Abstract
Purpose
Topical antibacterial agents, used as an off-label indication, are frequently administered pre- and postoperatively to prevent endophthalmitis. We compared topical treatment with fluoroquinolone (FQ) anti-infectives to non-FQ antibacterial agents to prevent Staphylococcus aureus endophthalmitis. We hypothesize that FQ anti-infectives are more effective than non-FQ antibacterial agents for preventing endophthalmitis.
Methods
Moxifloxacin 0.5%, ofloxacin 0.3%, gentamicin 0.3%, chloramphenicol 0.5%, polymyxin B/trimethoprim (10,000 units/mL/0.1%), povidone-iodine 5%, and saline were tested for topical treatment to prevent endophthalmitis. Topical treatment was applied every 15 min for 1 h (5 drops) to the left eye of 14 rabbits for each antibacterial agent and saline. After appropriate anesthesia, the anterior chambers were injected with 1×105 colony-forming units of a clinical endophthalmitis isolate of a S. aureus that was susceptible to all tested antibacterials. One drop was administered immediately and another 4 drops of topical treatment were applied over 24 h after injection. At 24 h postinjection, the eyes were graded for clinical signs of endophthalmitis (ocular discharge, conjunctivitis/scleral injection, limbal injection, hypopyon*, iritis*, anterior chamber cells*, anterior chamber flare*, corneal infiltration, and fibrin production*) using a severity scale (0–3). The indication of clinical endophthalmitis was a total score of >3.0 for the presentations marked with an asterisk. The data were analyzed using Fisher's Exact Randomization or Mann–Whitney nonparametric testing.
Results
Topical ofloxacin (14/14, 100% without endophthalmitis) and moxifloxacin (13/14, 93%) prevented the clinical presentation of endophthalmitis significantly more frequently (P=0.03, Fisher's Exact Test (FE)) than topical gentamicin (7/14, 50%), povidone iodine (4/14, 29%), chloramphenicol (0/14, 0%), polymyxin B/trimethoprim (0/14, 0%), and saline (0/14, 0%). The median total clinical scores for the ofloxacin (0.5) and moxifloxacin (0.8) groups were significantly (P=0.008, Mann–Whitney Test (MW)) lower than gentamicin (5.7), chloramphenicol (17.5), polymyxin B/trimethoprim (21.2), povidone-iodine (15.5), and saline (18.7).
Conclusions
The FQs, ofloxacin and moxifloxacin, were more effective in preventing endophthalmitis than the non-FQ antibacterial agents in a rabbit S. aureus endophthalmitis model. The observed results are consistent with the hypothesis that FQs penetrate into the anterior chamber at more effective levels than many of the common non-FQ antibacterial agents.
Introduction
The administration of topical antibacterial agents to prevent endophthalmitis is a common off-label practice used by ophthalmic surgeons during surgery. Povidone-iodine has been accepted to decrease the number of bacterial pathogens in the conjunctiva, eyelids, and surrounding ocular adnexa.1,2 Povidone-iodine eradicates bacterial contamination on the ocular surface, but does not penetrate into the intraocular chambers. Although some anti-infectives penetrate into the aqueous humor,3,4 there is no case-based evidence that topically applied anti-infectives decrease the incidence of postsurgical endophthalmitis. Barry et al. demonstrated in the European Society of Cataract and Refractive Surgeons study that intracameral cefuroxime significantly decreased the incidence of postsurgical endophthalmitis and that topical levofloxacin also decreased the incidence, but the decrease was not statistically significant.5 Romero et al. 6 and Garat et al.7 indicated that off-label intracameral cefazolin decreased the incidence of endophthalmitis. In animal studies, our group demonstrated the Proof of Principle that topical moxifloxacin 0.5% was able to prevent endophthalmitis and that a fourth generation fluoroquinolone (FQ) (moxifloxacin 0.5%) was more effective for preventing endophthalmitis than a third generation FQ (levofloxacin 0.5%).8,9
The elimination of topical contamination alone and the application of intraocular penetrating anti-infectives have not completely resolved the problem of postsurgical endophthalmitis. Until the final solution is found, new and enhanced contemporary antibacterial agents will need to be evaluated. Optimal topical agents should not only sterilize the ocular surface, but also penetrate into the aqueous humor at concentrations high enough to eliminate bacteria that enter the globe during surgery.
In the present rabbit study, we applied topical antibacterial agents before and postinjection of Staphylococcus aureus into the anterior chamber. The topical antibacterial agents were an antiseptic (povidone-iodine) and commonly used ophthalmic solutions (moxifloxacin, ofloxacin, gentamicin, chloramphenicol, and the polymyxin B/trimethoprim combination) used to treat infections. We hypothesize that FQ anti-infectives will be more effective in preventing endophthalmitis than an antiseptic and other commonly used antibacterial agents. To test our hypothesis, the rabbits were evaluated for clinical signs of endophthalmitis and intraocular bacterial growth, and the results were compared statistically for all the topical treatment groups.
Methods
A rabbit model was previously established by our laboratory to test topical anti-infectives to prevent endophthalmitis.8 The present study conformed to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research, and was approved by the University of Pittsburgh Institutional Animal Care and Use Committee (Protocol No. 0708986).
S. aureus endophthalmitis isolate
A S. aureus strain (E253) isolated from a clinical case of endophthalmitis was chosen from our clinical tissue bank that serves as a resource to validate laboratory susceptibility testing and monitoring of antibiotic resistance. The isolate was de-identified, stored at −80°C, and chosen based on its susceptibilities [minimum inhibitory concentrations (MICs)] to moxifloxacin 0.032 μg/mL, ofloxacin 0.38 μg/mL, gentamicin 0.75 μg/mL, chloramphenicol 8 μg/mL, and polymyxin B/trimethoprim (192 μg/mL, 2 μg/mL) (S. aureus is intrinsically resistant to polymyxin B). Povidone-iodine was deemed an antiseptic effective against S. aureus. It was the intent that the S. aureus be susceptible to all antibacterial agents tested in this study.
S. aureus E253 was retrieved from frozen storage and grown overnight at 37°C on trypticase soy agar supplemented with 5% sheep blood (BBL™, Becton, Dickinson, and Co., Sparks, MD). Overnight colonies were suspended in a trypticase soy broth (BBL, Becton, Dickinson, and Co.,) and the turbidity was determined spectrophotometrically at 650 nm. An optical density of 0.23 correlated to approximately 1×108 colony-forming units (CFU) per mL. The concentration based on the optical density was appropriately diluted in the sterile trypticase soy broth to provide the inoculum of 1×105 CFU/eye in 25 μL that was inoculated into the aqueous of all rabbit eyes. Colony counts were performed to confirm the actual CFU inoculated.
Treatment groups
Seven topical treatments groups were compared for the ability to prevent endophthalmitis: (1) moxifloxacin 0.5%, (Vigamox,7 moxifloxacin HCl ophthalmic solution; Alcon Laboratories, Ft. Worth, TX; Lot No. 115351F), (2) ofloxacin 0.3%, (ofloxacin ophthalmic solution USP; Falcon Pharmaceuticals Ltd; Lot No. 134614F), (3) gentamicin 0.3%, (gentamicin sulfate ophthalmic solution USP; Falcon Pharmaceuticals Ltd.; Lot No. 89058F), (4) chloramphenicol 0.5%, (Alcon Cusi, Barcelona, Spain; Lot No. 8ABG1B), (5) polymyxin B sulfate/trimethoprim sulfate 10,000 units/mL/0.1%, (Falcon Pharmaceuticals Ltd.; Lot No. 13843F), (6) povidone-iodine ophthalmic prep solution 5%, (Betadine, Alcon Laboratories; Lot No. KHE010), and (7) saline, 0.9% Sodium Chloride Injection USP (Baxter Healthcare Corp., Deerfield, IL). All antibacterial drugs were supplied by Alcon Laboratories in a secure, intact packaging. The injectable nonpreserved saline was obtained from the University of Pittsburgh Medical Center Central Pharmacy (Pittsburgh, PA).
Experimental protocol
A total of 98 rabbits (14 rabbits per group) were used in this study. The rabbits were equally divided into 7 trials consisting of 14 rabbits per trial and using 2 rabbits per antibacterial drug and saline control group. For each topical treatment, the left eye of 14 rabbits was administered 1 drop of drug every 15 min for 1 h (5 drops). Rabbits were systemically anesthetized with an intramuscular injection of ketamine (40 mg/kg) (Ketaject®, Phoenix Pharmaceuticals, St. Joseph, MO) and xylazine (4 mg/kg) (AnaSed®, Lloyd Laboratories, Shenandoah, IA) in the rear flank. Topical anesthesia (0.5% proparacaine) (Falcon Pharmaceuticals) was applied to each cornea before proptosing the globe with a soft-tipped dacron applicator. The anterior chamber was injected with 0.025 mL (25 μL) (1×105 CFU) of S. aureus E253 using a 30-gauge, ½ inch needle and 0.1-mL syringe through the central cornea without disturbing the intraocular structures. One drop of topical treatment was immediately applied and an additional 4 drops of topical treatment were applied over the following 24 h. The animals were given intramuscular ketoprofen (Ketofen®, Fort Dodge, IA) (1.5 mg/kg) to control potential discomfort over the following 24 h postinjection.
At 24 h post injection, the eyes were graded (ophthalmologist F.S.M.) in a masked fashion, where the clinical examiner did not know the topical treatment or any information concerning each specific rabbit. The examination was graded for clinical signs of endophthalmitis (ocular discharge, conjunctivitis/scleral injection, limbal injection, hypopyon, iritis, anterior chamber cells, anterior chamber flare, corneal infiltration, and fibrin production) using a severity scale (0–3).8,9 Following clinical examination, the rabbits were sacrificed with an overdose of Euthasol (Virbac AH, Inc., Fort Worth, TX), and the anterior chamber and vitreous were tapped using 23-gauge needles on tuberculin syringes. The number of viable S. aureus (CFU/mL) from the anterior chamber and vitreous was determined with standard bacterial colony counts.
Statistical analysis
The current study was performed in a total of 7 trials using 2 rabbits for each treatment group per trial. The results of the 7 trials were combined and the statistical analyses were performed.
The relative incidence of endophthalmitis was analyzed with the Fisher's Exact randomization test (www.langsrud.com/fisher.htm) based on slit lamp examination and/or positive cultures between the topical treatment groups. The graded clinical data were analyzed nonparametrically with the Mann–Whitney test (Minitab®, State College, PA). The designation of clinical endophthalmitis was based on the clinical judgment of the masked examiner and a total clinical score of 3.0 or greater based on the combined clinical scores of hypopyon, iritis, fibrin production, anterior chamber cells, and anterior chamber flare. The incidence of positive cultures, based on aqueous and/or vitreous isolation, between the topical treatment groups was analyzed with the Fisher's exact randomization test.
Results
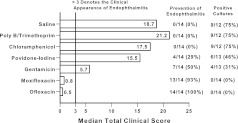
The actual S. aureus inocula injected ranged from 7.9×104 to 2.34×105 CFU per eye (mean±standard deviation=1.88±0.64×105 CFU/eye; median=1.88×105 CFU/eye) over the 7 trials. Figure 1 summarizes the data of rabbits administered topical antibacterials and challenged with S. aureus. Topical ofloxacin (14/14, 100%) and moxifloxacin (13/14, 93%) prevented the clinical presentation of endophthalmitis significantly more often (P=0.03, Fisher's Exact Test (FE)) than topical gentamicin (7/14, 50%), povidone-iodine (4/14, 29%), chloramphenicol (0/14, 0%), polymyxin B/trimethoprim (0/14, 0%), and saline (0/14, 0%). Gentamicin prevented endophthalmitis more often (P=0.005, FE) than saline, polymyxin B/trimethoprim, and chloramphenicol, but not more often (P=0.44, FE) than povidone-iodine. The prevention of endophthalmitis was statistically the same (P=0.09, FE) for saline, polymyxin B/trimethoprim, povidone-iodine, and chloramphenicol, and for ofloxacin and moxifloxacin (P=1.0, FE).
FIG. 1.
The bar chart summarizes the data of rabbits administered topical antibacterials and challenged with Staphylococcus aureus. The designation of clinical endophthalmitis was determined on the clinical judgment of the masked examiner and a Total Clinical Score of 3.0 or greater based on hypopyon, iritis, fibrin production, anterior chamber cells, and anterior chamber flare. Prevention of endophthalmitis is the number of rabbit eyes that topical treatment prevented endophthalmitis based on clinical and/or positive culture. Positive cultures are the number of eyes (aqueous and/or vitreous) that proved culture-positive for S. aureus.
The median Total Clinical Scores for the ofloxacin (0.5) and moxifloxacin (0.8) groups were significantly lower (P=0.008, Mann–Whitney Test (MW)) than gentamicin (5.7), chloramphenicol (17.5), polymyxin B/trimethoprim (21.2), povidone-iodine (15.5), and saline (18.7). The median Total Clinical Score for gentamicin was lower (P=0.007 MW) than the other non-FQs and saline. There was no clinical difference (P=0.07 MW) among the median Total Clinical Scores for saline, polymyxin B/trimethoprim, povidone-iodine, and chloramphenicol, and for ofloxacin and moxifloxacin (P=0.4 MW).
All rabbit eyes (aqueous and/or vitreous) that were culture-positive for S. aureus also presented with clinical signs of endophthalmitis. Ofloxacin (0/14) and moxifloxacin (0/14) were culture-positive significantly less frequently (P=0.04, FE) than gentamicin (4/13), povidone-iodine (6/13), polymyxin B/trimethoprim (9/12), chloramphenicol (9/12), and saline (9/12). There were fewer culture-positive eyes for gentamicin (P=0.047, FE) than saline, polymyxin B/trimethoprim, and chloramphenicol, and gentamicin was equivalent to povidone-iodine (P=0.22, FE). There was no significant difference in culture-positive eyes between ofloxacin (0/14) and moxifloxacin (0/14) (P=1.0, FE).
Discussion
The choice of topical antibacterial drugs to prevent endophthalmitis was the focus of this study. Based on the literature, there is no basis for the off-label application of topical antibacterial drugs to the eye during surgery to prevent bacterial endophthalmitis, but this is a common practice by ophthalmic surgeons as a prudent precaution to guard against postsurgical infection. Based on Speaker's study, the application of povidone-iodine is now a standard practice for preoperative ophthalmic surgery.1 The next question to address is whether sterilizing the ocular surface alone is more important than an antibacterial drug that both sterilizes the ocular surface and penetrates into the aqueous to eliminate any invading contamination that enters the eye. Reason would dictate that the added benefit of antibacterial penetration could optimize the prevention of postsurgical infection.
The design of our rabbit experiment was to evaluate common topical antibacterials under equal conditions against a S. aureus isolate that was cultured from endophthalmitis and was susceptible to all test agents (based on systemic susceptibility standards). Other bacterial isolates may have intrinsic resistance to some of the antibacterials tested, such as Pseudomonas aeruginosa to chloramphenicol and Streptococcus species to gentamicin. The concentration of bacteria injected into the anterior chamber produced consistent endophthalmitis in our model and should not be assumed to be the inoculum necessary to produce endophthalmitis during ophthalmic surgery. Our animal model like most animal models does not completely mimic the human condition. Our prime focus was penetration of an antibacterial through the cornea into the anterior chamber to prevent an infection. Increasing the variables to include intracameral injection of an antibiotic and ocular surface disinfection would complex the present study. The number of animals to complete such a study would be numerous when comparing multiple topical therapies.
Our previously established model demonstrated that moxifloxacin was effective in preventing endophthalmitis by a susceptible S. aureus isolate.8 We repeated the model design, and now also tested topical ofloxacin, gentamicin, chloramphenicol, polymyxin B/trimethoprim combination, and povidone-iodine to prevent endophthalmitis using the same S. aureus isolate. The importance of the present study is to demonstrate that topical antibacterials can enter the anterior chamber at concentrations to prevent endophthalmitis. We did not measure these concentrations because the limited amount of retrieved aqueous (0.1 mL without collapsing the anterior chamber) was used only to determine bacterial viability. There was not enough aqueous to determine both bacterial viability and antibacterial concentration without the risk of significant trauma to the integrity of the eye and reproducibility of the experiment. The aqueous concentrations of different classes of antibacterials cannot be compared because of the chemical structure. In addition, the information would not necessarily correlate meaningfully to the human clinical situation.
Our study determined that topical FQs, moxifloxacin, and ofloxacin, were more effective than topical non-FQs for preventing bacterial endophthalmitis. Ofloxacin, a second generation FQs, was as effective as moxifloxacin, a fourth generation FQ. Unfortunately, this may not be the case with many S. aureus isolates. Our laboratory (http://eyemicrobiology.upmc.com) and Major et al.10 have reported that FQ resistance is common with isolates of S. aureus. In addition, in our previous publication,9 we demonstrated that against a S. aureus isolate that was resistant in vitro to both moxifloxacin and levofloxacin (note: levofloxacin is the purified L-isomer of ofloxacin), only moxifloxacin was effective in preventing endophthalmitis. The coverage by moxifloxacin can be explained by the conveyance of resistance. Whereas, S. aureus requires 2 mutations to convey resistance to moxifloxacin, ofloxacin only requires a single mutation.11 Furthermore, moxifloxacin has been reported to be more potent than levofloxacin and ofloxacin with lower MICs against S. aureus isolated from endophthalmitis.12 Moxifloxacin has also been shown to have better intraocular penetration than other FQ anti-infectives.3,4
It was demonstrated by our current study that the topical non-FQs (gentamicin, chloramphenicol, and the polymyxin B/trimethoprim combination) and the povidone-iodine antiseptic were suboptimal for preventing endophthalmitis with some limited activity with gentamicin. These agents are excellent for reducing the bacteria on the ocular surface, and for treating conjunctivitis and possibly other superficial ocular infections, but they do not appear to penetrate through the cornea into the aqueous to prevent endophthalmitis.
An interesting finding in our study was that povidone-iodine provided some protection in preventing endophthalmitis as a topical antibacterial. The limited activity may be due to the entrance of povidone-iodine during the anterior chamber injection of S. aureus instead of penetrating through the cornea. The frequent dosing of the eye with povidone-iodine as performed in this study is not standard preoperative care. The dosing was performed for consistency in comparing all the antibacterial drugs under the same conditions. We do not recommend our dosing regimen for preoperative care until the appropriate studies of benefit are completed.
In summary, our data support the acceptance of the hypothesis that topical FQ anti-infectives are more effective in preventing endophthalmitis than many non-FQ antibacterial drugs. The impact of this study has less importance if sterilization of the ocular surface to prevent the entrance of bacteria into the anterior chamber is the only risk factor in the development of endophthalmitis. Postsurgical endophthalmitis has other risk factors, such as inadequate surgical preparation (i.e., eyelid cleansing, draping), poor surgical technique, and operating room breakdown, that could allow the entrance of bacteria into the eye. The application of topical anti-infectives to penetrate into the aqueous still appears to be a reasonable added safety measure to possibly guard against the development of bacterial endophthalmitis.
Acknowledgments
This study was funded by Alcon Laboratories, Inc. (Fort Worth, TX) with a research contract through the University of Pittsburgh, Pittsburgh, PA. The authors (R.P.K., E.G.R., R.M.Q.S., and F.S.M) have been paid independent consultant fees by Alcon Laboratories, Inc., and these fees were deemed not to produce a conflict of interest for the authors by the University of Pittsburgh, Pittsburgh, PA. Alcon Laboratories did not design, participate, collect data, or analyze the data for this study. The first author, Regis P. Kowalski, is fully responsible for the content of this manuscript. Additional support was provided in part by The Charles T. Campbell Foundation; The Pennsylvania Lions Club; National Institutes of Health Core grants P30EY008098 and A1085570 (Bethesda, MD); Eye and Ear Foundation of Pittsburgh, PA; and a Career Development Award from Research to Prevent Blindness (New York, NY).
Author Disclosure Statement
The authors were paid consultants of Alcon Laboratories, Ft. Worth, Texas.
References
- 1.Speaker M.G. Menikoff J.A. Prophylaxis of endophthalmitis with topical povidone iodine. Ophthalmology. 1991;98:1769–1775. doi: 10.1016/s0161-6420(91)32052-9. [DOI] [PubMed] [Google Scholar]
- 2.Ciula T.A. Starr M.B. Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002;109:13–24. doi: 10.1016/s0161-6420(01)00899-5. [DOI] [PubMed] [Google Scholar]
- 3.Kim D.H. Stark W.J. O'Brien T.P. Dick J.D. Aqueous penetration and biological activity of moxifloxacin 0.5% ophthalmic solution and gatifloxacin 0.3% solution in cataract surgery patients. Ophthalmology. 2005;112:1992–1996. doi: 10.1016/j.ophtha.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Solomon R. Donnenfeld E.D. Perry H.D. Snyder R.W. Nedrud C. Stein J. Bloom A. Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmology. 2005;112:466–469. doi: 10.1016/j.ophtha.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 5.Barry P. Seal D.V. Gettinby G. Lees F. Peterson M. Revie C.W. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery. J. Cataract Refract. Surg. 2006;32:407–410. doi: 10.1016/j.jcrs.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Romero P. Mendez I. Salvat M. Fernandez J. Almena M. Intracameral cefazolin as prophylaxis against endophthalmitis in cataract surgery. J. Cataract Refract. Surg. 2006;32:438–441. doi: 10.1016/j.jcrs.2005.12.135. [DOI] [PubMed] [Google Scholar]
- 7.Garat M. Moser C.L. Martin-Baranera M. Alonso-Tarres C. Alvarez-Rubio L. Prophylactic intracameral cefazolin after cataract surgery. Endophthalmitis risk reduction and safety results in a 6-year study. J. Cataract Refract. Surg. 2009;35:637–642. doi: 10.1016/j.jcrs.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Kowalski R.P. Romanowski E.G. Mah F.S. Yates K.A. Gordon Y.J. Topical prophylaxis with moxifloxacin prevents endophthalmitis in a rabbit model. Am. J. Ophthalmol. 2004;138:33–37. doi: 10.1016/j.ajo.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 9.Kowalski R.P. Romanowski E.G. Mah F.S. Sasaki H. Fukuda M. Gordon Y.J. A comparison of moxifloxacin and levofloxacin topical prophylaxis in a fluoroquinolone-resistant Staphylococcus aureus rabbit model. Jpn. J. Ophthalmol. 2008;52:211–216. doi: 10.1007/s10384-008-0530-1. [DOI] [PubMed] [Google Scholar]
- 10.Major J.C. Engelbert M. Flynn H.W. Miller D. Smiddy W.E. Davis J.L. Staphylococcus aureus endophthalmitis: antibiotic susceptibilities, methicillin resistance, and clinical outcomes. Am. J. Ophthalmol. 2010;149:278–283. doi: 10.1016/j.ajo.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz J. Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. J. Antimicrob. Chemother. 2003;51:1109–1117. doi: 10.1093/jac/dkg222. [DOI] [PubMed] [Google Scholar]
- 12.Mather R. Karenchak L.M. Romanowski E.G. Kowalski R.P. 4th Generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am. J. Ophthalmol. 2002;133:463–466. doi: 10.1016/s0002-9394(02)01334-x. [DOI] [PubMed] [Google Scholar]