Abstract
In 2006, Turner and colleagues (Behav Neurosci, 120:188–195) introduced the gap-startle paradigm as a high-throughput method for tinnitus screening in rats. Under this paradigm, gap detection ability was assessed by determining the level of inhibition of the acoustic startle reflex produced by a short silent gap inserted in an otherwise continuous background sound prior to a loud startling stimulus. Animals with tinnitus were expected to show impaired gap detection ability (i.e., lack of inhibition of the acoustic startle reflex) if the background sound containing the gap was qualitatively similar to the tinnitus pitch. Thus, for the gap-startle paradigm to be a valid tool to screen for tinnitus, a robust startle response from which to inhibit must be present. Because recent studies have demonstrated that the acoustic startle reflex could be dramatically reduced following noise exposure, we endeavored to 1) modify the gap-startle paradigm to be more resilient in the presence of hearing loss, and 2) evaluate whether a reduction in startle reactivity could confound the interpretation of gap prepulse inhibition and lead to errors in screening for tinnitus. In the first experiment, the traditional broadband noise (BBN) startle stimulus was replaced by a bandpass noise in which the sound energy was concentrated in the lower frequencies (5–10 kHz) in order to maintain audibility of the startle stimulus after unilateral high frequency noise exposure (16 kHz). However, rats still showed a 57% reduction in startle amplitude to the bandpass noise post-noise exposure. A follow-up experiment on a separate group of rats with transiently-induced conductive hearing loss revealed that startle reactivity was better preserved when the BBN startle stimulus was replaced by a rapid airpuff to the back of the rats neck. Furthermore, it was found that transient unilateral conductive hearing loss, which was not likely to induce tinnitus, caused an impairment in gap prepulse inhibition as assessed with the traditional BBN gap-startle paradigm, resulting in a false-positive screening for tinnitus. Thus, the present study identifies significant caveats of the traditional gap-startle paradigm, and describes experimental parameters using an airpuff startle stimulus which may help to limit the negative consequences of reduced startle reactivity following noise exposure, thereby allowing researchers to better screen for tinnitus in animals with hearing loss.
Keywords: acoustic startle reflex, gap detection, gap prepulse inhibition, hearing loss, tinnitus
1.0 INTRODUCTION
Subjective tinnitus is often described as a ringing or buzzing sensation in one or both ears or emanating from inside the head in the absence of an external sound. In the United States, approximately 25% of the adult population has experienced tinnitus, with nearly 8% reporting frequent bouts (Shargorodsky et al., 2010) and an estimated 1–2% suffering from severe, chronic, debilitating tinnitus (McCombe et al., 2001). According to a recent study, tinnitus is also a significant concern for members of the military; 49% of personnel exposed to blast trauma reported tinnitus as the primary audiologic complaint (Cave et al., 2007). In the general population, aging and noise exposure continue to be leading causes of hearing loss and tinnitus. Unfortunately for patients who develop persistent tinnitus, there are no widely accepted or FDA-approved treatments that completely abolish the phantom auditory perception.
In an effort to study the putative brain regions and neural mechanisms underlying tinnitus, several animal models have been developed, many of which require extensive animal training prior to tinnitus assessment (Bauer et al., 2001; Bauer et al., 1999; Guitton et al., 2003; Heffner, 2011; Heffner et al., 2002; Heffner et al., 2005; Jastreboff et al., 1988; Lobarinas et al., 2004; Lobarinas et al., 2006; Ruttiger et al., 2003; Yang et al., 2011). In 2006, Turner and colleagues introduced a high-throughput method for screening tinnitus in rats based on gap detection ability and its effect on the acoustic startle reflex (i.e., the large motoric response to a sudden, loud sound) (Turner et al., 2006). The dependent measure in this traditional gap-startle paradigm is the amplitude of the acoustic startle reflex elicited by a broadband noise (BBN), a reflex that can be suppressed when a silent gap inserted in an otherwise continuous background sound is detected prior to the presentation of the startle stimulus (Ison, 1982; Ison et al., 1991). Turner and colleagues hypothesized that animals would have poorer gap detection ability (as measured by a lack of gap prepulse inhibition of the acoustic startle reflex) if the background sound in which the gap was embedded was qualitatively similar to their tinnitus (i.e., tinnitus would effectively ‘fill in’ the gap). Several research groups, including our own, have since adopted the gap-startle paradigm to test for behavioral evidence of noise-induced tinnitus in rodents (Dehmel et al., 2012; Engineer et al., 2011; Holt et al., 2010; Kraus et al., 2010; Longenecker et al., 2011; Middleton et al., 2011; Turner et al., 2006; Wang et al., 2009; Zhang et al., 2011). In each of these studies, gap detection ability was determined based on the assessment of gap prepulse inhibition, which involved a calculation of the ratio of the startle amplitude generated during trials that contain a brief silent gap versus the amplitude of the startle response in trials without the preceding gap.
Ultimately, for the gap-startle paradigm to be a valid tool to screen for evidence of tinnitus, animals must not only be able to hear the background sound in which the gap is present but also react robustly to the acoustic startle stimulus. Therefore, to help preserve audibility, many researchers have elected to induce tinnitus by exposing animals to loud noise in only one ear (Dehmel et al., 2012; Kraus et al., 2010; Longenecker et al., 2011; Middleton et al., 2011; Turner et al., 2006; Wang et al., 2009; Zhang et al., 2011). However, despite using a unilateral noise exposure, a recent study on mice showed a considerable reduction (52%) in the acoustic startle reflex that persisted three months after exposure, even when hearing levels had recovered completely (Longenecker et al., 2011). Similar to a recent study (Engineer et al., 2011), our pilot testing revealed that some animals failed to startle following unilateral noise exposure, requiring that they be excluded from further tinnitus assessment, as any attempt to measure gap prepulse inhibition is rendered moot if the animals fail to generate a startle response. Excluding animals not only limits the high-throughput nature of the gap-startle paradigm, but could serve to eliminate animals that may indeed be experiencing tinnitus yet fail to startle. To avoid having to exclude animals, we sought to optimize the gap-startle paradigm to be more resilient to hearing loss.
In the present study, we conducted two separate experiments in which the startle stimulus of the gap-startle paradigm was modified in an effort to better preserve the startle response in rats with unilateral hearing loss. First, in rats exposed unilaterally to loud, high-frequency noise, the traditional BBN startle stimulus was replaced with a bandpass noise (5–10 kHz) in which the sound energy was concentrated at frequencies below the noise exposure (16 kHz) so that the startle stimulus would be more audible to the noise-exposed ear. In a follow-up experiment on a separate group of rats with unilateral conductive hearing loss via an earplug, the acoustic startle stimulus was replaced with a rapid airpuff delivered to the back of the rats neck to determine if the multimodal (auditory + tactile) nature of the airpuff would help preserve the startle reflex following unilateral hearing loss. Moreover, because a temporary earplug does not produce tinnitus in rats (Bauer et al., 2001), it was possible to evaluate whether unilateral hearing loss alone could confound the measures of gap prepulse inhibition and lead to a false-positive screening for tinnitus. Ultimately, this study reports experimental parameters which may help to optimize the gap-startle paradigm for tinnitus assessment in animal models, and alerts other investigators to the caveats we have discovered using this common tinnitus screening tool. Preliminary findings of this work were presented in abstract form at the annual meeting of the Association for Research in Otolaryngology (Lobarinas et al., 2012).
2.0 MATERIALS AND METHODS
2.1 Subjects
At total of 32 adult, male, albino Sprague Dawley SASCO rats (3–5 months, 325–450 g) were used in this study; 26 animals in Experiment 1 and six animals in Experiment 2. Rats were housed in Plexiglas cages, allowed free access to food and water, and were maintained on a normal 12 hour light/dark cycle in a temperature controlled room. All experimental procedures used in the present study were approved by the University at Buffalo- Institutional Animal Care and Use Committee (IACUC).
2.2 Testing Apparatus and General Procedures
Startle reflex testing was performed by placing each rat in an acoustically-transparent, wire-mesh (0.5 cm × 0.5 cm) cage (20 cm L, 7 cm W, 6 cm H) mounted on a Plexiglas base (20 cm × 10 cm) which rested on a pressure sensitive 35 mm piezoelectric transducer (MCM 28–745) that generated a voltage proportional to the magnitude of the startle response. Prior to animal testing, the baseline noise floor and waveform output of the startle platform were inspected using an oscilloscope and various weights (10–40 g) dropped from a fixed distance (3 cm). The startle platform was placed inside a custom-built, medium density fiber (MDF), sound-attenuating cubicle (57 cm L, 46 cm W, 46 cm H) that was lined with acoustic foam (noise floor <20 dB SPL at frequencies >4000 Hz). Sound stimuli were generated (TDT RX6, ~100 kHz sampling rate), amplified, and delivered via a free-field speaker (Fostex FT17H) placed above the startle platform (25 cm). The sound within the cubicle was calibrated using a Larson Davis sound level meter (SLM 824) and a ½ or ¼ inch condenser microphone. The output of the startle platform was amplified (Behringer ADA8000), digitized and low-pass filtered by an A/D converter (TDT RX8, ~6 kHz sampling rate), and stored on a computer for offline analysis.
2.3 Experiment 1
In pilot experiments using the gap-startle paradigm, a broadband noise (BBN) burst was used to elicit the acoustic startle reflex, as this is the startle stimulus that has been used in all previous reports (Dehmel et al., 2012; Engineer et al., 2011; Holt et al., 2010; Kraus et al., 2010; Longenecker et al., 2011; Middleton et al., 2011; Turner et al., 2008; Turner et al., 2006; Wang et al., 2009; Yang et al., 2007; Zhang et al., 2011). However, following unilateral high-frequency noise exposure, it was observed that, although hearing was preserved in the non-exposed ear, the startle response was abolished in many animals, requiring them to be excluded from any tinnitus assessment. We suspected that this impairment in startle reactivity occurred because the saliency of the high-frequency component of the BBN was reduced due to the profile of the hearing loss in the noise-exposed ear. Therefore, we replaced the traditional BBN with a bandpass noise (5–10 kHz) that concentrated the sound energy at lower frequencies so that the startle stimulus would remain audible to both ears after unilateral noise exposure. Experiment 1 presents data from 26 rats that were unilaterally exposed to a narrowband noise centered at 16 kHz and tested with the bandpass noise (5–10 kHz) startle stimulus before and after noise exposure (7–30 days post) to determine whether improving the audibility of the startle stimulus would help preserve a robust acoustic startle reflex in noise-exposed animals.
2.3.1 Experiment 1: Noise Exposure
The left ear of each rat (n=26) was exposed to a 120–126 dB SPL narrowband noise centered at 16 kHz (bandwidth=100 Hz) for 1 h. All subjects were anesthetized with isoflurane gas (5% induction, 1–2% maintenance), and placed on a temperature-controlled heating pad (37 °C) within a calibrated sound field. Narrowband noise was created from a high- and low-pass filtered Gaussian noise (TDT RP2.1), amplified (Crown XLS-202) and presented via a free-field speaker (Fostex FT17H horn tweeter) positioned 2 cm from the entrance of the left ear canal. The right (contralateral) ear was protected with a pediatric ear probe filled with plumbers tack; an approach that was shown in our previous study to prevent damage from the noise exposure (Kraus et al., 2010).
2.3.2 Experiment 1: Acoustic Startle Reflex as a Function of Presentation Level
To determine the effectiveness of a bandpass noise (5–10 kHz) in eliciting a robust startle response before and after noise exposure, the input-output function of the acoustic startle reflex was evaluated by randomly varying the intensity of the startle stimulus in a given testing session. The input-output schedule consisted of 100 trials with 10 presentations at each startle intensity level (70, 75, 80, 85, 90, 95, 100, 105, 110 and 115 dB SPL). The inter-trial interval (ITI) was also randomly varied (7–15 s). Sound was generated using Tucker Davis Technologies (TDT) RPVDS and a Real Time Processor (RP2.1). A Gaussian noise function was digitized with a sampling rate of 100 kHz, filtered, converted to analog, and presented through a high-frequency speaker (Fostex FT17H horn tweeter) in a calibrated sound field. Baseline behavioral testing of the bandpass noise input-output function consisted of three to five sessions conducted on non-consecutive days over a two week period. All baseline data were derived from the final baseline session, and compared to the corresponding results from the single post-noise session which occurred 7–30 days after noise exposure.
2.4 Experiment 2
Because in Experiment 1 it was found that replacing the traditional BBN with a bandpass noise still failed to generate a robust startle response in many of the noise-exposed rats (see section 3.2), we explored an alternative way to elicit a robust startle reflex that would be better preserved in rats with unilateral hearing loss. In addition to loud acoustic stimuli, it is well known that rodents exhibit a robust startle reflex when a rapid airpuff is delivered to the head and/or back of the neck (Varty et al., 1998; Varty et al., 1999). Based on these previous reports, the traditional gap-startle paradigm was modified by replacing the acoustic startle stimulus with an airpuff. In a separate group of rats (n=6), we investigated whether the multimodal (auditory + tactile) nature of the airpuff would elicit a robust startle response before and during unilateral conductive hearing loss, and the results were compared to those generated by the commonly-used BBN startle stimulus. An additional aim of Experiment 2 was to determine whether a reduction in startle reactivity could confound the interpretation of gap prepulse inhibition. Because temporary conductive hearing loss was not likely to induce tinnitus (Bauer et al., 2001), it was possible to evaluate if unilateral hearing loss alone impaired gap prepulse inhibition, resulting in a false-positive screening for tinnitus.
2.4.1 Experiment 2: Conductive Hearing Loss
To induce a conductive hearing loss, a small cotton otoblock was inserted into the left ear canal of each rat under light isoflurane anesthesia (1–1.5%), and then the canal was filled with a silicone elastomer (Kwik-Sil) via injection. The fast-drying elastomer provided a secure and tightly-sealed earplug that could not be removed by the rats as it was deeply inserted into the canal. The attenuation of the elastomer was verified by comparing pre-earplug auditory brainstem responses (ABRs) to those collected immediately after the ear plug was inserted (post-earplug ABRs). Note that throughout the manuscript, ‘post-earplug’ refers to conditions when the earplug was in place. The ABRs were obtained using a Tucker Davis Technologies System 3 Real Time Signal Processing System running BioSig32 and SigGen (Tucker Davis Technologies, Alachua, FL, USA). Rats were anesthetized with isoflurane (5% induction, 1–2% maintenance) for ABR threshold testing. Tone bursts (2 ms, 0.5 rise-fall time, 21/s) were presented at 6, 12, 16, 20, 24, and 32 kHz at intensities ranging from 10–100 dB SPL in 5 dB steps. Subdermal electrodes were placed at the ipsilateral pinna and the vertex, with an electrode at the contralateral pinna serving as a ground. The contralateral ear (non-silicone plugged) was occluded with a pediatric ear probe filled with plumbers tack during testing. ABR evoked potentials were averaged over 1024 repetitions, amplified (RA16PA, Tucker Davis Technologies), filtered (100– 3000 Hz bandpass) and digitized. Threshold at each frequency was defined as the lowest sound intensity to generate a visible waveform that was reproducible.
2.4.2 Experiment 2: Acoustic Startle Reflex as a Function of Presentation Level
As in Experiment 1, the input-output function of the acoustic startle reflex was evaluated, this time in response to a BBN burst (20 ms), by randomly varying the intensity of the BBN startle stimulus in a given testing session. Measurements were obtained before and after insertion of the earplug to assess the effects of a unilateral ear blockage on the acoustic startle reflex. The BBN was generated via the TDT RPVDS Gaussian noise function and was consistent with what others have reported in previous studies using the gap-startle paradigm (Dehmel et al., 2012; Engineer et al., 2011; Holt et al., 2010; Kraus et al., 2010; Longenecker et al., 2011; Middleton et al., 2011; Turner et al., 2008; Turner et al., 2006; Wang et al., 2009; Yang et al., 2007; Zhang et al., 2011).
2.4.3 Experiment 2: Airpuff Startle Reflex
The airpuff startle reflex was elicited with a brief (20 ms) airpuff (19 PSI) to the back of the neck delivered by triggering a solenoid air valve (Med Associates ESUB-PHM-276) placed 3 cm above the animal in the startle chamber. Although a concurrent acoustic startle sound was not presented via a speaker, the airpuff itself produced a broadband acoustic signal. The overall acoustic level of the airpuff was measured at 112 dB SPL (Larson Davis SLM824). Thus, the airpuff represented both an auditory and tactile stimulus.
2.4.4 Experiment 2: Gap Prepulse Inhibition of the Startle Reflex
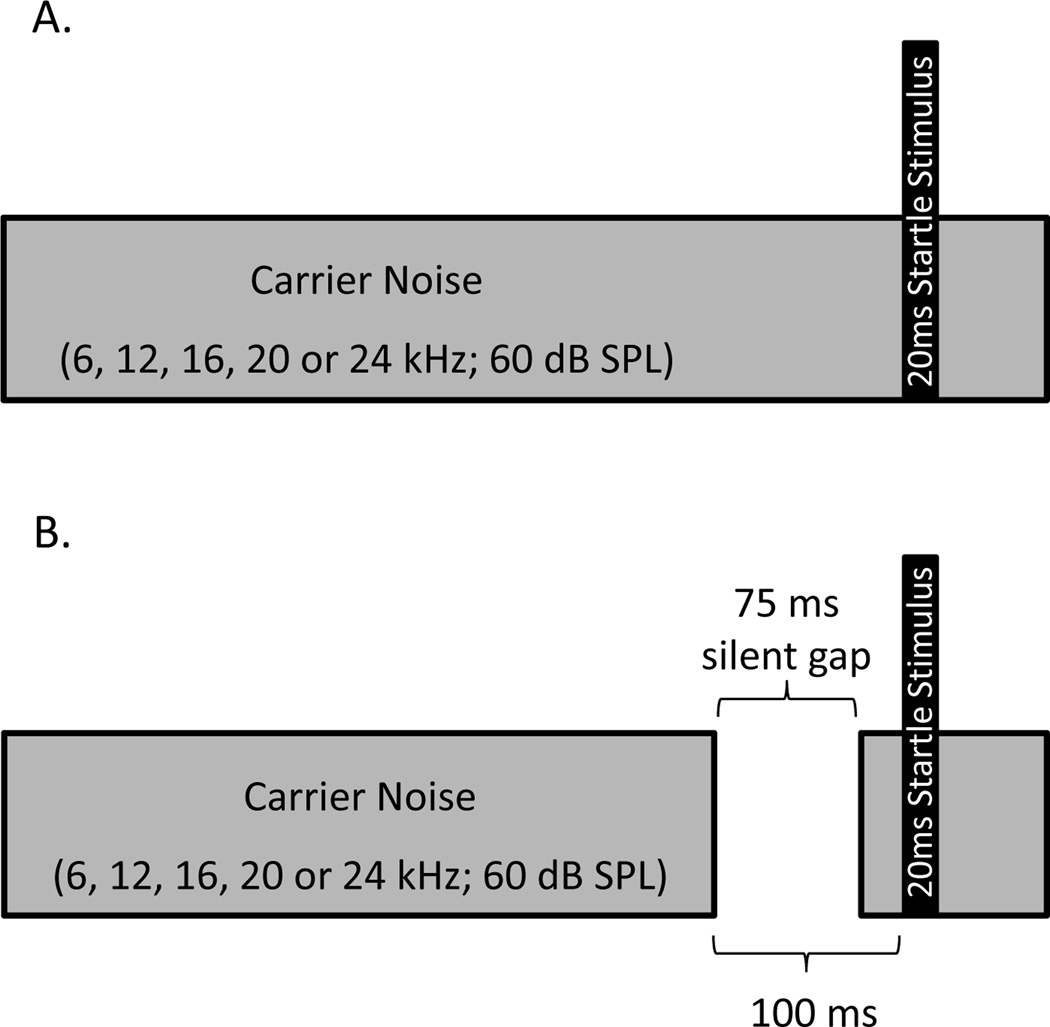
As described in the Introduction, it has been hypothesized that animals with tinnitus will demonstrate poorer gap detection ability that can be measured as a lack of suppression of the startle reflex during trials that contain a brief silent gap preceding the startle stimulus (i.e., tinnitus-positive rats will show impaired gap prepulse inhibition). As depicted in the general schematic in Figure 1, each trial of the gap-startle paradigm started with a background carrier sound that was presented for the duration of the ITI, and then a brief silent gap (75 ms) was inserted 100 ms prior to delivery of the startle stimulus (either BBN at 115 dB SPL or airpuff). The carrier sound was a narrowband noise centered at 6, 12, 16, 20, or 24 kHz (60 dB SPL; bandwidth ranged from 100–5000 Hz to account for differences in the critical band at the various carrier frequencies). Each session with either the BBN or airpuff startle stimulus consisted of 200 block-randomized trials (20 gap trials and 20 no-gap trials per frequency), and the ITI was randomly varied (7–15 seconds).
Figure 1.
Schematic representation of the gap-startle paradigm. For both no-gap (A) and gap (B) trials, a continuous background carrier noise (6, 12, 16, 20 or 24 kHz narrowband noise; 60 dB SPL) was presented for the duration of the inter-trial interval. The startle response was elicited with either a 20 ms broadband noise at 115 dB SPL or a 20 ms airpuff to the back of the neck (112 dB SPL). For gap trials (B) a 75 ms silent gap was inserted into the background carrier noise 100 ms prior to the startle stimulus.
2.4.5 Experiment 2: Timeline
During the first three weeks, rats underwent baseline behavioral testing for airpuff gap prepulse inhibition, BBN input-output function, and BBN gap prepulse inhibition, with three baseline measurements conducted each week for each of the behavioral tests. Following the completion of all baseline behavioral testing, ABRs were performed to assess baseline auditory thresholds. During the following week, unilateral conductive hearing loss was induced by plugging the left ear of each rat, and post-earplug ABRs were immediately performed to assess the level of attenuation produced by the earplug. Forty-eight hours after earplug insertion, rats underwent post-earplug behavioral testing for BBN input-output, and BBN- and airpuff gap prepulse inhibition. To ensure that all of the post-earplug behavioral tests occurred with a consistent level of conductive hearing loss, rats underwent all three post-earplug behavioral tests in the same day, with each test separated by several hours. All baseline data presented were derived from the third baseline session, and compared to the corresponding results from the single post-earplug session.
2.5 Data Analysis and Statistics
For both Experiment 1 and 2, the measurement window of each trial was from the end of the ITI (time 0) to 400 ms. Two epochs were analyzed for each trial. The root-mean-square (RMS) power of Epoch 1 was computed from 0–25 ms (post-ITI) and served as the noise floor. In sessions using an acoustic startle stimulus (which occurred at 100 ms post-ITI), the RMS power of Epoch 2 was computed from 110–210 ms (i.e., Epoch 2 began 10 ms after startle stimulus onset). Because of the longer delay inherent in the presentation of the airpuff, the time window of Epoch 2 was shifted to 120–220 ms for the airpuff sessions. In both the acoustic and airpuff sessions, the raw startle responses were derived from the RMS power of Epoch 2.
To be consistent with the majority of studies that have assessed gap prepulse inhibition using the gap-startle paradigm, we calculated the gap:no-gap ratio from the startle responses in the two conditions based on the following formula: gap:no-gap ratio = Gap / No-Gap, where No-Gap represented the average startle amplitude of the 20 trials that did not include a preceding gap, and Gap represented the startle amplitude of each of the 20 gap trials. Thus, for a given carrier frequency in a given session, 20 values were first generated, and then the mean of these values was calculated to describe the gap:no-gap ratio for the given carrier frequency. For example, a gap:no-gap ratio of 0.2 represented a session in which the mean response during the gap trials was only 20% of the amplitude of the mean response during the no-gap trials, whereas a gap:no-gap ratio of 1.0 would occur if the responses during the gap trials matched those of the no-gap trials. An animal was classified as having impaired gap prepulse inhibition at a particular carrier frequency if there was a statistically significant elevation in the gap:no-gap ratio during conductive hearing loss compared to the baseline level (i.e., post-earplug vs. baseline).
Statistical analyses were conducted on the data from Experiment 1 and 2 using either a two-way repeated measures analysis of variance (ANOVA), one-way repeated measures ANOVA, or paired t-test, depending on the comparison of interest (see Results section for the details of each specific comparison). All statistical comparisons used an alpha value of 0.05. When an ANOVA was used, post hoc testing was performed with Student-Newman-Keuls tests to avoid type I errors associated with multiple comparisons. Sigma Stat 3.5 was used for all statistical analyses. All results are presented as mean ± SEM.
3.0 RESULTS
3.1 Habituation of Startle Reflex at Baseline in Experiment 1 and 2
Based on pilot testing, we were aware that over multiple baseline sessions the startle response could be attenuated due to habituation. In the present study, the level of habituation was calculated in both Experiment 1 and 2, and the results provide the rationale for using only the final baseline session for comparison to the results post-noise exposure (Experiment 1) or post-earplug (Experiment 2) rather than averaging the results from the multiple baseline sessions. In Experiment 1, the group mean startle responses to the 115 dB SPL bandpass noise were greater during the first session compared to the final session (first: 1.57±0.15 V vs. final: 1.09±0.10 V; one-way repeated measures ANOVA, Student-Newman-Keuls test, P<0.001), yet there was no difference in the second to last session versus the final session (second to last: 1.02±0.09 vs. final: 1.09±0.11 V; Student-Newman-Keuls test, P>0.05), indicating that the responses had stabilized. In Experiment 2, the BBN startle responses during the no-gap trials at the various carrier frequencies did not differ between the first baseline session compared to the final baseline session except at the 20 kHz carrier frequency (first: 1.43±0.24 V vs. final: 1.00±0.21 V; two-way repeated measures ANOVA, Student-Newman-Keuls test, P<0.005). Furthermore, the airpuff startle responses during the no-gap trials did not differ between the first baseline session compared to the final baseline session at any of the various carrier frequencies (two-way repeated measures ANOVA, Student-Newman-Keuls test, P>0.05). These results suggest that the changes observed between the final baseline session and the single post-noise exposure session (Experiment 1) or post-earplug session (Experiment 2) were not likely to be influenced greatly by habituation that occurred between the final baseline session and the post-noise/earplug session.
3.2 Experiment 1: Effect of Unilateral Noise Exposure on Bandpass Noise Startle Reflex
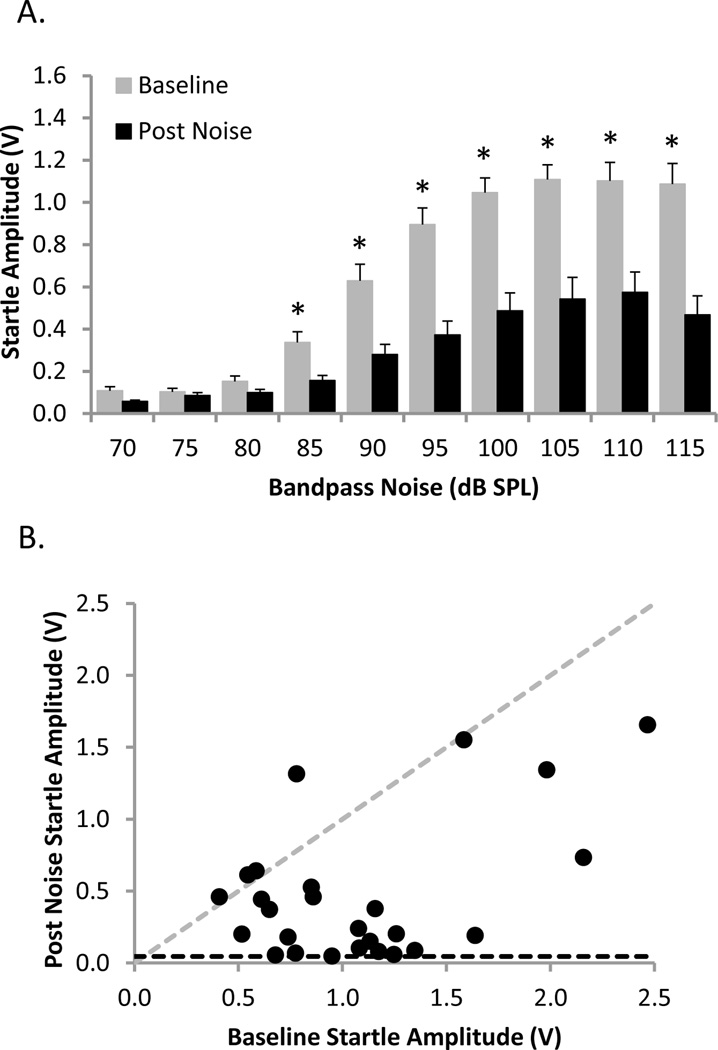
In all noise-exposed animals (n=26), sound overstimulation was presented unilaterally to ensure that the rats would retain normal hearing in one ear. Because the effect of 16 kHz noise exposure at 120–126 dB SPL was likely to result in damage in the mid- to high-frequency region of the cochlea, the acoustic startle stimulus was modified (i.e., bandpass noise at 5–10 kHz) so that it would be more audible to the noise-exposed ear than the traditional BBN. The magnitude of the mean startle response at baseline and post-noise exposure was determined as a function of the bandpass noise (5–10 kHz) stimulus intensity (70–115 dB SPL)(Figure 2A). A two-way repeated measures ANOVA found a significant interaction (P<0.001) between bandpass noise stimulus intensity and condition (baseline noise floor vs. baseline response vs. post-noise exposure noise floor vs. post-noise exposure response). Post hoc analysis revealed that the noise floor did not differ between baseline and post-noise exposure, and that the stimulus intensity did not affect the noise floor (Student-Newman-Keuls tests, P>0.05). The mean noise floor was 0.046±0.004 V at baseline and 0.046±0.006 V post-noise exposure. Additional post hoc analysis revealed that at baseline the startle responses were significantly above the noise floor at stimulus intensities from 85–115 dB SPL (Student-Newman-Keuls tests, P<0.001), whereas the startle responses post-noise exposure were significantly above the noise floor at intensities of 90–115 dB SPL (Student-Newman-Keuls tests, P<0.001). When the amplitude of the startle response was compared between the baseline and post-noise exposure conditions, unilateral noise exposure resulted in a significant reduction in the startle response at 85–115 dB SPL (e.g., at 115 dB: 1.09±0.10 V baseline vs. 0.47±0.09 V post-earplug; Student-Newman-Keuls tests, P<0.005). Furthermore, given the saturation of the input-output function in the post-noise condition (i.e., the response at 115 dB SPL was not different from the response at 110 dB SPL; Student-Newman-Keuls test, P>0.05), it is unlikely that any further increase in the intensity of the startle stimulus would have resulted in an appreciable increase in startle response.
Figure 2.
Unilateral noise exposure dramatically reduced the startle response to a bandpass noise (5–10 kHz) startle stimulus. (A) Group mean input-output function of the startle response to a bandpass noise of varying intensity (70–115 dB SPL) before (grey) and after (black) unilateral noise exposure. The startle amplitude was significantly greater than the noise floor at stimulus intensities of 85–115 dB SPL at baseline, and at stimulus intensities of 90–115 dB SPL following unilateral noise exposure (two-way repeated measures ANOVA and Student-Newman-Keuls tests, P<0.001; baseline noise floor: 0.046±0.004 V; post-noise exposure noise floor: 0.046±0.006 V). Noise exposure caused a significant reduction in the startle response from baseline at stimulus intensities of 85–115 dB SPL (two-way repeated measures ANOVA and Student-Newman-Keuls tests,* P<0.005). Values represent mean ± SEM for n = 26. (B) For each rat (single black circle), the startle response to a bandpass noise at 115 dB SPL post-noise exposure is plotted relative to its own baseline startle response at 115 dB SPL. If the post-noise exposure startle response was unchanged from baseline, the datum point fell on the dashed grey line of unity, whereas those values plotted below the line of unity represent rats whose startle response was reduced following unilateral noise exposure. For several rats, the startle response following unilateral noise exposure was reduced to the level of the noise floor (dashed black line).
In addition to determining the effect of unilateral noise exposure on the group mean startle response (Figure 2A), we investigated whether the loss of startle reactivity was consistent amongst animals. In Figure 2B, which plots each rats bandpass noise startle amplitude at 115 dB SPL post-noise exposure relative to its own baseline response at 115 dB SPL, it is apparent that the vast majority of animals showed a dramatic reduction in their acoustic startle reflex (i.e., the values fell well below the dashed line of unity, with several animals having a post-noise exposure response that was near the noise floor). Thus, despite modifying the acoustic startle stimulus so that its sound energy was concentrated at lower frequencies (5–10 kHz), the startle response was still reduced by more than 70% in nearly half (12/26) of the noise-exposed rats (Figure 2B).
3.3.1 Experiment 2: Unilateral Conductive Hearing Loss
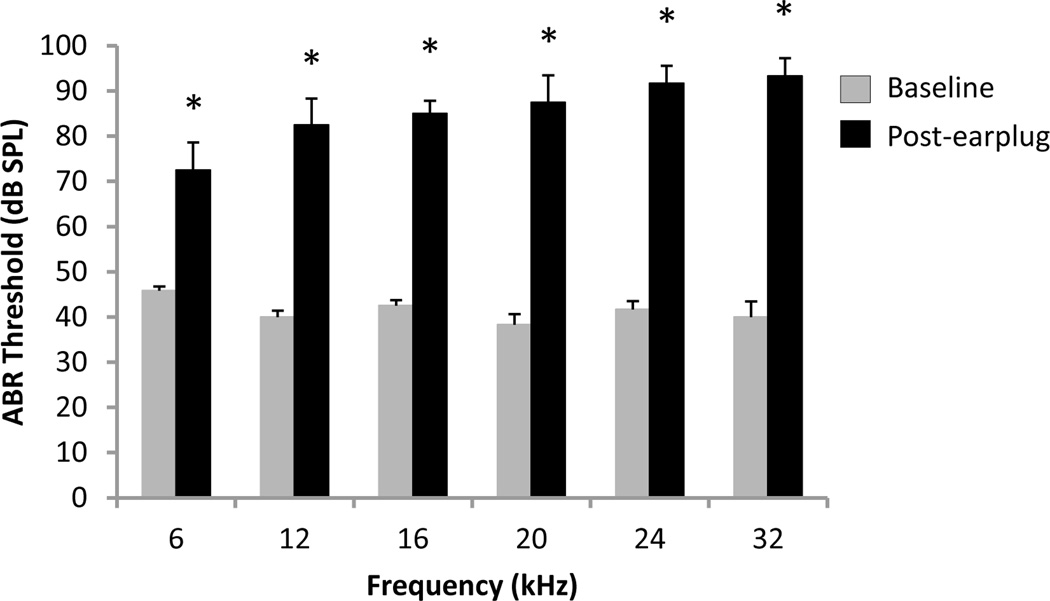
Conductive hearing loss was induced by filling the left ear of rats (n=6) with a silicone elastomer earplug. Figure 3 shows the mean ABR thresholds at 6–32 kHz at baseline and post-earplug. A two-way repeated measures ANOVA found a significant interaction (P<0.001) between frequency and condition on the ABR threshold. Post hoc analysis showed that the earplug caused a significant elevation in the ABR threshold at all frequencies (Student-Newman-Keuls tests, P<0.001), yet the post-earplug threshold at 6 kHz was significantly lower than the threshold at all of the other frequencies (Student-Newman-Keuls tests, P<0.01).
Figure 3.
Unilateral conductive hearing loss elevated ABR thresholds. The group mean ABR threshold post-earplug (black) was significantly elevated from baseline (grey) at all tested frequencies (6–32 kHz) (two-way repeated measures ANOVA and Student-Newman-Keuls post hoc tests, * P < 0.001). The average threshold shift generated by the earplug ranged from 27–53 dB SPL, with the threshold shift at 6 kHz significantly lower than the shift at higher frequencies (12–32 kHz) (one-way repeated measures ANOVA and Student-Newman-Keuls post hoc tests P< 0.01). Values represent mean ± SEM for n = 6.
3.3.2 Experiment 2: Effect of Unilateral Conductive Hearing Loss on BBN Startle Reflex
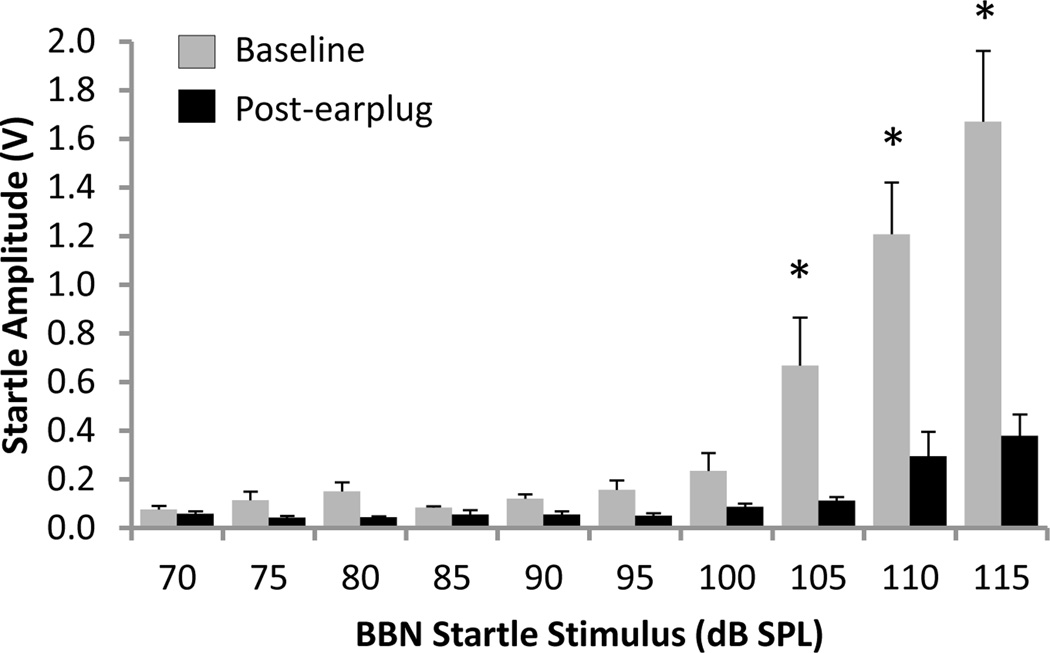
The input-output function of a BBN stimulus was assessed at baseline and during unilateral conductive hearing loss induced by the elastomer earplug. Figure 4 shows the mean startle amplitude to a BBN as a function of increasing stimulus presentation level (70–115 dB SPL) at both baseline and post-earplug. A two-way repeated measures ANOVA found a significant interaction (P<0.001) between stimulus intensity and condition (baseline noise floor vs. baseline response vs. post-earplug noise floor vs. post-earplug response) on the BBN startle amplitude. Post hoc analysis revealed that the noise floor was not different between the baseline and post-earplug sessions, and that the stimulus intensity did not affect the noise floor (Student-Newman-Keuls tests, P>0.05). The mean noise floor was 0.061±0.002 V at baseline and 0.043±0.002 V post-earplug. Additional post hoc analysis found that at baseline the startle response was above the noise floor at stimulus intensities from 105–115 dB SPL (Student-Newman-Keuls tests, P<0.001), whereas only the startle responses to the 110 and 115 dB SPL stimuli were above the noise floor during the post-earplug session (Student-Newman-Keuls tests, P<0.05). Finally, unilateral conductive hearing loss caused a significant reduction in the startle response at 105–115 dB SPL (e.g., at 115 dB: 1.67±0.29 V baseline vs. 0.38±0.09 V post-earplug; Student-Newman-Keuls tests, P<0.001). To summarize, despite a 77% reduction in amplitude caused by unilateral conductive hearing loss, the response to the 115 dB SPL stimulus exceeded the noise floor (i.e., the rats still startled, albeit modestly, to the loud BBN). Ultimately, these results highlight the importance of examining the input-output function of the startle response to evaluate whether the startle reflex is preserved after unilateral hearing loss.
Figure 4.
Input-output function of the startle response to a broadband noise (BBN) startle stimulus presented at varying intensity (70–115 dB SPL) before (grey) and during (black) unilateral conductive hearing loss. The group mean startle response was significantly above the noise floor at stimulus intensities of 105–115 dB SPL at baseline (two-way repeated measures ANOVA and Student-Newman-Keuls tests, P<0.001; baseline noise floor: 0.061±0.002 V), and at stimulus intensities of 110–115 dB SPL during unilateral conductive hearing loss (two-way repeated measures ANOVA and Student-Newman-Keuls tests, P<0.05; post earplug noise floor: 0.043±0.002 V). The startle amplitude during unilateral conductive hearing loss was significantly reduced from baseline for BBN startle stimuli at and above 105 dB SPL (two-way repeated measures ANOVA and Student-Newman-Keuls tests, * P < 0.001). Values represent mean ± SEM for n = 6.
3.3.3 Experiment 2: Effect of Unilateral Conductive Hearing Loss on Gap Detection Ability as Assessed with the BBN Gap-Startle Paradigm
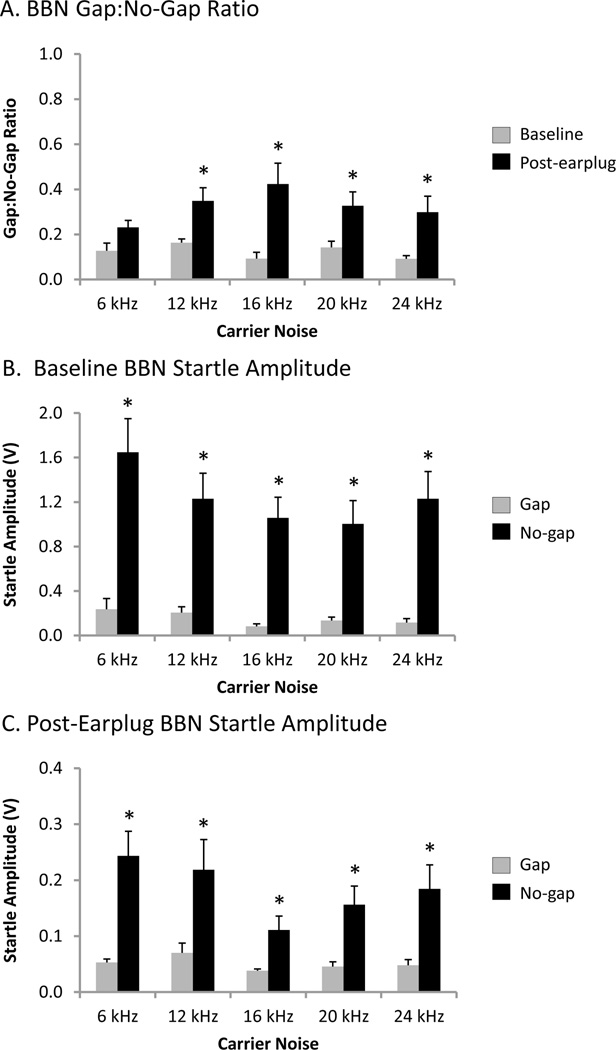
When the gap:no-gap ratio at baseline was compared to that post-earplug for each rat, all rats showed a statistically significant reduction post-earplug for at least two carrier frequencies (P<0.05, paired t-test for each rat). Out of the 30 baseline versus post-earplug comparisons made (six animals, each tested at five carrier frequencies), there were 18 instances when the gap:no-gap ratio during BBN stimulation was significantly elevated by unilateral conductive hearing loss: 3/6 rats at 6 kHz, 0/6 rats at 12 kHz, 6/6 rats at 16 kHz, 5/6 rats at 20 kHz and 4/6 rats at 24 kHz. Figure 5A shows the group mean gap:no-gap ratio during BBN stimulation when the background carrier sound varied from 6–24 kHz at baseline and post-earplug. A two-way repeated measures ANOVA found a significant main effect (P<0.001) for condition (baseline vs. post-earplug) on the gap:no-gap ratio, and post hoc analysis revealed that the gap:no-gap ratio was significantly elevated post-earplug at the 12, 16, 20 and 24 kHz carrier frequencies (Student-Newman-Keuls tests, P<0.005). Because it was unlikely that temporary unilateral conductive hearing loss induced tinnitus (Bauer et al., 2001), these findings are consistent with a false-positive screening for tinnitus as defined by a statistically significant reduction in gap prepulse inhibition, and identify a serious confound in using a measure of gap prepulse inhibition to screen for tinnitus in animals with unilateral hearing loss.
Figure 5.
Broadband noise (BBN) gap prepulse inhibition of the acoustic startle response (A) and raw startle amplitudes during gap and no-gap trials before (B) and during (C) unilateral conductive hearing loss. (A) During unilateral conductive hearing loss (black), the gap:no-gap ratio was significantly elevated compared to baseline (grey) at 12–24 kHz (two-way repeated measures ANOVA and Student-Newman-Keuls tests, * P<0.005). (B) At baseline, the group mean broadband noise startle amplitude during the gap trials (grey) was not significantly different from the noise floor (0.052±0.004 V) at any carrier noise frequency, whereas the startle amplitudes during no-gap trials (black) were above the noise floor at all frequencies. Additionally, the group mean broadband noise startle amplitude during gap trials was significantly lower than during no-gap trials at all carrier noise frequencies (two-way repeated measures ANOVA and Student-Newman-Keuls post hoc tests, * P<0.001). (C) During unilateral conductive hearing loss, the no-gap startle amplitudes were dramatically reduced from baseline (note the 5-fold reduction in ordinate scaling compared to panel B). The group mean startle amplitudes during the gap trials (grey) was not significantly different from the noise floor (0.037±0.002 V) at any carrier noise frequency, whereas the startle amplitudes during no-gap trials (black) were above the noise floor at all frequencies. Additionally, the group mean broadband noise startle amplitude during gap trials was significantly lower than during no-gap trials at all carrier noise frequencies (two-way repeated measures ANOVA and Student-Newman-Keuls post hoc tests, * P<0.05). Values represent mean ± SEM for n = 6.
In addition to calculating the gap:no-gap ratio, a statistical comparison was also made between the raw startle amplitudes during the gap trials versus no-gap trials at each carrier frequency, under the premise that a lack of statistical difference between these two stimulus conditions would represent impaired gap detection ability. As expected, at baseline, all of the individual rats demonstrated BBN startle amplitudes in the gap trials that were significantly lower than that of the no-gap trials at all carrier frequencies (P<0.05, paired t-test for each rat), indicating that the rats could detect the silent gaps in the background sounds. Figure 5B shows the group mean BBN startle amplitudes in the gap and no-gap trials at baseline when the background carrier sound varied from 6–24 kHz. A two-way repeated measures ANOVA found a significant interaction (P<0.001) between carrier noise frequency and stimulus condition (noise floor vs. gap trials vs. no-gap trials) on the BBN startle amplitude. Post hoc analysis revealed that the noise floor and the responses during the gap trials were not different from each other, and were not affected by the various carrier frequencies (Student-Newman-Keuls tests, P>0.05). In contrast, all of the responses in the no-gap trials exceeded the noise floor (mean: 0.052±0.004 V), and the startle amplitudes in the no-gap trials were significantly greater than that of the gap trials at all carrier frequencies (Student-Newman-Keuls tests, P<0.001; Figure 5B, compare black and grey bars). Furthermore, the startle amplitude in the no-gap trials at 6 kHz was significantly greater than at the other carrier frequencies (Student-Newman-Keuls tests, P<0.001), whereas the startle amplitude in no-gap trials at 20 kHz were smaller than at the 12 and 24 kHz carrier frequencies (Student-Newman-Keuls tests, P<0.05; Figure 5B, black bars).
During unilateral conductive hearing loss, three of the six rats tested showed a lack of statistical difference in the BBN startle amplitude between the gap and no-gap conditions for at least one carrier frequency (P>0.05, paired t-test for each rat). Figure 5C shows the effect of conductive hearing loss on the group mean BBN startle amplitudes in the gap and no-gap conditions when the background carrier sound varied from 6–24 kHz. Due to the dramatic reduction in startle amplitude compared to baseline, there is a 5-fold decrease in the scale of the ordinate in Figure 5C versus 5B. A two-way repeated measures ANOVA found a significant interaction (P<0.005) between carrier noise frequency and stimulus condition (noise floor vs. gap trials vs. no-gap trials) on the BBN startle amplitude during the post-earplug session. Similar to the results at baseline, post hoc analysis revealed that the noise floor and the responses during the gap trials were not different from each other, and were not affected by the various carrier frequencies (Student-Newman-Keuls tests, P>0.05). Additional post hoc analysis found that the startle amplitude in the no-gap trials at 6 kHz was significantly greater than at the 16, 20 and 24 kHz carrier frequencies (Student-Newman-Keuls tests, P<0.05), and the no-gap responses at 12 kHz were significantly greater than at the 16 and 20 kHz carrier frequencies (Student-Newman-Keuls tests, P<0.05), whereas the startle amplitude in no-gap trials at 16 kHz were smaller than at all of the other carrier frequencies (Student-Newman-Keuls tests, P<0.05; Figure 5C, black bars). Furthermore, the responses in the no-gap trials all exceeded the noise floor (mean: 0.037±0.002 V; Student-Newman-Keuls tests, P<0.05), despite a significant reduction in the startle amplitudes in the no-gap trials post-earplug (e.g., 90% reduction in no-gap startle amplitude at 16 kHz; 1.06±0.19 V baseline vs. 0.11±0.03 V post-earplug, paired t-test, P<0.005; compare black bar in 5B vs. 5C). Finally, the startle amplitudes in the no-gap trials were significantly greater than that of the gap trials at all carrier frequencies (Student-Newman-Keuls tests, P<0.05; Figure 5C, compare black and grey bars), indicating that the animals were still able to detect the gaps in the background sounds despite the large reduction in the raw startle amplitude.
3.3.4 Experiment 2: Effect of Unilateral Conductive Hearing Loss on Airpuff Startle Reflex
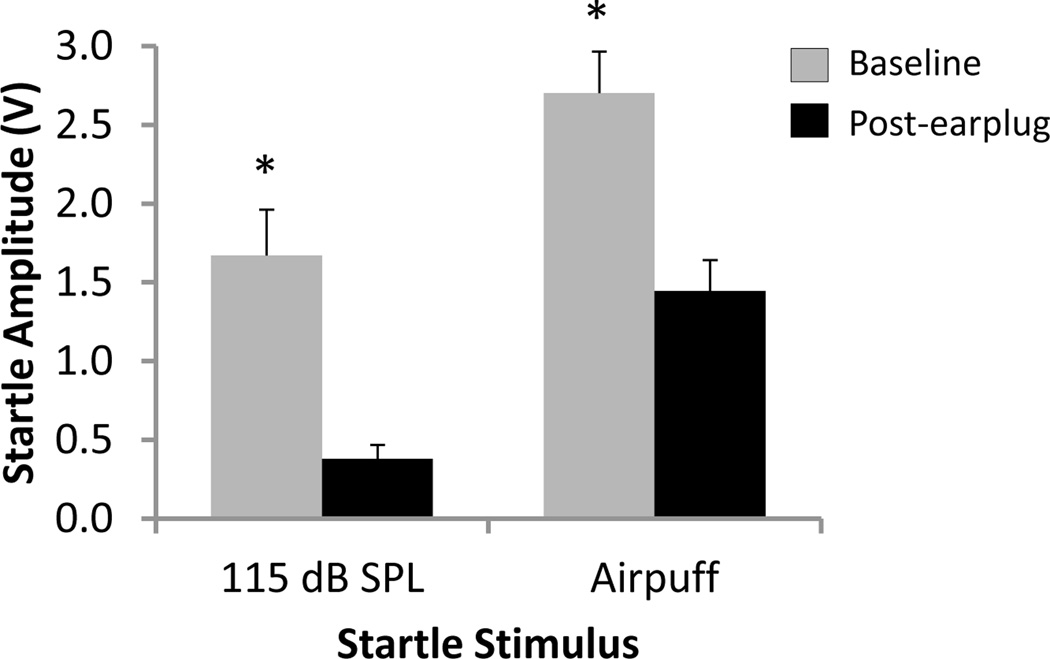
In addition to a loud acoustic stimulus, it is well established that rodents with normal hearing demonstrate a robust startle reflex when a rapid airpuff is delivered to the head and/or back of the neck (Varty et al., 1998; Varty et al., 1999). Figure 6 shows the group mean startle amplitudes elicited by either a BBN at 115 dB SPL or an airpuff stimulus in the same rats (n=6) at baseline and during conductive hearing loss. A two-way repeated measures ANOVA found a significant main effect for both stimulus (BBN vs. airpuff; P<0.001) and condition (baseline vs. post-earplug; P<0.005). Post hoc analysis revealed that at baseline the group mean airpuff startle amplitude was significantly greater than that of the BBN (2.70±0.26 V airpuff vs. 1.67±0.29 V BBN; Student-Newman-Keuls test, P<0.001; Figure 6, grey bars). This finding was not surprising given that it has been shown that combining concurrent acoustic and tactile stimulation (as would be the case in an airpuff stimulus) results in a greater startle reflex than either stimulus modality presented alone (Li et al., 1999). Additional post hoc analysis showed that conductive hearing loss significantly reduced the group mean startle response to both the BBN stimulus (77% reduction; 1.67±0.29 V baseline vs. 0.38±0.09 V post-earplug) and the airpuff stimulus (46% reduction; 2.70±0.26 V baseline vs. 1.45±0.20 V post-earplug)(Student-Newman-Keuls tests, P<0.001; Figure 6, black bars). Thus, when the earplug was inserted, the airpuff startle response remained significantly greater than that of the BBN (Student-Newman-Keuls test, P<0.001). It is likely that the startle response to the airpuff stimulus was better preserved than that of the BBN because the tactile component of the airpuff stimulus was not affected by unilateral conductive hearing loss.
Figure 6.
Startle responses to a 115 dB SPL broadband noise (BBN) startle stimulus and airpuff startle stimulus before (grey) and during (black) unilateral conductive hearing loss. The amplitude of the startle response elicited by the airpuff was significantly greater than that produced by the 115 dB SPL BBN startle stimulus at baseline and post-earplug (two-way repeated measures ANOVA and Student-Newman-Keuls post hoc tests, P<0.001). During unilateral conductive hearing loss, startle amplitude was significantly reduced from baseline for both the BBN and airpuff startle stimuli (two-way repeated measures ANOVA and Student-Newman-Keuls post hoc tests, * P<0.001). Values represent mean ± SEM for n = 6.
3.3.5 Experiment 2: Effect of Unilateral Conductive Hearing Loss on Gap Detection Ability as Assessed with the Airpuff Gap-Startle Paradigm
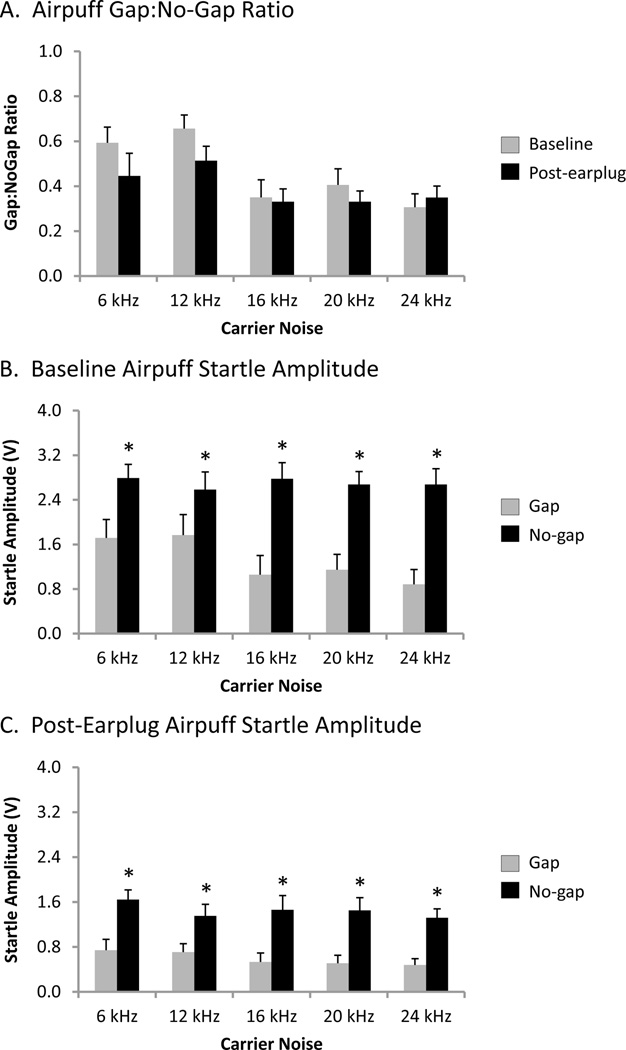
Using the airpuff startle stimulus, when the gap:no-gap ratio at baseline was compared to the post-earplug ratio for each rat (using paired t-tests for each carrier frequency), two rats showed an elevated gap:no-gap ratio after insertion of the earplug for at least one carrier frequency, whereas four of the six rats showed a decreased gap:no-gap ratio for at least one carrier frequency. Figure 7A shows the group mean gap:no-gap ratio during airpuff stimulation when the background carrier sound varied from 6–24 kHz at baseline and post-earplug. A two-way repeated measures ANOVA found a significant main effect (P<0.001) for carrier frequency on the gap:no-gap ratio. Post hoc analysis revealed that at baseline the group mean gap:no-gap ratio during airpuff stimulation was significantly greater at 6 and 12 kHz than 16, 20 and 24 kHz (Student-Newman-Keuls tests, P<0.001), whereas at post-earplug the group mean gap:no-gap ratio was significantly greater at 12 kHz versus 16, 20 and 24 kHz (Student-Newman-Keuls tests, P<0.005). Unlike BBN stimulation (Figure 5A), unilateral conductive hearing loss did not increase the group mean gap:no-gap ratio during airpuff stimulation at any carrier frequency (Student-Newman-Keuls tests, P>0.05; Figure 7A).
Figure 7.
Airpuff gap prepulse inhibition of the startle response (A) and raw startle amplitudes during gap and no-gap trials before (B) and during (C) unilateral conductive hearing loss. (A) The group mean airpuff gap:no-gap ratio did not differ between baseline and post-earplug conditions at any carrier noise frequency (two-way repeated measures ANOVA and Student-Newman-Keuls post hoc tests, P>0.05). At baseline (B) and during unilateral conductive hearing loss (C), the group mean airpuff startle amplitude during gap trials (grey) and no-gap trials (black) were above the noise floor (baseline: 0.043±0.004 V; post-earplug: 0.040±0.002 V) at all carrier noise frequencies. Furthermore, the group mean airpuff startle amplitude during gap trials was significantly lower than during no-gap trials at all carrier noise frequencies during baseline (B) as well as during unilateral conductive hearing loss (C) (two-way repeated measures ANOVAs and Student-Newman-Keuls post hoc tests, * P<0.005). Note the 10-fold increase in ordinate scaling in panel C versus Figure 5C. Values represent mean ± SEM for n = 6.
Figure 7B shows the group mean airpuff startle amplitudes in the gap and no-gap conditions at baseline when the background carrier sound varied from 6–24 kHz. A two-way repeated measures ANOVA found a significant interaction (P<0.001) between carrier noise frequency and stimulus condition (noise floor vs. gap trials vs. no-gap trials) on the airpuff startle amplitude. Post hoc analysis revealed that the responses in both the gap- and no-gap trials exceeded the noise floor at all carrier frequencies (Student-Newman-Keuls tests, P<0.005), and the noise floor was unaffected by carrier frequency (Student-Newman-Keuls tests, P>0.05; mean noise floor: 0.043±0.004 V). Additional post hoc analysis found that the airpuff startle amplitude in the gap trials at 6 and 12 kHz were significantly greater than at the other carrier frequencies (Student-Newman-Keuls tests, P<0.001), and the startle amplitude in gap trials at 24 kHz was smaller than at the 20 kHz carrier frequency (Student-Newman-Keuls tests, P<0.05; Figure 7B, grey bars). Finally, the airpuff startle amplitudes in the no-gap trials were significantly greater than that of the gap trials at all carrier frequencies (Student-Newman-Keuls tests, P<0.005; Figure 7B, compare black and grey bars), indicating that the rats could detect the gaps.
During conductive hearing loss, one of the six rats tested showed a lack of statistical difference in the airpuff startle amplitude between the gap and no-gap conditions at both 6 kHz (1.22±0.19 V gap trials vs. 1.53±0.16 V no-gap trials, P>0.05, paired t-test) and 12 kHz (1.12±0.17 V gap trials vs. 1.48±0.20 V no-gap trials, P>0.05, paired t-test). Figure 7C shows the effect of conductive hearing loss on the group mean airpuff startle amplitudes in the gap and no-gap conditions when the background carrier sound varied from 6–24 kHz (note the 10-fold increase in ordinate scaling in Figure 7C versus Figure 5C). A two-way repeated measures ANOVA found a significant interaction (P<0.005) between carrier noise frequency and stimulus condition (noise floor vs. gap trials vs. no-gap trials) on the airpuff startle amplitude during the post-earplug session. Similar to the results at baseline, post hoc analysis revealed that the airpuff startle responses in both the gap and no-gap trials exceeded the noise floor at all carrier frequencies (Student-Newman-Keuls tests, P<0.05), and the noise floor was unaffected by carrier frequency (Student-Newman-Keuls tests, P>0.05; mean noise floor: 0.040±0.002 V). Additional post hoc analysis found that the airpuff startle amplitude in the no-gap trials at 6 kHz were significantly greater than at the other carrier frequencies (Student-Newman-Keuls tests, P<0.05; Figure 7C, black bars), whereas the startle amplitude in the gap trials at 6 and 12 kHz were significantly greater than at 16, 20 and 24 kHz carrier frequencies, similar to the findings at baseline (Student-Newman-Keuls tests, P<0.01; Figure 7C, grey bars). Lastly, the airpuff startle amplitudes in the no-gap trials were significantly greater than that of the gap trials at all carrier frequencies (Student-Newman-Keuls tests, P<0.001; Figure 7C, compare black and grey bars), which indicates that the rats were able to detect the gaps in the background noises during unilateral conductive hearing loss.
4.0 DISCUSSION
In contrast to the overt behavioral training required to assess tinnitus in animals using conditioning paradigms, the gap-startle paradigm, first proposed by Turner and colleagues (2006), has offered researchers an alternative, seemingly high-throughput tool for tinnitus screening in animal models. Although the gap-startle paradigm intends to screen animals for tinnitus based on their gap detection ability, the dependent measure in the traditional paradigm is the amplitude of the acoustic startle reflex as generated by a loud BBN. Thus, a failure of the animal to startle following hearing loss renders any subsequent tinnitus screening moot. Because our pilot testing as well as a previous study found that startle reactivity was significantly reduced following unilateral noise exposure (Longenecker et al., 2011), we endeavored to better preserve the startle response in rats with unilateral hearing loss by modifying the startle stimulus from the traditional gap-startle paradigm.
In our first experiment, the traditional BBN startle stimulus was replaced with a bandpass noise in which the sound energy was concentrated in the lower frequencies (5–10 kHz), and the startle responses before and after unilateral noise exposure were compared. Despite optimizing the startle stimulus by having it be more audible to the noise-exposed ear, a significant decrease in the startle amplitude post-noise exposure was still observed (Figure 2A; 57% reduction at 115 dB SPL). This impairment in the acoustic startle response was similar to the 52% reduction reported recently for a group of mice unilaterally exposed to loud noise (116 dB SPL) (Longenecker et al., 2011). Further inspection of the present data revealed that unilateral noise exposure caused a >70% reduction in the acoustic startle response in nearly half (12/26) of the noise-exposed rats (Figure 2B). It is noteworthy that increasing the intensity of the stimulus above 115 dB SPL would not likely have improved startle reactivity because the input-output function of the response had saturated (Figure 2A).
Having observed that the acoustic startle reflex was dramatically reduced following unilateral noise exposure, we then performed a separate experiment designed to investigate the possible ramifications of reduced startle reactivity on the level of gap prepulse inhibition as measured by the gap:no-gap ratio, as these issues could confound tinnitus screening. By inserting an earplug into only one ear, we assessed the effects of temporary unilateral conductive hearing loss on the gap:no-gap ratio in rats that did not likely have tinnitus (Bauer et al., 2001). Unexpectedly, it was found that when the traditional BBN startle stimulus was used, the group mean gap:no-gap ratio at 12, 16, 20 and 24 kHz carrier frequencies was significantly elevated post-earplug (Figure 5A); findings consistent with a false-positive screening for tinnitus based on the statistical criterion used in previous studies (Longenecker et al., 2011; Zhang et al., 2011). In contrast, Turner et al. (2006) did not observe a significant increase in the gap:no-gap ratio at 10 kHz during a less severe earplug-induced conductive hearing loss (average threshold shift of 22 dB SPL; 5–35 dB SPL range across animals) than that observed in the present study (Figure 3). Unlike the present study, perhaps the less severe conductive hearing loss did not impair the acoustic startle reflex, thereby leaving the gap:no-gap ratio unaffected. However, it remains unknown how much unilateral hearing loss is required to impair startle reactivity as the raw startle amplitudes before or following insertion of the earplug were not reported by Turner and colleagues.
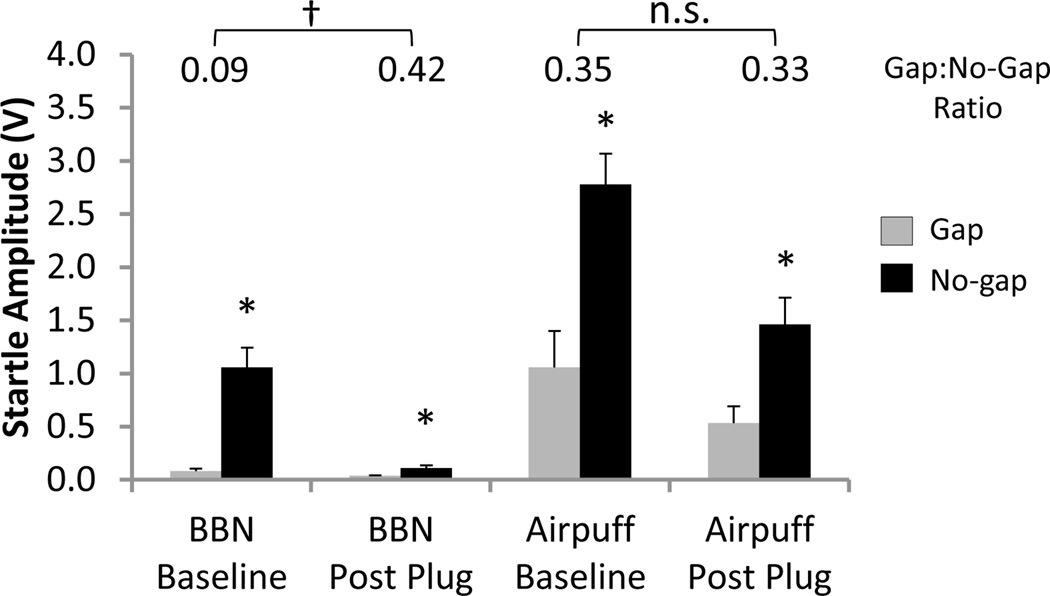
We suggest that the elevation in the gap:no-gap ratio at 12–24 kHz observed in the present study using the traditional BBN gap-startle paradigm was the consequence of a loss of startle reactivity following unilateral hearing loss, and not a gap detection deficit (or tinnitus). As shown in Figure 5C, the rats demonstrated a statistically significant inhibition of their startle responses during the gap trials as these responses were lower than those during the no-gap trials at all carrier frequencies, which is consistent with an ability to detect the gaps. Although not previously acknowledged in studies which have used the gap-startle paradigm to screen for tinnitus, an impairment in gap detection ability is not the only way to cause an elevation of the gap:no-gap ratio and thus meet the statistical criterion to be considered “tinnitus”. To generalize, consider two opposing scenarios in which the gap:no-gap ratio can be elevated compared to baseline levels; one which involves impaired gap detection ability, and another which does not. For example, if an animal indeed fails to detect the preceding gap, the response to the startle stimulus during gap trials would increase toward the amplitude of the response in the no-gap trials, thereby resulting in an elevated gap:no-gap ratio. However, in an alternative scenario, if an animal is still able to detect the gap post-hearing loss (i.e., the response during gap trials remains small, similar to baseline conditions), yet its startle response during the no-gap trials has decreased, the gap:no-gap ratio would also be elevated. As shown in Figure 8, it is clear that the gap:no-gap ratio at 16 kHz derived from the traditional gap-startle paradigm increased post-earplug, not because the animals failed to detect the gaps, but because the startle response during the no-gap trials decreased dramatically while the response in the gap trials remained unchanged at the level of the noise floor. These results highlight the problem with assuming that a reduction in gap prepulse inhibition as measured by an elevated gap:no-gap ratio was solely the result of a gap detection deficit. The caution against directly equating changes in the gap:no-gap ratio to gap detection ability is further evident when one considers that the trend for the earplug to decrease the gap:no-gap ratio in the airpuff gap-startle paradigm should not be ascribed to improved gap detection (Figure 7A). Put simply, would insertion of an earplug and listening with only one ear be expected to improve gap detection?
Figure 8.
Comparison of group mean gap and no-gap startle amplitudes for the 16 kHz carrier noise frequency during broadband noise (BBN) and airpuff gap prepulse inhibition testing at baseline and during unilateral earplug. For all conditions, the startle amplitude during the gap trials (grey bars) was significantly lower than during the no-gap trials (black bars)(two-way repeated measures ANOVA and Student-Newman-Keuls post hoc tests, * P<0.05). Above each pair of grey and black bars is the calculated gap:no-gap ratio, the measure traditionally used to assess gap detection ability in the gap- startle paradigm. The significant increase in the gap:no-gap ratio during BBN stimulation post-earplug compared to baseline is consistent with a false-positive for tinnitus (two-way repeated measures ANOVA and Student-Newman-Keuls post hoc test, † P<0.001). Given that the startle response in the gap trials at BBN Post Plug was significantly lower than that of the respective no-gap trials, the elevated gap:no-gap ratio was not the result of a gap detection deficit but rather the dramatic reduction in startle reactivity in the no-gap trials. Values represent mean ± SEM for n = 6. n.s., non-significant statistical difference.
Recall that the central hypothesis of the traditional gap-startle paradigm is that tinnitus impairs an animals gap detection ability, which can be measured as an increase in the gap:no-gap ratio (Dehmel et al., 2012; Engineer et al., 2011; Kraus et al., 2010; Longenecker et al., 2011; Middleton et al., 2011; Turner et al., 2006; Wang et al., 2009; Zhang et al., 2011). However, without statistically comparing the raw startle values in the gap and no-gap trials before and after attempts to induce tinnitus, it is not possible to evaluate whether or not an elevation in the gap:no-gap ratio occurred due to a gap detection deficit, or simply a loss of startle reactivity. Given the present findings that unilateral noise exposure (Figure 2) and conductive hearing loss (Figure 4) reduced startle reactivity, and the gap:no-gap ratio increased in the absence of a gap detection deficit (Figure 5), it becomes difficult to interpret previous studies in which it was reported that noise-exposed animals were experiencing tinnitus based solely on an increase in the gap:no-gap ratio at specific carrier frequencies. Perhaps similar to the present study in which unilateral conductive hearing loss resulted in a false-positive screening for tinnitus using the traditional gap-startle paradigm, the noise-exposed animals in previous studies demonstrated an elevated gap:no-gap ratio (and thus were classified as having tinnitus) due to a loss of startle reactivity that went unreported. Furthermore, although a frequency-specific elevation in the gap:no-gap ratio has been used in previous animal studies to classify the tinnitus pitch following noise exposure (Dehmel et al., 2012; Kraus et al., 2010; Longenecker et al., 2011; Middleton et al., 2011; Turner et al., 2006; Wang et al., 2009; Zhang et al., 2011), it is worth noting that we observed a similar finding in animals that were not likely experiencing tinnitus (Figure 5A, compare non-significant elevation at 6 kHz vs. significant elevation at 16 kHz). In the present study, the frequency-specific elevation in the gap:no-gap ratio was presumably due to differences in the severity of the reduction in the startle response in the no-gap trials at the various carrier frequencies (Figure 5C, black bars).
The results of the present study indicate that the traditional gap-startle paradigm is not resilient to unilateral hearing loss, and this leads to a confound in using the gap:no-gap ratio to assess tinnitus. Thus, we suggest that the traditional BBN startle stimulus be replaced with a rapid airpuff, as the startle response to this multimodal stimulus was much better preserved in animals with unilateral hearing loss (Figures 6–8). By using the more resilient airpuff stimulus, it is expected that fewer noise-exposed animals will need to be excluded from tinnitus screening because their startle response failed to exceed the noise floor. In addition to this experimental modification, we suggest that researchers revise the statistical analyses used to determine whether or not animals demonstrate a gap detection deficit post-noise exposure. Our results show that it is inappropriate to assume that a reduction in gap prepulse inhibition as measured by an elevated gap:no-gap ratio is solely the result of a gap detection deficit (Figure 5). Therefore, instead of comparing the gap:no-gap ratio before and after noise exposure (or between controls and noise-exposed animals), we suggest that a statistical comparison be performed between the raw startle amplitudes during the gap trials versus no-gap trials at each carrier frequency, as this approach will provide a better assessment of gap detection ability. For example, if a noise-exposed animal shows a statistically significant reduction in its startle response during the gap trials compared to the no-gap trials, we would argue that this animal is able to detect the gap in the background sound, irrespective of the calculated gap:no-gap ratio.
Although we have identified ways to optimize the gap-startle paradigm for tinnitus screening in animals, we suggest that there are two key issues that remain unresolved concerning the use of the gap-startle paradigm to screen for behavioral evidence of tinnitus. First, it is unknown how sensorineural hearing loss and the resulting plasticity throughout the brain affects the circuits mediating sensorimotor gating. Exposure to noise levels known to induce sensorineural hearing loss results in plasticity throughout the central auditory pathway (for review, see (Syka, 2002)) as well as in non-classical auditory structures such as those in the limbic system (Goble et al., 2009; Kraus et al., 2010; Wallhausser-Franke et al., 2003). Given the involvement of both auditory and non-auditory structures in the neural circuitry generating and regulating the startle reflex (for review, see (Swerdlow et al., 2000)), it remains to be determined if noise-induced plasticity within these structures confounds measures of gap prepulse inhibition using the gap-startle paradigm. Second, it is prudent to acknowledge that, to our knowledge, the working hypothesis that tinnitus effectively ‘fills in the gap’ has not been confirmed in humans. Certainly, this hypothesis will need to be abandoned if 1) gap detection ability as assessed with audiological testing is found to be normal in persons with tinnitus, or 2) the gap-startle paradigm fails to reveal a gap detection deficit (not simply an altered gap:no-gap ratio compared to controls) at only the specific frequencies matching the patients tinnitus pitch. However, should the gap-startle paradigm prove capable of objectively measuring tinnitus in humans, then we would encourage researchers using animal models to consider adopting the experimental parameters and analyses outlined in the present study to limit the confounding issue of reduced startle reactivity caused by hearing loss.
HIGHLIGHTS.
Gap-startle paradigm uses acoustic startle response (ASR) to screen rats for tinnitus
Noise exposure reduced ASR, which could confound tinnitus screening
Conductive hearing loss reduced ASR, leading to false-positive estimate of tinnitus
Airpuff startle response better preserved during conductive hearing loss than ASR
This study shows caveats and optimized parameters for the gap-startle paradigm
ACKNOWLEDGEMENTS
The authors wish to thank the following individuals for their valuable contributions to the successful completion of this project. As Director of the Center for Hearing and Deafness, Dr. Richard Salvi provided generous support, encouragement and guidance in all aspects of the study, and provided helpful comments on an earlier version of the manuscript. Daniel Stolzberg designed the custom software used for the gap-startle paradigm, and provided thought-provoking comments on the interpretation of the data. Carrie Shillitoe-Blair, Laura Lewicki and Carolyn Whitcomb provided technical assistance. Sarah Hayes received support from the National Defense Science and Engineering Graduate Fellowship through the U.S. Department of Defense. This work was supported in part by a generous research grant from the Tinnitus Research Initiative (EL). The project described was supported by Grant Number R03DC011374 (BLA) from the National Institute On Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Deafness and Other Communication Disorders or the National Institutes of Health.
ABBREVIATIONS
- ABR
auditory brainstem response
- BBN
broadband noise
- ITI
inter-trial interval
- SPL
sound pressure level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
EL, SHH and BLA designed the experiments, performed data collection and analysis, and wrote the manuscript. EL and SHH contributed equally to all aspects of the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol. 2001;2:54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg. 1999;121:457–462. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- Cave KM, Cornish EM, Chandler DW. Blast injury of the ear: clinical update from the global war on terror. Mil Med. 2007;172:726–730. doi: 10.7205/milmed.172.7.726. [DOI] [PubMed] [Google Scholar]
- Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus—possible basis for tinnitus-related hyperactivity? J Neurosci. 2012;32:1660–1671. doi: 10.1523/JNEUROSCI.4608-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble TJ, Moller AR, Thompson LT. Acute high-intensity sound exposure alters responses of place cells in hippocampus. Hear Res. 2009;253:52–59. doi: 10.1016/j.heares.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003;23:3944–3952. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE. A two-choice sound localization procedure for detecting lateralized tinnitus in animals. Behav Res Methods. 2011;43:577–589. doi: 10.3758/s13428-011-0061-4. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hear Res. 2002;170:83–95. doi: 10.1016/s0378-5955(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Koay G. Tinnitus and hearing loss in hamsters (Mesocricetus auratus) exposed to loud sound. Behav Neurosci. 2005;119:734–742. doi: 10.1037/0735-7044.119.3.734. [DOI] [PubMed] [Google Scholar]
- Holt AG, Bissig D, Mirza N, Rajah G, Berkowitz B. Evidence of key tinnitus-related brain regions documented by a unique combination of manganese-enhanced MRI and acoustic startle reflex testing. PLoS One. 2010;5:e14260. doi: 10.1371/journal.pone.0014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR. Temporal acuity in auditory function in the rat: reflex inhibition by brief gaps in noise. J Comp Physiol Psychol. 1982;96:945–954. [PubMed] [Google Scholar]
- Ison JR, O'Connor K, Bowen GP, Bocirnea A. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav Neurosci. 1991;105:33–40. doi: 10.1037//0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Brennan JF, Sasaki CT. An animal model for tinnitus. Laryngoscope. 1988;98:280–286. doi: 10.1288/00005537-198803000-00008. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yeomans JS. Summation between acoustic and trigeminal stimuli evoking startle. Neuroscience. 1999;90:139–152. doi: 10.1016/s0306-4522(98)00436-9. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear Res. 2004;190:109–114. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Yang G, Sun W, Ding D, Mirza N, Dalby-Brown W, Hilczmayer E, Fitzgerald S, Zhang L, Salvi R. Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol Suppl. 2006:13–19. doi: 10.1080/03655230600895408. [DOI] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol. 2011;12:647–658. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombe A, Baguley D, Coles R, McKenna L, McKinney C, Windle-Taylor P. Guidelines for the grading of tinnitus severity: the results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin Otolaryngol Allied Sci. 2001;26:388–393. doi: 10.1046/j.1365-2273.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108:7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttiger L, Ciuffani J, Zenner HP, Knipper M. A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: a new approach for an animal model on tinnitus. Hear Res. 2003;180:39–50. doi: 10.1016/s0378-5955(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82:601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol. 2008;17:S185–S192. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hauger RL, Geyer MA. Aging effects on the startle response and startle plasticity in Fischer F344 rats. Neurobiol Aging. 1998;19:243–251. doi: 10.1016/s0197-4580(98)00053-0. [DOI] [PubMed] [Google Scholar]
- Varty GB, Braff DL, Geyer MA. Is there a critical developmental 'window' for isolation rearing-induced changes in prepulse inhibition of the acoustic startle response? Behav Brain Res. 1999;100:177–183. doi: 10.1016/s0166-4328(98)00129-6. [DOI] [PubMed] [Google Scholar]
- Wallhausser-Franke E, Mahlke C, Oliva R, Braun S, Wenz G, Langner G. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res. 2003;153:649–654. doi: 10.1007/s00221-003-1614-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A. 2011;108:14974–14979. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang Y, Zhang X. Auditory cortex electrical stimulation suppresses tinnitus in rats. J Assoc Res Otolaryngol. 2011;12:185–201. doi: 10.1007/s10162-010-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]