Abstract
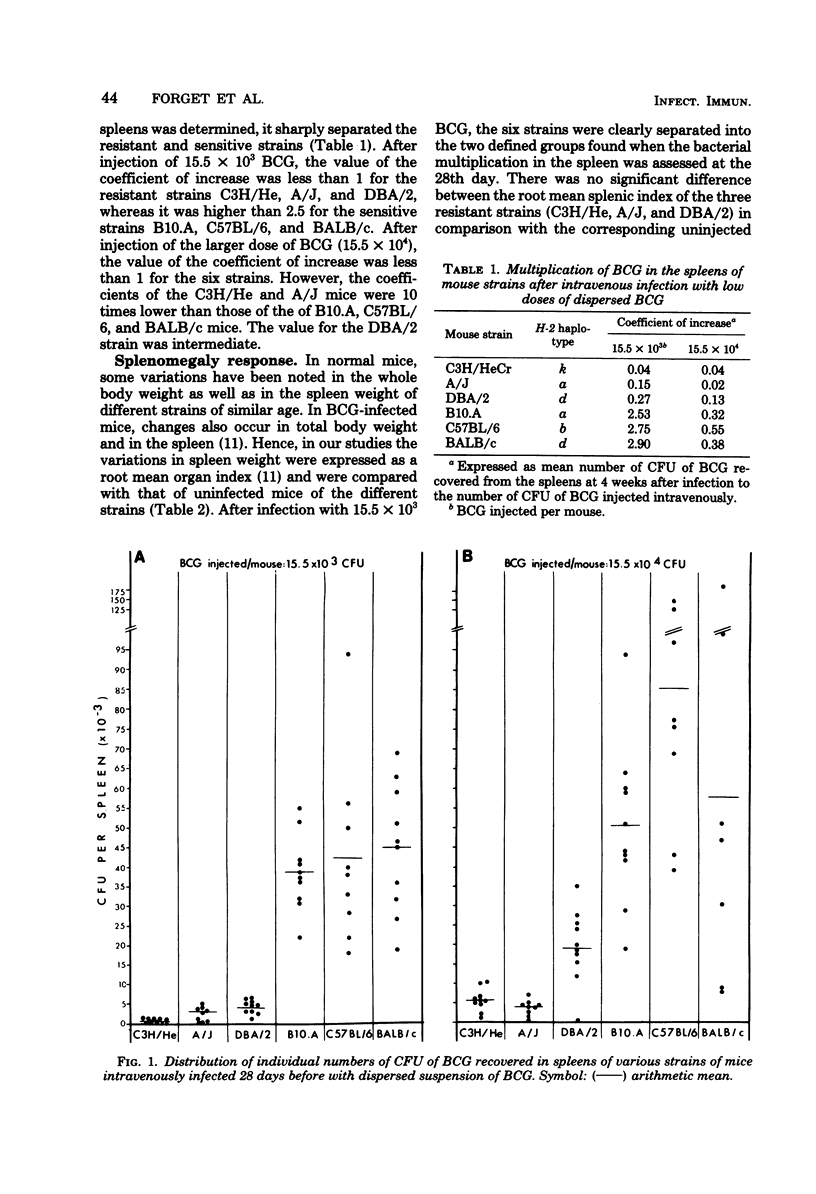
Intravenous infection of six inbred mouse strains with small doses of dispersed cells of Mycobacterium bovis BCG (15.5 × 103 or 15.5 × 104 colony-forming units) separated them into resistant (C3H/HeCr, A/J, and DBA/2) and sensitive (B10.A, C57BL/6, and BALB/c) strains as assessed by the magnitude of bacterial multiplication in the spleens at 28 days. The two groups were more sharply separated after infection with the lower dose of BCG (15.5 × 103 colony-forming units), which allowed for true multiplication of the bacteria in the spleens of permissive hosts, expressed as the ratio of the number of BCG recovered from the spleens to the number of BCG injected. This coefficient of increase was less than 1 in resistant strains, whereas it was higher than 2.5 in sensitive strains. Significant splenomegaly developed only in mice of the sensitive strains infected with BCG when compared with uninfected controls. There was no correlation between the magnitude of the delayed-type hypersensitivity (DTH) to BCG and susceptibility to infection: DTH was absent in both the sensitive and the resistant strains when the smaller dose of BCG was used for infection. Moreover, significant DTH was detected in animals of the most sensitive (BALB/c) as well as of the most resistant (C3H/HeCr) strain when the higher dose of BCG (15.5 × 104) was used for immunization. These results document significant genetic differences in the ability of inbred mice to inhibit bacterial multiplication after infection with small dispersed doses of BCG. Resistance to BCG multiplication, in this model, does not appear to be related to the establishment of DTH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman N., Cohen S., Yoshida T. Strain variations in murine MIF production. J Immunol. 1978 Jul;121(1):209–212. [PubMed] [Google Scholar]
- Allen E. M., Moore V. L., Stevens J. O. Strain variation in BCG-induced chronic pulmonary inflammation in mice. I. Basic model and possible genetic control by non-H-2 genes. J Immunol. 1977 Jul;119(1):343–347. [PubMed] [Google Scholar]
- Araujo F. G., Williams D. M., Grumet F. C., Remington J. S. Strain-dependent differences in murine susceptibility to toxoplasma. Infect Immun. 1976 May;13(5):1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENOIT J. C., PANISSET M. SURVIE ET MULTIPLICATION DU BCG ET DU BACILLE TUBERCULEUX CHEZ LA SOURIS. IV. SURVIE ET MULTIPLICATION DE SOUCHES-FILLES DE BCG EN FONCTION DU TEMPS ET DE LA DOSE. Acta Tuberc Pneumol Scand. 1963;43:125–136. [PubMed] [Google Scholar]
- BENOIT J. C., QUEVILLON M., PANISSET M. OBTENTION PAR FILTRATION DE BACILLES ISOL'ES, DE SUSPENSIONS CONTENANT DES AGR'EGATS BACILLAIRES. MISE AU POINT D'UNE M'ETHODE QUANTITATIVE. Rev Can Biol. 1963 Sep-Dec;22:437–445. [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol. 1977 Oct;30(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civil R. H., Mahmoud A. A. Genetic differences in BCG-induced resistance to Schistosoma mansoni are not controlled by genes within the major histocompatibility complex of the mouse. J Immunol. 1978 Mar;120(3):1070–1072. [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. I. Clinical histological evidence for varying susceptibility of mice to infection with Mycobacterium lepraemurium. Acta Pathol Microbiol Scand A. 1973 Jul;81(4):401–410. [PubMed] [Google Scholar]
- Collins F. M., Congdon C. C., Morrison N. E. Growth of mycobacterium bovis (BCG) in T lymphocyte-depleted mice. Infect Immun. 1975 Jan;11(1):57–64. doi: 10.1128/iai.11.1.57-64.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Miller T. E. Growth of a drug-resistant strain of Mycobacterium bovis (BCG) in normal and immunized mice. J Infect Dis. 1969 Nov;120(5):517–533. doi: 10.1093/infdis/120.5.517. [DOI] [PubMed] [Google Scholar]
- Forget A., Turcotte R., Benoit J. C., Borduas A. G. Enhancing effect of passive immunization with mycobacterial antibodies on humoral and cellular immunity in BCG-infected mice. Ann Immunol (Paris) 1978 Feb-Mar;129(2-3):255–265. [PubMed] [Google Scholar]
- Groves M. G., Osterman J. V. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. 1978 Feb;19(2):583–588. doi: 10.1128/iai.19.2.583-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Fimmel P. J., Roberts L. M., Keast D. Modification by soluble antigen of the immune response to mycobacterial infection. Infect Immun. 1977 Jun;16(3):904–909. doi: 10.1128/iai.16.3.904-909.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979 Jun;37(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Klesius P. H., Hinds S. E. Strain-dependent differences in murine susceptibility to coccidia. Infect Immun. 1979 Dec;26(3):1111–1115. doi: 10.1128/iai.26.3.1111-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. The effect of inoculum size on the immune response to BCG infection in mice. Immunology. 1971 Aug;21(2):369–381. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B., SMITH N., WELLS A. Q. The growth of intracellular tubercle bacilli in relation to their virulence. Am Rev Tuberc. 1954 Apr;69(4):479–494. doi: 10.1164/art.1954.69.4.479. [DOI] [PubMed] [Google Scholar]
- Medina S., Vas S. I., Robson H. G. Effect of nonspecific stimulation on the defense mechanisms of inbred mice. J Immunol. 1975 Jun;114(6):1720–1725. [PubMed] [Google Scholar]
- Morrison W. I., Roelants G. E., Mayor-Withey K. S., Murray M. Susceptibility of inbred strains of mice to Trypanosoma congolense: correlation with changes in spleen lymphocyte populations. Clin Exp Immunol. 1978 Apr;32(1):25–40. [PMC free article] [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Strain difference of delayed-type hypersensitivity to BCG and its genetic control in mice. Infect Immun. 1978 Dec;22(3):657–664. doi: 10.1128/iai.22.3.657-664.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. In vivo release of lymphokines in different strains of mice. Cell Immunol. 1980 Apr;51(1):173–178. doi: 10.1016/0008-8749(80)90247-6. [DOI] [PubMed] [Google Scholar]
- PIERCE-CHASE C. H., FAUVE R. M., DUBOS R. CORYNEBACTERIAL PSEUDOTUBERCULOSIS IN MICE. I. COMPARATIVE SUSCEPTIBILITY OF MOUSE STRAINS TO EXPERIMENTAL INFECTION WITH CORYNEBACTERIUM KUTSCHERI. J Exp Med. 1964 Aug 1;120:267–281. doi: 10.1084/jem.120.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE C. H., DUBOS R. J., SCHAEFER W. B. Multiplication and survival of tubercle bacilli in the organs of mice. J Exp Med. 1953 Feb 1;97(2):189–206. doi: 10.1084/jem.97.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. J. Expression of antibacterial resistance at the site of a delayed hypersensitivity reaction. Infect Immun. 1980 Jul;29(1):59–65. doi: 10.1128/iai.29.1.59-65.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature. 1974 Mar 22;248(446):345–347. doi: 10.1038/248345a0. [DOI] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- SEVER J. L., YOUMANS G. P. The enumeration of nonpathogenic viable tubercle bacilli from the organs of mice. Am Rev Tuberc. 1957 Feb;75(2):280–294. doi: 10.1164/artpd.1957.75.2.280. [DOI] [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Pearson J., Chirigos M. Virulence of six strains of Mycobacterium bovis (BCG) in mice. Infect Immun. 1973 Nov;8(5):736–742. doi: 10.1128/iai.8.5.736-742.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Stiffel C., Mouton D., Bouthillier Y., Decreusefond C., Biozzi G. Réponse du SRE au Mycobacterium tuberculosis (BCG) et au Corynebacterium parvum chez des souris de différentes lignées. J Reticuloendothel Soc. 1970 Feb;7(2):280–293. [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]
- Tokunaga T., Yamamoto S., Nakamura R. M., Kurosawa A., Murohashi T. Mouse-strain difference in immunoprophylactic and immunotherapeutic effects of BCG on carcinogen-induced autochthonous tumors. Jpn J Med Sci Biol. 1978 Apr;31(2):143–154. doi: 10.7883/yoken1952.31.143. [DOI] [PubMed] [Google Scholar]
- Turcotte R., Lafleur L., Labrèche M. Opposite effects of BCG on spleen and lymph node cells: lymphocyte proliferation and immunoglobulin synthesis. Infect Immun. 1978 Sep;21(3):696–704. doi: 10.1128/iai.21.3.696-704.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Moore R. N., Sipe J. D., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. I. In vivo studies. J Immunol. 1980 Apr;124(4):2004–2009. [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S., KANAI K. The difference in response of four strains of mice to immunization against tuberculous infection. Am Rev Respir Dis. 1959 Nov;80:753–756. doi: 10.1164/arrd.1959.80.5.753. [DOI] [PubMed] [Google Scholar]


