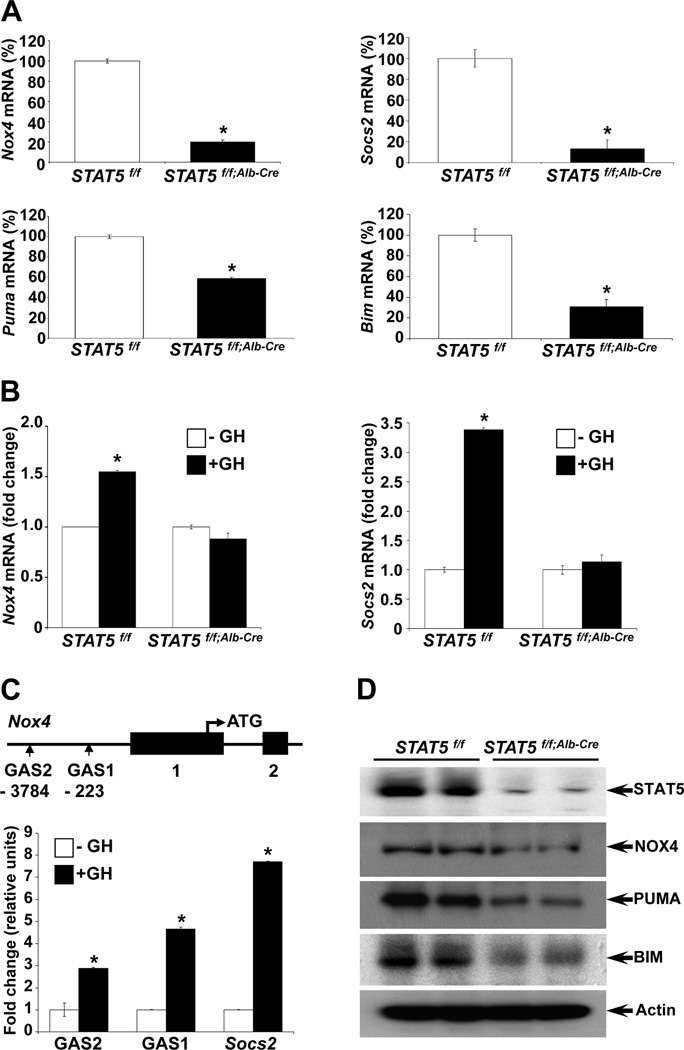
In a quest to gain further insight into STAT5’s role as tumor suppressor and understand underlying genetic pathways, we mined microarray-based expression data from liver tissue of control and liver-specific Stat5-null mice and from Stat5+/+ and Stat5−/− mouse embryonic fibroblasts (MEFs) (for GEO accession numbers see Materials and Methods). In addition to the reduced expression of genuine STAT5 target genes, such as Socs2, in Stat5-null liver tissue we observed a 2.5- and 3.6-fold reduction of Nox4 and Bim mRNA levels, respectively (Supporting Table 1). Similarly, expression of Nox4 in Stat5−/− MEFs was reduced 3.3-fold (Supporting Table 2). In addition, we observed a 5.7-fold reduction of Puma mRNA in Stat5−/− MEFs. While NOX4 (NADPH oxidase 4) is a reactive oxygen species (ROS) generating enzyme, BIM and PUMA are pro-apoptotic proteins. Quantitative RT-PCR and western blots confirmed GH- and STAT5-dependency of the Nox4, Puma and Bim genes in liver tissue. Nox4, Puma and Bim mRNA levels were reduced in Stat5-null livers (Fig. 1A). The Socs2 gene served as a positive control (Fig. 1A,B). NOX4, PUMA and BIM protein concentrations were also reduced in Stat5-null livers (Fig. 1D). Actin served as a loading control and the greatly reduced STAT5 levels verified the efficient deletion of the Stat5 locus. To establish GH-dependent expression in vivo, control and liver-specific Stat5-null mice were injected with GH followed by mRNA analyses. While GH treatment of control mice induced Nox4 mRNA levels, no such increase was observed in the absence of STAT5 (Supporting Table 1, Fig. 1B).
Figure 1.
STAT5 regulates Nox4 expression through STAT5 binding to conserved GAS sites in the Nox4 gene promoter in liver. (A) Expression of Nox4, Puma, Bim and Socs2 was analyzed by quantitative real-time PCR in liver tissue from Stat5f/f and Stat5f/f;Alb-Cre mice. Values are shown as means ± SD. (B) mRNA expression of Nox4 and Socs2 in Stat5f/f and Stat5f/f;Alb-Cre mice. Mice were injected with GH, tissue was harvested after four hours and RNA was analyzed by quantitative real-time PCR. All values represent means ± SD. (C) Schematic of the Nox4 gene. Vertical boxes indicate exons and conserved GAS sequences are marked. Chromatin immunoprecipitation (ChIP) analysis of STAT5 binding to the putative GAS sites. Stat5f/f mice were injected with GH, tissue was harvested after 45 minutes and binding to GAS sites was analyzed by quantitative real-time PCR. DNA was amplified from STAT5-precipitated complexes using specific primers spanning GAS motifs in the Socs2 and Nox4 genes. All values represent means ± SD from 3 independent experiments performed in triplicates. (D) Levels of NOX4, PUMA and BIM in liver tissue from Stat5f/f and Stat5f/f;Alb-Cre mice. Expression of NOX4, PUMA and BIM was determined by western blotting. *P < .05; compared with corresponding controls.
To determine whether STAT5 directly binds to, and thereby controls the Nox4 gene in liver, we scanned the promoter region for GAS motifs. ChIP analyses in Stat5-null livers confirmed GH-induced STAT5 binding to two GAS motifs in the Nox4 gene promoter (Fig. 1C). STAT5 binding to a GAS motif in the Socs2 gene promoter served as a positive control (Fig. 1C).
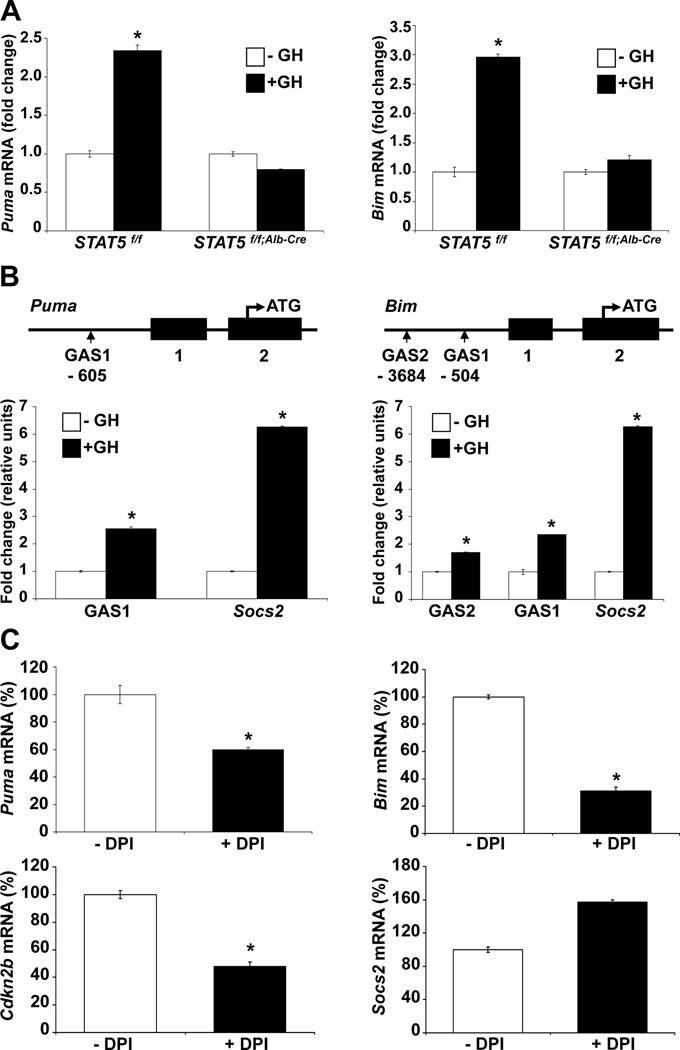
Similar to Nox4, GH-induced Puma and Bim expression in liver tissue was STAT5 dependent (Fig. 2A) and STAT5 bound to GAS motifs in the respective promoter regions as determined by ChIP analyses (Fig. 2B). Binding to the Socs2 gene promoter served as a positive control.
Figure 2.
STAT5 regulates expression of Puma and Bim through STAT5 binding to GAS sites in the Puma and Bim gene promoters in liver. (A) mRNA expression of Puma and Bim in Stat5f/f and Stat5f/f;Alb-Cre mice injected with GH. Mice were injected with GH and tissue was harvested after 4 hours. Expression of Puma and Bim was analyzed by quantitative real-time PCR in liver tissue from Stat5f/f and Stat5f/f;Alb-Cre mice. All values represent means ± SD. (B) Schematic of the Puma and Bim genes. Vertical boxes indicate exons and the location of conserved GAS sequences is shown. Chromatin immunoprecipitation (ChIP) analysis of STAT5 binding to the putative GAS sites. Stat5f/f mice were treated with GH and tissue was harvested after 45 minutes. Binding to GAS sites was analyzed by quantitative real-time PCR. DNA was amplified from STAT5-precipitated complexes using specific primers for known (Socs2) and suspected (Puma and Bim) GAS regions. All values represent means ± SD from 3 independent experiments performed in triplicates. (C) mRNA expression of Puma, Bim, Cdkn2b and Socs2 in immortalized wildtype hepatocyte of murine origin. The cells were treated with DPI for 2 hours. Expression of Puma, Bim, Cdkn2b and Socs2 mRNA was analyzed by quantitative real-time PCR. All values represent means ± SD from 3 independent experiments. *P < .05; compared with corresponding controls.
STAT5 does not control the anti-apoptotic genes Bcl2, Bcl2l1 and Mcl1
To determine whether STAT5 also controls expression of anti-apoptotic genes, we analyzed mRNA levels of the Bcl2, Bcl2l1 and Mcl1 genes in control and Stat5-null livers. The respective mRNA levels did not change significantly in the absence of STAT5 suggesting that these genes are not under STAT5 control (Supporting Fig. 1A). Moreover, Bcl2, Bcl2l1 and Mcl1 mRNA levels did not change upon acute GH treatment of mice (Supporting Fig. 1B). We also explored direct STAT5 binding to the respective genomic loci in MEFs through ChIP-seq analyses. Although GAS motifs were identified in the Bcl2, Bcl2l1 and Mcl1 gene promoters, no significant STAT5 binding was observed (Supporting Fig. 1C). In addition, no binding was observed in the miR15/16 locus. Binding to the promoter-bound GAS motif in the Socs2 gene served as a positive control.
Expression of Nox4 in MEFs is under STAT5 control
To gain mechanistic insight into the STAT5 control of Nox4, Puma and Bim and their interrelationship, we resorted to Stat5−/− MEFs and Stat5−/− MEFs ectopically expressing STAT5A (Stat5−/−; Stat5A) using a retroviral expression vector. This system also permitted us to study links between STAT5 and NOX4 promoted ROS production. Overexpression of STAT5A in Stat5−/− MEFs led to a further increase of Nox4 and Socs2 expression (Supporting Fig. 2A) and GH-induced expression of these genes was restored (Supporting Fig. 2B). STAT5-mediated induction of NOX4 was also observed at the protein level (Supporting Fig. 2E). To address whether the Nox4 gene is under direct GH/STAT5 control, Stat5+/+ and Stat5−/− MEFs were stimulated with GH. While Nox4 expression was induced 1.9-fold in Stat5+/+ MEFs, no induction was observed in Stat5−/− MEFs, (Supporting Fig. 3A). Similarly, Socs2 gene expression was not stimulated by GH in Stat5−/− MEFs (Supporting Fig. 3A). ChIP assays confirmed that STAT5 binds to the conserved proximal GAS motifs in the Nox4 gene promoter (Supporting Fig. 2C). STAT5 binding to the Socs2 gene promoter served as a positive control. Western blot analyses confirmed the reduction of NOX4 in Stat5−/− MEFs (Supporting Fig. 2D). NOX4 and BIM levels were increased in Stat5−/−; Stat5A MEFs as compared to parental Stat5−/− MEFs further supporting that STAT5 directly controls expression of these genes (Supporting Fig. 2E).
STAT5 controlled expression of Puma and Bim
Expression of Puma and Bim was STAT5-dependent and under GH control in MEFs (Supporting Fig. 3A). Western blot analyses confirmed the reduction of PUMA and BIM in Stat5−/− MEFs (Supporting Fig. 2D). Overexpression of STAT5A in Stat5−/− MEFs further increased Puma and Bim mRNA levels (Supporting Fig. 4A) and GH-dependent induction of Puma and Bim expression was observed in Stat5−/−; Stat5A but not in Stat5−/− MEFs carrying an empty control retrovirus (Supporting Fig. 4B). Tyrosine p-STAT5 was detected in GH stimulated Stat5+/+ MEFs (Supporting Fig. 3C) and elevated levels were observed in Stat5−/−; Stat5A MEFs (Supporting Fig. 3D). Levels of p-p53 were also increased in Stat5−/−; Stat5A MEFs as compared to parental Stat5−/− MEFs (Supporting Fig. 2E). Puma as a p53 target gene might be regulated by STAT5/p53 signaling.
One GAS motif was identified at position -605 in the Puma gene and two conserved GAS motifs were identified at positions -3684, and -540 in the Bim gene (Supporting Fig. 4C). ChIP analyses in Stat5+/+ MEFs confirmed GH-induced STAT5 binding to these GAS motifs (Supporting Fig. 4C). Binding to the Socs2 gene promoter served as a positive control.
To explore mechanistic links between p-p53 and expression of a subset of p53 target genes, we analyzed Stat5−/− and Stat5−/−; Stat5A MEFs. Expression of Bax, Fas, Noxa and Ataf was increased in Stat5−/−; Stat5A MEFs compared to Stat5−/− MEFs carrying an empty control retrovirus (Supporting Fig. 5). Expression of the p53 gene was not changed in Stat5−/−; Stat5A MEFs compared to Stat5−/− MEFs.
STAT5/NOX4-dependent regulation of ROS levels
To determine whether ROS generation is under direct STAT5/NOX4 control, Stat5+/+ and Stat5−/− MEFs were cultured and assayed for ROS using DCF-DA and lucigenin. DCF fluorescence, an indicator of ROS, was stronger in Stat5+/+ MEFs than in Stat5−/− MEFs ones (Supporting Fig. 6A). Treatment with H2O2 further increased the production of ROS in Stat5+/+ MEFs, as compared to Stat5−/− MEFs (Supporting Figs. 6A,7A). The Lucigenin chemiluminescent assays established that STAT5 deficiency led to a reduced level of intracellular ROS in MEFs (Supporting Fig. 6B). Treatment of Stat5+/+ MEFs with diphenylene iodonium (DPI), a NOX inhibitor, reduced ROS levels (Supporting Fig. 6A, 7B). Although DPI inhibits several NOX members, NOX4 is the only one expressed at appreciable levels in liver tissue. This suggests that ROS in MEFs originates from NOX4.
Puma and Bim are regulated by NOX4
To explore mechanistic links between STAT5A and NOX4 and expression of the Puma and Bim genes, we analyzed Stat5−/− MEFs in the absence and presence of retrovirally introduced STAT5 (Stat5−/−; Stat5A). Upon treatment of MEFs with DPI, expression of Puma and Bim was reduced only in MEFs expressing STAT5A (Supporting Fig. 6C). These data provide evidence that the Puma and Bim genes are regulated by STAT5 through NOX4 signaling. STAT5A-induced expression of the Cdkn2b gene, encoding a cell cycle inhibitor p15INK4B, was partially suppressed in the presence of DPI (Supporting Fig. 8A, B) suggesting the STAT5-target Cdkn2b is also under NOX4 control.
Treatment of MEFs with H2O2 further induced Puma mRNA levels in the presence of STAT5A but not in the absence of STAT5 (Supporting Fig. 6D). Simultaneous treatment with DPI led to a suppression of Puma expression (Supporting Fig. 6D). Cell survival in the presence of H2O2 was less affected in the absence of STAT5 (Supporting Fig. 6E). Simultaneous treatment with DPI led to a rebound of cell survival in the presence of STAT5A and to a lesser extent in the absence of STAT5 (Supporting Fig. 6E). These data suggest that STAT5/NOX4 signaling in MEFs controlled PUMA-induced apoptosis and p15INK4B-regulated cell cycle inhibition.
Puma and Bim are regulated by NOX4 in hepatocytes
To explore a possible relationship between STAT5/NOX4 and the Puma and Bim genes in hepatocytes, the cell line AML12 was treated with the NOX inhibitor DPI. This resulted in reduced levels of Puma and Bim mRNA (Fig. 2C). DPI treatment also resulted in decreased Cdkn2b expression. However, it did not change expression of the STAT5 target gene Socs2. Although DPI inhibits several NOX members, NOX4 is the only one family member expressed at appreciable levels in hepatocytes.24 These data imply that the direct STAT5 target gene Cdkn2b is also regulated by STAT5/NOX4 signaling.
As shown above, STAT5 did not bind to the Bcl2, Bcl2l1 and Mcl1 gene loci and expression was not controlled by STAT5 (Supporting Fig. 1A–C). To test whether these anti-apoptotic genes were regulated by NOX4, AML12 hepatocytes were treated with the NOX inhibitor DPI. Expression of Bcl2, Bcl2l1 and Mcl1 was similar in treated and untreated cells (Supporting Fig. 1D), suggesting that these genes are not under STAT5/NOX4 control.
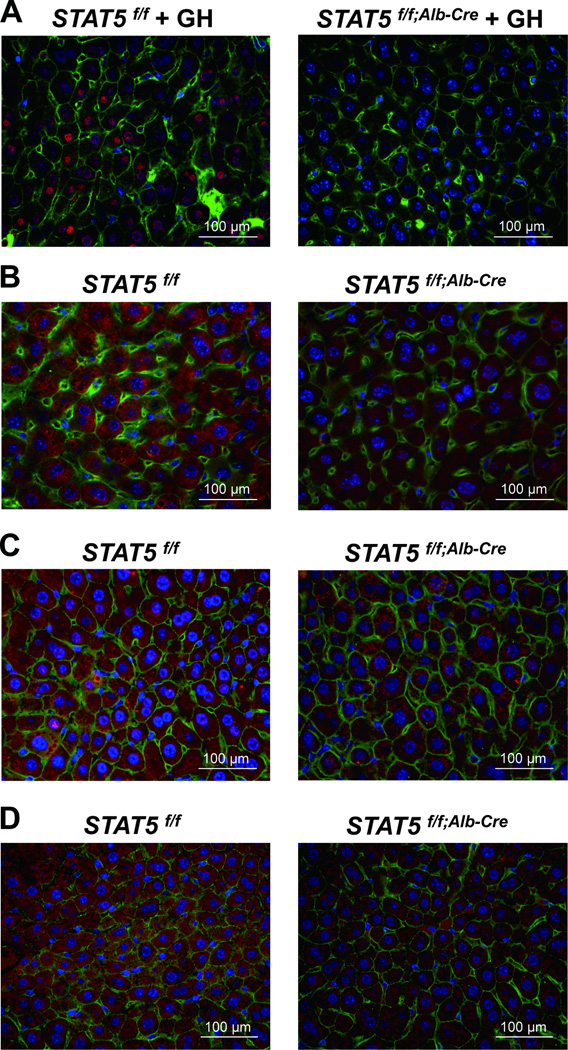
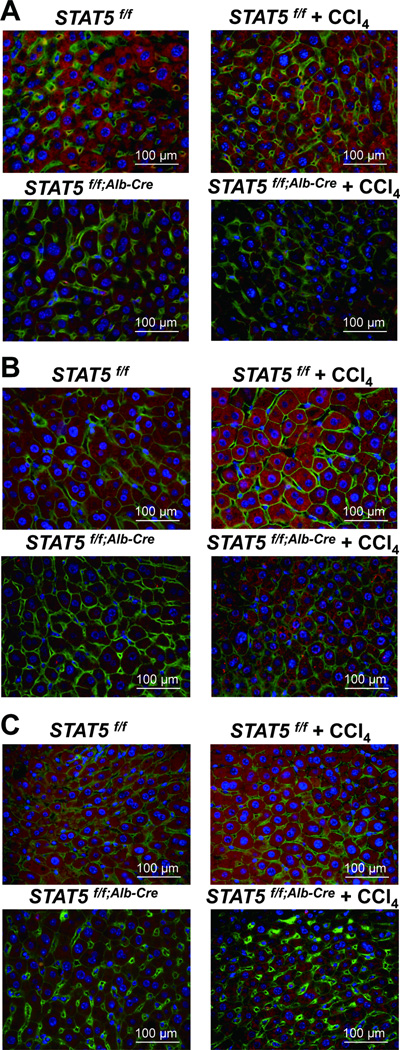
Immunohistochemistry was used as an independent means to corroborate the importance of STAT5 on the accumulation of NOX4, PUMA and BIM. NOX4, PUMA and BIM were observed in liver tissue of control mice (Fig. 3B–D, left panels) and at lower levels in liver-specific Stat5-null mice (Fig. 3B–D, right panels). GH-induced nuclear phospho-STAT5 staining was observed in control mice but not in the absence of STAT5 (Fig. 3A).
Figure 3.
Immunostaining of phospho-STAT5, NOX4, PUMA and BIM in Stat5f/f and Stat5f/f;Alb-Cre mice. (A) Livers from Stat5f/f and Stat5f/f;Alb-Cre mice were harvested after GH injection and analyzed for p-STAT5 expression using immunofluorescence staining with anti-p-STAT5 (red), anti-β-catenin (green) antibodies and DAPI (blue). (B–D) Livers from Stat5f/f and Stat5f/f;Alb-Cre mice were harvested and analyzed for NOX4 (B), PUMA (C) and BIM (D) expression using immunofluorescence staining with anti-NOX4 (red), anti-PUMA (red), anti-BIM (red), anti-β-catenin (green) antibodies and DAPI (blue).
STAT5-dependent regulation of hepatoprotective proteins
Since loss of STAT5 is correlated with the development of liver disease it is possible that STAT5 promotes the expression of hepatoprotective genes. We therefore analyzed whether the hepatoprotective genes Hnf6, Lifr, Egfr and Prlr were under GH/STAT5 control. While GH treatment of control mice induced Hnf6, Lifr, Egfr and Prlr mRNA levels, no such increase was observed in the absence of STAT5 (Supporting Fig. 9). Expression of Hnf6, Lifr, Egfr and Prlr mRNA was slightly, yet not significantly, reduced in liver-specific Stat5-null mice (Supporting Fig. 9). Thus, reduced levels of hepatoprotective proteins may contribute to the development of liver disease in liver-specific Stat5-null mice.
Loss of STAT5 promotes the development of HCC in liver-specific Stat5-null mice
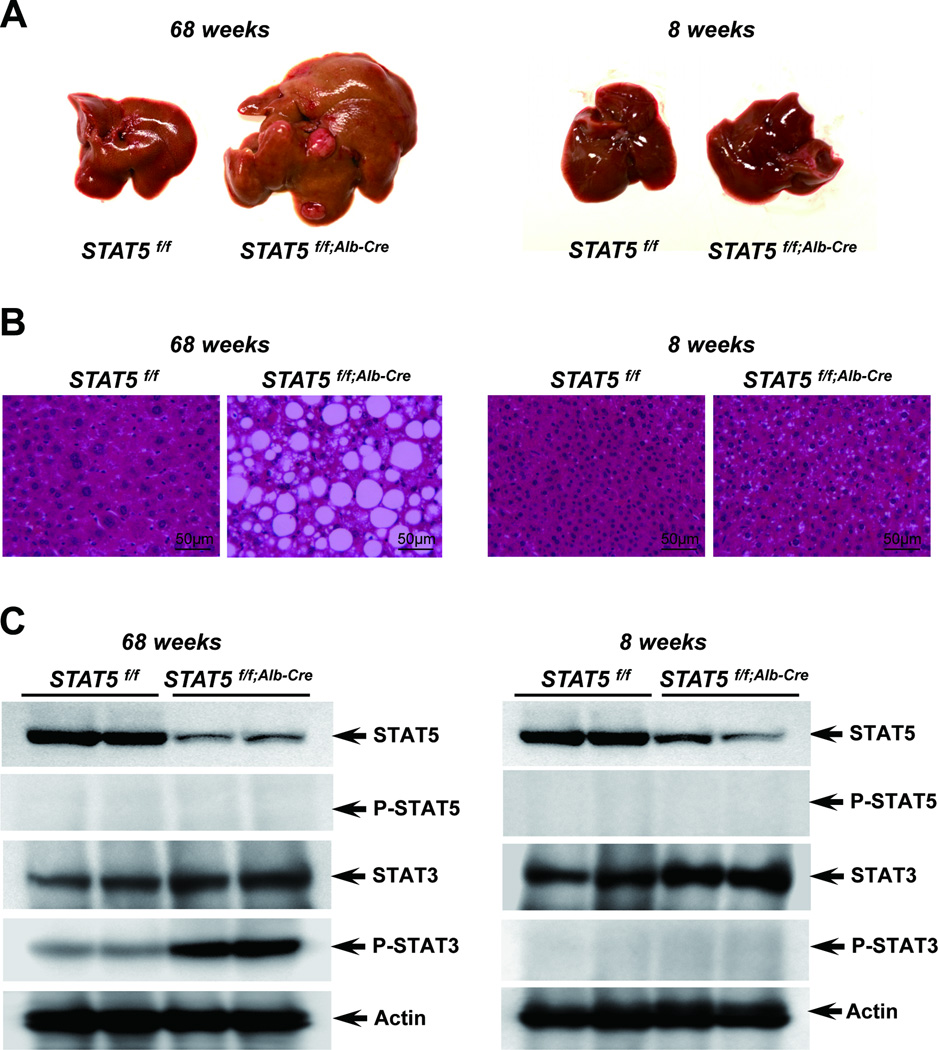
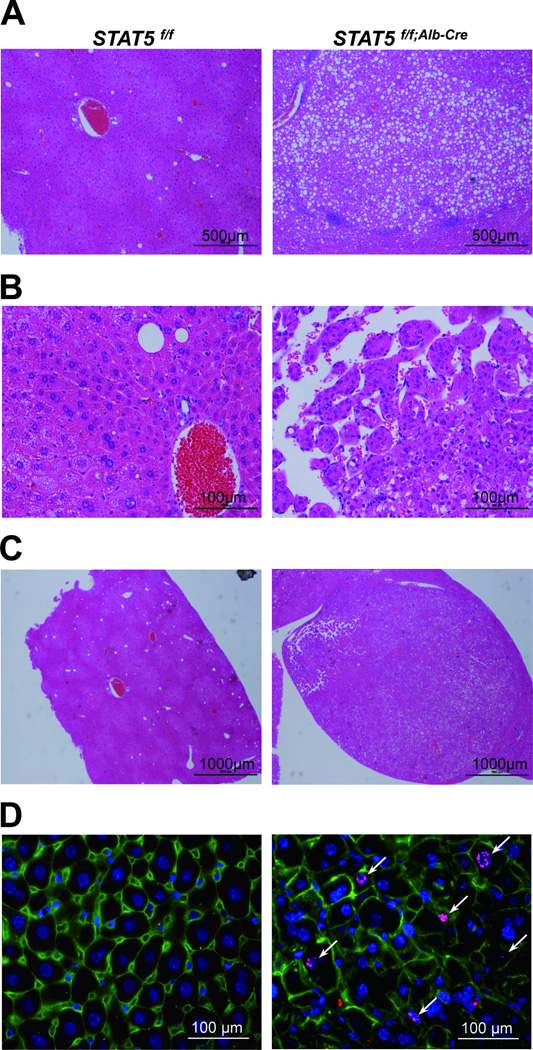
We had shown earlier that loss of STAT5 from liver tissue resulted in hepatosteatosis and HCC upon CCl4 exposure in 3 months old mice.3.25 To investigate whether loss of STAT5 can lead to the development of HCC without chemical injury, we analyzed control and liver-specific Stat5-null mice at 17 months of age. Severe heptosteatosis and HCC were observed in all four experimental mice analyzed but not in age-matched controls (Fig. 4, 5) and nodules were observed in two of the four mice. To investigate molecular consequences associated with the development of HCC, we analyzed p-STAT5 and p-STAT3 levels in control and liver-specific Stat5-null mice at 17 months of age. P-STAT3 levels were greatly elevated in liver-specific Stat5-null mice at 17 months of age (Fig. 4C) but not at 2 months. To determine whether loss of STAT5 correlated with increased cell proliferation, tissue sections were stained for phosphorylated histone H3 as a measure of cell proliferation (Fig. 5D). The number of phospho-Histone H3 positive nuclei in liver-specific Stat5-null mice at 17 months was higher than in age-matched controls.
Figure 4.
Loss of STAT5 induces development of tumor in Stat5f/f;Alb-Cre mice. (A) Liver of Stat5f/f and Stat5f/f;Alb-Cre mice at 17 months (left) and 2 months (right). (B) Hematoxylin and eosin (H&E) staining of liver sections from Stat5f/f and Stat5f/f;Alb-Cre mice. (C) Level of STAT5, p-STAT5, STAT3 and p-STAT3 in liver tissues from Stat5f/f and Stat5f/f;Alb-Cre mice. Expression of STAT5, p-STAT5, STAT3 and p-STAT3 was determined by western blotting.
Figure 5.
Histological analyses and immunostaining of phospho-Histone H3 in liver tissue from Stat5f/f and Stat5f/f;Alb-Cre mice. (A–C) Hematoxylin and eosin (H&E) staining of liver sections from Stat5f/f and Stat5f/f;Alb-Cre mice at 17 months of age. Hepatosteatosis (A) and hepatocellular carcinoma (HCC) (B) were only observed in liver-specific Stat5-null mice. Nodules were also observed only in liver-specific Stat5-null mice (C). (D) Liver tissue from 17 months old Stat5f/f and Stat5f/f;Alb-Cre mice was harvested and analyzed for phospho-Histone H3 using immunofluorescence staining with anti-phospho-Histone H3 (red), anti-β-catenin (green) antibodies and DAPI (blue).
As expected, levels of Nox4, Puma, Bim and Socs2 mRNA were reduced in 17 months old liver-specific Stat5-null mice compared to age matched controls (Supporting Fig. 10A). In contrast, and as expected, Bcl2l1 and Mcl1 mRNA levels were not altered (Supporting Fig. 10B). Unexpectedly, Bcl2 mRNA levels were increased in experimental mice (Supporting Fig. 10B).
CCl4 treatment results in STAT5-dependent increase of Puma and Bim
To further investigate whether CCl4 treatment contributes to the deregulation of Nox4, Puma and Bim we analyzed control and liver-specific Stat5-null mice at 3 months of age. CCl4 treatment induced Puma and Bim mRNA levels in control mice but not in liver-specific Stat5-null mice (Supporting Fig. 11). In contrast, no change of Nox4 expression was observed.
Using immunohistochemistry NOX4, PUMA and BIM were detected in liver tissue of control mice both in the absence and presence of CCl4 (Fig. 6A–C). In contrast, reduced NOX4, PUMA and BIM staining was observed in liver-specific Stat5-null mice in the absence and presence of CCl4 (Fig. 6A–C).
Figure 6.
Immunostaining of NOX4, PUMA and BIM in Stat5f/f and Stat5f/f;Alb-Cre mice injected with CCl4. (A–C) Liver tissue from Stat5f/f and Stat5f/f;Alb-Cre mice was harvested after 12 weeks of CCl4 injection and analyzed for expression of NOX4 (A), PUMA (B) and BIM (C) using immunofluorescence staining with anti-NOX4 (red), anti-PUMA (red), anti-BIM (red), anti-β-catenin (green) antibodies and DAPI (blue).
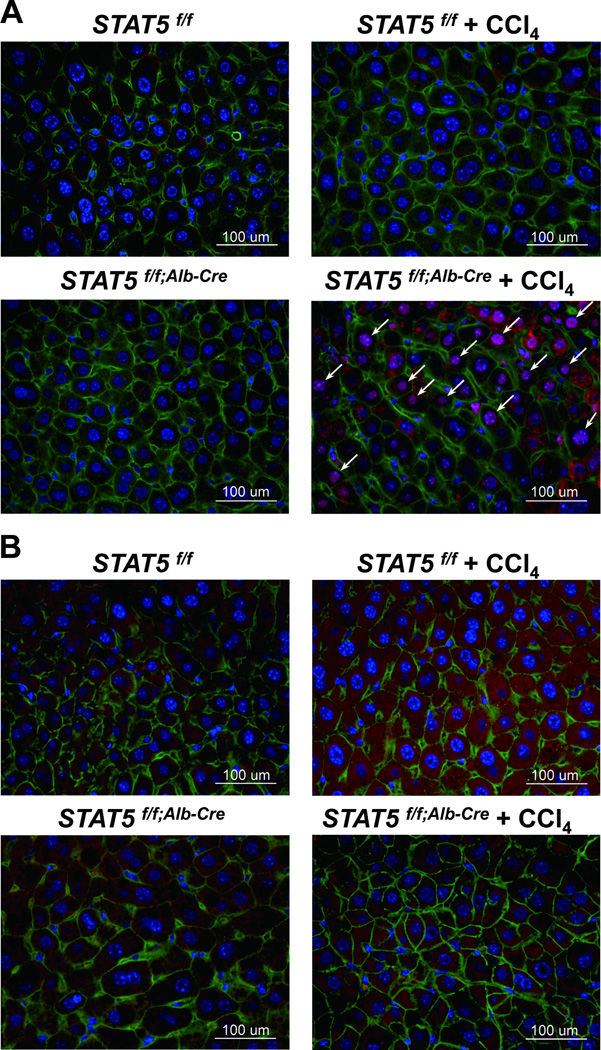
To establish whether loss of STAT5 and reduced levels of PUMA and BIM correlated with increased cell proliferation, we stained tissue sections for Ki-67 as a measure of cell proliferation (Fig. 7A). The number of Ki-67 positive cells increased in liver tissue of liver-specific Stat5-null mice that had been treated with CCl4 (Fig. 7A). In addition, activation of the apoptotic marker cleaved caspase-3 was decreased in liver tissue of Stat5-null mice treated with CCl4 compared to treated control mice (Fig. 7B). Levels of the pro-apoptotic protein BAX were decreased in liver tissue from Stat5-null mice compared to control mice (Supporting Fig. 12A,B). Proliferating cell nuclear antigen (PCNA), an indicator of cell proliferation, concentrations were elevated in liver-specific Stat5-null mice treated with CCl4 (Supporting Fig. 13A,B).
Figure 7.
Immunostaining of Ki-67 and cleaved caspase-3 in Stat5f/f and Stat5f/f;Alb-Cre mice injected with CCl4. (A) Liver tissue from Stat5f/f and Stat5f/f;Alb-Cre mice was harvested after 12 weeks of CCl4 injection and analyzed for Ki-67 using immunofluorescence staining with anti-Ki-67 (red), anti-β-catenin (green) antibodies and DAPI (blue). (B) Liver tissue from Stat5f/f and Stat5f/f;Alb-Cre mice was harvested after 12 weeks of CCl4 injection and analyzed for cleaved caspase-3 using immunofluorescence staining with anti-cleaved caspase-3 (red), anti-β-catenin (green) antibodies and DAPI (blue).
STAT5/NOX4-dependent regulation of apoptosis signaling in liver
To establish GH or TGF-β-dependent apoptosis signaling in vivo, control mice were injected with GH or TGF-β followed by protein and mRNA analyses. While GH treatment of control mice induced caspase3 activation and expression of Nox4, Puma and Bim, no such increase was observed in the absence of GH (Supporting Fig. 14A). TGF-β treatment of control mice, but not experimental mice, induced caspase3 activation and expression of Nox4, Puma and Bim mRNA levels (Supporting Fig. 14B). This suggests that caspase3 activation and expression of Puma and Bim by GH or TGF-β treatment induced apoptosis by STAT5/NOX4.
Discussion
While in many cell types the transcription factor STAT5 provides proliferative and survival cues by activating respective genetic programs, it serves as a bona fide tumor suppressor in liver tissue.3,25 Loss of STAT5 from liver tissue leads to hepatosteatosis and the development of HCC upon CCl4 treatment. In part STAT5’s function as tumor suppressor can be attributed to its ability to regulate the cell cycle control genes Cdkn2b and Cdkn1a.25 In addition, the presence of STAT5 also suppresses inappropriate cytokine-induced activation of STAT3, an oncoprotein in its own right.
We now provide evidence for additional venues used by STAT5 to control cell death and thus suppress the development of hepatocellular carcinoma. While CCl4 exposure is required to induce HCC in 3 months-old liver-specific Stat5-null mice, 17 months-old mice develop HCC in the absence of this chemical insult. Thus, loss of STAT5 by itself is sufficient to fundamentally alter cellular metabolism conducive to disease development. In this study we have identified and investigated additional STAT5 target genes whose deregulation likely contribute to the development of HCC in the absence of STAT5. Notably, STAT5 controls ROS production through the activation of the Nox4 gene and it activates the genes encoding the pro-apoptotic and tumor suppressive proteins PUMA and BIM. We therefore propose that STAT5 protects hepatocytes through several pathways, including the activation of cell death programs executed by NOX4, PUMA and BIM.
Studies on mice from which the genes encoding NOX4, PUMA and BIM had been deleted, as well as tissue culture cells expressing reduced levels of these proteins, provided sound evidence for these proteins in cell death programs. In hepatocytes, NOX4 is required for TGF-β -induced apoptosis19 and loss of NOX4 from lung epithelium is protective from TGF-β-induced apoptosis.26 In heart tissue, NOX4 protected cells from pressure overload-induced apoptosis.20 In addition to NOX4, NOX1 and NOX2 have also been linked to cell death in hepatocytes, as CCl4-dependent hepatic fibrosis and ROS generation were attenuated in the absence of the latter two isoforms.24,27,28 In addition BIM was also required for tumor cell apoptosis induced by a VEGF-A antagonist.29 Roles for BIM and PUMA in suppressing oncogenesis have been described for B cell leukemias30 and intestinal cells,31,32 respectively. In those cases, BIM and PUMA exerted a strong apoptotic effect and their loss lead to enhanced tumorigenesis.
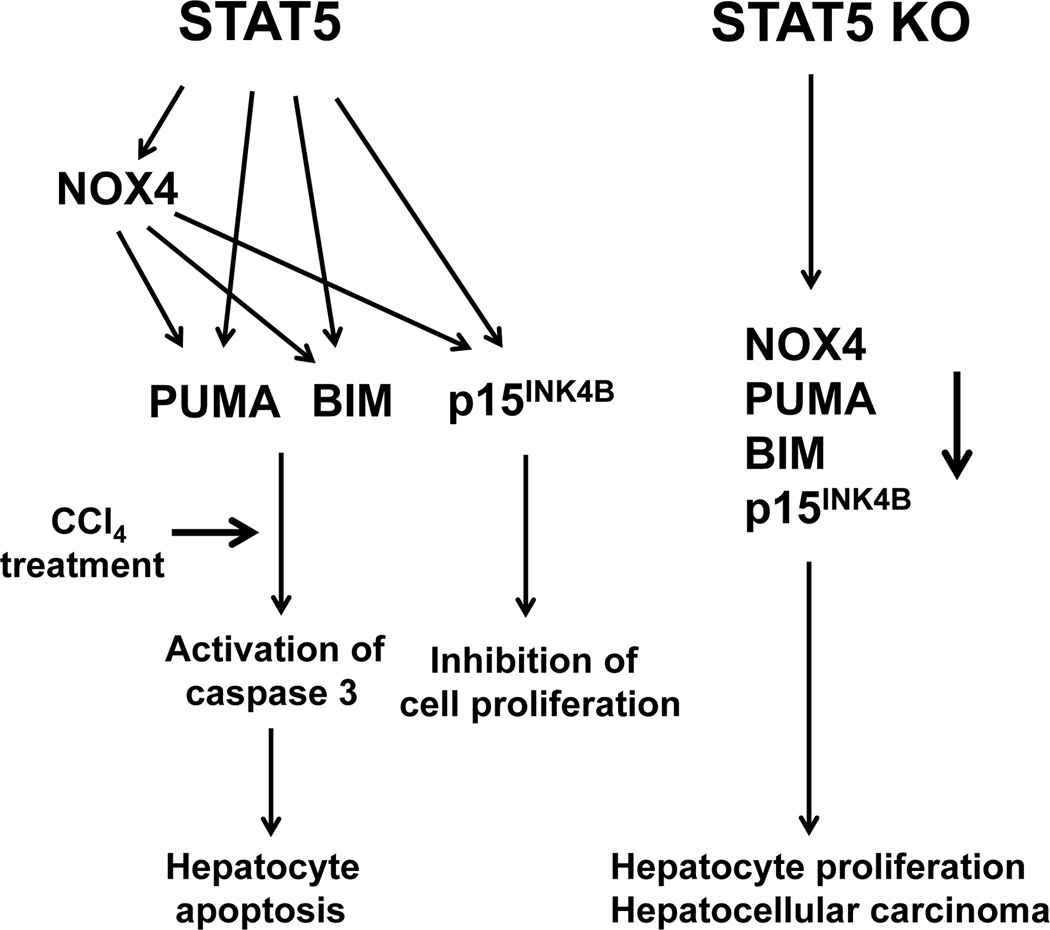
While STAT5 directly controls the expression of p15INK4B,25 PUMA and BIM (Fig. 8), it can also exert its function through activating another direct downstream target gene Nox4, which encodes NADPH oxidase 4, a key regulator of ROS.18,20 We further provide evidence for a direct link between NOX4 and PUMA and BIM. Inhibiting NOX4 activity led to decreased expression of PUMA and BIM and p15INK4B. The mechanism of this regulatory venue is still elusive.
Figure 8.
Proposed model of STAT5-regulated apoptosis in hepatocytes. Previous studies have shown that liver-specific STAT5-null mice develop hepatosteatosis and HCC upon treatment with CCl4. STAT5 regulates key cell cycle inhibitor and apoptotic genes. STAT5 directly activates the genes encoding NOX4, PUMA and BIM and the cell cycle inhibitor p15INK4B. NOX4 can also control PUMA, BIM and p15INK4B. We propose that loss of STAT5 induces hepatocyte proliferation and HCC upon CCl4 challenge as a result of decreased levels of PUMA, BIM and p15INK4B.
A picture is evolving that distinct signaling pathways emerging from STAT5 contribute to the protection of hepatocytes (Fig. 8). Hyperactive GH signaling imposed by a GH transgene promoted inflammatory liver cancer in mice and loss of STAT5 in these mice resulted in accelerated HCC.33 This study linked STAT5 to hepatoprotective genes and the aberrant activation of c-Jun in the absence of STAT5. Moreover, the combined loss of STAT5 and the glucocorticoid receptor (GR) resulted in the development of frank HCC.34 In that study development of HCC was associated with GH and insulin resistance and high ROS levels. Since NOX4, the enzyme generating ROS, is under STAT5 control, the source of ROS in the STAT5-GR double knock-out mice needs to be identified.
Although loss of STAT5 is sufficient to induce hepatosteatosis and HCC, it is not clear to what extent the loss of individual STAT5 executors, (NOX4, PUMA, BIM, p15INK4B) would sensitize hepatocytes to injury and lead to pathological changes. Lastly, the molecular basis of STAT5’s cell specificity, promoting proliferation in the hematopoietic system and apoptosis in liver, remains an enigma. Although STAT5 can activate genes controlling cell proliferation, survival and death, it is fair to propose that the relative activity of these pathways will determine whether STAT5 is an oncoprotein or a tumor suppressor.
Supplementary Material
Acknowledgement
This work was supported by the IRP of NIDDK and in part by the World Class University Program, Ministry of Education, Science and Technology, through the National Research Foundation of Korea (R31–10069), South Korea. LH is an adjunct member of the Department of Nanobiomedical Science and WCU Research Center for Nanobiomedical Science, Dankook University, Chungnam 330–740, Korea.
References
- 1.Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q, Wang HY, Liu X, Wasik MA. STAT5A is epigenetically silenced by the tyrosine kinase NPM1-ALK and acts as a tumor suppressor by reciprocally inhibiting NPM1-ALK expression. Nat Med. 2007;13:1341–1348. doi: 10.1038/nm1659. [DOI] [PubMed] [Google Scholar]
- 3.Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-beta and STAT3 activation. J Exp Med. 2009;206:819–831. doi: 10.1084/jem.20080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallette FA, Gaumont-Leclerc MF, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21:43–48. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrese V, Mallette FA, Deschenes-Simard X, Ramanathan S, Gagnon J, Moores A, et al. SOCS1 links cytokine signaling to p53 and senescence. Mol Cell. 2009;36:754–767. doi: 10.1016/j.molcel.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 6.Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, et al. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- 7.Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, Waxman DJ. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology. 2007;148:1977–1986. doi: 10.1210/en.2006-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karst AM, Dai DL, Martinka M, Li G. PUMA expression is significantly reduced in human cutaneous melanomas. Oncogene. 2005;24:1111–1116. doi: 10.1038/sj.onc.1208374. [DOI] [PubMed] [Google Scholar]
- 11.Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci USA. 2004;101:9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004;18:2095–2107. doi: 10.1101/gad.1204904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, et al. JNK1-dependent PUMA Expression Contributes to Hepatocyte Lipoapoptosis. J Biol Chem. 2009;284:26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, et al. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, et al. Fatal Hepatitis Mediated by Tumor Necrosis Factor TNFα Requires Caspase-8 and Involves the BH3-Only Proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmich K, Schlatter R, Corazza N, Sá Ferreira K, Ederer M, Brunner T, et al. Tumor necrosis factor α sensitizes primary murine hepatocytes to Fas/CD95-induced apoptosis in a Bim- and Bid-dependent manner. Hepatology. 2011;53:282–292. doi: 10.1002/hep.23987. [DOI] [PubMed] [Google Scholar]
- 18.Basuroy S, Tcheranova D, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase-derived reactive oxygen species, via endogenous carbon monoxide, promote survival of brain endothelial cells during TNF-α-induced apoptosis. Am J Physiol Cell Physiol. 2011;300:C256–C265. doi: 10.1152/ajpcell.00272.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernández M, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, Ozturk M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010;52:966–974. doi: 10.1002/hep.23769. [DOI] [PubMed] [Google Scholar]
- 23.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik YH, Iwaisako K, Seki E, Inokuchi S, Schnabl B, Osterreicher CH, et al. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730–1741. doi: 10.1002/hep.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu JH, Zhu BM, Wickre M, Riedlinger G, Chen W, Hosui A, et al. The transcription factors signal transducer and activator of transcription 5A (STAT5A) and STAT5B negatively regulate cell proliferation through the activation of cyclin-dependent kinase inhibitor 2b (Cdkn2b) and Cdkn1a expression. Hepatology. 2010;52:1808–1818. doi: 10.1002/hep.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375–1384. doi: 10.1053/j.gastro.2010.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui W, Matsuno K, Iwata K, Ibi M, Matsumoto M, Zhang J, et al. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology. 2011;54:949–958. doi: 10.1002/hep.24465. [DOI] [PubMed] [Google Scholar]
- 29.Naik E, O'Reilly LA, Asselin-Labat ML, Merino D, Lin A, Cook M, et al. Destruction of tumor vasculature and abated tumor growth upon VEGF blockade is driven by proapoptotic protein Bim in endothelial cells. J Exp Med. 2011;208:1351–1358. doi: 10.1084/jem.20100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ, et al. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest. 2011;121:1722–1732. doi: 10.1172/JCI42917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu W, Carson-Walter EB, Kuan SF, Zhang L, Yu J. PUMA suppresses intestinal tumorigenesis in mice. Cancer Res. 2009;69:4999–5006. doi: 10.1158/0008-5472.CAN-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedbichler K, Themanns M, Mueller KM, Schlederer M, Kornfeld JW, Terracciano LM, et al. Growth-hormone-induced signal transducer and activator of transcription 5 signaling causes gigantism, inflammation, and premature death but protects mice from aggressive liver cancer. Hepatology. 2012;55:941–952. doi: 10.1002/hep.24765. [DOI] [PubMed] [Google Scholar]
- 34.Mueller KM, Kornfeld JW, Friedbichler K, Blaas L, Egger G, Esterbauer H, et al. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology. 2011;54:1398–1409. doi: 10.1002/hep.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.