It's unknown how a single olfactory receptor per neuron is selected for expression. Sim et al. find that dREAM complex member Myb is required for the expression of CO2 receptors Gr21a and Gr63a and acts in opposition to H3K9 methyltransferase Su(var)3-9. Conversely, dREAM complex members Mip120 and E2F2 are required for Gr63a repression in inappropriate neurons. This study suggests a model whereby the dREAM complex plays a key role in epigenetically regulating olfactory receptors.
Keywords: Myb, odorant receptor, Gr63a, olfaction, Drosophila, chromatin
Abstract
In both mammals and insects, an olfactory neuron will usually select a single olfactory receptor and repress remaining members of large receptor families. Here we show that a conserved multiprotein complex, Myb–MuvB (MMB)/dREAM, plays an important role in mediating neuron-specific expression of the carbon dioxide (CO2) receptor genes (Gr63a/Gr21a) in Drosophila. Activity of Myb in the complex is required for expression of Gr63a/Gr21a and acts in opposition to the histone methyltransferase Su(var)3-9. Consistent with this, we observed repressive dimethylated H3K9 modifications at the receptor gene loci, suggesting a mechanism for silencing receptor gene expression. Conversely, other complex members, Mip120 (Myb-interacting protein 120) and E2F2, are required for repression of Gr63a in inappropriate neurons. Misexpression in mutants is accompanied by an increase in the H3K4me3 mark of active chromatin at the receptor gene locus. Nuclei of CO2 receptor-expressing neurons contain reduced levels of the repressive subunit Mip120 compared with surrounding neurons and increased levels of Myb, suggesting that activity of the complex can be regulated in a cell-specific manner. Our evidence suggests a model in which olfactory receptors are regulated epigenetically and the MMB/dREAM complex plays a critical role in specifying, maintaining, and modulating the receptor-to-neuron map.
The olfactory system detects a variety of volatile chemicals using a vastly differentiated array of sensory neurons that express transmembrane receptor proteins. Individual olfactory receptor (Or) genes are expressed in unique classes of olfactory receptor neurons (ORNs), forming a precise receptor-to-neuron map. Neurons expressing the same receptor send axonal projections to a specific area in the antennal lobe, a strategy conserved in insects and mammals (Couto et al. 2005; Mombaerts 2006).
A few principles are emerging to explain how a “one receptor per neuron” pattern is specified in sensory systems. In the Drosophila eye, interlocked feed-forward loops of transcriptional activation and repression can define expression of a rhodopsin gene in a cell type-specific manner (Johnston et al. 2011). Moreover, negative-feedback regulation from the expressed rhodopsin can provide additional exclusion mechanisms to ensure one rhodopsin per photoreceptor (Vasiliauskas et al. 2011). In the Drosophila olfactory system, receptor gene expression is driven by combinatorial codes of cis-acting sites that recruit transcriptional activators and repressors without negative feedback being involved (Ray et al. 2007, 2008; Tichy et al. 2008; Bai et al. 2009; Bai and Carlson 2010; Miller and Carlson 2010; Jafari et al. 2012). Based on these studies, transcription factors seem likely to act at two levels on olfactory receptor promoters: first to restrict expression in an organ-specific manner, and then within an organ to restrict expression to one class of neuron. By itself, this mechanism requires a transcription factor code exclusive to each of the 50 ORN classes. This would be biologically costly, and there are most likely additional mechanisms to ensure the precise expression patterns observed.
Recently, it has been shown that repressive chromatin plays an important role in olfactory receptor expression in mammals (McClintock 2010; Magklara et al. 2011). As olfactory neurons mature, entire blocks of OR genes are silenced by histone H3K9 di/trimethylation, and a single receptor allele per cell is thought to be activated by some unknown mechanism. The heterochromatin structure is proposed to maintain the remaining numerous OR genes in a stable “OFF” state for the remainder of the neuron's life. Active genes are accessible in an “ON” euchromatic state, accompanied by H3K4me3 histone modifications, but little is known about how chromatin in a specific receptor locus is selected to be turned ON. Such a bimodal chromatin structure can provide a restricted open template in a given cell type for subsequent selection of gene expression by combinatorial transcription factor codes. Although mature neurons are post-mitotic and lack the heritability aspect associated with classical epigenetic regulation, it has been argued that the stable chromatin status of a neuron could use the same epigenetic mechanisms seen in mitotic cells (Dulac 2010). We asked whether such a model of chromatin-mediated epigenetic gene regulation can also play a role in Drosophila in establishing the receptor-to-neuron map.
We focused on the Drosophila carbon dioxide (CO2) receptor to understand regulatory mechanisms due to its central role in insect olfaction. The receptor consists of a heterodimer of two seven-transmembrane proteins encoded by Gr21a and Gr63a that are selectively expressed in the antennal ab1C ORNs. These neurons form a labeled line circuit responsible for innate avoidance of CO2 in Drosophila (Suh et al. 2004; Jones et al. 2007; Kwon et al. 2007). The mosquito homologs Gr1, Gr2, and Gr3 are expressed in the maxillary palp (Lu et al. 2007) and are used to detect CO2 in exhaled air, the primary host-seeking cue for insects that transmit deadly human diseases (Gillies 1980).
The dREAM complex is composed of transcription factors and chromatin modifiers, including Myb, E2F2, DP, RBF1/2, Mip40 (Myb-interacting protein 40), Mip120, Mip130, and p55/Caf1, a histone chaperone. p55/Caf1 is also a member of nucleosome remodeling factor (NURF) and nucleosome remodeling and deacetylase (NuRD) complexes. In addition to these proteins, the larger Myb–MuvB (MMB) complex also contains Lin-52; Rpd3, a histone deacetylase; and L(3)mbt, a H4K20me1-binding protein (Korenjak et al. 2004; Lewis et al. 2004; Lipsick 2004; Trojer et al. 2007; Song et al. 2008; van den Heuvel and Dyson 2008; Clapier and Cairns 2009). The MMB/dREAM complex is thought to act as a molecular switch in certain aspects of development (Cayirlioglu et al. 2001; Beall et al. 2002) and could regulate expression and epigenetic control of cell cycle-specific and developmentally regulated genes (Dimova et al. 2003; Korenjak et al. 2004; Lewis et al. 2004; Georlette et al. 2007; Wen et al. 2008). We hypothesized that this complex may be part of the “elusive” mechanism that expresses a single receptor while excluding expression of all other members of the large gene family (McClintock 2010; Magklara et al. 2011).
Here we show that the MMB/dREAM protein complex plays a critical role in specifying the normal expression of Gr63a and Gr21a. Myb is required for expression in the antenna, and its absence substantially reduces activity of ab1C neurons and behavioral avoidance to low levels of CO2. Interestingly, the DNA-binding domain of Myb is not required for receptor regulation, consistent with the previous observation that Myb can bind to chromatin indirectly via other members of the MMB/dREAM complex (Andrejka et al. 2011). Expression of the receptor genes tested in the antenna show a pattern of repressive histone methylation (H3K9me2), an enrichment that has been previously seen in developing olfactory neurons in mammals (Magklara et al. 2011). A role for heterochromatin in this process is implied, since Su(var)3-9, an H3K9 histone methyltransferase, acts in opposition to Myb. Remarkably, changing levels of Myb activity in the adult affect expression of Gr63a. These results suggest that chromatin structure plays a role in modulation of a receptor after the formation of the receptor-to-neuron map. Promoter constructs inserted randomly into the genome mimic Gr63a and Gr21a expression in response to Myb, indicating that the chromatin changes are primarily dependent on local cis-acting sequences, rather than the neighboring chromatin state.
Another member of the complex, Mip120, acts in opposition to Myb by preventing Gr63a from being misexpressed in neurons of the peripheral sensory system and the brain. Loss of E2F2, another complex member, causes derepression of Gr63a in the neurons of the antenna and palp. Changing the stochiometric doses of E2F2 and Myb modulates Gr63a receptor expression and CO2 neuronal sensitivity in opposing manners. However, the role of the MMB/dREAM complex is not limited to the CO2 receptors. Novel olfactory responses are observed in mip130 mutant antenna, presumably caused by ectopic misexpression of another olfactory receptor gene. Together, our results provide evidence for combinatorial regulation of olfactory receptors by the multiprotein MMB/dREAM complex and chromatin, revealing mechanistic insights into how a sophisticated receptor-to-neuron map can be generated, maintained, and modulated.
Results
Expression of Gr63a and Gr21a depends on Myb, a member of the MMB/dREAM complex
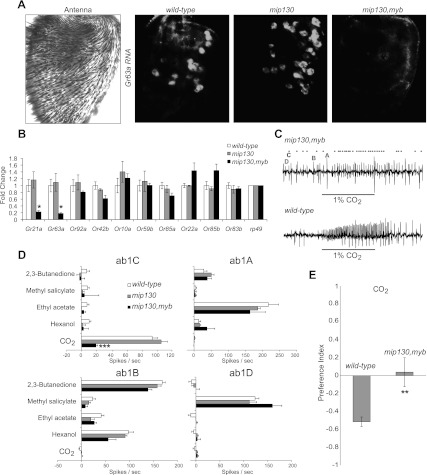
We performed an in-depth analysis of the regulatory sequences of the CO2 receptors Gr21a and Gr63a in search of predicted or known protein-binding sites. We identified an interesting multiprotein complex called MMB/dREAM, which binds strongly to the Gr63a promoter in chromatin immunoprecipitation (ChIP) combined with DNA microarray (ChIP–chip) experiments (Supplemental Fig. S1A; Georlette et al. 2007). The myb-null mutant is larval-lethal, so in order to test whether members of the MMB/dREAM complex have any effect on the expression of Gr63a in adults, we analyzed mip130,myb double mutants that are adult-viable (Manak et al. 2002; Beall et al. 2004). We compared these double-mutant animals with mip130 single-mutant and wild-type animals. Gr63a expression was significantly reduced in mip130,myb mutant antennae but not in mip130 antennae, as shown by whole-mount RNA in-situ hybridization (Fig. 1A) and quantitative RT–PCR (qRT–PCR) (Fig. 1B). A similar effect was observed for Gr21a, which is closely related and shares significant sequence similarity to Gr63a in the region bound by the MMB/dREAM complex (Fig. 1B; Supplemental Fig. S1A,B). Other large basiconic receptor genes and the broadly expressed Or83b/Orco coreceptor showed no significant change in expression (Fig. 1B). These experiments indicate that Myb is required for the normal expression of Gr63a and Gr21a.
Figure 1.
Myb is required for response to CO2. (A) Whole-mount antennae showing a bright-field microscopy image (left) and Gr63a RNA in situ hybridization (right). Male flies of the following genotypes were used: wild-type (wCanton-S), mip130 mutant (mip1301–36), and mip130,myb double mutant (mip1301–36,mybMH107). (B) qRT–PCR using antennal cDNA of indicated genotypes to measure expression of Or genes found in large basiconic sensilla using rp49 as a normalizer. n = 3 independent experiments per genotype. (C) Representative single-sensillum electrophysiological traces of the ab1 sensillum when wild-type or mip130,myb double-mutant flies were exposed to 1% CO2. Characteristic spikes of the four neurons are indicated by their respective letters (A–D). Each spike belonging to the C neuron is denoted by a black dot in the mutant trace. (D) Electrophysiological responses of the four ab1 neurons to the indicated diagnostic odors. n = 10 recordings per genotype. (E) Preference index to measure behavioral responses of indicated flies to 0.33% CO2 in a T-maze. n = 10 trials per genotype (∼40 flies per trial). Data are presented as mean ± SEM. Two-tailed Student's t-test was used: (*) P < 0.05; (**) P < 0.01; (***) P < 0.001.
Both Gr63a and Gr21a are necessary for formation of a functional CO2 receptor and for neuronal response to CO2 (Jones et al. 2007; Kwon et al. 2007). As predicted by our studies of gene expression, the mip130,myb mutants showed a substantially reduced CO2 response in the ab1C neurons (Fig. 1C,D). This phenotype is highly specific, and odorant responses of neurons in the other three classes (A, B, and D) housed within the ab1 sensillum were unaffected (Fig. 1D). Interestingly, the ab1C neuron itself was still present in the mutant, as shown by stereotypic action potentials of the appropriate amplitude (Fig. 1C). Together, these data imply that Myb is required specifically for expression of Gr63a and Gr21a in the ab1C neurons but not for the development of the neuron itself.
Drosophila melanogaster have an innate avoidance to CO2, which is found in stress odor and unripe fruit (Suh et al. 2004; Faucher et al. 2006). The avoidance to 0.33% CO2 was abolished in the mip130,myb mutant flies, indicating that the reduction of Gr63a and Gr21a expression affects behavior (Fig. 1E). However, at a higher dose of CO2, the avoidance response was still observed (data not shown), as we would expect from the residual electrophysiological activity.
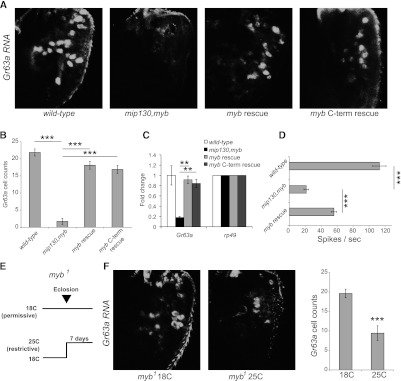
Pan-neuronal reintroduction of Myb in mip130,myb mutant animals using elav-GAL4 to drive transgenic UAS-RFP∷myb+ cDNA rescued Gr63a expression, as observed by both RNA in-situ hybridization (Fig. 2A,B) and RT–PCR (Fig. 2C). Similar results were also observed for Gr21a (Supplemental Fig. S1B). The electrophysiological response to CO2 was also partially rescued in the ab1C sensillum (Fig. 2D). Ectopic expression of myb+ did not drive additional ectopic Gr63a expression, suggesting that Myb alone is insufficient for receptor expression (Supplemental Fig. S4C).
Figure 2.
Myb activates Gr63a expression post-developmentally via the MMB/dREAM complex. (A) Whole-mount male antennae showing Gr63a RNA in situ hybridization on wild-type, mip130,myb double mutant, myb rescue (mip1301–36,mybMH107; elav-GAL4/+; UAS-RFP∷myb+/+), and myb C-term rescue (mip1301–36,mybMH107; myb promoter-GFP∷mybC-terminal/+). (B) Number of Gr63a-expressing cells in genotypes indicated in A. n = 9–13 antennae per genotype. (C) qRT–PCR using antennal cDNA to measure Gr63a expression in the indicated genotypes. n = 3 biological replicates. (D) Electrophysiological responses to 1% CO2 in wild-type, double-mutant, and myb rescue genotypes. n = 8–24 per genotype. (E) Schematic of culturing temperature-sensitive myb1 males at 18°C (permissive) and shifting them to 25°C (restrictive) post-eclosion. (Top panel) Control myb1 flies were kept at 18°C throughout the entire period. (F) Whole-mount antenna Gr63a RNA in situ hybridization on myb1 flies. n = 23–31 per condition. Data are presented as mean ± SEM. Two-tailed Student's t-test was used: (**) P < 0.01; (***) P < 0.001.
Myb regulates CO2 receptor expression via the MMB/dREAM complex
Interestingly, we found that a truncated version of Myb containing only the C-terminal domain also rescued Gr63a and Gr21a expression (Fig. 2A–C; Supplemental Fig. S1B). Despite lacking all three repeats of the highly conserved Myb DNA-binding domain (Peters et al. 1987), this Myb C-terminal domain is sufficient to assemble into the MMB/dREAM complex and localize to specific regions in the genome to regulate gene expression (Wen et al. 2008; Andrejka et al. 2011). Thus, the rescue of receptor expression in the absence of the highly conserved Myb DNA-binding domain suggests that Myb is acting via the complex at the receptor loci.
Since the myb-null mutant is adult-lethal (Manak et al. 2002), we initially investigated the mip130,myb double mutant. We chose not to analyze myb− clones, since loss of myb during pupal development can cause defects such as incomplete cell division (Katzen and Bishop 1996), which could confound results in mature ORNs. To test the effect of loss of Myb alone on Gr63a expression, we used a temperature-sensitive myb1 mutant that is viable if raised to adulthood at the permissive temperature (18°C) (Katzen and Bishop 1996). This myb1 allele has a point mutation in the functionally important C-terminal domain, disrupting protein function at the restrictive temperature (25°C) (Katzen et al. 1998). Flies were cultured at the permissive temperature and, upon reaching adulthood, were transferred to the restrictive temperature (Fig. 2E). There was a significant reduction in Gr63a expression at the restrictive temperature (Fig. 2F; Supplemental Fig. S4A). Interestingly, at the restrictive temperature, the number of Gr63a+ cells per antenna followed a bimodal distribution (Supplemental Fig. S4A), and we conjecture that this may suggest a stochastic process with two discrete chromatin states influenced in part by the dose of Myb protein. We cannot, however, rule out other possibilities. These results indicate that the complex is required post-developmentally to maintain receptor gene expression.
A heterochromatin signature in odorant receptor genes
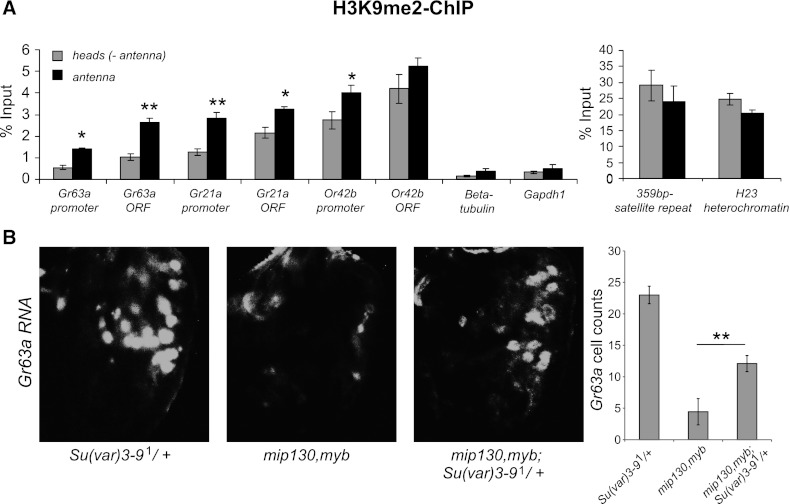
Epigenetic control of odor receptor gene expression has recently been shown in mice where unexpressed OR genes are repressed by H3K9 dimethylation in olfactory stem cells and by trimethylation in mature olfactory neurons (Magklara et al. 2011). To test whether repressive chromatin is also found at Drosophila olfactory receptor genes, we performed ChIP-PCR on the adult antenna using antibodies specific for the H3K9me2 mark. Since individual odor receptor genes are expressed in a very small fraction of antennal olfactory neurons, we expect that >98% of ORNs will potentially display repressive marks in receptor gene loci. We observed enrichment of the H3K9me2 mark at Gr21a, Gr63a, and another receptor gene, Or42b, in the antenna compared with head tissue (Fig. 3A). Negative controls such as β-tubulin and Gapdh1 did not show a high level, as would be expected for broadly expressed genes, while positive controls such as satellite repeats (Rudolph et al. 2007) and pericentric heterochromatin (Zhang et al. 2008) showed the expected high levels for H3K9me2 (Fig. 3A). A widely used antibody against H3K9me3 showed nonspecific binding when tested on a peptide array (data not shown), so we do not present data from those ChIP experiments. The H3K9me2-repressive chromatin structure at odor receptor genes is similar to that observed in mammalian olfactory stem cells and raises the interesting possibility that the MMB/dREAM complex may also play a conserved role in its regulation.
Figure 3.
Myb opposes a repressive chromatin modifier. (A) ChIP using antibodies to H3K9me2 on wild-type antennae or heads without antennae. qRT–PCR was performed for indicated regions. n = 3–4 biological replicates. (B) Whole-mount antennae showing Gr63a RNA in situ hybridization on heterozygous Su(var)3-91/+, mip130,myb double mutant, and heterozygous mip130,myb; Su(var)3-91/+. n = 9–21 per genotype. Data are presented as mean ± SEM. Two-tailed Student's t-test was used: (*) P < 0.05; (**) P < 0.01; (***) P < 0.001.
Epigenetic regulation of receptor expression by MMB/dREAM
Because ab1C neurons make up a small fraction of the total cell population (∼20–30 cells per antenna), it is not feasible to measure H3K9 methylation status exclusively in these cells. However, in Drosophila, it is known that the majority of H3K9me2 methylation marks are catalyzed by the histone methyltransferase Su(var)3-9 (Rea et al. 2000; Ebert et al. 2004). To test whether regulation of Gr63a expression by Myb involves H3K9 methylation via Su(var)3-9, we tested for a genetic interaction between the two. Upon halving the gene dosage of Su(var)3-9 in the mip130,myb mutant, we found a partial rescue of Gr63a expression (Fig. 3B). Halving the gene dosage of Su(var)3-9 alone did not affect Gr63a expression (Fig. 3B). Thus, Myb opposes the repressive effects of H3K9 histone methyltransferase Su(var)3-9 in Gr63a regulation. Taken together, our data indicate that the MMB/dREAM complex is required to affect permissive chromatin at the Gr63a locus in the ab1C ORNs.
Myb also acts on transgenic receptor–promoters inserted randomly in the genome
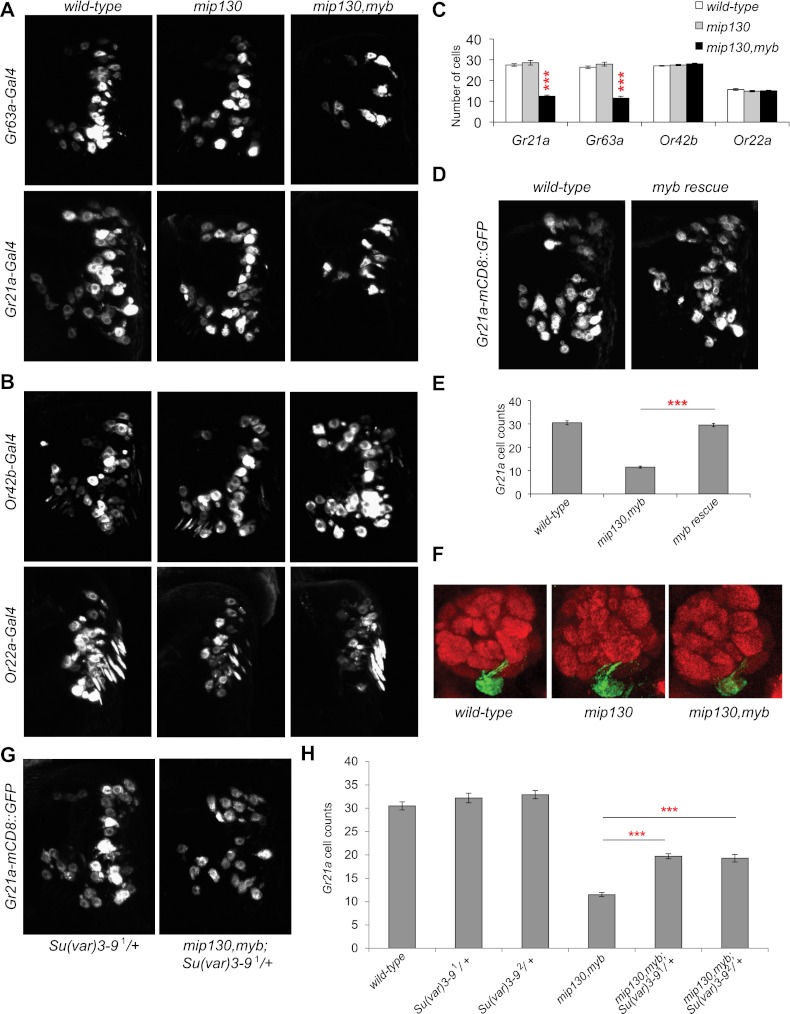
In Drosophila and mammals, transgenes containing short Or gene promoter sequences can reproduce the spatial expression patterns of the endogenous receptor (Vassalli et al. 2002; Rothman et al. 2005; Ray et al. 2007, 2008). To test whether short promoters are sufficient to recapitulate regulation of the Gr63a locus by Myb, we used a 2.64-kb Gr63a promoter-GAL4 to drive expression of a GFP reporter (UAS-mCD8∷GFP) in wild-type and mip130,myb mutant flies. We found a significant decrease in the number of GFP-expressing neurons (Fig. 4A,C), consistent with the expression of endogenous Gr63a RNA. Likewise, a Gr21a-GAL4 reporter labeled fewer neurons in the double mutant (Fig. 4A,C). In contrast, Or42b-GAL4 and Or22a-GAL4 showed no significant difference in GFP+ cell numbers (Fig. 4B,C), again consistent with the expression of endogenous genes. A direct promoter-GFP fusion (Gr21a promoter-mCD8∷GFP) was used to demonstrate that expression is rescued by introducing elav-GAL4; UAS-RFP∷myb+ in the double-mutant flies (Fig. 4D,E). This reporter was also used to demonstrate genetic opposition between mip130,myb and Su(var)3-9, as seen with Gr63a RNA in situ hybridization (Fig. 4G,H). Thus, the MMB/dREAM complex affects the chromatin state of both the endogenous and transgenic promoters of Gr63a and Gr21a integrated randomly at other regions of the genome.
Figure 4.
Promoters of Gr63a and Gr21a are important for Myb regulation (A,B) Whole-mount antennae showing neurons expressing GAL4-responsive membrane targeted GFP (UAS-mCD8∷GFP) with Gr63a or Gr21a promoter-GAL4s (A) or controls, Or42b or Or22a promoter-GAL4s (B), in wild-type, mip130 mutant, and mip130,myb double mutant. (C) Counts of GFP+ cells for A and B. n = 10 per genotype. (D) Whole-mount antennae showing neurons expressing a different Gr21a promoter direct fusion transgene (Gr21a promoter-mCD8∷GFP) and myb rescue (mip130,myb; Gr21a promoter-mCD8∷GFP/elav-GAL4; UAS-RFP∷myb+). (E) Counts of GFP+ cells for D. n = 10 per genotype. (F) Immunofluorescence using anti-GFP (green) and anti-nc82 (neuropil marker; red) antibodies on antennal lobes of wild-type, mip130 mutant, and mip130,myb double mutant with Gr63a-GAL4; UAS-mCD8∷GFP. (G) Whole-mount antennae showing neurons expressing Gr21a promoter-mCD8∷GFP in Su(var)3-91/+ and mip130,myb;Su(var)3-91/+. (H) Counts of GFP+ cells for G and Su(var)3-92, a second null allele. n = 10 per genotype. Data are presented as mean ± SEM. Two-tailed Student's t-test was used: (***) P < 0.001.
ORNs expressing a particular olfactory receptor project their axons to a distinct glomerulus in the antennal lobe (Couto et al. 2005), and transcription factors such as pdm3 and acj6 have dual roles in mediating both Or gene expression and ORN axonal projection (Clyne et al. 1999; Komiyama et al. 2004; Tichy et al. 2008). Using Gr63a-GAL4, UAS-mCD8∷GFP, which still had some residual expression in the absence of both Myb and Mip130, we showed that the axonal targeting of ab1C neurons to the V glomerulus was unaffected (Fig. 4F).
We asked whether myb has genetic interactions with other genes known to affect Gr63a expression. The microRNA mir-279 and its regulator, prospero, have been shown to play a developmental role in repressing the formation of ectopic CO2 neurons in the maxillary palp (Cayirlioglu et al. 2008; Hartl et al. 2011). Using Gr21a promoter-mCD8∷GFP to test for a genetic interaction between myb and mir-279, we found that the number of CO2 ORNs was similar between mip130,myb; mir-279 triple mutants and mir-279 single mutants in the antenna and maxillary palp (Supplemental Fig. S2A,B), suggesting that ectopic cells in the maxillary palp in the mir-279 mutant do not require Myb for expression. In addition, myb expression was normal in mir-279 mutant maxillary palps (Supplemental Fig. S2C), while mir-279 expression was normal in mip130,myb mutant antenna (Supplemental Fig. S2D), indicating lack of genetic interaction. mir-279 appears to play a developmental role in preventing the birth of CO2-like neurons in the maxillary palp (Cayirlioglu et al. 2008; Hartl et al. 2011), while Myb plays a role in the expression of Gr63a and Gr21a in the mature ORN.
Repression of receptor expression in inappropriate cells by Mip120
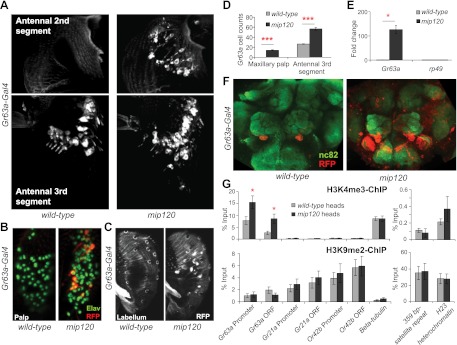
The specification of olfactory receptor gene expression requires activation in the correct neurons as well as silencing in other neuronal classes. The Mips of the MMB/dREAM complex have previously been shown to have a repressive role antagonistic to Myb (Korenjak et al. 2004; Lewis et al. 2004), raising the possibility that these proteins might mediate repression of Gr63a in inappropriate neurons. Wild-type flies express Gr63a only in the ab1C neurons of the third antennal segment. In contrast, we found that mip120 mutant flies misexpressed Gr63a promoter-GAL4 in other classes of sensory neurons, including the second antennal segment (hearing) (Fig. 5A), maxillary palp (olfactory) (Fig. 5B,D,E), and labellum (taste) (Fig. 5C). Gr63a was also overexpressed in approximately twice the number of ORNs in the large basiconic region of the third antennal segment (Fig. 5A,D).
Figure 5.
Mip120 is required to repress Gr63a misexpression. (A) Whole-mount antennae of wild-type and mip120 mutant (mip12067-21-6/mip12067-9A-9) expressing Gr63a-GAL4, UAS-mCD8∷chRFP. (B) Immunofluorescence using anti-Elav (pan-neuronal marker; green) and anti-RFP (red) of whole-mount maxillary palps of the genotypes indicated in A. (C) Whole-mount labellum of the indicated genotypes. (D) Number of Gr63a-expressing cells in A and B. n = 10 antennae or maxillary palps per genotype. (E) qRT–PCR using maxillary palp cDNA of indicated genotypes to measure Gr63a misexpression in the palp. n = 3 independent experiments per genotype. (F) Immunofluorescence using antibodies to nc82 (green) and RFP (red) on whole-mount brains of the genotypes indicated in A. (G) ChIP using antibodies to H3K4me3 or H3K9me2 on wild-type and mip120 mutant heads. n = 5–6 biological replicates per genotype. Data are presented as mean ± SEM. Two-tailed Student's t-test was used: (*) P < 0.05; (***) P < 0.001.
Consistent with these ectopic expression patterns, mip120 mutant antennal lobes showed innervation by Gr63a promoter-expressing neurons of multiple glomeruli in addition to the V glomerulus (Fig. 5F; Supplemental Fig. S3A,B). Gr63a was also misexpressed in a large number of additional neurons in the brain (Fig. 5F). Using single-sensillum electrophysiology, we tested whether the misexpression of Gr63a in ORNs of the maxillary palps can impart responsiveness to CO2, and found that the neurons were not responsive to 1% CO2 (n = 6 neurons) (data not shown), suggesting that other required components of the CO2 detection machinery, such as Gr21a or Gαq, may not be misexpressed in the same neurons of the palps (Yao and Carlson 2010). Indeed, whole-mount in situ hybridizations showed, on average, only one cell positive for Gr21a RNA in the maxillary palps of mip120 mutants (n = 6 palps). The olfactory responses to a diagnostic five-odor panel were normal from each of the six classes of ORNs (n = 3 for each class), suggesting that there is no obvious developmental defect in the maxillary palps of the mip120 mutants. Taken together, these results indicate that the role of Mip120 in the MMB/dREAM complex is to restrict Gr63a expression to the correct antennal segment by mediating silencing in other sensory tissues.
Since misexpression of Gr63a is widespread across the head, it provides us with sufficient starting material to directly test by ChIP-PCR whether there are changes in the chromatin corresponding to derepression of Gr63a in the mip120 mutant heads. We found a significant increase in H3K4me3 that marks active chromatin at the Gr63a locus, while the levels of H3K9me2 remained unaffected (Fig. 5G). These results indicate that in the absence of Mip120, ectopic expression of Gr63a is associated with enrichment of H3K4me3, further emphasizing the combinatorial role of the MMB/dREAM complex in epigenetic regulation of Gr63a.
Refinement of Or gene expression by Mip130
Mip130 is a generally repressive MMB/dREAM complex member (Wen et al. 2008). However, unlike mip120 mutants, mip130 flies did not show an obvious misexpression phenotype for Gr63a (Fig. 1A,B). One possibility could be that mip130 instead plays a role in refining expression of other receptor gene families, such as ionotropic or odorant receptors, which also require precise expression in a one receptor per neuron fashion. In order to test this, we used a diagnostic odor panel and screened eight classes of ORNs in the antennae of the mip130 mutant flies using single-sensillum electrophysiology. Indeed, one of the eight classes, the ab3A neuron, had an unusual odor response profile in the mip130 antenna (Fig. 6F; Supplemental Fig. S6). The ab3A neuron in the mip130 mutant antenna detected diagnostic odors characteristic of its endogenous receptor, Or22a (such as pentyl acetate), but also showed strong responses to methyl salicylate, ethyl acetate, and anisole, odors that are normally not detected by this neuron (Fig. 6F). Or22a was still expressed in the mutant antenna (Figs. 4B, 6G). However, gained odor responses do not match that of any characterized odorant receptors (Hallem and Carlson 2006; Kreher et al. 2008). A possible interpretation of these results is that the novel odor responses are conferred by the misexpression of an uncharacterized Or gene specifically in the ab3A cells of the mip130 mutant flies. These results suggest that the MMB/dREAM complex and its effect on chromatin structure may play an important role in the differential expression of receptor genes in a variety of olfactory neurons.
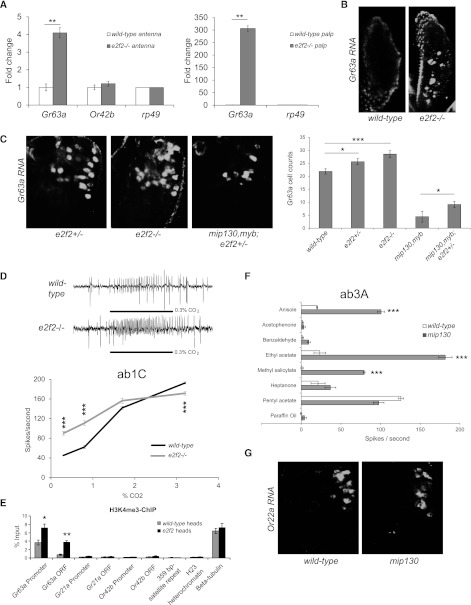
Figure 6.
MMB/dREAM has broader roles in olfactory receptor regulation. (A) qRT–PCR using antennal (left panel) or maxillary palp (right panel) cDNA of wild-type or e2f2 mutant (e2f21–188/e2f2329) to measure expression of Gr63a or control Or42b. n = 3 independent experiments per genotype. (B) Whole-mount maxillary palp showing Gr63a RNA in situ hybridization in wild-type and e2f2 mutant. (C) Whole-mount antennae showing Gr63a RNA in situ hybridization on heterozygous e2f2329/+ and homozygous e2f21–188/e2f2329 mutant flies and mip130,myb; e2f2329/+ flies. n = 9–15 per genotype. Cell counts are shown in the right panel. (D, top) Representative electrophysiological traces of ab1 sensillum when e2f2 mutant flies were exposed to 0.3% CO2. (Bottom) Dose response of e2f2 mutant ab1C neuron to CO2. n = 6. (E) ChIP using antibodies to H3K4me3 on wild-type and e2f2 mutant heads. n = 3 biological replicates per genotype. (F) Electrophysiological responses of mip130 mutant ab3A neuron to diagnostic odors. n = 5 recordings per genotype. (G) Whole-mount antennae showing Or22a RNA in situ hybridization on wild-type and mip130 mutant. Data are presented as mean ± SEM. Two-tailed Student's t-test was used: (*) P < 0.05; (**) P < 0.01; (***) P < 0.001.
Modulation of CO2 neuronal sensitivity by MMB/dREAM
Another member of the MMB/dREAM complex, E2F2, has also been shown to play a repressive role (Lewis et al. 2004). The e2f2 mutant flies showed a significant increase in the levels of Gr63a in both the antenna and the maxillary palp (Fig. 6A), whereas Or42b showed no significant change in expression by RT–PCR (Fig. 6A). These observations were validated by RNA in situ hybridization experiments (Fig. 6B). Gr21a was also sporadically misexpressed in the maxillary palps of the e2f2 mutants with detectable RNA in an average of two cells per palp (n = 6). However, a thorough survey using single-sensillum electrophysiology of the maxillary palps showed that neurons were unresponsive to CO2 stimuli of up to 4.8% (n = 25 neurons) (data not shown). Characteristic olfactory responses to each of the six classes of ORNs were identifiable (n = 3 for each class), suggesting that there is no obvious developmental defect in the e2f2 mutant maxillary palps.
We also found that halving the gene dosage of e2f2 partially suppressed the mip130,myb phenotype in Gr63a expression (Fig. 6C). In addition, heterozygous e2f2 flies in an otherwise wild-type genetic background also showed an increase of Gr63a expression compared with wild-type flies (Fig. 6C). The genetic opposition of e2f2 to myb is consistent with our expectation that e2f2 has a repressive regulatory role in the MMB/dREAM complex (Wen et al. 2008). Interestingly, heterozygous mip130,myb female flies also showed an intermediate Gr63a expression relative to wild-type and homozygous mip130,myb females (Supplemental Fig. S4B), suggesting that the stoichiometric dose of each MMB/dREAM component could play a critical role in gene regulation.
The olfactory response of a neuron may depend on not only the identity of the receptor being expressed, but also the level of receptor expression (Tanoue et al. 2008). Since little is known about mechanisms that ensure appropriate levels of receptor expression, we wanted to investigate whether the doses of individual MMB/dREAM members, some with opposing effects, could play a role in receptor modulation. Using single-sensillum electrophysiology to probe CO2 response in the ab1C sensillum, we found that the electrophysiological response was affected in opposite directions by the dose of myb and e2f2. In Figure 2D, we show that the electrophysiological response to CO2 was substantially lowered in the myb,mip130 flies and partially rescued when myb was expressed using a pan-neuronal driver, elav-GAL4. Conversely, the dose response to CO2 showed that the response was significantly increased in the e2f2 mutant flies at the lower concentrations (Fig. 6D). These results demonstrate that the levels of CO2 receptor expression affect the sensitivity of the neuron and indicate that the MMB/dREAM complex can act to mediate a gain-control mechanism for the olfactory neuron by integrating doses of positive (Myb) and negative (E2F2) subunits.
In the absence of E2F2, the misexpression of Gr63a is associated with a concomitant enrichment of the H3K4me3 mark in the heads (Fig. 6E). This pattern is similar to what we found in mip120 mutants, suggesting a correlation between misexpression and chromatin changes.
MMB/dREAM complex members are present in ORN nuclei
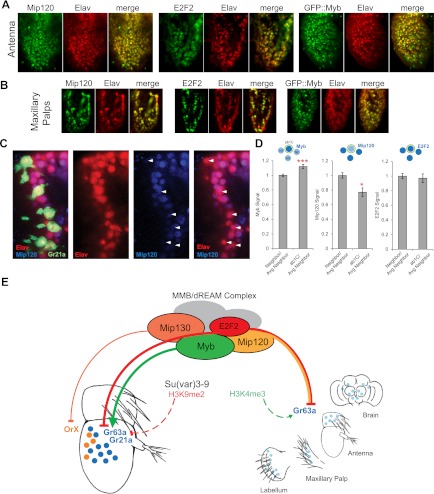
The genetic analysis demonstrates roles for several members of the MMB/dREAM complex, such as Myb, Mip120, Mip130, and E2F2, in the ORN nuclei. We generated antibodies against the two repressive members, Mip120 and E2F2, to characterize their expression. The specificity of the antibodies was validated by the absence of staining in mip120 or e2f2 mutants (Supplemental Fig. S7B). We found that both proteins were detected inside the nuclei of a large fraction of olfactory neurons of the antenna and the maxillary palps, which were costained with anti-Elav, a pan-neuronal marker (Fig. 7A,B). To map expression of Myb, we used a genomic construct encoding a Myb-GFP fusion protein and found that this protein was also present in a large fraction of the olfactory neuron nuclei (Fig. 7A,B). Taken together, these experiments demonstrate that these nuclear proteins are present in ORNs and persist post-developmentally.
Figure 7.
MMB/dREAM in olfactory organs and model for olfactory receptor expression. Confocal micrographs of antennae (A) and maxillary palp (B)s using anti-Mip120 and anti-Elav in wild-type flies (left panels), anti-E2F2 and anti-Elav in wild-type flies (middle panels), or anti-GFP and anti-Elav in myb promoter-GFP∷Myb flies (right panels). (C) Representative confocal micrograph from a Gr21a promoter-mCD8∷GFP antenna stained with anti-CD8, anti-Elav, and anti-Mip120 antibodies. Overlays of all three (left) and of anti-Elav with anti-Mip120 (right) are provided. Arrowheads indicate nuclei of Gr21a+ ab1C neurons. (D) Schematic showing cells that were selected for analysis of nuclear stain signal intensity and mean Myb, Mip120, or E2F2 signal intensity in ab1C or a neighboring nucleus as a ratio of the mean signal in the neighboring three cells. For Myb signal, n = 48 for neighboring cells and n = 16 for ab1C cells. For Mip120 signal, n = 70 for neighboring cells and n = 25 for ab1C cells. For E2F2 signal, n = 29 for neighboring cells and n = 13 for ab1C cells. Error bars indicate SEM. (*) P < 0.05; (***) P < 0.001. (E) Olfactory receptors have a H3K9me2-repressive chromatin mark (red dashed line). Expression of Gr63a/Gr21a receptors in the ab1C neurons of antennae requires Myb (green arrow), and Myb opposes histone methyltransferase Su(var)3-9. Conversely, repression of Gr63a in other sensory organs and the brain requires Mip120 and E2F2 (combined red and orange line). The absence of Mip120 or E2F2 causes misexpression and increased H3K4me3 chromatin mark at Gr63a gene (green dashed arrow). E2F2 also has a repressive modulatory effect on Gr63a expression in ab1C cells (solo red line), while Mip130 represses expression of an unknown olfactory receptor in the antenna (dark-orange line).
Since the members of the complex are present in several antennal neurons, we asked whether the MMB/dREAM complex could be differentially regulated to achieve neuron-specific selectivity. We performed antibody staining and demonstrated that Myb, the activator of the complex, was consistently higher in ab1C nuclei compared with surrounding neurons. Interestingly, a repressive subunit of the complex, Mip120, was present at lower levels in the ab1C neurons as compared with neighboring neurons. E2F2 levels appeared similar between ab1C and its neighbors (Fig. 7C,D). In total, we observed that the Myb:Mip120 ratio in ab1C nuclei is ∼45% higher compared with neighboring nuclei. The simplest interpretation of these results is that the ab1C nuclei are able to express the CO2 receptors by virtue of having higher levels of activating Myb and lower levels of the repressive Mip120 subunit of the dREAM complex, while the neighboring cells with lower Myb and higher Mip120 levels cannot.
Discussion
Heterochromatin-like repression of odor receptors in Drosophila
A central question in olfactory receptor regulation is how a neuron expresses a single member of a large gene family while excluding other members. One model emerging from mammals suggests that histone H3K9 di/trimethylation maintains the odor receptor family members in a generalized repressed state and that expression of a single receptor may be induced by removal of these repressive marks. Here we observed that a pattern of repressive H3K9me2 chromatin exists in the promoters and gene bodies of olfactory receptors of Drosophila, similar to the olfactory stem cells in mammals. In mammals, activating H3K4me3 is found at gene loci in the specific cells where a receptor is expressed (Magklara et al. 2011). Although a similar cell-sorting experiment is not technically feasible in Drosophila, we did find a similar H3K4me3-mediated derepression of the Gr63a gene when it is misexpressed in the mip120 mutant brain. Although the olfactory receptor gene families in mammals and insects are not evolutionarily related, both systems appear to use repressive chromatin in solving part of the one receptor per neuron problem.
MMB/dREAM epigenetically regulates receptor gene expression
Establishing one receptor per neuron likely involves two processes: the initial selection of a receptor for expression while keeping all other receptors repressed, and the maintenance of this expression profile throughout the life of the neuron. Here we show that the multiprotein MMB/dREAM complex in flies has a role in this process. Myb, the permissive member of the complex, is required for maintaining Gr63a/21a expression in the antenna, genetically opposing the H3K9 methyltransferase Su(var)3-9. Repressive members Mip120 and E2F2 are required for maintaining repression of Gr63a in other sensory organs. This bears a resemblance to other epigenetic complexes, such as the Polycomb group (PcG) and Trithorax group (TrxG) proteins, which can stably maintain repressive and active chromatin, respectively, at Hox gene loci during embryonic development (Schuettengruber et al. 2011).
The roles of other members of the MMB/dREAM complex in receptor gene regulation will be very interesting to pursue, especially the roles of the chromatin-modifying proteins found associated with the complex: Rpd3 (a histone deacetylase), L(3)mbt (a H4K20me1-binding protein) (Trojer et al. 2007), and p55/Caf1 (a histone chaperone) (Song et al. 2008). It will be intriguing to study in depth how MMB/dREAM and chromatin remodeling can participate in epigenetically regulating olfactory receptor expression. Analysis of the mip130 mutant raises the possibility that members of the complex may regulate the odorant receptor gene family members in the antenna as well.
Myb is required in the mature neuron
It has recently been demonstrated that chromatin modification at Notch target loci via Hamlet plays a role in cell fate determination in the developing sensillum (Endo et al. 2011). Our findings are distinct from this pathway, as Myb is required post-mitotically. Loss of Myb does not perturb formation of the C neuron in ab1 sensilla or axonal targeting to the V glomerulus. The only detectable defect is the loss of Gr63a and Gr21a expression, which is rescued when myb is expressed with elav-GAL4, a promoter only active in mature neurons. Furthermore, Myb is required to maintain expression of Gr63a in the adult, as demonstrated with the temperature shift experiments using the myb1 allele. Taken together, our data suggest that Myb participates in receptor gene expression after the final neuronal cell division and is required continuously to maintain expression of the CO2 receptors throughout the life of the neuron.
MMB/dREAM acts on Gr63a and Gr21a promoters
The presence of the MMB/dREAM complex at the Gr63a promoter was identified by ChIP–chip from a Drosophila cell line (Georlette et al. 2007), and this region of the promoter, including a consensus complex-binding sequence, is partly conserved upstream of Gr21a as well. Because the regulatory effect of the complex is reported reliably by both Gr63a and Gr21a promoter–GAL4 constructs, this opens the possibility of more detailed characterization of predicted MMB/dREAM-binding sites through mutational analysis of the promoters. In mice, the property of singular expression is also retained by short transgenic promoters of OR genes inserted outside of their endogenous loci (Serizawa et al. 2003; Lewcock and Reed 2004). It is possible that these DNA sequences contain instructive cues that are responsible for enrichment of repressive H3K9 methylation, which can be conveniently examined using the fly model.
Evolutionary conservation and odor receptor gene expression
Unlike D. melanogaster, which avoid CO2, mosquitoes are strongly attracted to it via orthologous receptors (Gr1, Gr2, and Gr3) in the maxillary palps. Compared with the 12 Drosophila species, Myb and Mip130 are well conserved in Anopheles gambiae (malaria), Aedes aegypti (dengue and yellow fever), and Culex quinquefasciatus (filariasis and West Nile), while Mip120 and E2F2 are more poorly conserved (Supplemental Fig. S5A,B). We predict that differences in Mip120 and E2F2 activity in mosquitoes might provide a template for evolutionary differences in CO2 receptor expression in the maxillary palps. Alternatively, changes in cis-regulatory sequences upstream of the CO2 receptor gene orthologs could be responsible for differences in expression across species. Since the CO2 receptor plays a central role in the ability of mosquitoes to find humans and transmit diseases, our findings could have a broader impact for human health. Knowledge about the mechanisms of CO2 receptor gene expression and the evolution of its expression patterns could have important implications in controlling mosquito-borne diseases such as malaria and dengue fever that affect hundreds of millions of people.
The MMB/dREAM complex is also conserved in mammals as well (Litovchick et al. 2007; Pilkinton et al. 2007; Schmit et al. 2007). There are three mammalian homologs of the Drosophila myb: A-Myb/MYBL1, B-Myb/MYBL2, and c-Myb/MYB (Lipsick 2004). In fact, there are some indications that A-Myb and c-Myb may have a role in the mouse olfactory system. A-myb is expressed in the olfactory epithelium, while c-myb mutant mice have smaller olfactory bulbs (Trauth et al. 1994; Malaterre et al. 2008).
One receptor per neuron: combination of chromatin accessibility and transcription regulation
Previous studies investigating Or gene expression have reported that a combinatorial code of cis-regulatory elements and transcription factors can specify restricted expression domains in olfactory neuron classes. For example, organ-specific, region-specific, and neuron-specific regulatory elements have been identified for Or genes expressed in the maxillary palps (Ray et al. 2007, 2008). Given that a receptor gene is expressed in only one class of olfactory neurons (∼20 cells) out of >100,000 neurons in the organism, it is very unlikely that each of ∼50 ORN classes would possess a unique transcription factor code that is not recapitulated elsewhere in the nervous system. A recent study reported that seven transcription factors from different protein families could combinatorially regulate the expression of several olfactory receptors in the adult antenna (Jafari et al. 2012). The MMB/dREAM complex appears to function in a different fashion, as it integrates the effect of transcriptional activators, repressors, and chromatin-modifying proteins in a single complex and acts as an adaptable molecular switch for Or expression. A key question is how various regulatory mechanisms are able to act in a neuron-specific manner to achieve selective receptor expression. Most known factors that are required for olfactory receptor expression, like POU, HLH, HTH, and Zn finger transcription factors, are expressed far more broadly than the cells where they are required for receptor expression (Ray et al. 2007, 2008; Tichy et al. 2008; Bai et al. 2009; Bai and Carlson 2010; Miller and Carlson 2010; Jafari et al. 2012).
A possible model supported by our observations is that most receptor genes are in a repressed chromatin state in neurons, and selective opening of chromatin structure in certain cells at a specific receptor locus provides a permissive template for transcription factors to drive transcription of the receptor (Fig. 7E). After one set of factors initiates receptor gene choice, epigenetic complexes such as MMB/dREAM would be recruited to the locus in order to maintain stable expression. Two layers of regulatory mechanisms could restrict expression patterns in only those cells where both pathways are active. Complexes such as MMB/dREAM, which contain epigenetic modulators along with transcriptional activators and repressors, would be exceptionally well suited to initiate, maintain, and modulate expression. Other complexes, like MMB/dREAM, may also be involved in Or gene regulation. While Myb itself seems to be specific to the CO2 receptors among the eight classes of neurons that we surveyed, there are ∼40 additional ORN classes in the adult and ∼21 in the larvae that we have yet to examine. We speculate that while repressive chromatin may be a general strategy for receptor regulation, the dREAM complex could be one of many factors that interact with it and influence the final outcome of receptor expression.
The selectivity question is particularly interesting for the MMB/dREAM complex, since it is the first multiprotein complex shown to be involved in regulation of receptor gene expression and contains both permissive and repressive subunits. We show that ab1C nuclei contain significantly higher Myb and lower Mip120 than neighboring neurons, implying that the stoichiometry of the complex can determine whether a gene will be active or not. The mechanism underlying this differential regulation of Myb and Mip120 may be the key to achieving neuron-specific expression of olfactory receptors.
We also demonstrate that expression of Gr63a can be modulated in the adult stage by the activity of Myb. The level of E2F2 affects Gr63a expression in the opposite direction of Myb activity. Changes in levels of Gr63a expression via the MMB/dREAM complex alter the sensitivity to a CO2 stimulus significantly. This finding could provide a molecular template for modulation of specific chemosensory receptors in the differentiated olfactory system without perturbation of the precise receptor-to-neuron map. Such receptor-specific plasticity could be important for modulating receptor expression and neuronal sensitivity to various changes in the physiological state, such as feeding, mating, and circadian rhythms, or to external stimuli such as changes in environmental conditions.
Materials and methods
qRT–PCR
Total RNA was isolated from 100 antennae or maxillary palps by Trizol extraction (Invitrogen). cDNA was generated using SuperScript III reverse transcriptase (Invitrogen). One microliter of the resulting cDNA, fast SYBR Green PCR master mix (Applied Biosystems), and gene-specific primers were combined in a 20-μL reaction on 96-well plates. Primer sequences are provided (Supplemental Fig. S8). The SYBR Green reaction was then used for qPCR on the StepOnePlus real-time PCR system (Applied Biosystems). Also see the Supplemental Material.
ChIP
Ten fly heads or 100 antennae were used per ChIP sample. Chromatin was sonicated to obtain 300- to 500-base-pair (bp) DNA fragments and incubated with Dynabeads (Invitrogen) and 2 μg of antibodies to H3K9me2 (ab1220) or H3K4me3 (ab8580). Specificity of anti-H3K9me2 antibody was validated using a peptide array (Supplemental Fig. S7A). Immunoprecipitated chromatin was eluted off the beads and purified. One microliter of ChIP DNA was used for each qPCR reaction on the StepOnePlus real-time PCR system (Applied Biosystems). Primer sequences are provided (Supplemental Fig. S8). Also see the Supplemental Material.
Immunofluorescence
Immunostaining was performed on brains as described previously (Lee and Luo 1999) and on maxillary palps and antennae with 3% Triton X-100 permeabilization instead of 0.3%, as stated in the protocol. Primary antibodies were mouse anti-Elav (1:5 dilution; Developmental Studies Hybridoma Bank), mouse anti-nc82 (1:5; Developmental Studies Hybridoma Bank), chicken anti-GFP (1:2000; ab13970), rat anti-CD8 (1:100; Invitrogen), rabbit anti-Myb (1:200) (Manak et al. 2002), rabbit anti-RFP (1:500; gift from S. Heidmann), rabbit anti-Mip120 (1:500), and rabbit anti-E2F2 (1:100). Also see the Supplemental Material.
Single-sensillum electrophysiology
Recordings were obtained as described previously (Turner and Ray 2009).
RNA in situ hybridization
Procedures were performed as described previously (Goldman et al. 2005). Antenna whole-mount RNA in situs were performed using ∼900-bp antisense RNA–digoxigenin probes in fixed tissue. Probes were detected using an anti-digoxigenin fragment conjugated to an alkaline phosphatase. Signal was developed using Fast Red and imaged using a Zeiss LSM 560 confocal microscope with a 543-nm laser and 560LP filter.
Behavior
T-maze assays were performed as described previously (Turner and Ray 2009), except that 0.33% CO2 was used.
Statistics
Statistical analysis was done using unpaired two-tailed Student's t-test using R software.
Acknowledgments
We thank Shane McInally for help with electrophysiology; Laura Andrejka for making anti-E2F2 and anti-Mip120 antibodies; Ze Yang and Or Gozani for help with validating anti-H3K9me2 antibody specificity; Mattias Alenius for providing Or-mCD8∷GFP flies; Pelin Cayirlioglu Volkan for providing mir-279 mutant flies; Michael Botchan for providing mip120 and mip130 mutant flies; Robert J. Duronio for providing e2f2 mutant flies; Anupama Dahanukar for providing Gr63a-GAL4 flies; Alisa Katzen and Michael Bishop for providing the myb1 flies; Liqun Luo Margaret T. Fuller, Arend Sidow, Li Zhu, Ying Peng, Liang Liang, David Luginbuhl, Allyson Campbell Spence, Alejandro Sánchez Alvarado, and Christi Scott for helpful discussions; and members of the Lipsick and Ray laboratories for reagent support and manuscript suggestions. This work was partially supported by funds provided by the Whitehall Foundation (to A.R.), USPHS grant RO1CA128836 (to J.S.L.), and the Agency for Science, Technology, and Research in Singapore (to C.K.S.). C.K.S. conceived the initial project; planned and performed immunohistochemical, PCR, ChIP, and promoter expression experiments; and cowrote the manuscript. S.P. planned and performed behavior and RNA in situ experiments, analyzed the data, and helped write the manuscript. S.T. performed electrophysiology experiments. J.S.L. provided conceptual and technical guidance, and A.R. managed the project, provided guidance, and cowrote the manuscript.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.201665.112.
References
- Andrejka L, Wen H, Ashton J, Grant M, Iori K, Wang A, Manak JR, Lipsick JS 2011. Animal-specific C-terminal domain links myeloblastosis oncoprotein (Myb) to an ancient repressor complex. Proc Natl Acad Sci 108: 17438–17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Carlson JR 2010. Distinct functions of acj6 splice forms in odor receptor gene choice. J Neurosci 30: 5028–5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Goldman AL, Carlson JR 2009. Positive and negative regulation of odor receptor gene choice in Drosophila by acj6. J Neurosci 29: 12940–12947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall EL, Manak JR, Zhou S, Bell M, Lipsick JS, Botchan MR 2002. Role for a Drosophila Myb-containing protein complex in site-specific DNA replication. Nature 420: 833–837 [DOI] [PubMed] [Google Scholar]
- Beall EL, Bell M, Georlette D, Botchan MR 2004. Dm-myb mutant lethality in Drosophila is dependent upon mip130: Positive and negative regulation of DNA replication. Genes Dev 18: 1667–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayirlioglu P, Bonnette PC, Dickson MR, Duronio RJ 2001. Drosophila E2f2 promotes the conversion from genomic DNA replication to gene amplification in ovarian follicle cells. Development 128: 5085–5098 [DOI] [PubMed] [Google Scholar]
- Cayirlioglu P, Kadow IG, Zhan X, Okamura K, Suh GS, Gunning D, Lai EC, Zipursky SL 2008. Hybrid neurons in a microRNA mutant are putative evolutionary intermediates in insect CO2 sensory systems. Science 319: 1256–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR 2009. The biology of chromatin remodeling complexes. Annu Rev Biochem 78: 273–304 [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, Carlson JR 1999. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron 22: 339–347 [DOI] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ 2005. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol 15: 1535–1547 [DOI] [PubMed] [Google Scholar]
- Dimova DK, Stevaux O, Frolov MV, Dyson NJ 2003. Cell cycle-dependent and cell cycle-independent control of transcription by the Drosophila E2F/RB pathway. Genes Dev 17: 2308–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C 2010. Brain function and chromatin plasticity. Nature 465: 728–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert A, Schotta G, Lein S, Kubicek S, Krauss V, Jenuwein T, Reuter G 2004. Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev 18: 2973–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K, Karim MR, Taniguchi H, Krejci A, Kinameri E, Siebert M, Ito K, Bray SJ, Moore AW 2011. Chromatin modification of Notch targets in olfactory receptor neuron diversification. Nat Neurosci 15: 224–233 [DOI] [PubMed] [Google Scholar]
- Faucher C, Forstreuter M, Hilker M, de Bruyne M 2006. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol 209: 2739–2748 [DOI] [PubMed] [Google Scholar]
- Georlette D, Ahn S, MacAlpine DM, Cheung E, Lewis PW, Beall EL, Bell SP, Speed T, Manak JR, Botchan MR 2007. Genomic profiling and expression studies reveal both positive and negative activities for the Drosophila Myb MuvB/dREAM complex in proliferating cells. Genes Dev 21: 2880–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MT 1980. The role of carbon dioxide in host-finding by mosquitoes: A review. Bull Entomol Res 70: 525–532 [Google Scholar]
- Goldman AL, Van der Goes van Naters W, Lessing D, Warr CG, Carlson JR 2005. Coexpression of two functional odor receptors in one neuron. Neuron 45: 661–666 [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR 2006. Coding of odors by a receptor repertoire. Cell 125: 143–160 [DOI] [PubMed] [Google Scholar]
- Hartl M, Loschek LF, Stephan D, Siju KP, Knappmeyer C, Kadow IC 2011. A new Prospero and microRNA-279 pathway restricts CO2 receptor neuron formation. J Neurosci 31: 15660–15673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S, Alkhori L, Schleiffer A, Brochtrup A, Hummel T, Alenius M 2012. Combinatorial activation and repression by seven transcription factors specify Drosophila odorant receptor expression. PLoS Biol 10: e1001280 doi: 10.1371/journal.pbio.1001280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ Jr, Otake Y, Sood P, Vogt N, Behnia R, Vasiliauskas D, McDonald E, Xie B, Koenig S, Wolf R, et al. 2011. Interlocked feedforward loops control cell-type-specific Rhodopsin expression in the Drosophila eye. Cell 145: 956–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB 2007. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445: 86–90 [DOI] [PubMed] [Google Scholar]
- Katzen AL, Bishop JM 1996. myb provides an essential function during Drosophila development. Proc Natl Acad Sci 93: 13955–13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen AL, Jackson J, Harmon BP, Fung SM, Ramsay G, Bishop JM 1998. Drosophila myb is required for the G2/M transition and maintenance of diploidy. Genes Dev 12: 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Carlson JR, Luo L 2004. Olfactory receptor neuron axon targeting: Intrinsic transcriptional control and hierarchical interactions. Nat Neurosci 7: 819–825 [DOI] [PubMed] [Google Scholar]
- Korenjak M, Taylor-Harding B, Binne UK, Satterlee JS, Stevaux O, Aasland R, White-Cooper H, Dyson N, Brehm A 2004. Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell 119: 181–193 [DOI] [PubMed] [Google Scholar]
- Kreher SA, Mathew D, Kim J, Carlson JR 2008. Translation of sensory input into behavioral output via an olfactory system. Neuron 59: 110–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR 2007. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci 104: 3574–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L 1999. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461 [DOI] [PubMed] [Google Scholar]
- Lewcock JW, Reed RR 2004. A feedback mechanism regulates monoallelic odorant receptor expression. Proc Natl Acad Sci 101: 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PW, Beall EL, Fleischer TC, Georlette D, Link AJ, Botchan MR 2004. Identification of a Drosophila Myb–E2F2/RBF transcriptional repressor complex. Genes Dev 18: 2929–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsick JS 2004. synMuv verite–Myb comes into focus. Genes Dev 18: 2837–2844 [DOI] [PubMed] [Google Scholar]
- Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, Chen R, Washburn MP, Liu XS, DeCaprio JA 2007. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell 26: 539–551 [DOI] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJ, Takken W, Carlson JR, et al. 2007. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol 17: 1533–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magklara A, Yen A, Colquitt BM, Clowney EJ, Allen W, Markenscoff-Papadimitriou E, Evans ZA, Kheradpour P, Mountoufaris G, Carey C, et al. 2011. An epigenetic signature for monoallelic olfactory receptor expression. Cell 145: 555–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaterre J, Mantamadiotis T, Dworkin S, Lightowler S, Yang Q, Ransome MI, Turnley AM, Nichols NR, Emambokus NR, Frampton J, et al. 2008. c-Myb is required for neural progenitor cell proliferation and maintenance of the neural stem cell niche in adult brain. Stem Cells 26: 173–181 [DOI] [PubMed] [Google Scholar]
- Manak JR, Mitiku N, Lipsick JS 2002. Mutation of the Drosophila homologue of the Myb protooncogene causes genomic instability. Proc Natl Acad Sci 99: 7438–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock TS 2010. Achieving singularity in mammalian odorant receptor gene choice. Chem Senses 35: 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Carlson JR 2010. Regulation of odor receptor genes in trichoid sensilla of the Drosophila antenna. Genetics 186: 79–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P 2006. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol 22: 713–737 [DOI] [PubMed] [Google Scholar]
- Peters CW, Sippel AE, Vingron M, Klempnauer KH 1987. Drosophila and vertebrate myb proteins share two conserved regions, one of which functions as a DNA-binding domain. EMBO J 6: 3085–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkinton M, Sandoval R, Colamonici OR 2007. Mammalian Mip/LIN-9 interacts with either the p107, p130/E2F4 repressor complex or B-Myb in a cell cycle-phase-dependent context distinct from the Drosophila dREAM complex. Oncogene 26: 7535–7543 [DOI] [PubMed] [Google Scholar]
- Ray A, van der Goes van Naters W, Shiraiwa T, Carlson JR 2007. Mechanisms of odor receptor gene choice in Drosophila. Neuron 53: 353–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, van der Goes van Naters W, Carlson JR 2008. A regulatory code for neuron-specific odor receptor expression. PLoS Biol 6: e125 doi: 10.1371/journal.pbio.0060125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, et al. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406: 593–599 [DOI] [PubMed] [Google Scholar]
- Rothman A, Feinstein P, Hirota J, Mombaerts P 2005. The promoter of the mouse odorant receptor gene M71. Mol Cell Neurosci 28: 535–546 [DOI] [PubMed] [Google Scholar]
- Rudolph T, Yonezawa M, Lein S, Heidrich K, Kubicek S, Schafer C, Phalke S, Walther M, Schmidt A, Jenuwein T, et al. 2007. Heterochromatin formation in Drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog SU(VAR)3-3. Mol Cell 26: 103–115 [DOI] [PubMed] [Google Scholar]
- Schmit F, Korenjak M, Mannefeld M, Schmitt K, Franke C, von Eyss B, Gagrica S, Hanel F, Brehm A, Gaubatz S 2007. LINC, a human complex that is related to pRB-containing complexes in invertebrates regulates the expression of G2/M genes. Cell Cycle 6: 1903–1913 [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Martinez AM, Iovino N, Cavalli G 2011. Trithorax group proteins: Switching genes on and keeping them active. Nat Rev Mol Cell Biol 12: 799–814 [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H 2003. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science 302: 2088–2094 [DOI] [PubMed] [Google Scholar]
- Song JJ, Garlick JD, Kingston RE 2008. Structural basis of histone H4 recognition by p55. Genes Dev 22: 1313–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ 2004. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431: 854–859 [DOI] [PubMed] [Google Scholar]
- Tanoue S, Krishnan P, Chatterjee A, Hardin PE 2008. G protein-coupled receptor kinase 2 is required for rhythmic olfactory responses in Drosophila. Curr Biol 18: 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy AL, Ray A, Carlson JR 2008. A new Drosophila POU gene, pdm3, acts in odor receptor expression and axon targeting of olfactory neurons. J Neurosci 28: 7121–7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth K, Mutschler B, Jenkins NA, Gilbert DJ, Copeland NG, Klempnauer KH 1994. Mouse A-myb encodes a trans-activator and is expressed in mitotically active cells of the developing central nervous system, adult testis and B lymphocytes. EMBO J 13: 5994–6005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojer P, Li G, Sims RJ 3rd, Vaquero A, Kalakonda N, Boccuni P, Lee D, Erdjument-Bromage H, Tempst P, Nimer SD, et al. 2007. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell 129: 915–928 [DOI] [PubMed] [Google Scholar]
- Turner SL, Ray A 2009. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature 461: 277–281 [DOI] [PubMed] [Google Scholar]
- van den Heuvel S, Dyson NJ 2008. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 9: 713–724 [DOI] [PubMed] [Google Scholar]
- Vasiliauskas D, Mazzoni EO, Sprecher SG, Brodetskiy K, Johnston RJ Jr, Lidder P, Vogt N, Celik A, Desplan C 2011. Feedback from rhodopsin controls rhodopsin exclusion in Drosophila photoreceptors. Nature 479: 108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli A, Rothman A, Feinstein P, Zapotocky M, Mombaerts P 2002. Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron 35: 681–696 [DOI] [PubMed] [Google Scholar]
- Wen H, Andrejka L, Ashton J, Karess R, Lipsick JS 2008. Epigenetic regulation of gene expression by Drosophila Myb and E2F2–RBF via the Myb–MuvB/dREAM complex. Genes Dev 22: 601–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CA, Carlson JR 2010. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J Neurosci 30: 4562–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin N, Carroll PM, Chan G, Guan B, Xiao H, Yao B, Wu SS, Zhou L 2008. Epigenetic blocking of an enhancer region controls irradiation-induced proapoptotic gene expression in Drosophila embryos. Dev Cell 14: 481–493 [DOI] [PMC free article] [PubMed] [Google Scholar]