Abstract
Purpose
The objective of this study was to determine the ocular bioavailability of hesperidin and hesperetin, especially with respect to their distribution into the posterior segment of the eye, following systemic and topical administration in rabbits.
Methods
Hesperidin and hesperetin were administered either intravenously or topically to male New Zealand white (NZW) rabbits. Vitreous humor and plasma samples were collected after intravenous administration and analyzed to estimate the concentrations of the parent compounds and their metabolites. Ocular tissue concentrations, obtained on topical administration of hesperidin and hesperetin, were also determined.
Results
In the systemic circulation, hesperidin and hesperetin were rapidly metabolized into their glucuronides, which are extremely hydrophilic in nature. Vitreal samples did not demonstrate any detectable levels of hesperidin/hesperetin following intravenous administration. Topical administration produced significant concentrations of hesperidin/hesperetin in all the ocular tissues tested at the 1 and 3 hours time points postdosing, with hesperetin showing higher levels compared to hesperidin. However, only low levels were generated in the vitreous humor. Inclusion of a penetration enhancer, benzalkonium chloride (BAK), improved the back-of-the-eye hesperetin levels.
Conclusions
Ocular delivery of hesperidin/hesperetin via the systemic route does not seem to be feasible considering the rapid generation of the hydrophilic metabolites. Topical application appears to be more promising and needs to be further developed/refined.
Introduction
Diseases of the posterior segment of the eye such as diabetic retinopathy (DR) and diabetic macular edema (DME) are the leading causes of acquired blindness, especially in Western countries.1 Literature suggests that the bioflavonoid hesperidin and its aglycone hesperetin have pharmacological activities that may be beneficial in the prevention or treatment of DR, DME, and other ocular disorders.
What makes hesperidin and hesperetin particularly attractive for the treatment of DR is their effect on ocular blood flow and vascular permeability. Chiou and Xu demonstrated that hesperidin and, especially, hesperetin produce marked increase in ocular blood flow and retinal function recovery following retinal ischemia.2 Additionally, these compounds have been demonstrated to be effective in the treatment of chronic venous insufficiency.3,4 Reports also suggest that hesperidin can prevent microvascular leakage through their capillary wall-strengthening action: hesperidin methyl chalcone, given intravenously, significantly inhibited the macromolecular permeability-increasing effect of bradykinin, LTB4, and histamine.5 Furthermore, hesperidin and hesperitin can reduce platelet aggregation, a factor involved in the blockage of retinal blood vessels.6,7 Both compounds have also demonstrated potential in protecting against free-radical induced damage to the retinal neuronal cells, protecting the health of retinal vascular cells and inhibiting inflammatory mechanisms that can lead to the induction of angiogenesis. In a study by Xiaoting et al.,8 hesperidin was able protect the retinal cells by inhibiting nitric oxide production, through inducible nitric oxide synthase inhibition. Recent studies also demonstrate their antiangiogenic activity through the inhibition of the expression of platelet-endothelial cell adhesion molecule-19 and hypoxia-inducible factor-1α,10 which have been implicated in vasculogenesis and angiogenesis. Additionally, hesperidin and hesperetin demonstrate strong antioxidant properties. A number of researchers have studied the antioxidant activity or radical scavenging properties, both in vitro and in vivo, of hesperidin and hesperetin using a variety of assay systems. Findings from these studies indicate that both hesperidin and hesperetin possess strong antioxidant activity and also neuroprotective properties arising from their antioxidant characteristics. Hesperidin11 also exhibits a significant anti-inflammatory activity by modulating the prostaglandin synthesis and the COX-2 gene expression pathway.
Pharmacology of these compounds in relation to ocular diseases has been recently reviewed.12 Taken together, hesperidin may prove to be a very important therapeutic candidate in the treatment of DR.
Currently, hesperidin is used as a dietary supplement to improve blood flow and for its vasoprotective properties, and is commercially available as an oral formulation. Ameer et al.13 reported that following oral administration of hesperidin, hesperetin concentration in the urine was measured and cumulative urinary recovery of hesperetin indicated low bioavailability (<25%). To be orally absorbed, hesperidin needs to be first converted into hesperetin.14 It has been established that intestinal beta-glucosidase and/or alpha-rhamnosidase converts hesperidin into hesperetin. Hesperetin then undergoes phase-II metabolism to yield hesperetin-glucoronides as the major metabolites and some hesperetin-sulfates. Additionally, hesperetin may be converted into eriodictyol.15–17 Thus, several factors come into play to limit oral bioavailability of hesperidin, including first-pass metabolism,15–17 poor water solubility, and precipitation, in an acidic environment.18
Drug delivery to the eye, in comparison to other parts of the body, poses significant challenges because of the presence of various barriers, which are inherent and unique to the anatomy and physiology of the eye. The general routes of administration for ocular drug delivery include topical, periocular, intravitreal, systemic, and oral routes. A topically administered drug may penetrate into the posterior chamber tissues, such as the retina, through corneal or noncorneal routes.19 Transcorneal absorption represents the major route of penetration for most therapeutic agents. However, several studies demonstrate that the noncorneal route is also significant, wherein the drug molecule penetrates into the intraocular tissues via diffusion across the conjunctiva and sclera.19,20 For an orally administered drug, it has to first reach the systemic circulation and then the eye. To move from the vascular bed into the ocular tissue, the molecule needs to permeate across the blood–retinal barrier (BRB), formed by the retinal pigmented epithelium (RPE) and the endothelial cells of the retinal blood vessels. The diffusion limiting capabilities of the BRB have been well established for both hydrophilic (limited by tight junctions) as well as lipophilic (through efflux mechanism) compounds.21
The long-term goal of this project is to achieve therapeutically optimal hesperidin/hesperetin concentrations at the target site, that is, posterior segment of the eye. The ocular bioavailability of hesperidin and its aglycone hesperetin, through any route of administration, has not been reported yet. In this study, our major objective was to evaluate the systemic and topical routes for the delivery of hesperidin and hesperetin to the posterior segment of the rabbit eye. The feasibility of the oral route for the delivery of the bioflavonoids into the ocular tissues may be speculated/extrapolated from the results obtained with the intravenous administration study. Thus, the aim of this study was to determine plasma and vitreal kinetics of hesperidin and hesperetin following systemic and topical administration.
Methods
Animals
Studies were conducted using male New Zealand white (NZW) rabbits, procured from Myrtle's Rabbitry (Thompson Station, TN). Experiments conformed to the tenets of the Association for Research in Vision and Ophthalmology statement on the Use of Animals in Ophthalmic and Vision Research and followed the University of Mississippi Institutional Animal Care and Use Committee approved protocol. Each experiment was conducted at least in quadruplicates.
Materials
Hesperidin, hesperetin, beta-glucuronidase (Type HP-2, from Helix pomatia), hydroxylpropyl beta-cyclodextrin (HP-β-CD), randomly methylated beta-cyclodextrin (RM-β-CD), and microsomes (obtained from rat liver, pooled) were purchased from Sigma-Aldrich (St. Louis, MO). Ketamine hydrochloride and xylazine were procured from Fort Dodge Animal Health (Fort Dodge, IA) and Lloyd Laboratories (Shenandoah, IA), respectively. Pentobarbital was obtained from Virbac AH, Inc. (Fort Worth, TX). Microdialysis probes (CMA/20; 20,000 Da molecular mass and 10-mm shaft) were obtained from CMA/Microdialysis, Inc. (North Chelmsford, MA). Acetonitrile [(high-performance liquid chromatography (HPLC) grade], methanol (HPLC grade), Ortho-phosphoric acid, dimethyl sulfoxide (DMSO), benzalkonium chloride (BAK), propylene glycol, hydrochloric acid, formic acid, and sodium hydroxide were purchased from Thermo Fisher Scientific (Waltham, MA) and used as such.
In vitro probe recovery
Microdialysis probe recovery was performed following a previously published report.22,23 Briefly, recovery values were obtained by placing the probe in an isotonic phosphate-buffered saline (IPBS; pH 7.4) at 37°C, containing a known concentration of the compound (hesperidin or hesperetin). The probe was perfused with sterile IPBS at a flow rate of 3 μL/min, and the dialysate was collected every 20 min. Relative recovery was calculated using eq. (1):
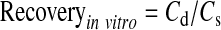 |
(1) |
where Cd is the concentration of the respective compound in the dialysate and Cs is the concentration in IPBS. The concentration of the compound in the vitreous humor samples was calculated by dividing the dialysate concentration by the probe-specific in vitro recovery factor, obtained as described above. The recovery factor for each probe is individually determined before and after the experiment, and the in vivo samples obtained from each probe are uniquely coded. For the probes to be considered intact during the experimental procedure, the relative standard deviation between the recovery factors at the start and at the end of the study should not be greater than 5%. The mean recovery factor for each probe was then used to obtain the actual vitreous humor levels from the vitreous humor sample concentrations.
Intravenous administration study
Probe implantation
Probes were implanted following previously published reports.22,23 Briefly, rabbits (weighing 2–2.5 kg) were anesthetized using ketamine (35 mg/kg)/xylazine (3.5 mg/kg) administered intramuscularly, and were maintained under anesthesia throughout the duration of the experiment (ketamine/xylazine administered intramuscularly every 40 min). Before probe implantation, 1% tropicamide was applied topically to dilate the pupil. A 22-gauge needle was then inserted into the posterior chamber of the eye. The point of insertion was approximately 3 mm below the corneal-scleral limbus. The needle was withdrawn, and the vitreal probe was implanted immediately. The position of the probe was adjusted so that the semipermeable membrane was in the mid-vitreous section. The probes were continuously perfused with sterile IPBS (pH 7.4) at a flow rate of 3 μL/min, using a CMA/100 microinjection pump (CMA/Microdialysis, Inc.). After probe implantation, animals were allowed to stabilize for 2 h before the administration of the respective compounds. Vitreal samples were collected every 20 min for 10 h. Samples were collected in microcentrifuge tubes and stored at −80°C until further analysis. At the end of the study, animals were euthanized, under deep anesthesia, with an overdose of sodium pentobarbital administered through the marginal ear vein.
Intravenous administration of hesperidin/hesperetin
After anesthetizing the rabbits, a 21–25-gauge catheter was placed in the central ear artery for blood collection. Once the catheter was appropriately placed, heparin solution was injected into the catheter to maintain patency. Subsequently, the microdialysis probe was implanted; following its stabilization period, 0.5 mL of hesperidin or hesperetin solution, formulated in DMSO:propylene glycol:IPBS (pH 7.4) (40:40:20), was administered intravenously through the marginal ear vein [20 mg/kg body weight (BW)]. Four animals were used for each compound (n=4). Blood samples were withdrawn and collected in heparinized vials, predose and approximately at 10, 15, 30, 60, 90, 120, 240, 360, and 480 min postdosing. Plasma was separated from the whole blood by centrifugation at 13,000 rpm for 10 min at 4°C (accuSpin Micro 17R; Thermo Fisher Scientific) and was stored at −80°C until further analysis.
Topical administration study
In our previous studies, we found that both compounds were highly insoluble in water.24,25 To improve the solubility of hesperidin and hesperetin, HP-β-CD and RM-β-CD were used. These cyclodextrins (CDs) have been used in ophthalmic formulations and differ with respect to the drug-CD complex formation and may show differences in their effect on ocular penetration.26 Thus, both CDs were tested in vivo with respect to their effect on the back-of-the-eye delivery. However, RM-β-CD was used only for hesperetin, as this compound demonstrated better inherent tissue permeability than hesperidin. Hesperidin solution (1% w/v) for topical instillation was prepared by dissolving it in 100 μL of 1 N sodium hydroxide and adding this solution to 10% HP-β-CD (prepared in IPBS, pH 7.4, containing 0.1% HPMC). Final pH was adjusted to 7.4 using 1 N hydrochloric acid. The hesperetin solution was prepared by dissolving it in 100 μL of 1 N sodium hydroxide and adding this solution to 10% HP-β-CD or 10% RM-β-CD (prepared in IPBS, pH 7.4, containing 0.1% Hydroxypropyl methylcellulose) and the final pH was adjusted to 7.4 using 1 N hydrochloric acid. In the case of hesperetin, an additional solution formulation, which included 0.05% BAK, was also tested.
Rabbits were anesthetized as described in the microdialysis probe implantation section and were maintained under anesthesia throughout the duration of the experiment. Following anesthesia, hesperidin or hesperetin solution (100 μL of 1% w/v) was administered topically into the cul-de-sac of 1 eye of the rabbit and held for 30 s. Rabbits were sacrificed at the 1 and 3 h time points postdosing, eyes were collected and ocular tissues (cornea, iris-ciliary body, lens, retina-choroid, sclera, aqueous humor, and vitreous humor) were isolated, weighed, and stored at −80°C until further analysis. Four animals were used for each compound (n=4).
Analytical methods
Liquid chromatography/mass spectrometry (LC-ESI-TOF)
The liquid chromatography system used was an Agilent Series 1100 comprising of the following modular components: quaternary pump, a vacuum solvent microdegasser, an autosampler with a 100-well tray. The mass spectrometric analysis was performed by using the LC-ESI-TOF (Model No. G1969A; Agilent Technologies, Palo Alto, CA) equipped with an Electrospray Ionization source. All acquisitions were performed under a positive ionization mode with a capillary voltage of 3000 V. Nitrogen was used as the nebulizer gas (35 psig) as well as the drying gas at 11 L/min at a temperature of 350°C. The voltage of Photomultiplier, fragmentor, and skimmer was set at 850, 100, and 60 V, respectively. Full scan mass spectra were acquired from m/z 200–1000. Data acquisition and processing was done using the Analyst™ QS software (Agilent Technologies).
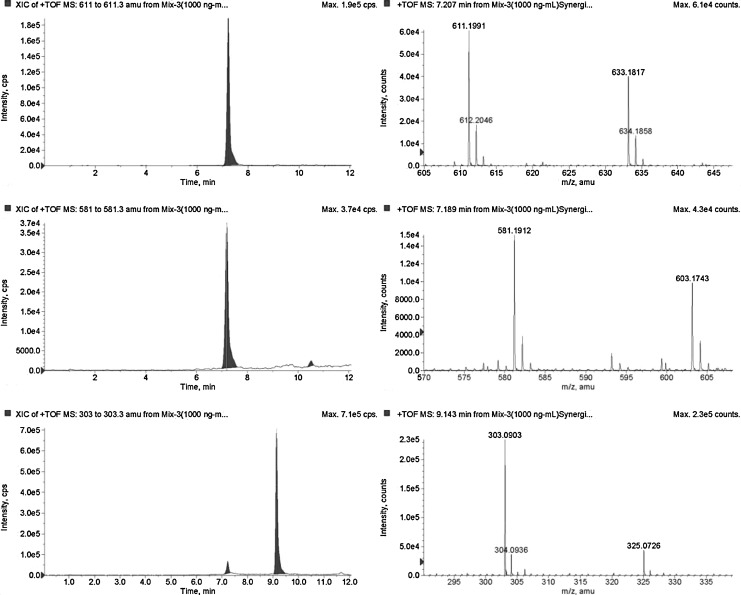
Chromatographic separation was achieved on a synergi Hydro-RP; 100×2.0 mm I.D.; 4 μm particle size (Phenomenex, Torrance, CA). The column was equipped with a guard column (Supelco, Bellefonte, PA). The mobile phase consisted of ammonium formate (20 mM) with 0.1% formic acid (A), and acetonitrile with 0.1% formic acid (B) at a flow rate of 0.3 mL/min, with the following gradient elution: 0 min, 95% A/5% B to 100% B over 10 min. Each run was followed by a 5-min wash with 100% B and an equilibration period of 11 min with 95% A/5% B. The total run time for analysis was 10 min. Sample (10 μL) was injected and peaks were assigned with respect to the mass of the compounds and comparison of the retention times. This method involved the use of the [M+H]+ ions in the positive ion mode with extractive ion monitoring. In the positive ion mode, the protonated species [M+H]+ at m/z 611.1991 for hesperidin, 303.0869 for hesperetin, and 581.19 for naringin (internal standard) were observed (Fig. 1).
FIG. 1.
Selected ion chromatogram and mass spectra of standard compounds (1 μg/mL).
Plasma sample analysis
Hesperidin and hesperetin were previously reported to undergo phase-II metabolism to form hesperetin-glucuronide.27,28 However, hesperetin-glucuronide standard is commercially not available; therefore, the plasma samples were run in duplicates with and without the addition of 40 μL of 15,000 U/mL of beta-glucuronidase (HP-2 type solution from Helix pomatia) in the 0.1 mM acetate buffer (pH 5.0). Following the addition of the beta-glucuronidase, samples were incubated at 37°C for 2 h, to convert the conjugated metabolite to parent hesperetin. Later, 30 μL of internal standard (naringin 10 μg/mL) was added to both samples and mixed thoroughly. To this mixture, 330 μL of acetonitrile:methanol (50:50) was added to precipitate the proteins and centrifuged (accuSpin micro 17R; Thermo Fisher Scientific) at 12,000 rpm for 20 min at 4°C. Supernatant was collected and analyzed for hesperidin/hesperetin content using liquid chromatography/mass spectrometry (LC-MS). Calibration standards (1–1000 ng/mL) were prepared by spiking known amount of hesperidin/hesperetin, with internal standard, into blank rabbit plasma.
Ocular tissue sample analysis
To a weighed quantity of the respective ocular tissue (cornea, iris-ciliary, lens, sclera, and choroid-retina), 60 μL of the internal standard (naringin 10 μg/mL) was added and mixed thoroughly. To this, 3 mL of the acetonitrile:methanol (50:50) mixture was added and homogenized using a Tissuemiser (Thermo Fisher Scientific). The homogenate was centrifuged (accuSpin micro 17R; Thermo Fisher Scientific) at 12,000 rpm for 20 min at 4°C. Supernatant was collected and analyzed for content using LC-MS. Calibration standards (1–1000 ng/g) were prepared by spiking a known amount of hesperidin/hesperetin, with internal standard, into respective blank rabbit ocular tissue homogenate. In sample analysis, if the concentrations are above the calibration range, dilutions were done to analyze the sample concentration.
Microdialysis sample analysis
Hesperidin and hesperetin content in the microdialysis samples obtained from the intravenous administration study was analyzed using an HPLC method. An HPLC system equipped with Waters 600 pump controller, 2470 dual wavelength UV detector, refrigerated 717 plus auto-sampler, and Agilent 3394B integrator was used. The detector was operated at 284 nm. The mobile phase consisted of 20 mM monobasic potassium phosphate (pH adjusted to 2.5 with ortho-phosphoric acid) and acetonitrile in a ratio of 75:25 and 50:50 for hesperidin and hesperetin, respectively. The flow rate was maintained at 1 mL/min. A Phenomenex Luna, 250×4.6 mm, 5 μ, C18(2) column was used. Samples were injected (30 μL) on to the column as such. Calibration standards were in the range of 20–15,000 ng/mL for hesperetin and 50–15,000 ng/mL for hesperidin.
Data analysis
Pharmacokinetic parameters were determined using WinNonlin® (version 5.2; Pharsight, Mountain View, CA). Terminal slopes of the vitreous concentration–time profile were estimated by log-linear regression, and the apparent elimination rate constant (k) was derived from the slope. Elimination half-life (t1/2) was calculated from the equation:
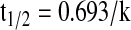 |
(2) |
The area under the vitreal concentration–time curve from time 0 to time t was calculated by the linear trapezoidal method and extrapolated to infinity according to equation (3):
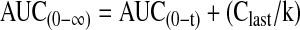 |
(3) |
Results
Intravenous administration
Hesperidin and hesperetin are reported to form hesperetin-glucuronide in vivo and an objective of this project was to estimate the concentration of this metabolite in the plasma samples. Since hesperetin-glucuronide standard is commercially not available, experimental conditions for the conversion of the hesperetin-glucuronide in the plasma samples to hesperetin with the aid of the deconjugating enzyme, beta-glucuronidase, were optimized. For this, hesperetin-glucuronide was initially formed using rat liver microsomes. Formation of the glucuronides and their reconversion to hesperetin was confirmed analytically. Similar procedures have been reported for the estimation of hesperetin-glucuronide content in the plasma samples in the literature.29,30
Systemic pharmacokinetics of hesperidin
Hesperidin was administered intravenously at a dose of 20 mg/kg BW to anesthetized rabbits. Plasma samples without beta-glucuronidase treatment did not show any hesperetin. Only hesperidin levels were observed in these samples. However, plasma samples treated with the beta-glucuronidase enzyme exhibited levels of hesperetin along with the parent hesperidin. This suggests that, following systemic administration, hesperidin exists in the plasma as an unchanged parent and as its phase-II metabolite, hesperetin-glucuronide.
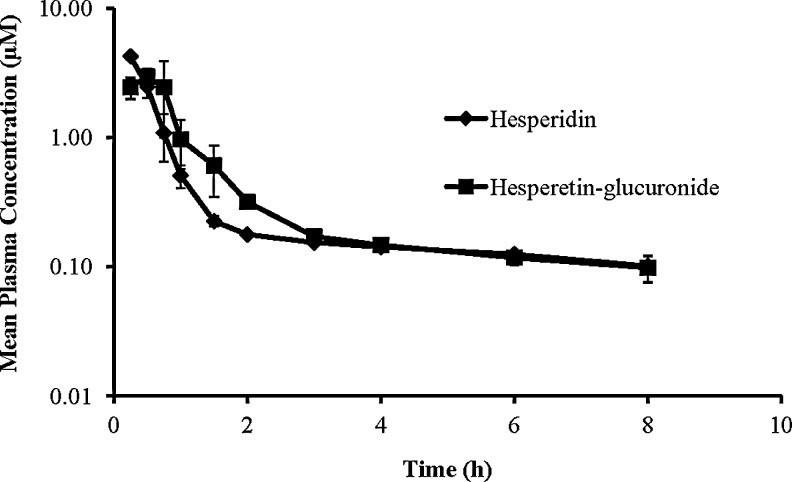
Observed plasma concentration–time profiles of hesperidin and the metabolite hesperetin-glucuronide is presented in Fig. 2. It should be noted that the concentrations (μM) of hesperetin in the enzyme-treated plasma samples actually represents the concentrations of the metabolite, hesperetin-glucuronide. Systemic pharmacokinetic parameters of hesperidin and regenerated hesperetin were calculated using noncompartmental analysis and have been presented in Table 1. It is apparent from the results obtained that hesperidin's plasma half-life (6.74 h) is relatively short and that the plasma levels decrease rapidly. The metabolite, hesperetin-glucuronide, also demonstrated a very short half-life, around 5.16 h. Systemic clearance of hesperidin was found to be 6.69±0.58 L/h/kg.
FIG. 2.
Mean plasma concentration–time profile of hesperidin and hesperetin-glucuronide (estimated as hesperetin after the treatment of the plasma samples with beta-glucuronidase to convert hesperetin-glucuronide to hesperetin), generated in vivo, in the rabbits following intravenous administration of hesperidin at 20 mg/kg body weight (n=4).
Table 1.
Calculated Pharmacokinetic Parameters from the Plasma Concentration–Time Profile of Hesperidin and Hesperetin-Glucuronide (Estimated as Hesperetin After the Treatment of the Plasma Samples with Beta-Glucuronidase to Convert Hesperetin-Glucuronide to Hesperetin), Following Intravenous Administration of Hesperidin at 20 mg/kg
| PK parameter | Hesperidin | Hesperetin-glucuronide |
|---|---|---|
| Half-life (h) | 6.74±0.79 | 5.16±1.56 |
| Co (μM) | 7.47±1.64 | |
| AUC last (h μM) | 4.12±0.26 | 3.99±0.43 |
| AUC inf. (h μM) | 4.92±0.38 | 4.57±0.44 |
| Vz (L/kg) | 64.58±3.70 | |
| Cl (L/h/kg) | 6.69±0.58 | |
| MRT (h) | 4.22±0.67 | 3.63±1.27 |
Noncompartmental analysis was followed for calculating PK parameters.
Values represent mean±SD (n=4).
PK, pharmacokinetic; AUC, area under the curve; Vz, volume of distribution; Cl, clearance; MRT, mean residence time; SD, standard deviation.
Systemic pharmacokinetics of hesperetin
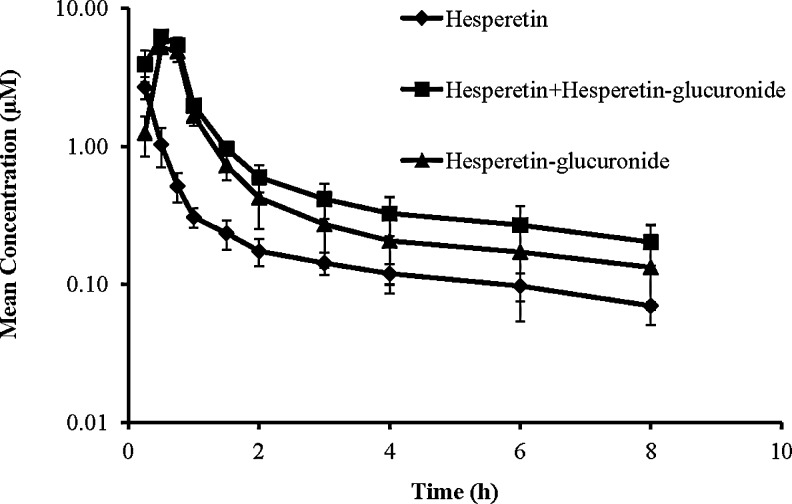
Hesperetin was administered intravenously at a dose of 20 mg/kg to the anesthetized rabbits. Blood samples were collected to estimate the systemic availability of hesperetin and its metabolite, hesperetin-glucuronide. The first set of plasma samples that were analyzed directly, without treatment with the beta-glucuronidase enzyme, showed hesperetin concentrations. The hesperetin concentrations in the second set, where the plasma samples were treated with the beta-glucuronidase enzyme, were significantly higher compared to first sample set. This suggests that hesperetin undergoes metabolism in vivo and forms hesperetin-glucuronide. Thus, treatment of the plasma samples with the beta-glucuronidase enzyme resulted in higher levels of hesperetin. To estimate the concentration of the metabolite, hesperetin-glucuronide, hesperetin concentrations (μM) from the first sample set were subtracted from that obtained in the second set. The plasma concentration–time profile of hesperetin and its metabolite, hesperetin-glucuronide has been presented in Fig. 3. Pharmacokinetic parameters were calculated using noncompartmental analysis and have been presented in Table 2. The biological half-life (4.6 h) and MRT (3.17 h) of hesperetin-glucuronide were consistent with that obtained with hesperidin administration (5.16 and 3.63 h, respectively).
FIG. 3.
Mean plasma concentration–time profile of hesperetin (from direct analysis) and hesperetin-glucuronide (estimated as hesperetin after the treatment of the plasma samples with beta-glucuronidase to convert hesperetin-glucuronide to hesperetin) following intravenous administration of hesperetin at a dose of 20 mg/kg in rabbits (n=4).
Table 2.
Calculated Pharmacokinetic Parameters from Plasma Concentration–Time Profile of Hesperetin (From Direct Analysis) and Hesperetin-Glucuronide (Estimated as Hesperetin After the Treatment of the Plasma Samples with Beta-Glucuronidase to Convert Hesperetin-Glucuronide to Hesperetin), Following Intravenous Administration of Hesperidin at 20 mg/kg
| PK parameter | Hesperetin | Hesperetin-glucuronide |
|---|---|---|
| Half-life (h) | 5.32±0.62 | 4.61±0.62 |
| Co (μM) | 7.35±1.71 | |
| AUC last (h μM) | 3.01±0.29 | 5.45±1.38 |
| AUC inf. (h μM) | 3.49±0.37 | 6.14±1.69 |
| Vz (L/kg) | 145.60±8.84 | |
| Cl (L/h/kg) | 19.09±1.99 | |
| MRT (h) | 3.60±0.21 | 3.17±0.39 |
Noncompartmental analysis was followed for calculating PK parameters.
Values represent mean±SD (n=4).
Vitreal bioavailability
Vitreal microdialysis was carried out to estimate the amount of hesperidin or hesperetin reaching the vitreous humor following intravenous administration. However, these samples did not exhibit any detectable levels of the compounds, indicating that their vitreal bioavailability through the systemic route is negligible.
Topical administration
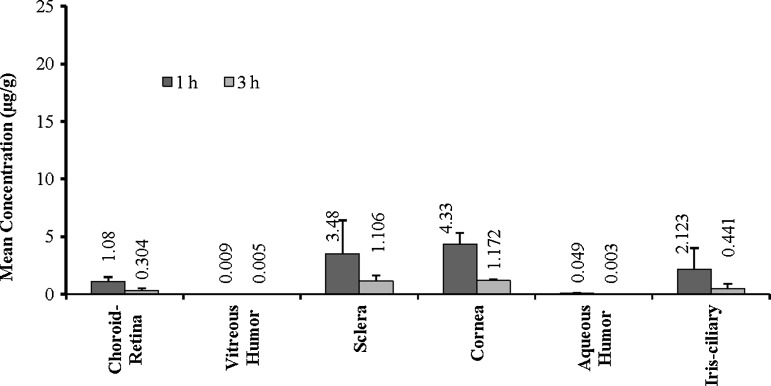
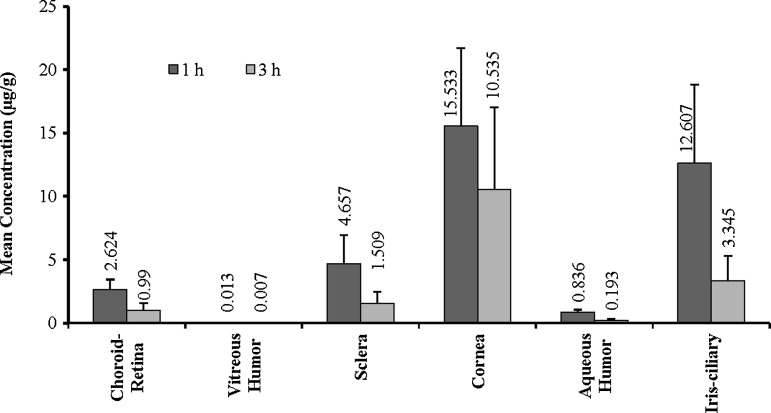
Experiments were carried out in anesthetized rabbits and the eyes were collected at 1 and 3 h postadministration. The ocular tissues were isolated and analyzed for hesperidin or hesperetin. Concentrations of hesperidin and hesperetin observed in the ocular tissues following topical administration are presented in Figs. 4 and 5, respectively. Hesperidin and hesperetin levels were observed in all the ocular tissues tested. However, very low concentrations were detected in the retina-choroid and vitreous humor, the target sites.
FIG. 4.
Levels of hesperidin observed in the rabbit ocular tissues at 1 and 3 h following topical administration of hesperidin [100 μL of 1% w/v solution containing HP-β-CD (10% w/v), HPMC (0.1% w/v), 1 N NaOH (0.05% v/v), and 1 N HCl (0.05% w/v), IPBS pH 7.4 (q.s.)]. Values represent mean±SD (n=4). HP-β-CD, hydroxylpropyl beta-cyclodextrin; HPMC, hydroxypropyl methylcellulose; IPBS, isotonic phosphate-buffered saline; SD, standard deviation.
FIG. 5.
Levels of hesperetin observed in the rabbit ocular tissues at 1 and 3 h following topical administration of hesperetin [100 μL of 1% w/v solution containing HP-β-CD (10% w/v), HPMC (0.1% w/v), 1 N NaOH (0.05% v/v), and 1 N HCl (0.05% w/v), IPBS pH 7.4]. Values represent mean±SD (n=4).
Effect of the solubilizers (HP-β-CD and RM-β-CD) and the penetration enhancer/preservative, BAK, on hesperetins' ocular penetration following topical administration was also studied. The results from these experiments have been presented in Tables 3 and 4. Hesperetin concentrations achieved in the retina with HP-β-CD (2.6 μg/g of tissue) was not significantly different from that observed with RM-β-CD (2.5 μg/g of tissue)-based formulations. However, higher hesperetin levels were obtained in the vitreous humor with RM-β-CD compared to HP-β-CD. Inclusion of BAK, in HP-β-CD and RM-β-CD formulations, improved the penetration of hesperetin into the ocular tissues. The effect of BAK was significantly more pronounced in the presence of HP-β-CD compared to RM-β-CD.
Table 3.
Hesperetin Concentrations Observed in the Rabbit Ocular Tissues at 1 h Following Topical Administration of 100 μL of Hesperetin Solution (1% w/v) Containing HP-β-CD (10% w/v), HPMC (0.1% w/v), 1 N NaOH (0.05% v/v), 1 N HCl (0.05% w/v), Benzalkonium Chloride (0 or 0.05% w/v) IPBS pH 7.4
| HP-β-CD | HP-β-CD:BAK | Fold difference | |
|---|---|---|---|
| Aqueous humor (μg/mL) | 0.84±0.21 | 1.06±0.21 | 1.26 |
| Vitreous humor (μg/mL) | 0.013±0.01 | 0.15±0.03 | 9.62 |
| Cornea (μg/g of tissue) | 15.53±8.15 | 27.05±6.53 | 1.74 |
| Iris-ciliary (μg/g of tissue) | 12.61±8.21 | 27.10±13.61 | 2.15 |
| Retina (μg/g of tissue) | 2.62±0.81 | 18.39±4.86 | 7.01 |
| Sclera (μg/g of tissue) | 4.66±2.29 | 10.25±3.78 | 2.20 |
Values represent mean±SD (n=4).
HP-β-CD, hydroxylpropyl beta-cyclodextrin; Hydroxypropyl methylcellulose; IPBS, isotonic phosphate-buffered saline; BAK, benzalkonium chloride.
Table 4.
Hesperetin Concentrations Observed in the Rabbit Ocular Tissues at 1 h Following Topical Administration of 100 μL of Hesperetin Solution (1% w/v) Containing RM-β-CD (10% w/v), HPMC (0.1% w/v), 1 N NaOH (0.05% v/v), 1 N HCl (0.05% w/v), Benzalkonium Chloride (0 or 0.05% w/v) IPBS pH 7.4
| RM-β-CD | RM-β-CD:BAK | Fold difference | |
|---|---|---|---|
| Aqueous humor (μg/mL) | 0.06±0.03 | 0.23±0.14 | 3.59 |
| Vitreous humor (μg/mL) | 0.05±0.02 | 0.14±0.02 | 2.64 |
| Cornea (μg/g of tissue) | 5.13±1.86 | 17.62±7.07 | 3.44 |
| Iris-ciliary (μg/g of tissue) | 3.46±2.32 | 6.40±2.16 | 1.85 |
| Retina (μg/g of tissue) | 2.50±1.04 | 5.62±0.15 | 2.25 |
| Sclera (μg/g of tissue) | 1.32±0.56 | 5.42±1.17 | 4.10 |
Values represent mean±SD (n=4).
RM-β-CD, randomly methylated beta-cyclodextrin.
Discussion
Both hesperidin and hesperetin are established antioxidant and anti-inflammatory agents and have also been reported to increase retinal blood flow and decrease microvasculature permeability. Considering their favorable pharmacological properties, these are potential candidates for the prevention or treatment of diseases of the posterior segment of the eye like DR and DME.12 However, for hesperidin and hesperetin to be effective in DR they must penetrate efficiently into the ocular tissues. Drug delivery to the deeper ocular tissues poses significant challenges because of the presence of various physiological barriers protecting the eye. The BRB, comprised of the endothelial cells of the retinal capillaries and the epithelial cells of the RPE, acts as a significant barrier to the passage of molecules from the systemic circulation into the retinal tissue. The barrier characteristic is dependent on the physiochemical properties of the diffusing molecule and its interaction with different influx and efflux transporters expressed on the BRB.21 In this current investigation, delivery of hesperidin and hesperetin to the posterior segment of the eye was investigated through the systemic and topical administration routes in anesthetized rabbits. Effective concentration/dose of hesperidin and hesperetin for various pharmacological activities has been reported by several researchers. These compounds exhibited dose-dependent activities in different in vitro (1–100 μM)9,10,31–36 and in vivo studies (10–80 mg/kg BW). Importantly, several studies indicate that hesperetin is more active than hesperidin.37–41
In our studies, male NZW rabbits were used. Rabbits have been widely used to study ocular pharmacokinetics of drugs.42–46 Although some differences between the rabbit and human eye do exist, for example, volume of vitreous humor and minimal neural retinal vasculature, pharmacokinetic behavior of drugs in this model extrapolates well with that observed in humans. However, the structural differences need to be kept in mind while evaluating the data.
Vitreal microdialysis technique was used to sample the vitreous humor following intravenous administration of the investigational compounds. Blood samples were also collected to measure the systemic exposure of hesperidin/hesperetin and their metabolite. Hesperidin or hesperetin was not detected in the microdialysis samples collected from the vitreous humor, indicating limited or negligible ocular bioavailability of these molecules. This could be attributed to several factors. First, as indicated earlier, ocular bioavailability from the systemic circulation is limited by the presence of the BRB. Second, hesperidin was seen to undergo rapid bioreversion in the plasma to its hydrophilic metabolite, hesperetin-glucuronide. In fact, hesperidin exists in the plasma primarily as the glucuronide metabolite (Fig. 2). The BRB structure is such that passage of hydrophilic molecules is severely limited by the cellular tight junctions present on the BRB components.47,48 Thus, in view of the hydrophilic characteristics of hesperetin-glucuronide, diffusion of this metabolite across the BRB would be severely restricted. With respect to hesperetin, better permeation across the BRB could be expected, since hesperetin is more lipophilic (LogP 2.9) in nature compared to hesperidin (LogP 1.78).24,25 This is also suggested by our earlier reports demonstrating hesperetins' greater in vitro permeability (2.37×10−6 cm/s) across the sclera-choroid-RPE compared to that of hesperidins' (0.82×10−6 cm/s).24,25 Additionally, in our previous study with quinidine, which has a similar LogP (2.6) and molecular weight (324.417 g/mol), significant levels were obtained in the vitreous humor through the systemic route of administration.49 However, in this study, it is apparent from the plasma hesperetin concentration–time profile that high plasma hesperetin concentrations are not maintained long enough to drive permeation across the BRB into the vitreous humor. Again, this is probably because of the rapid in vivo metabolism of hesperetin into its hydrophilic glucuronide metabolite (Fig. 3). Therefore, permeation of these hydrophilic molecules across the BRB is a limiting factor.
In addition to the above-discussed factors, the extrapolated mean plasma concentration of hesperidin and hesperetin at time zero, the maximum concentration following intravenous administration, was around 7.47 and 7.35 μM (Tables 1 and 2), respectively. Considering their previously reported in vitro permeability values (0.82×10−6 and 2.37×10−6 cm/s for hesperidin and hesperetin, respectively), across the sclera-choroid-RPE,24 which would be an underestimation of the actual in vivo barrier characteristics, very little, if any, levels would be expected in the vitreous humor. Additionally, plasma levels of both parent molecules and their metabolites fall rapidly (shorter biological half-lives; 4.6–6.7 h).
Overall, it is thus not surprising that hesperetin concentrations were not detectable in the vitreous humor. Rapid decline in plasma concentrations and conversion into hesperetin-glucuronide in the systemic circulation are the primary factors limiting ocular bioavailability. The microdialysis technique may be a limitation with respect to the minimum concentrations detectable in the vitreous humor, in this particular study. However, the results clearly suggest that oral administration is not a feasible approach for delivering hesperidin/hesperetin into the retinal tissues, the target sites, because first-pass metabolism would significantly decrease the plasma hesperetin concentrations attained. The pharmacological activities of the metabolite, hesperetin glucuronide, have not been thoroughly investigated and, thus, there utility is unclear.
Penetration of hesperidin/hesperetin into the ocular tissues following topical administration was estimated next, in the anesthetized rabbits. To avoid the limitations of the microdialysis technique, animals were sacrificed at each time point and the vitreal samples were collected for analysis.
Results from the topical administration studies indicate that significant concentrations of the parent compounds were generated in the cornea, sclera, iris-ciliary body, and aqueous humor for both compounds (Figs. 4 and 5). This is consistent with the corneal penetration pathway. With respect to the posterior segment of the eye (choroid-retina and vitreous humor), higher hesperidin/hesperetin concentrations were obtained in the choroid-retina tissue compared to the vitreous humor. From these results, and from our earlier findings with respect to the in vitro ocular permeability coefficients,24,25 it is apparent that the sclera is not a significant diffusional barrier and that hesperidin/hesperetin are able to reach the choroid-retina. However, they are not able to efficiently permeate across the BRB into the inner retinal tissues. Moreover, from a therapeutic standpoint, concentration of these compounds declined rapidly from almost all ocular tissues. For example, in the choroid-retina there was more than 50% decrease in concentration within 2 h.
Comparing the levels of hesperidin and hesperetin obtained in the ocular tissues tested, it was evident that hesperetin demonstrated greater in vivo permeability (Figs. 4 and 5). This could be because of the contrasting physicochemical properties of the administered compounds. It is well recognized that transcorneal and transchoroid-retina transport favors relatively lipophilic molecules.19 In this case, hesperetin is more lipophilic compared to hesperidin. Additionally, the molecular size of hesperetin is much smaller compared to hesperidin. These factors explain the better in vivo permeability values of hesperetin.
However, the vitreal levels achieved, even with hesperetin and the topical administration route, is subtherapeutic.9,31 To improve penetration into the deeper ocular tissues, effect of solubilizers (CDs) and BAK (used as a penetration enhancer) in the topical formulation was studied at the 1 h time point postdosing (Tables 3 and 4). CDs are a group of cyclic oligosaccharides that have been shown to improve solubility of a multitude of poorly soluble compounds, through the formation of inclusion complexes. Chemically modified CDs, like HP-β-CD and RM-β-CD, have been extensively studied for drug delivery purposes. Several reviews have been published on the applications of CDs in ocular drug delivery.26,50 In this study, hesperetin concentrations achieved in the vitreous humor and the choroid-retina, from HP-β-CD- and RM-β-CD-based ophthalmic solutions, were similar. BAK, a preservative, has often been investigated as a penetration enhancer in ocular preparations.51–54 In the present study, inclusion of BAK in the HP-β-CD- and RM-β-CD-based formulations improved the penetration of hesperetin into the ocular tissues. Interestingly, BAK was observed to be more effective when combined with HP-β-CD in terms of fold-increase in hesperetin concentrations produced in the retina-choroid and the vitreous humor.
In view of the results from the topical administration study, it is evident that the intravenous administration would have generated even lower ocular tissue concentrations, if any, and thus could not be detected in the vitreous humor by the microdialysis technique. A separate study evaluating the ocular tissue concentrations generated at 30 min postintravenous administration was debated, but was thought to be pointless, in view of the fact that when administered orally very little, if any, of the parent drug would be present in the plasma.
Conclusions
In conclusion, ocular bioavailability of hesperidin or hesperetin is limited or negligible through the intravenous route of administration. Based on the intravenous administration study results, it can be speculated or hypothesized that the ability of these compounds to reach the deeper ocular tissues following oral administration, considering the reported high intestinal and hepatic first-pass metabolism of these molecules, will be very poor. On the other hand, following topical administration, significant concentrations of hesperidin and hesperetin were observed in the ocular tissues. However, very low levels were obtained in the vitreous humor and the compounds were rapidly cleared from the ocular tissues. Inclusion of the penetration enhancer, benzalkonium chloride, improved the hesperetin levels in the back-of-the eye tissues. Thus, further studies attempting to improve penetration into the back-of-the eye, through the topical administration route, with the aid of controlled release formulation approaches is warranted.
Acknowledgments
This project was supported in part by Grant Numbers, P20RR021929 from the National Center for Research Resources (NIH/NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Eye Institute, National Institutes of Health. We appreciate the support and technical help extended by the animal facility staff, in particular Dr. Harry Fyke (University attending veterinarian) and Penni Bolton (animal facility supervisor).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ciulla T.A. Amador A.G. Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26:2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 2.Chiou G.C. Xu X.R. Effects of some natural flavonoids on retinal function recovery after ischemic insult in the rat. J. Ocul. Pharmacol. Ther. 2004;20:107–113. doi: 10.1089/108076804773710777. [DOI] [PubMed] [Google Scholar]
- 3.Calderone V. Chericoni S. Martinelli C., et al. Vasorelaxing effects of flavonoids: investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch. Pharmacol. 2004;370:290–298. doi: 10.1007/s00210-004-0964-z. [DOI] [PubMed] [Google Scholar]
- 4.Manthey J.A. Biological properties of flavonoids pertaining to inflammation. Microcirculation. 2000;7:S29–S34. [PubMed] [Google Scholar]
- 5.Bouskela E. Cyrino F.Z. Marcelon G. Inhibitory effect of the Ruscus extract and of the flavonoid hesperidine methylchalcone on increased microvascular permeability induced by various agents in the hamster cheek pouch. J. Cardiovasc. Pharmacol. 1993;22:225–230. doi: 10.1097/00005344-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Korthuis R.J. Gute D.C. Adhesion molecule expression in postischemic microvascular dysfunction: activity of a micronized purified flavonoid fraction. J. Vasc. Res. 1999;36(Suppl 1):15–23. doi: 10.1159/000054070. [DOI] [PubMed] [Google Scholar]
- 7.McGregor L. Bellangeon M. Chignier E. Lerond L. Rousselle C. McGregor J.L. Effect of a micronized purified flavonoid fraction on in vivo platelet functions in the rat. Thromb. Res. 1999;94:235–240. doi: 10.1016/s0049-3848(98)00216-3. [DOI] [PubMed] [Google Scholar]
- 8.Xiaoting L. Xiangyun Z. Shumei L. Minghua D. Liang X. Effect of hesperidin on expression of inducible nitric oxide synthase in cultured rabbit retinal pigment epithelial cells. Adv. Exp. Med. Biol. 2010;664:193–201. doi: 10.1007/978-1-4419-1399-9_22. [DOI] [PubMed] [Google Scholar]
- 9.Choi E.J. Kim G.D. Chee K.M. Kim G.H. Effects of hesperetin on vessel structure formation in mouse embryonic stem (mES) cells. Nutrition. 2006;22:947–951. doi: 10.1016/j.nut.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Choi I.Y. Kim S.J. Jeong H.J., et al. Hesperidin inhibits expression of hypoxia inducible factor-1 alpha and inflammatory cytokine production from mast cells. Mol. Cell. Biochem. 2007;305:153–161. doi: 10.1007/s11010-007-9539-x. [DOI] [PubMed] [Google Scholar]
- 11.Hirata A. Murakami Y. Shoji M. Kadoma Y. Fujisawa S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005;25:3367–3374. [PubMed] [Google Scholar]
- 12.Majumdar S. Srirangam R. Potential of the bioflavonoids in the prevention/treatment of ocular disorders. J. Pharm. Pharmacol. 2010;62:951–965. doi: 10.1211/jpp.62.08.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ameer B. Weintraub R.A. Johnson J.V. Yost R.A. Rouseff R.L. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin. Pharmacol. Ther. 1996;60:34–40. doi: 10.1016/S0009-9236(96)90164-2. [DOI] [PubMed] [Google Scholar]
- 14.Manach C. Morand C. Gil-Izquierdo A. Bouteloup-Demange C. Remesy C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003;57:235–242. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 15.Garg A. Garg S. Zaneveld L.J. Singla A.K. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. 2001;15:655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 16.Brand W. Boersma M.G. Bik H., et al. Phase II metabolism of hesperetin by individual UDP-glucuronosyltransferases and sulfotransferases and rat and human tissue samples. Drug Metab. Dispos. 2010;38:617–625. doi: 10.1124/dmd.109.031047. [DOI] [PubMed] [Google Scholar]
- 17.Breinholt V.M. Offord E.A. Brouwer C. Nielsen S.E. Brosen K. Friedberg T. In vitro investigation of cytochrome P450-mediated metabolism of dietary flavonoids. Food Chem. Toxicol. 2002;40:609–616. doi: 10.1016/s0278-6915(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 18.Gil-Izquierdo A. Gil M.I. Tomas-Barberan F.A. Ferreres F. Influence of industrial processing on orange juice flavanone solubility and transformation to chalcones under gastrointestinal conditions. J. Agric. Food. Chem. 2003;51:3024–3028. doi: 10.1021/jf020986r. [DOI] [PubMed] [Google Scholar]
- 19.Ghate D. Edelhauser H.F. Ocular drug delivery. Expert Opin. Drug Deliv. 2006;3:275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 20.Maurice D.M. Drug delivery to the posterior segment from drops. Surv. Ophthalmol. 2002;47(Suppl 1):S41–S52. doi: 10.1016/s0039-6257(02)00326-0. [DOI] [PubMed] [Google Scholar]
- 21.Duvvuri S. Majumdar S. Mitra A.K. Drug delivery to the retina: challenges and opportunities. Expert Opin. Biol. Ther. 2003;3:45–56. doi: 10.1517/14712598.3.1.45. [DOI] [PubMed] [Google Scholar]
- 22.Majumdar S. Hippalgaonkar K. Srirangam R. Vitreal kinetics of quinidine in rabbits in the presence of topically coadministered P-glycoprotein substrates/modulators. Drug Metab. Dispos. 2009;37:1718–1725. doi: 10.1124/dmd.108.026450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srirangam R. Hippalgaonkar K. Majumdar S. Intravitreal kinetics of hesperidin, hesperetin, and hesperidin G: effect of dose and physicochemical properties. J. Pharm. Sci. 2012;101:1631–1638. doi: 10.1002/jps.23047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majumdar S. Srirangam R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: a natural bioflavonoid. Pharm. Res. 2009;26:1217–1225. doi: 10.1007/s11095-008-9729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srirangam R. Majumdar S. Passive asymmetric transport of hesperetin across isolated rabbit cornea. Int. J. Pharm. 2010;394:60–67. doi: 10.1016/j.ijpharm.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loftssona T. Jarvinen T. Cyclodextrins in ophthalmic drug delivery. Adv. Drug Deliv. Rev. 1999;36:59–79. doi: 10.1016/s0169-409x(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Kanaze F.I. Bounartzi M.I. Georgarakis M. Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007;61:472–477. doi: 10.1038/sj.ejcn.1602543. [DOI] [PubMed] [Google Scholar]
- 28.Erlund I. Meririnne E. Alfthan G. Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001;131:235–241. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto H. Ikoma Y. Sugiura M. Yano M. Hasegawa Y. Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J. Agric. Food Chem. 2004;52:6653–6659. doi: 10.1021/jf0491411. [DOI] [PubMed] [Google Scholar]
- 30.Gardana C. Guarnieri S. Riso P. Simonetti P. Porrini M. Flavanone plasma pharmacokinetics from blood orange juice in human subjects. Br. J. Nutr. 2007;98:165–172. doi: 10.1017/S0007114507699358. [DOI] [PubMed] [Google Scholar]
- 31.Choi E.M. Lee Y.S. Effects of hesperetin on the production of inflammatory mediators in IL-1beta treated human synovial cells. Cell Immunol. 2010;264:1–3. doi: 10.1016/j.cellimm.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Kuntz S. Wenzel U. Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999;38:133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 33.Kalpana K.B. Devipriya N. Srinivasan M. Menon V.P. Investigation of the radioprotective efficacy of hesperidin against gamma-radiation induced cellular damage in cultured human peripheral blood lymphocytes. Mutat. Res. 2009;676:54–61. doi: 10.1016/j.mrgentox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Kalpana K.B. Srinivasan M. Menon V.P. Evaluation of antioxidant activity of hesperidin and its protective effect on H2O2 induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol. Cell Biochem. 2009;323:21–29. doi: 10.1007/s11010-008-9960-9. [DOI] [PubMed] [Google Scholar]
- 35.Chen M.C. Ye Y.Y. Ji G. Liu J.W. Hesperidin upregulates heme oxygenase-1 to attenuate hydrogen peroxide-induced cell damage in hepatic L02 cells. J. Agric. Food Chem. 2010;58:3330–3335. doi: 10.1021/jf904549s. [DOI] [PubMed] [Google Scholar]
- 36.Cos P. Calomme M. Sindambiwe J.B., et al. Cytotoxicity and lipid peroxidation-inhibiting activity of flavonoids. Planta Med. 2001;67:515–519. doi: 10.1055/s-2001-16472. [DOI] [PubMed] [Google Scholar]
- 37.Akiyama S. Katsumata S. Suzuki K. Ishimi Y. Wu J. Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010;46:87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoclet J.C. Chataigneau T. Ndiaye M., et al. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Balakrishnan A. Menon V.P. Antioxidant properties of hesperidin in nicotine-induced lung toxicity. Fundam. Clin. Pharmacol. 2007;21:535–546. doi: 10.1111/j.1472-8206.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 40.Gandhi C. Upaganalawar A. Balaraman R. Protection against in vivo focal myocardial ischemia/reperfusion injury-induced arrhythmias and apoptosis by hesperidin. Free Radic. Res. 2009;43:817–827. doi: 10.1080/10715760903071656. [DOI] [PubMed] [Google Scholar]
- 41.Choi E.J. Ahn W.S. Neuroprotective effects of chronic hesperetin administration in mice. Arch. Pharm. Res. 2008;31:1457–1462. doi: 10.1007/s12272-001-2130-1. [DOI] [PubMed] [Google Scholar]
- 42.Kim H. Csaky K.G. Gravlin L., et al. Safety and pharmacokinetics of a preservative-free triamcinolone acetonide formulation for intravitreal administration. Retina. 2006;26:523–530. doi: 10.1097/00006982-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Burgalassi S. Cappello B. Chetoni P. Iervolino M. Monti D. Saettone M.F. Rufloxacin eyedrops: effect of different formulations on ocular pharmacokinetics in rabbits. Eur. J. Ophthalmol. 2006;16:311–317. doi: 10.1177/112067210601600219. [DOI] [PubMed] [Google Scholar]
- 44.Gomes dos Santos A.L. Bochot A. Doyle A., et al. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. J. Controlled Release. 2006;112:369–381. doi: 10.1016/j.jconrel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Iyer M.N. He F. Wensel T.G. Mieler W.F. Benz M.S. Holz E.R. Clearance of intravitreal moxifloxacin. Invest. Ophthalmol. Vis. Sci. 2006;47:317–319. doi: 10.1167/iovs.05-1124. [DOI] [PubMed] [Google Scholar]
- 46.Robinson M.R. Lee S.S. Kim H., et al. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp. Eye Res. 2006;82:479–487. doi: 10.1016/j.exer.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Kuppermann B.D. Loewenstein A. Drug delivery to the posterior segment of the eye. Dev. Ophthalmol. 2010;47:59–72. doi: 10.1159/000320074. [DOI] [PubMed] [Google Scholar]
- 48.Loftsson T. Sigurdsson H.H. Konradsdottir F. Gisladottir S. Jansook P. Stefansson E. Topical drug delivery to the posterior segment of the eye: anatomical and physiological considerations. Pharmazie. 2008;63:171–179. [PubMed] [Google Scholar]
- 49.Hippalgaonkar K. Srirangam R. Avula B. Khan I.A. Majumdar S. Interaction between topically and systemically coadministered P-glycoprotein substrates/inhibitors: effect on vitreal kinetics. Drug Metab. Dispos. 2010;38:1790–1797. doi: 10.1124/dmd.110.032672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaur I.P. Chhabra S. Aggarwal D. Role of cyclodextrins in ophthalmics. Curr. Drug Deliv. 2004;1:351–360. doi: 10.2174/1567201043334623. [DOI] [PubMed] [Google Scholar]
- 51.Okabe K. Kimura H. Okabe J., et al. Effect of benzalkonium chloride on transscleral drug delivery. Invest. Ophthalmol. Vis. Sci. 2005;46:703–708. doi: 10.1167/iovs.03-0934. [DOI] [PubMed] [Google Scholar]
- 52.Majumdar S. Hippalgaonkar K. Repka M.A. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int. J. Pharm. 2008;348:175–178. doi: 10.1016/j.ijpharm.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Sasaki H. Yamamura K. Mukai T., et al. Modification of ocular permeability of peptide drugs by absorption promoters. Biol. Pharm. Bull. 2000;23:1524–1527. doi: 10.1248/bpb.23.1524. [DOI] [PubMed] [Google Scholar]
- 54.Sasaki H. Nagano T. Yamamura K. Nishida K. Nakamura J. Ophthalmic preservatives as absorption promoters for ocular drug delivery. J. Pharm. Pharmacol. 1995;47:703–707. doi: 10.1111/j.2042-7158.1995.tb06726.x. [DOI] [PubMed] [Google Scholar]