Rosbash and colleagues identify deubiquitylation of the Drosophila circadian clock protein CLK as a key means of regulating circadian transcriptional activity. The ubiquitin-specific protease USP8, which itself exhibits high-amplitude circadian rhythmicity, deubiquitylates CLK and inhibits CLK/CYC-mediated gene activation at the end of a transcription cycle. Regulation of the ubiquitin modification during the daily cycle influences the balance between CLK mono- and polyubiquitylation states, thereby modulating fly locomotor activity via CLK/CYC-dependent transcription.
Keywords: CLOCK, USP8, circadian rhythm, Drosophila, transcription, deubiquitylation
Abstract
A conserved transcriptional feedback loop underlies animal circadian rhythms. In Drosophila, the transcription factors CLOCK (CLK) and CYCLE (CYC) activate the transcription of direct target genes like period (per) and timeless (tim). They encode the proteins PER and TIM, respectively, which repress CLK/CYC activity. Previous work indicates that repression is due to a direct PER–CLK/CYC interaction as well as CLK/CYC phosphorylation. We describe here the role of ubiquitin-specific protease 8 (USP8) in circadian transcriptional repression as well as the importance of CLK ubiquitylation in CLK/CYC transcription activity. usp8 loss of function (RNAi) or expression of a dominant-negative form of the protein (USP8-DN) enhances CLK/CYC transcriptional activity and alters fly locomotor activity rhythms. Clock protein and mRNA molecular oscillations are virtually absent within circadian neurons of USP8-DN flies. Furthermore, CLK ubiquitylation cycles robustly in wild-type flies and peaks coincident with maximal CLK/CYC transcription. As USP8 interacts with CLK and expression of USP8-DN increases CLK ubiquitylation, the data indicate that USP8 deubiquitylates CLK, which down-regulates CLK/CYC transcriptional activity. Taken together with the facts that usp8 mRNA cycles and that its transcription is activated directly by CLK/CYC, USP8, like PER and TIM, contributes to the transcriptional feedback loop cycle that underlies circadian rhythms.
Circadian rhythms of animal behavior, physiology, and metabolism are an evolutionary adaptation to the rotation of the earth. There is an underlying transcriptional feedback mechanism, which is modulated by the post-translation modification of key transcription factors. In Drosophila, the transcription factors CLOCK (CLK) and CYCLE (CYC) activate the transcription of period (per) and timeless (tim) and other direct target genes during the day. At night, PER and TIM then enter the nucleus and inhibit their own transcription. The activity of CLK/CYC is regulated by a direct interaction with these negative regulators as well as by post-translational modifications, with a focus to date on PER phosphorylation (Allada and Chung 2010; Doherty and Kay 2010). Specifically, PER and TIM are gradually hyperphosphorylated by different kinases during the early night (Price et al. 1998; Martinek et al. 2001; Lin et al. 2002; Chiu et al. 2011) and then interact with and/or sequester the CLK/CYC complex, which inhibits its transcription activity (Menet et al. 2010). In the morning after exposure to light, PER and TIM are degraded, which liberates and reactivates CLK/CYC. In addition, hyperphosphorylation of CLK appears to coincide with low transcription activity, suggesting that direct phosphorylation–dephosphorylation may also modulate CLK/CYC activity.
Other modifications also occur on core clock proteins. BMAL1, the mammalian ortholog of CYC, is acetylated by its partner, CLK, at the single lysine residue K572 with a timing corresponding to the repression phase of circadian transcription of clock-controlled genes (Hirayama et al. 2007; Nakahata et al. 2008). BMAL1 also exhibits a circadian pattern of sumoylation and ubiquitylation, which parallels its activation in mouse livers (Cardone et al. 2005; Lee et al. 2008); these dual modifications are essential for CLK/BMAL1 transcriptional activation.
Ubiquitylation of a protein substrate results from the antagonistic functions of ubiquitin ligases and deubiquitylating enzymes. Polyubiquitylation is usually associated with proteasome-mediated proteolysis, whereas monoubiquitylation can regulate the cellular localization, trafficking, and even transcriptional activity of a protein (Komander and Rape 2012). Proteolysis is intimately connected to the circadian molecular cycle due to the daily degradation that many circadian proteins undergo. Indeed, work over the last two decades has identified specific E3 ligases required for this proteasome-mediated degradation. For example, Drosophila SLIMB and JETLAG are involved in the degradation of PER and TIM, respectively (Grima et al. 2002; Ko et al. 2002; Koh et al. 2006). The mouse F-box protein FBXL3, a component of the SKP1–CUL1–F-box-protein (SCF) E3 ubiquitin ligase complex, mediates the degradation of transcription repressors PER1 and PER2 and/or CRY1 and CRY2 (Busino et al. 2007; Godinho et al. 2007; Siepka et al. 2007). Unlike their mammalian orthologs, the protein levels of CLK and CYC do not oscillate across a circadian cycle, and there are no reports of post-translational modifications of these proteins other than phosphorylation.
In contrast to ubiquitin E3 ligases, deubiquitylating enzymes are less well studied. This protease family removes the ubiquitin moiety from substrates, which are generally linked to their stabilization and/or recycling. A large number of these proteins belong to the ubiquitin-specific protease (USP) family, which can recognize a variety of substrates and therefore function in many essential pathways within cells, such as protein degradation, localization, and transcriptional regulation. In response to oxidative stress, deubiquitylation of the transcription factor FOXO4 by USP7 represses FOXO transcriptional activity in mammals without affecting its degradation (van der Horst et al. 2006). In Drosophila, USP7 contributes to the Pc-mediated silencing of the homeotic genes through modulation of histone H2 deubiquitylation (van der Knaap et al. 2010). In contrast, UBP8, a component of the yeast SAGA complex, deubiquitylates H2B and stimulates transcription by enhancing Lys4 trimethylation of H3. Also notable is mammalian usp2; its mRNA cycles, and its transcription is under CLOCK/BMAL1 control (Oishi et al. 2003, 2005). USP2 was shown more recently to stabilize BMAL1 and CRY1 by deubiquitylation in mouse livers (Scoma et al. 2011; Tong et al. 2012). USP8/UBPY, another deubiquitylating enzyme in the USP family, functions in the endocytosis and endosome sorting of ligand-activated receptor tyrosine kinases (RTKs) in mammals (Mizuno et al. 2005; Wright et al. 2011), and a knockout of the usp8 gene causes developmental lethality in Drosophila as well as in mammals (Niendorf et al. 2007; Mukai et al. 2010). usp8 mRNA always appeared near the top of the lists of cycling mRNAs in several early microarray studies of Drosophila head RNA (Claridge-Chang et al. 2001; McDonald and Rosbash 2001; Ueda et al. 2002) and has been shown more recently to also cycle within circadian neurons (Kula-Eversole et al. 2010).
In this study, we address the function of USP8 in Drosophila circadian rhythms and show that it deubiquitylates CLK in a circadian manner and contributes to cyclical repression of core clock transcription. Loss of function (RNAi) or expression of a dominant-negative form of the protein (USP8-DN) not only enhances CLK/CYC transcriptional activity, but also alters fly locomotor activity rhythms. This is especially prominent in circadian neurons within which clock protein and mRNA molecular oscillations are virtually absent in the USP8-DN flies. Furthermore, CLK ubiquitylation cycles robustly in wild-type flies and peaks coincident with maximal CLK/CYC transcription. As USP8 interacts with CLK and expression of USP8-DN increases CLK ubiquitylation, the data indicate that USP8 deubiquitylates CLK, which down-regulates CLK/CYC transcriptional activity. Taken together with the facts that usp8 mRNA cycles and that its transcription is activated directly by CLK/CYC, the data indicate a new function for USP8: It negatively regulates its own transcription and that of other direct target genes, thereby contributing to the circadian transcriptional feedback cycle.
Results
The usp8 short isoform is a CLK direct target
As described above, usp8 mRNA is one of the very few Drosophila mRNAs other than core clock gene mRNAs that cycle robustly in fly heads as well as in purified circadian neurons. For this reason as well as the lack of a robust role for ubiquitylation in the fly circadian system, we decided to investigate the function and regulation of usp8. We first asked whether usp8 is a direct target of the CLK/CYC complex by assaying CLK occupancy by chromatin immunoprecipitation (ChIP) and tiling arrays (ChIP–chip). The chromatin was made from fly heads collected at different times during a light–dark (LD) cycle (Abruzzi et al. 2011).
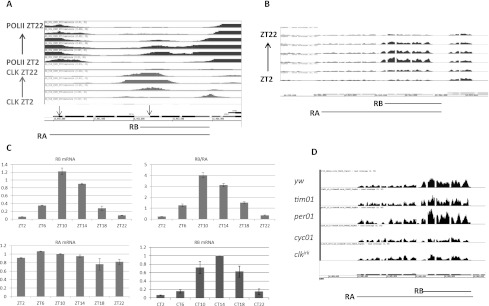
usp8 is a very robust CLK target gene and manifests canonical CLK cycling on its target sites exactly like core clock genes (rank #639). Moreover, there is a cycling RNA polymerase II (Pol II) signal near the promoter of the short isoform of usp8 mRNA. In contrast, there is a noncycling Pol II and an absence of CLK signal near the promoter of the long isoform (Fig. 1A). Consistent with these isoform differences, RNA sequencing (RNA-seq) (Fig. 1B) and quantitative RT–PCR (qRT–PCR) of mRNA from both LD and constant darkness (DD) conditions (Fig. 1C) show that only the short isoform is cycling, with a temporal profile similar to those of per and tim mRNAs (Menet et al. 2010).
Figure 1.
usp8 transcription is under clock control. (A) CLK and Pol II ChIP at six time points (ZT2–ZT22) from fly heads were performed as in Abruzzi et al. (2011). The CLK and Pol II occupancy at the usp8 promoter regions were visualized using the integrated gene browser (IGB; Affymetrix). RA and RB are two isoforms of the usp8 gene. USP8-PA contains 896-amino-acid residues; USP8-PB has 367-amino-acid residues and a unique 29-amino-acid N terminus. Both isoforms share the same enzymatic domain. The black arrows above the usp8 gene diagram indicate the start codon. (B) RNA-seq of usp8 mRNA at six time points from fly heads was performed as in Rodriguez et al. (2012). (C) Real-time PCR analysis of RA and RB isoforms of usp8 from head total RNA. The Y-axis represents the relative value of usp8 mRNAs normalized to that of rpl32 mRNA. (D) RNA-seq of usp8 mRNA at ZT16 in yw as well as the clock mutants tim01, per01, cyc01, and clkjrk.
The short isoform of usp8 mRNA is most abundant in head RNA; its expression level is approximately fourfold to fivefold higher than the long isoform at times of maximal expression level; e.g., at Zeitgeber time 10 (ZT10). Importantly, levels of this isoform are high in mutants of the repressor genes tim and per and low in clkjrk and cyc01 mutants; levels of the long isoform are relatively unaffected (Fig. 1D). Taken together, the results indicate that CLK/CYC specifically regulates the transcription of the short form of usp8 mRNA, resulting in a cycling mRNA.
USP8 is important for locomotor activity rhythms in vivo
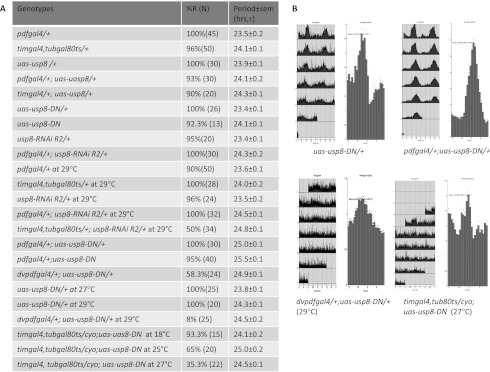
To investigate the contribution of USP8 to circadian rhythms, we took advantage of the GAL4-UAS system and several usp8 RNAi strains (Dietzl et al. 2007; Mukai et al. 2010) to selectively knock down the expression of USP8 within circadian neurons and analyze the resulting effects on locomotor activity rhythms. One RNAi line, uas-usp8-RNAi R2, encodes a dsRNA construct against both usp8 isoforms (Mukai et al. 2010) and consistently exhibited long periods when expressed with a pdfgal4 driver (∼1 h longer than control flies) (Fig. 2A). This strategy restricts expression of the RNAi construct to PDF neurons, which include the principal circadian pacemaker neurons (Stoleru et al. 2004). With the timgal4 driver, expression of the usp8-RNAi R2 construct in all circadian neurons as well as additional locations (Kaneko and Hall 2000) caused premature mortality (data not shown), consistent with the essential nature of the gene (Mukai et al. 2010).
Figure 2.
usp8 mutant flies exhibit abnormal locomotor activity. (A) Behavioral analysis of fly strains expressing usp8 RNAi or uas-usp8-DN constructs by different gal4 drivers. Flies were entrained for 3–4 d in LD cycles and released into DD for at least 5 d at 25°C unless indicated. (%R) Percentage of rhythmic flies; (N) number of flies assayed. (B) Actograms and average actograms under DD were shown for flies expressing USP8-DN driven by pdfgal4, dvpdfgal4 and timgal4, tubgal80ts drivers (Levine et al. 2002).
To overcome this developmental lethality, we took advantage of a timgal4, tubgal80ts driver to repress UAS expression at a permissive temperature (18°C) until the adult stage, when the temperature was raised to the nonpermissive temperature (29°C) to allow expression (McGuire et al. 2003). Almost 50% of the flies were arrhythmic with this strategy compared with 96%–100% rhythmicity of control flies. The remaining rhythmic flies have long periods, an average of 1 h longer than control flies (Fig. 2A).
To further examine the effect of USP8 knockdown, we examined fly locomotor activity with another USP8 RNAi line (Bloomington HMS01898). This construct was expressed in a somewhat broader set of circadian neurons with the dvpdfgal4 driver; i.e., all PDF cells, LNds, and the PDF-negative fifth s-LNv (Bahn et al. 2009). These flies also exhibit long periods, 1.3-h longer than control flies (Supplemental Fig. 1).
To confirm the importance of USP8 to fly locomotor activity rhythms, we constructed a dominant-negative form of USP8, USP8-DN. It harbors a point mutation within its catalytic domain (C572A), which was reported to render it inactive in mammalian systems (Mizuno et al. 2005; Mukai et al. 2010). We first restricted expression of USP8-DN to PDF neurons. Most pdfgal4; uas-usp8-DN flies showed robust rhythms with a 25-h period, ∼1.5 h longer than the uas-usp8-DN and pdfgal4 control flies (Fig. 2A,B, top panels). This result was similar to but stronger than the RNAi result shown above.
Expression of USP8-DN with the dvpdfgal4 driver either significantly reduced rhythmicity (58.3% at 25°C) or almost completely abolished it (8% at 29°C) (Fig. 2A,B, bottom left panel). We attempted to do similar USP8-DN experiments with the even broader timgal4 driver, but a preliminary experiment indicated that expression in all clock neurons once again conferred developmental lethality (data not shown). To circumvent this issue, we again used the timgal4, tubgal80ts driver combination and a temperature shift of adult flies from 18°C to 25°C or 27°C.
In contrast to the permissive temperature in which timgal4, tubgal80ts; uas-usp8-DN flies exhibited normal locomotor activity rhythms and period length, raising the temperature had profound effects on fly behavior. For example, a temperature shift to 25°C or 27°C dramatically reduced the percentage of rhythmic flies from 80%–90% to 65% (25°C) or 35% (27°C) (Fig. 2A,B, bottom right panel). Furthermore, the remaining rhythmic flies had very weak rhythms. Somewhat surprisingly, overexpression of wild-type USP8 in either pacemaker neurons (pdfgal4) or all clock neurons (timgal4) had no effect on behavior (data not shown). Taken together, the data indicate that USP8 contributes to circadian rhythms.
USP8 is required for molecular oscillations of PER and TIM
Since the timgal4, tubgal80ts; uas-usp8-DN (tim>DN) flies are arrhythmic or have weak rhythms, we hypothesized that molecular oscillations might be disrupted and even the cause of the disrupted rhythms under free-running conditions (DD). Under normal LD conditions, however, we did not observe dramatic changes in CLK, PER, and TIM protein levels or cycling in USP8-DN flies, most likely because light can trigger efficient TIM and PER degradation and override or compensate for a modest issue with transcriptional regulation. To circumvent this light issue and address molecular oscillations that might be more relevant to the behavioral phenotypes described above (in DD), we assayed these clock proteins by Western blotting during the first day of DD (DD1).
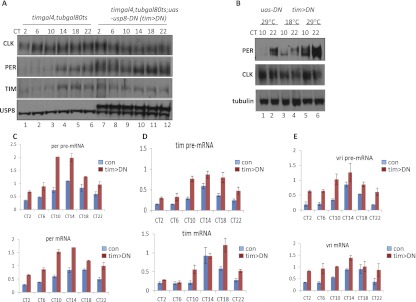
As previously reported (Zeng et al. 1996), control flies have very low levels of PER and TIM between circadian time 12 (CT2) and CT10, which increase dramatically throughout the subjective night in head extracts (Fig. 3A,B). Strikingly, PER and TIM did not manifest these low levels in the early–mid-subjective day (CT6–CT10) in the tim>DN strain, resulting in much less cycling (Fig. 3A [cf. lanes 8,9 and 2,3], B [cf. lanes 5 and 1,3]). Consistent with its normal behavior phenotype (data not shown), overexpression of wild-type USP8 has little effect on PER cycling (Supplemental Fig. 2). In contrast, CLK protein levels as well as its phosphorylation remain quite similar to the control strain (Fig. 3A,B; Supplemental Fig. 3).
Figure 3.
The levels of PER and TIM and their RNA were increased in the tim>DN flies. (A) Control driver flies (timgal4, tubgal80ts) and tim>DN flies were entrained for three LD cycles at 29°C and collected at the first day of DD (DD1). Protein levels from head extracts at six time points were analyzed by Western blotting. Note: The lower USP8 band is the endogenous wild-type (WT) USP8, and the higher band is the USP8-DN band. (B) tim>DN flies were entrained for three LD cycles at permissive (18°C) and nonpermissive (29°C) temperatures and collected at DD1. As a control, the uas-usp8-DN flies (uas-DN) were entrained at 29°C. Proteins from head extract at CT10 and CT22 were analyzed as in A. (C–E). Real-time PCR analysis of per (C), tim (D), and vri (E) mRNA and pre-mRNA in tim>DN and control (uas-DN) flies. Flies were entrained as in A and B. Total RNA was prepared from six time points on DD1 and analyzed by real-time PCR with specific primers for per, tim, and vri genes.
To determine whether this change of protein expression is due to effects of the tim>DN on CLK/CYC transcription, we examined per, tim, and vri pre-mRNA as well as mRNA expression during the first subjective day of DD. Previous work has demonstrated that pre-mRNA profiles are quite similar to other transcriptional assays, such as nuclear run-ons, and can be used to approximate transcription (Menet et al. 2010).
Expression of USP8-DN caused a twofold to fourfold increase in pre-mRNA levels of all three CLK direct target circadian genes relative to the control strain (Fig. 3C–E, top). The mRNA levels of per and vri also increased in the tim>DN flies (Fig. 3C,E, bottom). For unknown reasons, the increase in tim mRNA levels is less consistent and can only be reliably seen at certain time points (Fig. 3D, bottom). The results indicate that reducing USP8 activity increases the transcription of many CLK/CYC direct target genes and suggests that USP8 functions to repress CLK/CYC transcription.
USP8 represses CLK/CYC transcriptional activation by direct deubiquitylation of CLK
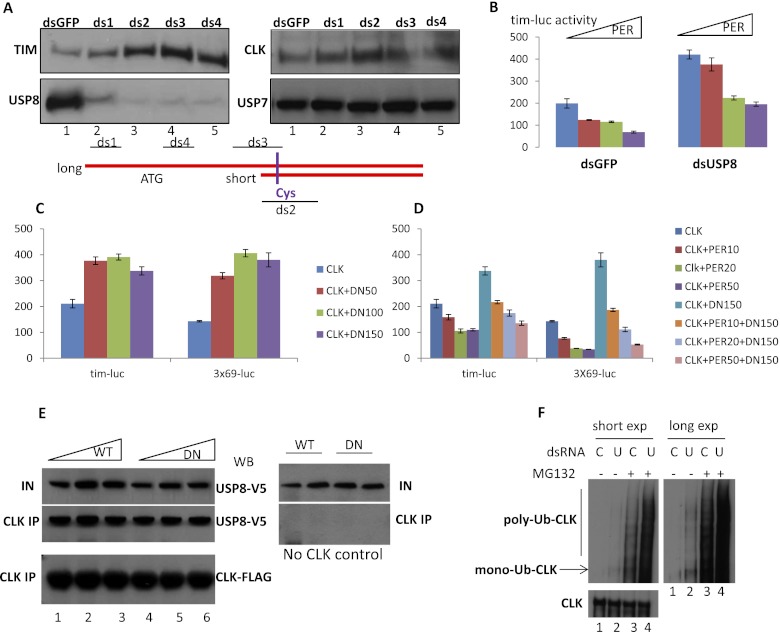
To address more directly the role of USP8 in modulating CLK/CYC transcription, we used three different S2 cell assays. First, we examined the extent to which four different USP8 dsRNAs affect endogenous TIM expression in a stable cell line expressing CLK (pMT-3XFlag-clk-HBH; leaky expression without copper addition). Three out of four dsRNAs efficiently knocked down endogenous USP8 (ds2–4) (Fig. 4A), and these three most strongly increased endogenous TIM expression. In contrast, there was no strong or systematic effect on the levels of CLK and USP7, the latter known for its effect on H2B deubiquitylation (Fig. 4A, lanes 3–5).
Figure 4.
USP8 inhibits CLK/CYC transcription activity in S2 cells. (A) The pMT-3XFlag-CLK-HBH S2 stable line was incubated with four dsRNAs against USP8 (ds1, ds2, ds3, and ds4) for 2 d. The presence of TIM, USP8, CLK, and USP7 in whole-cell extract was shown by Western blotting. Antibody for USP8 was raised to recognize the full-length (long) isoform only (Mukai et al. 2010). Note that ds2 and ds3 were able to knock down both the long and short isoforms of usp8 mRNA. dsRNA for GFP (dsGFP) was used as the control. (B) Repression of PER on CLK/CYC transcription activation of a luciferase gene fused to the timeless promoter (tim-luc) when cells were incubated with USP8 dsRNA (ds3). The values at the Y-axis are the normalized luciferase activity. Four different amounts of pAct-per plasmids (0, 10, 25, and 50 ng) were cotransfected with 5 ng of pAct-clk plasmid after 2 d of incubation with dsRNA. Cells were harvested 2 d after transfection. Results were averaged from two experiments with duplicates. (C) The effect of USP8-DN on CLK/CYC transcription activation of tim-luc and 3X69-luc reporters. Different amounts of pAct-usp8-DN (0, 50, 100, and 150 ng) were cotransfected as in A. (D) Normalized luciferase activity of tim-luc and 3X69-luc when pAct-per and pAct-usp8-DN were cotransfected. (E) Coimmunoprecipitation of USP8 with CLK. pAct-usp8-V5 or pAct-usp8-DN-V5 plasmids (150, 300, and 600 ng) were transfected into pMT-3XFlag-clk-HBH S2 stable cells. CLK was immunoprecipitated using anti-Flag beads. The presence of USP8 in the input and immunoprecipitation was visualized by anti-V5 Western blotting. A negative immunoprecipitation control without CLK expression is shown in the right panel. (F) The effect of USP8 knockdown on CLK ubiquitylation. pMT-3XFlag-clk-HBH cells were incubated with USP8 dsRNAs (ds2 and ds3) for 2 d. Cells were harvested 4 h after addition of 50 uM MG132 or DMSO. CLK was immunoprecipitated as in E. Ubiquitylated CLK was visualized by an FK2 monoclonal antibody recognizing both mono- and polyubiquitylated proteins (Enzo Life Sciences). The same blot is shown as both a short and long exposure. (U) USP8 dsRNA; (C) control GFP dsRNA; (mono-Ub-CLK) monoubiquitylated CLK; (poly-Ub-CLK) polyubiquitylated CLK.
Second, we tested whether this effect of USP8 knockdown on endogenous TIM expression reflects an increase in tim transcription and whether PER can still repress CLK/CYC transcriptional activation under these conditions. To this end, we assayed CLK/CYC-mediated luciferase expression from a reporter construct expressing luciferase under the control of the tim promoter (tim-luc) (Darlington et al. 1998; Nawathean and Rosbash 2004). S2 cells were cotransfected with pAct-clk as well as dsRNA for either USP8 (ds3) or GFP (control). Indeed, transfection with USP8 dsRNA substantially increased luciferase levels compared with control cells (Fig. 4B, blue), and increasing amounts of a PER-expressing plasmid still gave rise to a dose-dependent reduction in luciferase levels.
Third, we assayed the effect of USP8-DN on CLK/CYC transcription. Increasing amounts of a USP8-DN-expressing plasmid were transfected with pAct-clk and either of two circadian reporters in S2 cells: the tim-luc reporter described above or a reporter that expresses luciferase under the control of a minimal per promoter (3x69-luc) (Hao et al. 1997). With both reporters, USP8-DN led to a dose-dependent increase in luciferase activity (Fig. 4C). This increased CLK/CYC transcription activity is also sensitive to PER repression (Fig. 4D). The three S2 cell transcription activation assays indicate that USP8 functions to repress CLK/CYC activity, at least in S2 cells.
Since USP8 is a ubiquitin-specific protease, we designed two experiments to test whether CLK is ubiquitylated and a substrate of USP8. We first assayed whether USP8 and CLK can interact in vitro. Plasmids expressing V5-tagged USP8 (both wild-type and DN versions) were transfected into the pMT-3XFlag-clk-HBH stable S2 cells. CLK was immunoprecipitated using anti-Flag beads (Sigma), after which both the input and the immunoprecipitates were examined via anti-V5 Western blotting for USP8. Both wild-type and DN forms of USP8 were present in the CLK immunoprecipitates (Fig. 4E, lanes 1–6, left panel), indicating that USP8 can interact with CLK at least in S2 cells.
Next, we tested whether CLK ubiquitylation is affected by depletion of USP8. pMT-clk stable S2 cells were transfected with either USP8 dsRNAs or control dsRNAs (GFP). CLK was immunoprecipitated as in Figure 4E, and the pellets analyzed by Western blotting with anti-ubiquitin or anti-Flag (CLK) reagents.
CLK protein levels are not affected by USP8 knockdown (Fig. 4F, bottom panel), whereas CLK ubiquitylation (most likely mono-Ub) is increased dramatically (Fig. 4F, top, lanes 1,2, arrow). Furthermore, addition of USP8 dsRNA as well as MG132, a broad proteasome inhibitor, led to the accumulation of more mono-Ub as well as poly-Ub CLK compared with treatment by USP8 dsRNA or MG132 alone (Fig. 4F, top, cf. lanes 4 and 2,3). The results indicate that CLK is ubiquitylated and that Ub-CLK is turned over by the proteasome as well as deubiquitylated by USP8 in S2 cells.
CLK ubiquitylation in fly heads
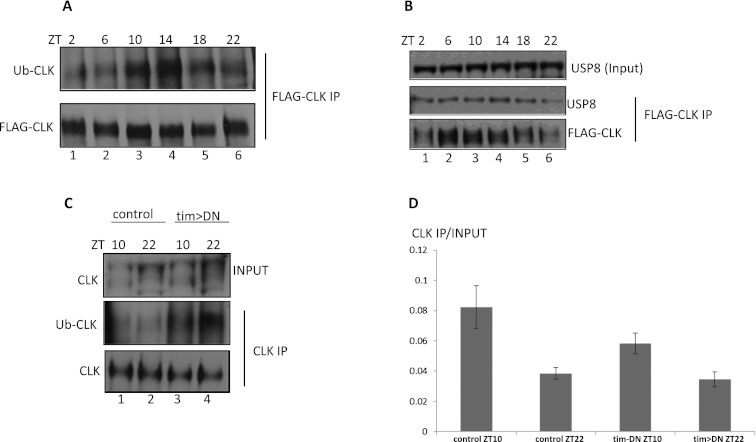
Is CLK also ubiquitylated and regulated by USP8 in flies? To address these questions, we generated a transgenic fly strain expressing a 3XFlag tagged clk14.8 transgene that contains the full-length CLK genomic sequence (3XFlag-CLK fly) and functions identically to the wild-type gene (see the Materials and Methods; data not shown). Fly head extracts were prepared from flies entrained in 12:12 LD cycles, and CLK was immunoprecipitated by anti-Flag beads. The resulting pellets were analyzed via Western blotting with either an FK2 anti-ubiquitin antibody or anti-Flag to detect Ub-CLK or CLK, respectively.
Maximal CLK ubiquitylation occurs at ZT10–ZT14 (Fig. 5A, lanes 3,4, left panel), a time coincident with maximal CLK/CYC transcription activation and CLK binding to chromatin (Abruzzi et al. 2011). We also assayed USP8, and it is associated with CLK at all six time points. There appears to be a higher ratio of USP8 to CLK at ZT18–ZT2 (Fig. 5B; Supplemental Fig. 4), approximately coincident with maximal transcriptional repression. In contrast, PER appears not to interact with USP8 (Supplemental Fig. 5).
Figure 5.
(A) CLK ubiquitylation is cycling in fly head extract. 3XFlag-CLK flies were entrained for three LD cycles and collected at six time points. CLK was precipitated by anti-Flag beads. CLK and ubiquitylated CLK present in the immunoprecipitates were visualized by Western blotting with an anti-Flag and an FK2 ubiquitin antibody, respectively. (Ub-CLK) Ubiquitylated CLK. (B) USP8 was coimmunoprecipitated with CLK. CLK immunoprecipitation from 3XFlag-CLK flies was performed as in A. The presence of USP8 in the CLK precipitates is shown by anti-USP8 Western blotting. (C) CLK ubiquitylation was increased in the tim>DN flies. tim>DN and uas-DN control flies were entrained for three LD cycles at 29°C. Extracts from fly heads collected at ZT10 and ZT22 were subjected to CLK immunoprecipitation with guinea pig anti-CLK antibody (Houl et al. 2008). Ubiquitylated CLK was shown by anti-ubiquitin Western blotting. (D) CLK binding at the E-box of the tim gene in tim>DN flies. CLK ChIP in tim>DN and uas-DN control flies at ZT10 and ZT22 was performed with guinea pig anti-CLK antibody. The occupancy of CLK at the tim E-box was measured by qPCR with primers within the E-box. The Y-axis value was calculated by the ratio of chromatin immunoprecipitated by CLK to the input. Results were averaged from two experiments.
Because CLK ubiquitylation is relatively low in the late night to early morning (ZT18–ZT2) (Fig. 5A), we speculate that USP8 functions to remove ubiquitin from CLK and contributes to transcriptional inhibition at these times. Therefore, inhibition of USP8 may give rise to increased CLK ubiquitylation. To test this hypothesis, we immunoprecipitated CLK with a CLK polyclonal antibody (Houl et al. 2006) and probed the Western blot with a ubiquitin antibody using tim>DN and uas-usp8-DN (control) fly head extracts.
Only low levels of CLK ubiquitylation were detected in the control flies at both ZT10 and ZT22 (Fig. 5C, lanes 1,2), most likely due to the relatively inefficient immunoprecipitation with the polyclonal antibody. More importantly, CLK immunoprecipitates from tim>DN head extract had much higher levels of ubiquitylated CLK (Fig. 5C, lanes 3,4), with stronger signals at ZT22 than at ZT10. This indicates that USP8 indeed deubiquitylates CLK in fly heads.
To address whether expression of USP-DN affects CLK binding to core clock gene promoters, we performed an anti-CLK ChIP with the same strains. CLK binding to the tim E-box does not change significantly (Fig. 5D), suggesting that CLK ubiquitylation impacts transcriptional activation rather than DNA binding.
To further characterize CLK ubiquitylation, we performed a genome-wide ubiquitylated CLK binding on chromatin at ZT10 (approximate peak of maximal clock gene transcription) and ZT22 (approximate peak of minimal transcription) by sequential ChIP (seq-ChIP) in extracts from 3XFlag-CLK fly heads. Seq-ChIP has been successfully used to show the co-occupancy of two transcription factors and the modification of a transcription factor or histone at a specific gene locus (Geisberg and Struhl 2004; Huang et al. 2006; Metivier et al. 2008; Katan-Khaykovich and Struhl 2011). First, CLK-bound chromatin was precipitated with anti-Flag beads. Proteins were eluted from the beads and subjected to anti-Ub immunoprecipitation, and both chromatin fractions analyzed with Drosophila Tiling 2.0 arrays (Affymetrix).
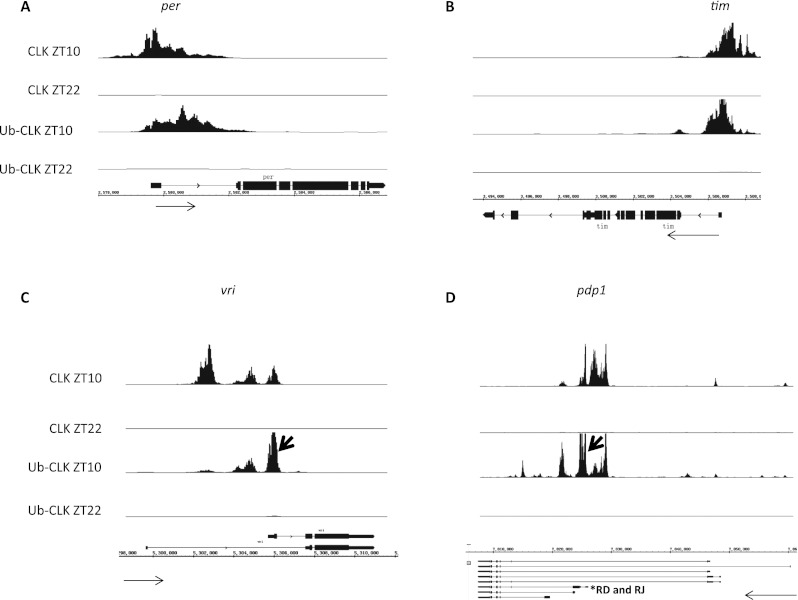
As expected, there was a relatively high CLK signal at the promoters of four canonical clock genes (per, tim, vri, and pdp1) at ZT10, with much less signal at ZT22. Despite the difference in the tagged gene and antibody, the CLK patterns on these genes are similar to those previously reported with a V5-CLK transgene (Abruzzi et al. 2011). The ubiquitylated patterns are also similar, including much more signal at ZT10 than at ZT22 (Fig. 6), suggesting that CLK is broadly ubiquitylated on clock gene promoters.
Figure 6.
Ubiquitylated CLK binding on the promoter regions of per, tim, vri, and pdp1 genes. Ubiquitylated CLK binding on chromatin was measured by seq-ChIP. CLK ChIP was performed at ZT10 and ZT22 in 3XFlag-CLK flies. The elution from CLK ChIP was subjected to anti-ubiquitin immunoprecipitation. The precipitated DNA was analyzed by Drosophila Tiling 2.0 array (Affymetrix). The CLK and ubiquitylated CLK binding on the promoters of per (A), tim (B), vri (C), and pdp1 (D) were visualized by IGB browser. Thick black arrows indicate the peaks of ubiquitylated CLK binding near the promoter region of vri and pdp1 cycling isoform. Asterisk indicates the RD and RJ isoforms of pdp1, which share the same transcription start site and promoter and only differ by one small exon (42 base pairs). Thin black arrows indicate 5′-to-3′ transcription direction.
A more detailed comparison of the ZT10 Ub-CLK peaks with the CLK peaks indicates little or no difference on tim (Fig. 6A), whereas the Ub-CLK distribution on per is somewhat shifted toward the transcribed region (Fig. 6B). There is, however, a more striking difference between the CLK and Ub-CLK patterns on vri (Fig. 6C). The most upstream peak of CLK binding, which is the strongest and derives principally from the eye (Abruzzi et al. 2011), is essentially absent in the Ub-CLK-binding profile. The middle Ub-CLK peak is comparable with its relative strength as a CLK peak. In contrast, the Ub-CLK signal is strongest at the most downstream CLK peak. Its position coincides well with the start site of the short vri isoform, which is under CLK control in circadian neurons (Cyran et al. 2003).
A similar situation occurs at the promoter of pdp1. The expression of the pdp1-D and pdp1-J isoforms is cyclic and important for maintaining rhythms in clock neurons (Cyran et al. 2003; Zheng et al. 2009). The peaks of CLK that correspond to these two transcription start sites have a substantial Ub-CLK signal. In contrast, the two more upstream peaks of CLK on the pdp1 promoter are more prominent and eye-specific but with rather little Ub-CLK signal (Abruzzi et al. 2011). Based on the vri and pdp1 patterns, Ub-CLK binding may be important for isoform-specific gene expression within clock neurons.
USP8 is essential for molecular oscillations within pacemaker neurons
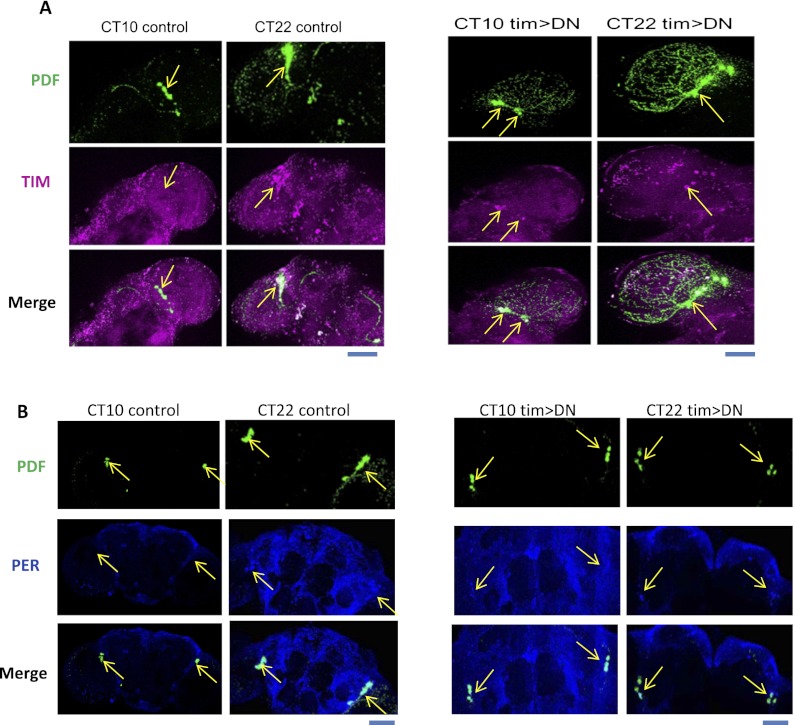
Clock gene molecular oscillations in pacemaker neurons underlie rhythmic behavior. Does USP8 have a particularly strong effect on molecular oscillations within these neurons? Since timgal4, tubgal80ts; uas-usp8-DN (tim>DN) flies are largely arrhythmic, we used immunostaining to examine TIM and PER levels in PDF neurons at peak and trough times, CT10 and CT22, respectively. As expected, we observed normal TIM and PER oscillations in uas-usp8-DN control flies (Curtin et al. 1995; Shafer et al. 2002): TIM and PER proteins are undetectable at CT10, whereas high TIM and PER signals are observed at CT22 (Fig. 7A,B, right panels). In contrast, expression of USP8-DN dramatically increases TIM and PER levels at CT10 within PDF neurons; they are comparable with the signals observed in tim>DN flies at CT22 (Fig. 7A, arrows point to PDF neurons). This indicates that expression of USP8-DN virtually eliminates the normal oscillations of TIM and PER in PDF neurons even more dramatically than what is observed by Western blotting of extracts from the same flies (Fig. 3).
Figure 7.
PER and TIM oscillations in PDF neurons are disrupted in tim>DN flies. Representative staining results of TIM (A) and PER (B) in uas-usp8-DN (uas-DN) control and tim>DN brains. Fly heads were collected at CT10 and CT22 of DD1 after 3 d of LD entrainment at 29°C. Brains were immunostained by anti-PDF (green), anti-TIM (magenta), and anti-PER (blue) antibodies. PDF neurons were labeled by PDF (green). At CT10 in the tim>DN brains, TIM and PER staining signals remained high. Bar, 50 μm. Yellow arrows point to PDF neurons.
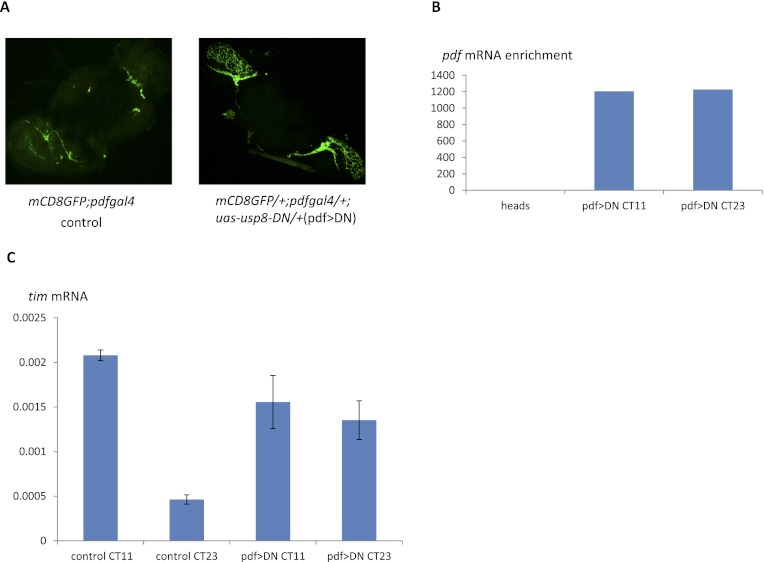
To determine whether the effect of USP-DN on TIM and PER oscillations in PDF neurons is due to transcription, we purified GFP-labeled PDF neuron RNA from mCD8-GFP; pdfgal4, uas-usp8-DN (pdf>DN) flies as previously described (Nagoshi et al. 2009; Kula-Eversole et al. 2010). PDF neurons expressing USP8-DN are morphologically normal (Fig. 8A) and appropriately enriched in pdf mRNA (Fig. 8B). Consistent with published results from LD conditions (Kula-Eversole et al. 2010), tim mRNA levels at CT11 are four to five times of those at CT23 within wild-type PDF neurons. In striking contrast, tim mRNA from pdf>DN neurons are almost at the same levels at these same circadian times. This indicates that expression of USP8-DN within PDF neurons disrupts tim RNA cycling, suggesting that this is the cause of the increased PER and TIM levels at CT10 as well as the poor rhythms of these files. We conclude that USP8 contributes to the temporal regulation of CLK/CYC transcriptional activity, which is particularly important for molecular oscillations within clock neurons.
Figure 8.
tim mRNA in PDF neurons is not cycling in tim>DN flies. (A) PDF neurons are GFP-labeled in the control and pdf>DN brains. Dissected brains were directly visualized by GFP microscope. (B) PDF mRNA is highly enriched in PDF neurons from pdf>DN brains. pdf>DN flies were entrained for three LD cycles at 25°C and collected at CT11 and CT23 of DD1. PDF neurons were manually sorted from papain-treated brains. PDF mRNA was analyzed by qPCR. The enrichment was calculated by PDF mRNA signals normalized to rpl32 signals. (C) tim mRNA from PDF neurons remained high at CT11 in pdf>DN brains. Control flies and pdf>DN flies were entrained, and PDF neurons were isolated as in B. tim mRNA level was assayed by qPCR. The Y-axis values are relative tim mRNA signals normalized to rpl32. Results were averaged from three experiments.
Discussion
In this study, we addressed the function of the deubiquitylating enzyme USP8 in Drosophila circadian rhythms. Although usp8 mRNA was one of the top cyclers identified in head RNA more than a decade ago (McDonald and Rosbash 2001), it was only recently identified as one of the few non-clock gene mRNAs that cycles in circadian neuron RNA as well as in head RNA (Kula-Eversole et al. 2010). This revived our interest in understanding its role in circadian rhythms. The data here indicate that USP8 deubiquitylates CLK, which likely contributes to inhibiting clock gene transcription at the appropriate trough times in the circadian cycle. This regulation appears particularly important for clock neurons because interference of USP8 function by either RNAi knockdown or expression of USP8-DN dramatically alters fly locomotor activity, ranging from long periods to complete arrhythmicity (Fig. 2).
The expression of USP8-DN also had dramatic effects on the oscillations of both tim and per mRNAs and proteins. For example, expression of USP8-DN under the control of the tim driver increases the RNA levels of per, tim, and vri at almost all times of the circadian cycle. Effects on pre-mRNA were similar and included the usual trough times, from late night to early morning (CT22–CT6) (Fig. 3C–E). The increased levels of mRNA presumably contribute to the increase in PER and TIM in head extracts at CT6–CT10, a time when there is no more than a trace visible in wild-type extracts (Fig. 3A,B). However, we note that per and tim RNAs and proteins are still cycling in the USP8-DN-expressing flies, in apparent conflict with the stronger behavioral phenotypes of these mutant flies. We suspected that this inconsistency reflects at least in part the heterogeneity of fly head tissues; i.e., a substantial circadian contribution from the eyes might mask a quantitatively more important contribution of USP8 within circadian neurons. Indeed, expression of USP8-DN has a much more dramatic effect on the levels of PER and TIM at CT10 and tim mRNA at CT22 in PDF neurons (Figs. 7, 8), reflecting and perhaps even causing the long period or arrhythmicity of these flies (Fig. 2).
Most USP family proteins stabilize their substrates by preventing proteasome-mediated degradation and therefore enhancing protein levels (Reyes-Turcu et al. 2009). Yet USP8-DN increases CLK activity without affecting CLK levels. The same conclusion results from the S2 cell experiments: RNAi knockdown of endogenous USP8 or transfection of USP8-DN enhanced CLK/CYC transcription activity, which is still sensitive to PER repression (Fig. 4B,C). Based on the results from flies as well as S2 cells, USP8 probably functions as an inhibitor of CLK/CYC transcriptional activation.
Inhibition of USP8 causes an increase in CLK ubiquitylation, mostly monoubiquitylation (Figs. 4, 5). This presumably reflects the interaction of CLK with USP8 in S2 cells and fly heads (Fig. 5B); i.e., the direct deubiquitylation of CLK. Interestingly, CLK ubiquitylation levels cycle and peak at ZT10–ZT14, when maximal transcription occurs (Fig. 5A). Moreover, expression of USP8-DN dramatically increased the level of CLK ubiquitylation at ZT10 and ZT22, especially at ZT22 (Fig. 5C). Because CLK binding at the E-box of the tim promoter did not change dramatically between the two strains (and perhaps even in the opposite direction) (Fig. 5D), it is likely that CLK monoubiquitylation does not enhance DNA binding, but rather potentiates transcriptional activity. Monoubiquitylation of a transcription factor has been shown to help recruit other transcription factors and even Pol II to a promoter (Salghetti et al. 2001; Kodadek 2010).
Ubiquitylation may be mechanistically related to the “black widow” model of transcription activation, in which transcription factors on chromatin are actively degraded by the proteasome (Tansey 2001). This may apply to mammalian BMAL1, the ortholog of CYC, suggesting that the same turnover mechanism may be relevant to CLK/CYC in flies (Lee et al. 2008; Stratmann et al. 2012). A maximally active chromatin-bound CLK may therefore be monoubiquitylated, which can then experience two inhibitory fates. It can be further ubiquitylated, perhaps directly on chromatin, which may then require proteasome-mediated degradation to allow replacement by another maximally active monoubiquitylated CLK, or it can be deubiquitylated by USP8. The former may predominate at times of maximal transcription so that rapid recycling of CLK/CYC by the proteasome maintains maximal levels of monoubiquitylated CLK and maximal transcription rates. In the late night–early morning, deubiquitylation by USP8 may predominate and help minimize clock gene transcription. It is notable that these are circadian times when cycling usp8 mRNA levels are maximal. However, it is possible that regulation of an E3 ligase also contributes to the cycling of CLK ubiquitylation.
The importance of differential ubiquitylation is reinforced by the different ubiquitylated CLK-binding patterns on some clock genes; i.e., vri and pdp1. The fact that ubiquitylated CLK occurs preferentially close to the transcription start sites of these genes (Fig. 6; data not shown) suggests that CLK monoubiquitylation may help recruit factors to drive transcription; Pol II or factors associated with transcription initiation are good candidates. In this view, the eye-specific vri and pdp1 CLK-binding sites with poor ubiquitylation may reflect CLK-binding sites without these cofactors, allowing deubiquitylation to predominate. The relatively poor CLK ubiquitylation at these sites may also indicate a relationship with other transcription factors. Put otherwise, deubiquitylated CLK may play a more modest role at these sites and partner with (unidentified) factors that contribute most of the transcriptional activation potential, perhaps within certain eye cell types.
Nonetheless, the fact that CLK deubiquitylation by USP8 appears maximal at the end of the transcriptional cycle suggests that deubiquitylated CLK is associated with changes in chromatin structure and/or transcription complexes at this time. Indeed, most CLK is sequestered by PER in an off-DNA inhibitory complex at the end of the cycle (Menet et al. 2010), suggesting that this complex contains substantial levels of deubiquitylated CLK. PER may enhance USP8 activity on CLK or inhibit CLK monoubiquitylation within the PER–CLK complex. Alternatively, PER may function strictly to inhibit DNA binding, suggesting that CLK monoubiquitylation and even deubiquitylation are predominantly on-DNA events.
It is interesting that the original analysis of cycling mRNAs within PDF neurons indicated that the circadian amplitudes of most clock gene mRNAs were dramatically enhanced compared with their amplitudes in head RNA (Kula-Eversole et al. 2010). This is consistent with the enhanced effect of USP8 inhibition of mRNA cycling in PDF neurons compared with the effects on head RNA, suggesting that USP8 function may contribute to this enhanced amplitude of clock gene cycling. A challenge for the future will be to understand how and why USP8 functions differentially within circadian neurons. It will also be important to integrate this goal with the ability to assay CLK/CYC binding and even ubiquitylated CLK binding as a function of circadian time within PDF neurons.
Materials and methods
Fly strains
All gal4 and gal80ts drivers used in this study have been described previously: timgal4 (Kaneko et al. 2000), pdfgal4 (Stoleru et al. 2004), dvpdfgal4 (Bahn et al. 2009), tubgal80ts (McGuire et al. 2003), usp8-RNAi R2 (Mukai et al. 2010), and usp8-RNAi (HMS01889, Bloomington). uas-usp8-DN transgenic flies were generated by injecting yw embryos with pUAST-usp8-DN plasmid (BestGene, Inc.). The pUAST-usp8-DN plasmid was generated by cloning usp8 coding sequence with cysteine at residue 572 mutated to alanine into pUAST plasmid. uas-usp8 wild-type and uas-usp8-RB-DN (short isoform) transgenic flies were generated by injecting yw embryos with pUAST-uas-usp8 and pUAST-uas-usp8-RB-DN plasmids. 3XFlag-CLK14.8 transgenic flies were generated by injecting yw embryos with pCasPer4.0 3XFlag-clk14.8 plasmid. The pCasPer4.0 3XFlag-clk14.8 plasmid was constructed previously (Kadener et al. 2008) with the following modification: Sequence encoding 3XFlag peptides was inserted in-frame before the ATG codon of clk 14.8-kb fragment.
Locomotor behavior
Young male flies were entrained and monitored for 3–4 d in LD conditions, followed by at least 5 d in DD using Trikinetics Drosophila activity monitors. Behavior analyses were performed with a signal processing toolbox (Levine et al. 2002) using MATLAB software.
ChIP–chip and seq-ChIP
Anti-CLK and anti-Pol II ChIPs were performed as previously described (Abruzzi et al. 2011) with slight modifications: Fly heads were quickly ground using a mortar on dry ice. The resulting powder was transferred to a glass dounce homogenizer and homogenized in 5 vol of homogenization buffer (10 mM Hepes-KOH at pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 0.8 M sucrose, 0.5 mM EDTA, 1 mM DTT, 1× protease inhibitor, 1× phosphatase inhibitor cocktail). Homogenates were loaded on equal volumes of sucrose cushion buffer (with 1.0 M sucrose and 10% glycerol in the homogenization buffer) and centrifuged in a HB-6 rotor (Sorvall) at 11,000 rpm for 10 min at 4°C. Pelleted nuclei were cross-linked in 1% formaldehyde for 15 min at room temperature and sonicated in 0.5 mL of lysis buffer (20 mM Tris-HCl at pH 7.5, 150 mM NaCl, 10% glycerol, 1% NP-40, 1 mM DTT, PI and PPase inhibitors).
Seq-ChIP was performed according to published protocols (Geisberg and Struhl 2004) with the following modification: Chromatin immunoprecipitated by anti-Flag beads was eluted by 150 μL of ChIP elution buffer (50 mM Tris–HCl at pH 7.5, 10 mM EDTA, 1% SDS) for 10 min at 65°C. Elutes (135 μL) were adjusted to 1.5 mL by adding lysis buffer (20 mM Tris-HCl at pH 7.5, 10% glycerol, 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 10 mM N-ethylmaleimide) and incubated with 5 μL of FK2 ubiquitin antibody and 30 μL of protein G magnetic beads (Invitrogen) for 2 h at room temperature in the presence of 5 mg/mL BSA and 50 μg/mL Escherichia coli tRNA. Precipitated chromatin was eluted by ChIP elution buffer, decross-linked for 6 h at 65°C, and purified by a Qiagen PCR purification kit. Tiling arrays and data analysis were performed as previously described (Abruzzi et al. 2011).
Western blotting and immunoprecipitation
S2 cell and fly head extracts were prepared in lysis buffer (20 mM Tris-HCl at pH 7.5, 10% glycerol, 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 10 mM N-ethylmaleimide) supplemented by protease inhibitor cocktail and phosphatase inhibitor cocktail. Extracts were subjected to mild sonication. For experiments of phosphatase treatment, phosphatase inhibitor cocktail was omitted. Clear and denatured lysates were resolved by NuPAGE Novex 3%–8% Tris-Acetate gel (Invitrogen). Protein transfer was performed by using the iBlot dry blotting system (Invitrogen). Protein bands were visualized by an ECL reagent kit according to the manufacturer's manual. For CLK immunoprecipitation, 25 μL of M2 anti-Flag beads (Sigma) were incubated with extracts for 2 h at 4°C. Proteins were eluted by 1× SDS loading buffer for 5 min at 95°C. Antibodies used for Western blotting were as follows: anti-CLK (Houl et al. 2008), anti-PER (Dembinska et al. 1997), anti-TIM (Zeng et al. 1996), anti-USP8 (Mukai et al. 2010), anti-USP7 (van der Knaap et al. 2005), anti-Flag (Sigma), anti-V5 (Invitrogen), and Fk2 anti-ubiquitin (Enzo Life Sciences).
S2 cell transfection, dsRNA systhesis, RNAi treatment, and luciferase activity assay
S2 cells were maintained in insect tissue culture medium (HyClone) supplemented with 10% fetal bovine serum and antibiotics. Transfection was performed at 60%–70% confluence with 10 μL of cellfectin II (Invitrogen) per well in a six-well plate or 75 μL of cellfectin in a 75-cm2 flask. We performed dsRNA synthesis and RNAi treatment of S2 cells according to the protocol described previously (Nawathean et al. 2005). For luciferase activity experiments, 50 ng of luciferase firefly reporter and 100 ng of pCopia-Renilla luciferase reporter (transfection control) were always included in the transfection.
RNA analysis by real-time PCR
Total RNA was prepared from adult heads using Trizol reagent (Invitrogen) and was DNase-treated using RQ1 DNase (Promega) according to the manufacturer's protocols. Two micrograms of RNA was used for RT–PCR using SuperScript II (Invitrogen) and random primers (Promega). The resulting cDNA was used as a template in qPCR using the Syber Master Mix (Qiagen) and a Rotorgene qPCR machine (Qiagen). Primer sequences used for qRT–PCR are available on request. Total RNA purification and amplification from PDF neurons were performed according to published protocols (Nagoshi et al. 2009).
Immunostaining
Immunostaining of Drosophila brains using anti-PER, anti-PDF (Shafer et al. 2002), and anti-TIM (Tang et al. 2010) was performed as described previously. Dissected brains were incubated with PER antibody (1:1000) for two overnights at 4°C or with rat anti-TIM (1:1000) overnight at 4°C.
Acknowledgments
We thank Paul Hardin for generously providing the CLK antibody, and Satoshi Goto for providing the USP8 antibody and usp8 RNAi strain. We thank Sebastian Kadener, Sean Bradley, Jerome Menet, and members of the Rosbash laboratory for valuable advice and comment. This research was supported by the Howard Hughes Medical Institute and NIH grants (P01 NS44232 to M.R.).
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.200584.112.
References
- Abruzzi KC, Rodriguez J, Menet JS, Desrochers J, Zadina A, Luo W, Tkachev S, Rosbash M 2011. Drosophila CLOCK target gene characterization: Implications for circadian tissue-specific gene expression. Genes Dev 25: 2374–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Chung BY 2010. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol 72: 605–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn JH, Lee G, Park JH 2009. Comparative analysis of Pdf-mediated circadian behaviors between Drosophila melanogaster and D. virilis. Genetics 181: 965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Draetta GF, Pagano M 2007. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 316: 900–904 [DOI] [PubMed] [Google Scholar]
- Cardone L, Hirayama J, Giordano F, Tamaru T, Palvimo JJ, Sassone-Corsi P 2005. Circadian clock control by SUMOylation of BMAL1. Science 309: 1390–1394 [DOI] [PubMed] [Google Scholar]
- Chiu JC, Ko HW, Edery I 2011. NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell 145: 357–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW 2001. Circadian regulation of gene expression systems in the Drosophila head. Neuron 32: 657–671 [DOI] [PubMed] [Google Scholar]
- Curtin K, Huang ZJ, Rosbash M 1995. Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron 14: 365–372 [DOI] [PubMed] [Google Scholar]
- Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, Hardin PE, Young MW, Storti RV, Blau J 2003. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112: 329–341 [DOI] [PubMed] [Google Scholar]
- Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TDL, Weitz CJ, Takahashi JS, Kay SA 1998. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 280: 1599–1603 [DOI] [PubMed] [Google Scholar]
- Dembinska ME, Stanewsky R, Hall JC, Rosbash M 1997. Circadian cycling of a PERIOD-β-galactosidase fusion protein in Drosophila: Evidence for cyclical degradation. J Biol Rhythms 12: 157–172 [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156 [DOI] [PubMed] [Google Scholar]
- Doherty CJ, Kay SA 2010. Circadian control of global gene expression patterns. Annu Rev Genet 44: 419–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisberg JV, Struhl K 2004. Cellular stress alters the transcriptional properties of promoter-bound Mot1–TBP complexes. Mol Cell 14: 479–489 [DOI] [PubMed] [Google Scholar]
- Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, Romero MR, et al. 2007. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316: 897–900 [DOI] [PubMed] [Google Scholar]
- Grima B, Lamouroux A, Chelot E, Papin C, Limbourg-Bouchon B, Rouyer F 2002. The F-box protein slimb controls the levels of clock proteins period and timeless. Nature 420: 178–182 [DOI] [PubMed] [Google Scholar]
- Hao H, Allen DL, Hardin PE 1997. A circadian enhancer mediates PER-dependent mRNA cycling in Drosophila melanogaster. Mol Cell Biol 17: 3687–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P 2007. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 450: 1086–1090 [DOI] [PubMed] [Google Scholar]
- Houl JH, Yu W, Dudek SM, Hardin PE 2006. Drosophila CLOCK is constitutively expressed in circadian oscillator and non-oscillator cells. J Biol Rhythms 21: 93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houl JH, Ng F, Taylor P, Hardin PE 2008. CLOCK expression identifies developing circadian oscillator neurons in the brains of Drosophila embryos. BMC Neurosci 9: 119 doi: 10.1186/1471-2202-9-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Perez-Burgos L, Placek BJ, Sengupta R, Richter M, Dorsey JA, Kubicek S, Opravil S, Jenuwein T, Berger SL 2006. Repression of p53 activity by Smyd2-mediated methylation. Nature 444: 629–632 [DOI] [PubMed] [Google Scholar]
- Kadener S, Menet JS, Schoer R, Rosbash M 2008. Circadian transcription contributes to core period determination in Drosophila. PLoS Biol 6: e119 doi: 10.1371/journal.pbio.0060119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Hall JC 2000. Neuroanatomy of cells expressing clock genes in Drosophila: Transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 422: 66–94 [DOI] [PubMed] [Google Scholar]
- Kaneko M, Park J, Cheng Y, Hardin P, Hall J 2000. Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J Neurobiol 43: 207–233 [DOI] [PubMed] [Google Scholar]
- Katan-Khaykovich Y, Struhl K 2011. Splitting of H3–H4 tetramers at transcriptionally active genes undergoing dynamic histone exchange. Proc Natl Acad Sci 108: 1296–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HW, Jiang J, Edery I 2002. Role for Slimb in the degradation of Drosophila Period protein phosphorylated by Doubletime. Nature 420: 673–678 [DOI] [PubMed] [Google Scholar]
- Kodadek T 2010. No splicing, no dicing: Non-proteolytic roles of the ubiquitin-proteasome system in transcription. J Biol Chem 285: 2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Zheng X, Sehgal A 2006. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science 312: 1809–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D, Rape M 2012. The ubiquitin code. Annu Rev Biochem 81: 203–229 [DOI] [PubMed] [Google Scholar]
- Kula-Eversole E, Nagoshi E, Shang Y, Rodriguez J, Allada R, Rosbash M 2010. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc Natl Acad Sci 107: 13497–13502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lee Y, Lee MJ, Park E, Kang SH, Chung CH, Lee KH, Kim K 2008. Dual modification of BMAL1 by SUMO2/3 and ubiquitin promotes circadian activation of the CLOCK/BMAL1 complex. Mol Cell Biol 28: 6056–6065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC 2002. Signal analysis of behavioral and molecular cycles. BMC Neurosci 3: 1 doi: 10.1186/1471-2202-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JM, Kilman VL, Keegan K, Paddock B, Emery-Le M, Rosbash M, Allada R 2002. A role for casein kinase 2α in the Drosophila circadian clock. Nature 420: 816–820 [DOI] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS, Young MW 2001. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105: 769–779 [DOI] [PubMed] [Google Scholar]
- McDonald MJ, Rosbash M 2001. Microarray analysis and organization of circadian gene expression in Drosophila. Cell 107: 567–578 [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL 2003. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302: 1765–1768 [DOI] [PubMed] [Google Scholar]
- Menet JS, Abruzzi KC, Desrochers J, Rodriguez J, Rosbash M 2010. Dynamic PER repression mechanisms in the Drosophila circadian clock: From on-DNA to off-DNA. Genes Dev 24: 358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, et al. 2008. Cyclical DNA methylation of a transcriptionally active promoter. Nature 452: 45–50 [DOI] [PubMed] [Google Scholar]
- Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M 2005. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell 16: 5163–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai A, Yamamoto-Hino M, Awano W, Watanabe W, Komada M, Goto S 2010. Balanced ubiquitylation and deubiquitylation of Frizzled regulate cellular responsiveness to Wg/Wnt. EMBO J 29: 2114–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi E, Sugino K, Kula E, Okazaki E, Tachibana T, Nelson S, Rosbash M 2009. Dissecting differential gene expression within the circadian neuronal circuit of Drosophila. Nat Neurosci 13: 60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P 2008. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134: 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawathean P, Rosbash M 2004. The doubletime and CKII kinases collaborate to potentiate Drosophila PER transcriptional repressor activity. Mol Cell 13: 213–223 [DOI] [PubMed] [Google Scholar]
- Nawathean P, Menet JS, Rosbash M 2005. Assaying the Drosophila negative feedback loop with RNA interference in s2 cells. Methods Enzymol 393: 610–622 [DOI] [PubMed] [Google Scholar]
- Niendorf S, Oksche A, Kisser A, Lohler J, Prinz M, Schorle H, Feller S, Lewitzky M, Horak I, Knobeloch KP 2007. Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol Cell Biol 27: 5029–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G, Ohkura N, Azama T, Mesaki M, Yukimasa S, et al. 2003. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem 278: 41519–41527 [DOI] [PubMed] [Google Scholar]
- Oishi K, Ohkura N, Amagai N, Ishida N 2005. Involvement of circadian clock gene Clock in diabetes-induced circadian augmentation of plasminogen activator inhibitor-1 (PAI-1) expression in the mouse heart. FEBS Lett 579: 3555–3559 [DOI] [PubMed] [Google Scholar]
- Price JL, Blau J, Rothenfluh-Hilfiker A, Abodeely M, Kloss B, Young MW 1998. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94: 83–95 [DOI] [PubMed] [Google Scholar]
- Reyes-Turcu FE, Ventii KH, Wilkinson KD 2009. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 78: 363–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Menet JS, Rosbash M 2012. Nascent-seq indicates widespread cotranscriptional RNA editing in Drosophila. Mol Cell 47: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salghetti SE, Caudy AA, Chenoweth JG, Tansey WP 2001. Regulation of transcriptional activation domain function by ubiquitin. Science 293: 1651–1653 [DOI] [PubMed] [Google Scholar]
- Scoma HD, Humby M, Yadav G, Zhang Q, Fogerty J, Besharse JC 2011. The de-ubiquitinylating enzyme, USP2, is associated with the circadian clockwork and regulates its sensitivity to light. PLoS ONE 6: e25382 doi: 10.1371/journal.pone.0025382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Rosbash M, Truman JW 2002. Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. J Neurosci 22: 5946–5954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS 2007. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129: 1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M 2004. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431: 862–868 [DOI] [PubMed] [Google Scholar]
- Stratmann M, Suter DM, Molina N, Naef F, Schibler U 2012. Circadian Dbp transcription relies on highly dynamic BMAL1–CLOCK interaction with E boxes and requires the proteasome. Mol Cell 48: 1–11 [DOI] [PubMed] [Google Scholar]
- Tansey WP 2001. Transcriptional activation: Risky business. Genes Dev 15: 1045–1050 [DOI] [PubMed] [Google Scholar]
- Tang CH, Hinteregger E, Shang Y, Rosbash M 2010. Light-mediated TIM degradation within Drosophila pacemaker neurons (s-LNvs) is neither necessary nor sufficient for delay zone phase shifts. Neuron 66: 378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Buelow K, Guha A, Rausch R, Yin L 2012. USP2a deubiquitinates and stabilizes the circadian protein CRY1 in response to inflammatory signals. J Biol Chem 287: 25280–25291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda HR, Matsumoto A, Kawamura M, Iino M, Tanimura T, Hashimoto S 2002. Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J Biol Chem 277: 14048–14052 [DOI] [PubMed] [Google Scholar]
- van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice MM, Burgering BM 2006. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol 8: 1064–1073 [DOI] [PubMed] [Google Scholar]
- van der Knaap JA, Kumar BR, Moshkin YM, Langenberg K, Krijgsveld J, Heck AJ, Karch F, Verrijzer CP 2005. GMP synthetase stimulates histone H2B deubiquitylation by the epigenetic silencer USP7. Mol Cell 17: 695–707 [DOI] [PubMed] [Google Scholar]
- van der Knaap JA, Kozhevnikova E, Langenberg K, Moshkin YM, Verrijzer CP 2010. Biosynthetic enzyme GMP synthetase cooperates with ubiquitin-specific protease 7 in transcriptional regulation of ecdysteroid target genes. Mol Cell Biol 30: 736–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MH, Berlin I, Nash PD 2011. Regulation of endocytic sorting by ESCRT-DUB-mediated deubiquitination. Cell Biochem Biophys 60: 39–46 [DOI] [PubMed] [Google Scholar]
- Zeng H, Qian Z, Myers MP, Rosbash M 1996. A light-entrainment mechanism for the Drosophila circadian clock. Nature 380: 129–135 [DOI] [PubMed] [Google Scholar]
- Zheng X, Koh K, Sowcik M, Smith CJ, Chen D, Wu MN, Sehgal A 2009. An isoform-specific mutant reveals a role of PDP1ɛ in the circadian oscillator. J Neurosci 29: 10920–10927 [DOI] [PMC free article] [PubMed] [Google Scholar]