Abstract
Although there is a strong relationship between depression and smoking, most nicotine dependence treatment trials exclude depressed smokers. Our objective was to determine if bupropion improves abstinence rates and abstinence-associated depressive symptoms when added to transdermal nicotine replacement therapy (NRT) and group cognitive behavioral therapy (CBT) in smokers with unipolar depressive disorder (UDD). Adult smokers with current (n=90) or past (n=109) UDD were randomly assigned to receive bupropion or placebo added to NRT and CBT for 13 weeks. In the primary analysis, with dropouts considered smokers, 36% (35/97) of those on bupropion and 31% (32/102) on placebo attained biochemically-validated 7-day point-prevalence abstinence at end of treatment (NS). Because of a high drop out rate (50%) and a significant difference in abstinence status at dropout by treatment group, a traditional intent-to-treat (ITT) analysis with last observation carried forward imputation of abstinence status was performed. In this secondary analysis, 56% (54/97) of those on bupropion and 41% (42/102) on placebo met criteria for abstinence at end of trial, Chi2=4.18, p=0.04. NRT usage and absence of a co-morbid anxiety disorder predicted abstinence. Abstinence was associated with increased depressive symptoms, regardless of bupropion treatment. Thus, in the primary analysis, bupropion neither increased the efficacy of intensive group CBT and NRT for smoking cessation in smokers with UDD nor prevented abstinence-associated depressive symptoms. Bupropion appeared to provide an advantage for smoking cessation for those who remained in the trial. The dropout rate was high and was characterized by higher prevalence of current comorbid anxiety disorder. Given the high abstinence rate achieved with CBT plus NRT, a ceiling effect related to the high level of intervention received by all subjects may have prevented an adequate test of bupropion.
Keywords: Depression, Major Depressive Disorder, Unipolar Depressive Disorder, Nicotine, Smoking Cessation, Bupropion, Cognitive Behavioral Therapy, Nicotine Replacement Therapy, Relapse
Introduction
Although there is a strong association between unipolar depressive disorders (UDD) and tobacco smoking, with few exceptions,1 nicotine dependence treatment trials have excluded depressed smokers. Those with major depressive disorder (MDD) are more likely to smoke than those without an affective disorder,2 and severity of nicotine dependence is positively correlated with depressive symptom ratings.3 Many2,4,5 but not all6 studies have suggested that smokers with past MDD are more likely to fail smoking cessation efforts than those without such history, an effect that is stronger in those with recurrent MDD,7,8 and in women.9 Negative affect and depressive symptoms prior to a cessation attempt are strongly associated with failure at smoking cessation,10 and post-cessation negative affect11 and increase in depressive symptoms12 are associated with relapse to smoking after initial abstinence,13 particularly in smokers with past MDD.14,15
Past history of MDD is also associated with mood disturbance16,17 or relapse to MDD following smoking cessation in many2,4,13,18–21 but not all22,23 studies, particularly in the absence of antidepressant treatment.5 High baseline depression symptoms and rapid increase in depressive symptoms following cessation may be predictive of recurrence of MDD with abstinence.20
Because smokers with UDD may respond to nicotine dependence treatments differently than those without,24,25 some have highlighted the need for treatments tailored to the needs of smokers with depressive disorders.1,26 Recent work has examined factors associated with acceptance of smoking cessation treatment among currently depressed smokers and found current level of depressive symptoms to be unrelated to readiness to quit smoking.27 In fact, 24% of currently depressed smokers were ready to make a smoking cessation attempt in the next month.28,29 Bupropion, an antidepressant medication considered a first-line treatment for smoking cessation,30 is also effective for smoking cessation and prevention of relapse to smoking in patients with past MDD,31 and has been associated with reduction in depressive symptoms during a smoking cessation attempt in highly dependent smokers.32 All previous smoking cessation trials of bupropion have excluded smokers with significant depressive symptoms, thereby excluding perhaps more than 40% of “real world” smokers.
To investigate whether bupropion would be effective for smokers with current depressive illness, we conducted a study in which smokers with current or past UDD (major depressive disorder, dysthymia, and minor depression), who were not currently being treated with anti-depressant medications, entered treatment with standard group cognitive behavioral therapy (CBT) and nicotine patch and were randomly assigned to double-blind treatment with adjunctive placebo or bupropion-SR. This allowed us to test the hypothesis that treating depressive symptoms or preventing their emergence in individuals with current or past history of depression can help these smokers to quit smoking and maintain abstinence. Our primary hypotheses were that 1) bupropion-SR would be associated with higher point-prevalence abstinence rates than placebo when added to CBT and nicotine replacement therapy (NRT) in smokers with current or past UDD, 2) that abstinence would be associated with worsening of depressive symptoms and 3) that bupropion treatment would be associated with a lower rate of worsening of depressive symptoms than placebo.
Methods
The study was approved by the Human Research Committee of the Massachusetts General Hospital. Participants were recruited by advertisement and physician referral and signed informed consent after full explanation of study procedures and potential risks. Eligible participants were persons aged 18–70 years who smoked ≥10 cigarettes per day for ≥2 years prior to enrollment and met DSM-IV criteria for lifetime diagnosis of a unipolar depressive disorder (major depression, minor depression, or dysthymic disorder), according to the Structured Clinical Interview for DSM-IV-Axis I Disorders/Patient Edition (SCID-I/P).33 Exclusion criteria included substance use disorder other than nicotine or caffeine in the past 6 months, lifetime DSM-IV diagnosis of organic mental disorder, delusional disorder, schizophrenia, bipolar disorder, psychotic disorder not elsewhere classified, eating disorder, or antisocial personality disorder, and people currently using nicotine-containing products, psychotropic medications, including antidepressant medications, or behavioral smoking cessation treatments.
The study used a block randomization based on the following components: level of nicotine dependence (high vs. low as determined by the Fagerstrom Test of Nicotine Dependence score of 7 or higher for high level of dependence),34 history of failed prior attempts to quit with an adequate trial of nicotine replacement therapy and/or CBT by self-report (yes or no), and either current or past UDD as determined by a research psychiatrist. All subjects received 13 sessions of group CBT, 8 weeks of NRT and 12 weeks of either bupropion-SR or identical placebo during the 13-week acute treatment phase. Participants received treatment free of charge and were not paid in any way for their participation.
Interventions
All participants received trans-dermal nicotine patches at a dose of 21 mg per day from study weeks 2–6, 14 mg per day for study weeks 7 and 8, and 7 mg per day for study weeks 9 and 10. Participants were encouraged to comply with this regimen and to return used and unused patches and to report difficulties they might have experienced with patch use. Half of the subjects received bupropion-SR, 150 mg per day for 3 days then 150 mg bid, and half received identical placebo tablets under double-blind conditions. The study psychiatrist met individually with each subject on a weekly basis to review study medication utilization, record and manage adverse events, and perform the clinician-rated evaluations. The cognitive-behavioral therapist (JP, AF) conducted 13 weekly CBT meetings with groups of up to 6 patients and instructed the patients on how to use the nicotine patch. The CBT intervention utilized a multi-component group therapy approach.35,36 CBT sessions were held each week at a time held constant for each cohort. During the first session, general information regarding the study and nicotine dependence was provided and group participants discussed their motivations to quit smoking. The group therapist explained the rationale behind the cognitive-behavioral intervention, and subjects were prepared for the quit day. During the second session, specific cognitive-behavioral suggestions and instructions for the use of the nicotine patch were provided. Subjects were instructed to apply the nicotine patch for the first time on the day following the second session and to stop smoking that day. The remaining CBT meetings (sessions 3–13) emphasized cognitive-behavioral strategies for the maintenance of abstinence, with discussions on weight control, exercise, assertiveness, cognitive-restructuring, and self-reward.
Outcome Measures
The primary outcome measure was the rate of 7-day point prevalence abstinence at the end of treatment, as defined by a self-report of tobacco abstinence for the past 7 days and an expired air carbon monoxide (CO) concentration of < 9 ppm at study week 13. End-expiratory CO was measured following a ten-second breath-hold using a P.K. Morgan CO analyzer (Chatham, Kent, UK). Secondary outcomes were 4-week continuous abstinence in study weeks 10–13 and worsening of depressive symptoms among those with low depressive symptom scores at enrollment. The 6-item Hamilton Rating Scale for Depression (HAM-D-6) was performed at baseline and each week. Based on the finding that, on average, mean post-treatment scores on the HAM-D-6 correspond to 56% of mean total scores of the 17-item version of the Hamilton Rating Scale for Depression (HAM-D-17),37 a cutoff point of ≤4 on the HAM-D-6 was used as an indicator of low depressive symptoms at baseline and was assumed to be analogous to the standard cutoff point of ≤7 on the HAM-D-17.38 The rate of conversion from ≤4 to >4 on the HAM-D-6 was then calculated for those in each treatment group and by abstinence status.
Statistical Methods
Student’s t tests and Chi-square tests were used to investigate baseline differences by randomization status. Chi-square tests were used to evaluate differences in abstinence rates by randomization status. In preplanned analyses, dropouts were considered to be smokers. Because there was high rate of dropout and a significant difference in abstinence status at the last visit by randomization status, we conducted secondary analyses using a conventional last observation carried forward (LOCF) analysis, in which abstinence status at the end of treatment was imputed from the abstinence status at the time of dropout. We then conducted a sensitivity analysis in which subjects who were abstinent at the time of dropout were contacted for self-report of duration of abstinence following dropout from the trial.
RESULTS
Two hundred and fifty-five subjects signed informed consent and were enrolled in the study. Fifty-six subjects withdrew consent prior to study intervention and were not included in the analysis. One hundred ninety-nine subjects were randomized, received at least one week of the study interventions, and were included in the analyses. Subjects were randomized according to current (n=90) or past (n=109) UDD, high (n=106) vs. low (n=93) degree of nicotine dependence and history of past failed attempt to quit smoking with NRT or bupropion (n=67) or not (n=132) and were randomly assigned to receive bupropion (n=97) or placebo (n=102) added to NRT and group CBT for 13 weeks. Of the 90 subjects with current UDD, 76% (68/90) met criteria for MDD, 16% (14/90) for minor depressive disorder and 11% (10/90) for dysthymia. Mean Hamilton Depression 17-item Scale (HAMD-17) score at screening for those subjects with current UDD was 15.43 (SD=5.56). Baseline participant characteristics are shown in Table 1a and b.
Table 1.
| Table 1a. Baseline Demographic Characteristics | |||
|---|---|---|---|
| All Subjects (n=199) | Bupropion (n=97) | Placebo (n=102) | |
| Age | 43 (11) | 43 (12) | 43 (10) |
| Male (no., %) | 102 (51%) | 47 (48%) | 55 (53%) |
| Expired Air CO (ppm) | 17 (11) | 16 (11) | 18 (11) |
| Cigarettes per Day | 25 (11) | 24 (11) | 25 (10) |
| FTND | 5.8 (2.2) | 5.9 (2.3) | 5.7 (2.2) |
| Age at Onset of Smoking | 20.8 (7.9) | 20.8 (8.1) | 20.8 (7.7) |
| Pack Years | 29 (24) | 28 (25) | 30 (23) |
| Past Failed Cessation Attempt (no., %) | 75 (38%) | 37 (38%) | 38 (37%) |
| Comorbid Anxiety Disorder (Lifetime or Current) (no., %) | 79 (40%) | 40 (41%) | 39 (38%) |
| HAMD-17 Score at Screening | 10.6 (6.3) | 9.9 (5.5) | 11.2 (6.9) |
| Self-Report of Prior Depressive Episodes | 4.0 (7.1) | 5.2 (9.7) | 3.0 (3.4) |
| Table 1b. Baseline Demographic Characteristics by Completer Status | |||
|---|---|---|---|
| Dropout (n=100) | Completer (n=99) | ||
| Current Unipolar Depressive Disorder at Randomization | 44 (44%) | 46 (47%) | |
| Current Anxiety Disorder** | 46 (46%) | 33 (33%) | |
| Randomization to Bupropion | 46 (46%) | 51 (52%) | |
| High Level of Nicotine Dependence (FTND>5) | 51 (51%) | 55 (56%) | |
| Previous Cessation Attempt | 37 (37%) | 38 (38%) | |
| Gender: Male | 49 (49%) | 53 (54%) | |
| Age | 42.0 (10.4) | 44.1 (11.7) | |
| Years of Regular Smoking | 22 (12) | 23 (12) | |
| Age of Initiation of Smoking | 20.7 (8.9) | 20.9 (6.7) | |
| CO at Baseline (ppm) | 17 (11) | 17 (11) | |
| Cigarettes per Week at Baseline | 170 (71) | 174 (79) | |
| Total Number of Patches Used per Week in Trial* | 4.1 (2.2) | 4.8 (1.7) | |
| Tobacco Withdrawal Score | 11.8 (5.1) | 10.4 (5.2) | |
| HAMD-17 Score at Screening | 11.1 (6.6) | 9.9 (6.0) | |
| Self-Report of Prior Depressive Episodes | 3.9 (5.8) | 4.1 (8.2) | |
p<0.025
p<0.06
Seventy-two percent of participants, (143/199), achieved 7-day point-prevalence abstinence at least once during the study intervention, and if participants who dropped out of the trial were considered to be smokers at endpoint, 34% (67/199) met criteria for 7-day point-prevalence abstinence at the end of treatment. NRT use was associated with abstinence such that for each patch used, the odds ratio of 7-day point prevalence abstinence at end of treatment was 1.06 (95% CI: 1.03–1.08, p<0.001). Lifetime anxiety disorder diagnosis was associated with failure to attain abstinence at the end of treatment: 23% (18/79) of participants with an anxiety disorder achieved abstinence at the end of treatment vs. 41% (49/120) of those without an anxiety disorder (Chi2= 6.9, p<0.01). An effect of a comorbid anxiety disorder on ability to attain abstinence was seen, particularly in those 111 subjects with high depression symptoms (HAMD-6 >4) at screen. Thirty-seven of these subjects (33%) were abstinent and 74 subjects (67%) were not abstinent at the end of trial. Of the 74 subjects who were not abstinent, half (37) had an anxiety disorder and half did not. Of those who did achieve abstinence, 9 (24%) had an anxiety disorder and 28 (76%) did not, Chi2=6.701, p=0.009.
The mean HAMD score at screen of those who attained 7-day point prevalence abstinence at end of treatment was 9.80 (6.33) vs 10.94 (6.30) for those who did not attain abstinence, t=1.09, p=0.278. There was no significant relationship between number of self-reported prior episodes of unipolar depressive disorder and abstinence in this study.
Effect of Bupropion on Abstinence
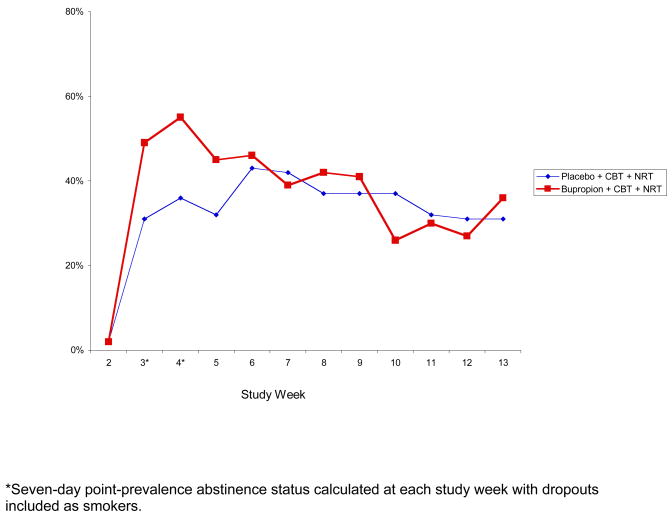
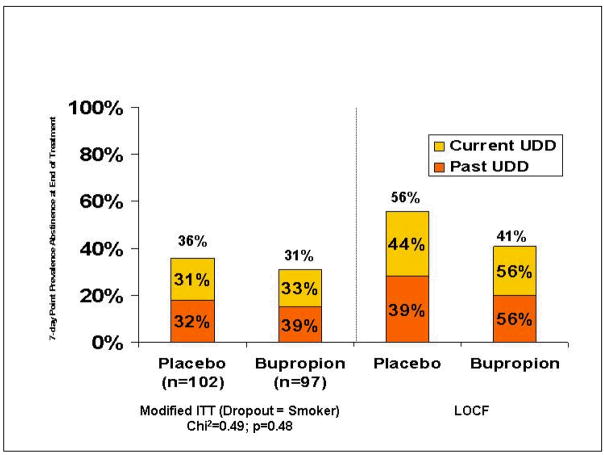
Using the modified intent-to-treat (ITT) approach in which dropouts were considered to be smokers, 7-day point prevalence abstinence rates at the end of the trial were 36% (37/97) in the bupropion + NRT + CBT group and 31% (32/102) in the placebo + NRT + CBT group, Chi2=0.49, ns (Figures 1 and 2).
Figure 1.
Seven-Day Point Prevalence Abstinence Rates by Study Medication and Week
Figure 2.
Effect of Bupropion Added to CBT and NRT on Abstinence Rates (%) at End of Treatment
Abstinence Rates by Depression Status at Randomization
There was no effect of depression status at randomization on abstinence status at endpoint, (32% (29/90) with current UDD were abstinent vs. 35% (38/109) with past UDD, Chi2=0.15, ns). Among subjects with current UDD, 33% (15/45) of those randomized to bupropion and 31% (14/45) of those on placebo attained 7-day point prevalence abstinence at the end of the trial, Chi2=0.05, ns. Among those with past UDD, 39% (20/52) of those on bupropion were abstinent at the end of the trial compared to 32% (18/57) of those on placebo, Chi2=0.57, ns.
Abstinence Associated with Increase in Depressive Symptoms
Seventy-four percent (63/85) of those who entered the trial with low depressive symptoms (HamD-6 ≤4) achieved 7-day point-prevalence abstinence during the trial. Among those with low baseline depressive scores and abstinence during the trial, 56% (35/63) reported high ratings of depression symptoms (HAMD-6 >4) 1 or more weeks following the initial week of abstinence. Only 27% (6/22) of those with low depressive symptoms who did not achieve abstinence subsequently experienced high ratings of depressive symptoms (Chi2=4.60, p=0.032). Bupropion was not protective against this abstinence-associated increase in depression symptoms as 50% (17/34) of those on bupropion vs. 62% (18/29) of those on placebo who entered the trial with HAMD-6 ≤4 and achieved abstinence reported a subsequent roughening of depressive symptoms as evidenced by HAM-D-6 score >4, Chi2=0.923, p=0.34.
Dropouts During Active Treatment
Fifty percent of those who entered randomized treatment dropped out before the end of the 13-week intervention. There was a trend for those randomized to bupropion to remain in the trial longer (mean= 6.5 weeks, SD=3.4) than those on placebo (mean=5.3 weeks, SD=3.6), t=1.57, p<0.12. There was also a trend toward a greater rate of lifetime anxiety disorders among subjects who dropped out of the trial, 46% (46/100), compared to completers, 33% (33/99), Chi2= 3.334, p<0.06 (Table 1b). There was no effect of current vs. past depressive disorder or any other measured baseline characteristic on dropout. Subjects who completed the trial used significantly more transdermal nicotine patches per week while in the trial compared to those who dropped out, (mean=4.8 (1.7) vs. 4.1 (2.2), t=2.26, p<0.025). Those who dropped out had no greater increase in depressive or nicotine withdrawal symptoms from baseline to the last measurement than those who completed the trial. Change in depression symptom ratings on the HAM-D from baseline to last visit was −2.4 (4.6) for those who completed the trial and −2.0 (4.5) in those who dropped out of the trial. Change in nicotine withdrawal symptom ratings from baseline to last study visit was 2.7 (5.5) in completers and 3.6 (6.1) in those who dropped out.
Secondary analysis of effect of bupropion on abstinence
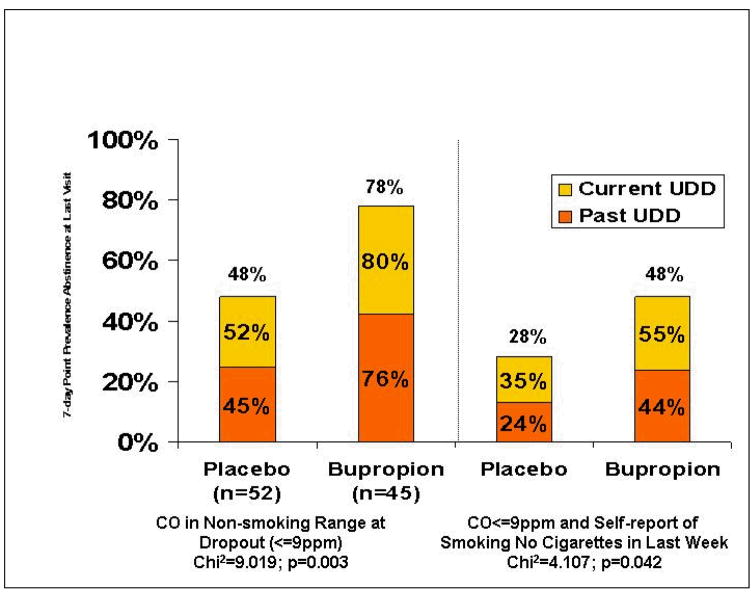
It is conventional in smoking cessation studies to classify dropouts as smokers at time points after dropout because it is assumed that participants drop out because they have been unable to quit smoking during the trial or because they have resumed smoking at the time of dropout. This results in a conservative estimate of abstinence rates. It is not known if those with UDD may be more likely to drop out of a smoking cessation trial for reasons other than resumption of smoking, such as apathy, anhedonia or depressed mood. In this study in smokers with UDD, many were abstinent at the time of dropout; 78% (35/45) of those on bupropion had CO levels in the non-smoking range (<=9 ppm) on the visit prior to dropout vs. 48% (25/52) of those receiving placebo, Chi2=9.02, p<0.003, and significantly more dropouts in the bupropion group met full 7-day point-prevalence abstinence criteria (self-report of no cigarettes in the past 7 days and expired air CO <=9 ppm) on the visit prior to dropout vs. those on placebo, 48% (22/45) vs. 28% (15/52), Chi2=4.107, p<0.042 (Figure 3).
Figure 3.
Abstinence status at time of drop-out in bupropion and placebo groups.
Because of this large difference in abstinence status at dropout by medication status, and the large number of dropouts in the study (100/199), imputation of non-abstinent smoking status for all dropouts may create a bias in the analysis against finding an effect of bupropion. Thus, a second, exploratory analysis was performed using a traditional last observation carried forward (LOCF) method for imputation of missing data after drop-out, in which abstinence status at time of drop-out was imputed. According to this traditional ITT or LOCF approach, 48% of participants (90/199) met criteria for 7-day point-prevalence abstinence at endpoint, 50% (45/90) with current UDD and 47% (51/109) with past UDD. In the LOCF analysis, 56% (54/97) of those in the bupropion group and 41% (42/102) on placebo met criteria for 7-day point prevalence abstinence at end of trial, Chi2=4.18, p=0.04. (Figure 3) Among those with current UDD, 56% (25/45) of those on bupropion and 44% (20/45) of those on placebo attained 7-day point prevalence abstinence. Among those with past UDD, 56% (29/52) of those on bupropion and 39% (22/57) of those on placebo met abstinence criteria at endpoint, Chi2=3.22, p<0.072. A scripted, follow-up telephone interview was conducted in those who were abstinent at drop-out to assess duration of abstinence and reason for drop-out. Sixty-three percent reported that they were abstinent at drop out and remained abstinent for ≥6 months. Participants reported that they dropped out primarily due to time demands of the study, increased work responsibilities or scheduling conflicts. No participants reported dropping out due to negative experiences during the trial or adverse events.
Follow-up Data
Because of the high rate of drop-out in the 12-month follow-up period of this trial, we were unable to assess long term abstinence rates or the risk of relapse to MDD associated with tobacco abstinence in this sample.
Discussion
To our knowledge, this is the first study to compare pharmacotherapy for nicotine dependence in smokers with current UDD. In this study, all participants expressed the intention to quit smoking and all received intensive treatment with transdermal NRT, weekly group CBT and brief physician visits. The rate of smoking cessation in the placebo + NRT + CBT group was high, and bupropion did not provide significant, additional benefits for smoking cessation in this setting using the pre-specified modified ITT analysis, although it did appear to provide greater benefit than placebo using a traditional ITT analysis. Even with the less conservative analytic approach, bupropion did not have its greatest effect in those with current UDD at randomization, although this does not rule out an effect on reducing depressive symptoms that may have emerged with abstinence. Importantly, attainment of 7-day point prevalence abstinence during the trial was associated with significantly increased incidence of switch from low to high depressive symptoms, and bupropion was not protective against this abstinence-associated roughening of depressive symptoms.
Limitations
Participants who were taking antidepressant medications were excluded in order to evaluate the value of adding the antidepressant, bupropion, to nicotine dependence treatment in those with depression. Thus the findings are generalizable to those with UDD not requiring current antidepressant treatment. The attrition rate was high; 22% of those who were enrolled dropped out before receiving study interventions, and 50% (100/199) of those who initiated treatment dropped out before the end of study interventions. This may reflect the inherent difficulty of smoking cessation for people with depressive disorders and particularly those with depression and co-morbid anxiety disorders. Other factors that may have played an important role in the high rate of dropout were the requirement that participants wait until a cohort of 3–6 participants were enrolled to begin treatment and the fact that participants were not compensated in any way for their participation other than to receive free treatment that was available elsewhere free of charge at the time. Incentives may be needed to retain people with UDD in a smoking cessation program.
The drop-out rates were similar between bupropion and placebo groups, but, because significantly more participants assigned to bupropion were abstinent at the time of drop-out, the modified intent-to-treat analytic approach, in which dropouts are considered to be smokers, is likely to be conservative in its assessment of any benefit of bupropion when added to NRT and CBT. The LOCF approach, in which participants who are abstinent at the time of drop-out are assumed to maintain abstinence despite discontinuation of treatment, is likely to overestimate the abstinence rates at end of treatment. The true benefit of bupropion when added to NRT + CBT is likely to be an in increase abstinence rates of between the 5% observed with the modified ITT analysis and 15% observed with the LOCF approach. This is supported by the self-report data collected after drop-out that indicated that 63% of subjects who were abstinent at time of dropout were abstinent 6 months later. Because of the high drop-out rate in the follow-up period and the significant potential for bias in interpretation of the data from the remaining participants, we were unable to assess the effect of treatment on long-term abstinence, the effect of abstinence on subsequent relapse to MDD in the one year follow-up period or any protective effect of bupropion for these events.
Previous studies have indicated that cognitive behavioral therapy is effective for smoking cessation in smokers with a history of MDD.8,9,39,40 All participants in this trial received intensive therapy that included weekly group CBT, brief weekly physician visits and NRT, possibly creating a ceiling effect. Because there was no placebo only or medication management only arm, and all participants received two active treatments in CBT and NRT, the estimate of the benefit of bupropion for smoking cessation in people with past or current unipolar depressive disorders is limited to the setting of an intensive regimen of NRT, weekly group CBT and physician visits. These data do not support the hypothesis that the addition of bupropion would be especially helpful to smokers with current, clinically significant depressive symptoms.
Lastly, without 12- month follow up data in a majority of participants, the public health impact of the interventions cannot be assessed.
Conclusion
In this double-blind, placebo-controlled study, smokers with past or current UDD achieved reasonably high abstinence rates; approximately one third of participants were abstinent at the end of treatment using the modified ITT, and about half using the traditional ITT method. However, abstinence was associated with roughening of depressive symptoms in those who entered the trial with low depressive symptoms. Co-morbid anxiety was associated with both a greater likelihood of dropping out of the study and lower likelihood of achieving abstinence, particularly among those with higher depressive symptoms at study entry. Using the modified ITT, bupropion did not add significantly to the efficacy of intensive group CBT and NRT for smoking cessation or for abstinence-associated depressive symptoms. Bupropion appeared to provide an advantage for smoking cessation rates using a traditional ITT analysis during active treatment. Given the high abstinence rate achieved in the CBT plus NRT plus placebo group, a ceiling effect related to the high level of intervention received by all subjects may have prevented an adequate test of bupropion.
Acknowledgments
This work was supported by NIDA 5R01DA011512 (Dr. Fava) and NIDA 1K23DA00510-01 (Dr. Evins). GlaxoSmithKline provided sustained release bupropion and identical placebo.
We would like to thank Drs. Elena Tomba and Timothy Petersen for their assistance with this project.
Footnotes
These data have been presented in part at the 2005 Annual Meeting of the American College of Neuropsychopharmacology and have not been previously published.
References
- 1.Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: A randomized clinical trial. Am J Public Health. 2006;96:1808–14. doi: 10.2105/AJPH.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glassman AH, Helzer JE, Covey LS, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–9. [PubMed] [Google Scholar]
- 3.Lerman C, Audrain J, Orleans CT, et al. Investigation of mechanisms linking depressed mood to nicotine dependence. Addict Behav. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 4.Glassman AH. Cigarette smoking: Implications for psychiatric illness. Amer J Psychiatry. 1993;150:546–53. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- 5.Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Compr Psychiatry. 1990;31:350–4. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- 6.Hitsman B, Borrelli B, McChargue DE, et al. History of depression and smoking cessation outcome: A meta-analysis. J Consult Clin Psychol. 2003;71:657–63. doi: 10.1037/0022-006x.71.4.657. [DOI] [PubMed] [Google Scholar]
- 7.Covey LS, Glassman AH, Stetner F, et al. Effect of history of alcoholism or major depression on smoking cessation. Am J Psychiatry. 1993;150:1546–7. doi: 10.1176/ajp.150.10.1546. [DOI] [PubMed] [Google Scholar]
- 8.Haas AL, Munoz RF, Humfleet GL, et al. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. J Consult Clin Psychol. 2004;72:563–70. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- 9.Hall SM, Reus VI, Munoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55:683–90. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- 10.Acton GS, Kunz JD, Wilson M, et al. The construct of internalization: Conceptualization, measurement, and prediction of smoking treatment outcome. Psychol Med. 2005;35:395–408. doi: 10.1017/s0033291704003083. [DOI] [PubMed] [Google Scholar]
- 11.Hall SM, Munoz RF, Reus VI, et al. Mood management and nicotine gum in smoking treatment: A therapeutic contact and placebo-controlled study. J Consult Clin Psychol. 1996;64:1003–9. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- 12.Killen JD, Fortmann SP, Kraemer HC, et al. Interactive effects of depression symptoms, nicotine dependence and weight change on late smoking relapse. J Consult Clin Psychol. 1996;64:1060–67. doi: 10.1037//0022-006x.64.5.1060. [DOI] [PubMed] [Google Scholar]
- 13.Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. Am J Psychiatry. 1997;154:263–5. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- 14.Hall SM, Munoz RF, Reus VI, et al. Nicotine, negative affect, and depression. J Consult Clin Psychol. 1993;61:761–7. doi: 10.1037//0022-006x.61.5.761. [DOI] [PubMed] [Google Scholar]
- 15.Thorsteinsson HS, Gillin JC, Patten CA, et al. The effects of transdermal nicotine therapy for smoking cessation on depressive symptoms in patients with major depression. Neuropsychopharmacology. 2001;24:350–8. doi: 10.1016/S0893-133X(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 16.Breslau N, Kilbey MM, Andreski P. Nicotine withdrawal symptoms and psychiatric disorders: Findings from epidemiologic studies. Am Journ Psych. 1992;149:464–69. doi: 10.1176/ajp.149.4.464. [DOI] [PubMed] [Google Scholar]
- 17.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives General Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 18.Stage KB, Glassman AH, Covey LS. Depression after smoking cessation: Case reports. J Clin Psychiatry. 1996;57:467–9. doi: 10.4088/jcp.v57n1005. [DOI] [PubMed] [Google Scholar]
- 19.Glassman AH, Covey LS, Stetner F, et al. Smoking cessation and the course of major depression: A follow-up study. Lancet. 2001;357:1929–32. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- 20.Borrelli B, Niaura R, Keuthen NJ, et al. Development of major depressive disorder during smoking-cessation treatment. J Clin Psychiatry. 1996;57:534–8. doi: 10.4088/jcp.v57n1106. [DOI] [PubMed] [Google Scholar]
- 21.Tsoh JY, Humfleet GL, Munoz RF, et al. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157:368–74. doi: 10.1176/appi.ajp.157.3.368. [DOI] [PubMed] [Google Scholar]
- 22.Kahler CW, Brown RA, Ramsey SE, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. J Abnorm Psychol. 2002;111:670–5. doi: 10.1037//0021-843x.111.4.670. [DOI] [PubMed] [Google Scholar]
- 23.Prochaska JJ, Hall SM, Tsoh JY, et al. Treating tobacco dependence in clinically depressed smokers: Effect of smoking cessation on mental health functioning. Am J Public Health. 2008;98:446–48. doi: 10.2105/AJPH.2006.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Covey LS, Glassman AH, Stetner F. Naltrexone effects on short-term and long-term smoking cessation. J Addict Dis. 1999;18:31–40. doi: 10.1300/J069v18n01_04. [DOI] [PubMed] [Google Scholar]
- 25.Hitsman B, Pingitore R, Spring B, et al. Antidepressant pharmacotherapy helps some cigarette smokers more than others. J Consult Clin Psychol. 1999;67:547–54. doi: 10.1037//0022-006x.67.4.547. [DOI] [PubMed] [Google Scholar]
- 26.Lerman C, Caporaso N, Main D, et al. Depression and self-medication with nicotine: The modifying influence of the dopamine d4 receptor gene. Health Psychol. 1998;17:56–62. doi: 10.1037//0278-6133.17.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Haug NA, Hall SM, Prochaska JJ, et al. Acceptance of nicotine dependence treatment among currently depressed smokers. Nicotine Tob Res. 2005;7:217–24. doi: 10.1080/14622200500055368. [DOI] [PubMed] [Google Scholar]
- 28.Prochaska JJ, Rossi JS, Redding CA, et al. Depressed smokers and stage of change: Implications for treatment interventions. Drug Alcohol Depend. 2004;76:143–51. doi: 10.1016/j.drugalcdep.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Tsoh JY, Hall SM. Depression and smoking: From the transtheoretical model of change perspective. Addict Behav. 2004;29:801–5. doi: 10.1016/j.addbeh.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 30.A clinical practice guideline for treating tobacco use and dependence: A us public health service report. The tobacco use and dependence clinical practice guideline panel, staff, and consortium representatives. JAMA. 2000;283:3244–54. [PubMed] [Google Scholar]
- 31.Cox LS, Patten CA, Niaura RS, et al. Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. J Gen Intern Med. 2004;19:828–34. doi: 10.1111/j.1525-1497.2004.30423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerman C, Niaura R, Collins BN, et al. Effect of bupropion on depression symptoms in a smoking cessation clinical trial. Psychol Addict Behav. 2004;18:362–6. doi: 10.1037/0893-164X.18.4.362. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for dsm-iv axis i disorders--patient edition (scid-i/p, version 2.0) Journal. 1995 [Google Scholar]
- 34.Heatherton TF, Kozlowski LT, Frecker RC, et al. The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. Brit J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 35.Liese BS. The kufp five-visit quit-smoking program: An office-based smoking cessation protocol. Kansas Medicine. 1993;94:294–98. [PubMed] [Google Scholar]
- 36.Liese BS, Govaker DA. The five-session quit smoking clinic. Kansas Medicine. 1987;88:291–93. [PubMed] [Google Scholar]
- 37.Licht RW, Bech P. Why the hamilton depression rating scale endures. Am J Psychiatry. 2005;162:2394–5. doi: 10.1176/appi.ajp.162.12.2394-b. author reply 97–8. [DOI] [PubMed] [Google Scholar]
- 38.Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence Arch Gen Psychiatry. 1991;48:851–5. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 39.Kahler CW, Strong DR, Niaura R, et al. Hostility in smokers with past major depressive disorder: Relation to smoking patterns, reasons for quitting, and cessation outcomes. Nicotine Tob Res. 2004;6:809–18. doi: 10.1080/1462220042000282546. [DOI] [PubMed] [Google Scholar]
- 40.Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62:141–6. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]