Abstract
Data suggest that risk factors for ovarian carcinoma vary by histologic type, but findings are inconsistent. We prospectively evaluated risk factors by histological subtypes of incident ovarian cancer (n = 849) in a cohort of 169,391 women in the NIH-AARP Diet and Health Study. We constructed Cox models of individual exposures by comparing case subtypes to the entire non-case group and assessed P-heterogeneity in case-case comparisons using serous as the reference category. Substantial risk differences between histologic subtypes were observed for menopausal hormone therapy (MHT) use, oral contraceptive (OC) use, parity, and body mass index (P-heterogeneity=0.01, 0.03, 0.05, 0.03, respectively). MHT users were at increased risk for all histologic subtypes except for mucinous carcinomas, where risk was reduced (relative risk (RR)=0.37; 95% confidence interval (CI): 0.18, 0.80). OC users were only at significantly decreased risk for serous cancers (RR=0.69; 95% CI: 0.55, 0.85). Although parity was inversely associated with risk of all subtypes, the RRs ranged from 0.28 (clear cell) to 0.83 (serous). Obesity was a significant risk factor only for endometrioid cancers (RR=1.64; 95% CI: 1.00, 2.70). Our findings support a link between etiological factors and histological heterogeneity in ovarian carcinoma.
Keywords: reproductive factors, non-reproductive factors, histology, ovarian cancer, prospective study
Ovarian cancer is the most lethal gynecologic malignancy.1 Hypotheses regarding the etiology of ovarian carcinogenesis include incessant ovulation, and exposure to elevated levels of gonadotropins, sex-steroid hormones, and inflammatory cytokines,2 but the etiology of ovarian cancer is complex and poorly understood. Many studies have shown that subtypes of ovarian carcinomas have different molecular, pathological, and clinical characteristics,3-5 suggesting that histological diversity of epithelial ovarian carcinoma may constitute several distinct entities instead of representing one disease. Approximately 90% of ovarian malignant tumors are carcinomas, which include four major histologic subtypes: serous, mucinous, endometrioid, and clear cell, with majority of ovarian carcinomas classified as serous.5-6
However, the relationships between ovarian cancer risk factors and ovarian cancer histology remains unclear. Many epidemiologic studies,7-11 including a pooled analysis of 10 case-control studies, have identified multi-parity as a protective factor, but other studies,12-14 including a recent prospective study, have reported a decreased risk only for endometrioid subtype and a possible increased risk for mucinous carcinomas. Menopausal hormone therapy (MHT) use has been shown to be more strongly positively associated with endometrioid cancers in some,12, 14-15 but not all previous7, 10, 12 studies. Among non-reproductive exposures evaluated, studies have reported no consistent differences according to histological subtypes by body mass index (BMI),10-11, 14, 16-20 physical activity,14, 16, 21-22 alcohol use,10, 16, 23-24 or smoking status.10-11, 14, 16, 25-30 Although these studies suggest that some associations differ by subtype, the data are inconsistent. Due to limited case numbers and frequently missing histologic information in many studies, most risk factors have not been sufficiently studied according to ovarian carcinoma subtypes.
To further assess these relationships, we examined risk factors for ovarian carcinoma by histological subtype in the large prospective NIH-AARP Diet and Health Study with on average 10 years of follow-up.
MATERIAL AND METHODS
Study population
The NIH-AARP Diet and Health Study design and methodology have been described in detail elsewhere.31 In brief, the NIH-AARP Diet and Health Study was established in 1995–1996 by inviting 3.5 million AARP members ages 50-71 years in six states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia and Detroit, Michigan) to complete a baseline questionnaire. A total of 617,119 self-administered questionnaires were mailed back, of which 566,401 were non-duplicate and satisfactorily completed.
We excluded study participants who were male (n = 339,669); reported a previous diagnosis of epithelial ovarian cancer (n = 80), history of oophorectomy (n = 52,335) or unknown oophorectomy status (n = 4,535); died of ovarian cancer (histology not available) (n=308); developed borderline or non-epithelial ovarian cancer during follow-up (n=85); or died, moved out of the study area, or dropped out of the study before study entry (n = 12). The resulting cohort consisted of 169,391 women. The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the U.S. National Cancer Institute.
Exposure assessment
The baseline questionnaire ascertained self-report of demographic factors, anthropometric measures, lifestyle factors, and personal and family medical history. Frequency of physical activities that lasted at least 20 minutes and caused increased in breathing or heart rate or caused sweating at work or home was assessed. Based on self-reported weight and height, we calculated each individual's BMI (kg/m2). For analysis, we grouped BMI as less than 30 kg/m2 and 30 kg/m2 or greater. In addition, we grouped all non-White races (Black, Hispanic, Asian, Pacific Islander, American Indian or Alaska Native) into one group because of the small numbers of each specific non-White race. Female study participants were additionally asked to provide information on reproductive and menstrual history, basic information (ever/never and duration) about any oral contraceptive (OC) and MHT use, according to defined response categories. To determine whether study participants were menopausal, they were asked at what age they had their last menstrual period, and, if periods had stopped, whether menopause was natural or due to surgery or radiation/chemotherapy. Study participants were also asked whether they had a hysterectomy or surgery that involved removal of one or both ovaries.
Ascertainment of ovarian cancer
Cohort members were followed through the U.S. Postal Service national database for address changes and the U.S. Social Security Administration Death Master File and the National Death Index Plus for updated vital status. Incident ovarian cancers were identified by probabilistic linkages with cancer registries in the original recruitment areas. To capture cases who moved out of the study area, we expanded our cancer catchment areas to two common states of relocation (Arizona and Texas). The completeness of case ascertainment in this cohort has been reported previously, with an estimated sensitivity of approximately 90% and specificity of 99.5% with respect to identification of cases by cancer registry linkage. Follow-up time was defined as time from the baseline questionnaire return (between 1995 and 1996) until diagnosis of any ovarian cancer, date of death, the date moved out of registry ascertainment area, or last follow-up (December 31, 2006).
All incident cases of ovarian cancer were considered eligible for this analysis, regardless of history of cancer in other sites. During the follow-up, 1,004 study subjects were diagnosed with incident ovarian cancer, of which 849 were included in the analysis after the exclusions described in the previous section were made. Using histology codes from the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), we classified ovarian cancer into serous (codes 8441, 8460, 8461), endometrioid (codes 8380, 8381, 8560, 8570), mucinous (codes 8470, 8471, 8480, 8481), clear cell (codes 8310, 8313), and other epithelial (codes 8010, 8020, 8021, 8050,8070, 8120, 8140, 8240, 8246, 8255, 8260, 8323, 8440, 8450, 8490, 8562) carcinoma types. Histology of the ovarian cancers was available from the cancer registries. Stage was available for 56% (N=471) and grade for 72% (N=613) of ovarian cancer cases included in this analysis. Stage was not provided by all states; among the cancer registries that provided stage information, stage was available on 72% of cases included in this analysis.
Statistical analysis
We used Cox proportional hazards regression to estimate relative risks (RR) and 95% confidence intervals (CI) with age as the time metric. We built a parsimonious regression model by adding ovarian cancer risk factors that were considered a priori important potential confounders.
Multivariable models included the following covariates: age (continuous), OC use (ever/never), parity (yes/no), and MHT use (ever/never). For covariates with missing data, women were coded into a separate category. Ever/never use of OC and years of OC use were not adjusted for each other because the two variables are highly correlated. Similarly, ever/never use of MHT and years of MHT use as well as parity (yes/no) and number of live births were not adjusted for the other.
We explored risk factor associations for serous, endometrioid, mucinous, clear cell, and other epithelial carcinoma subtypes. We constructed five separate Cox models for each exposure of interest by stratifying the analysis by histologic subtype and comparing each case subtype to the entire non-case group. We used the same multivariable model for the various histological subtypes to ease interpretation. Differences in risk estimates across histologic subtype were assessed using P-values from unconditional logistic regression models that treated serous carcinomas as the reference category and excluded non-cases. We entered any categorical variables as a single continuous parameter, rather than dummy variables of each category separately, in the models to calculate P-heterogeneity. We present this P-heterogeneity as the main analysis, but we also applied a method used to account for competing risks when there is more than one type of outcome.32 In our analysis, a diagnosis of one histological subtype of ovarian carcinoma precluded a case from being diagnosed as a different histological subtype of ovarian carcinoma.
For the latter method of assessing heterogeneity, we created five duplicate data sets to produce one record for each subtype and treated the outcome of four of the five records as a non-event. We estimated the histology-specific risk, the probability of failure due to histology of interest, by censoring each histologic subtype at the time when it occurred. This model thus assumed different associations by histologic subtype. We compared this model with a model with a single estimate for all cases regardless of subtype by using the likelihood ratio test to determine the significance of heterogeneity by subtypes for each potential ovarian cancer risk factors. Furthermore, as a sensitivity analysis, we limited our analysis to first primary ovarian cancer cases (N=697) as our case group (i.e. cases with previous cancers at other sites before the ovarian cancer diagnosis were excluded).
For all analyses, P-values of ≤ 0.05 were considered statistically significant. All tests of statistical significance were two-sided. Analyses were performed using SAS software release 9.1.3 (SAS Institute, Cary, NC).
RESULTS
A total of 169,391 women contributed 1,657,966.8 person-years, including an average period of 5.1 years from enrollment to diagnosis for cases and 9.8 years of observation time for non-cases. The mean (standard deviation) of ages at enrollment were 62.8 (5.3) years for cases vs. 61.8 (5.4) for non-cases; ages at exit comparable values were 67.9 (6.0) years for cases and 71.6 (5.7) for non-cases. Most women were white race/ethnicity (90%), had at least a high school education (68%), and were postmenopausal at the time of study entry (94%).
Characteristics of the study population and of the cases by histological subtypes are presented in Table 1. Of the 849 epithelial ovarian cancer cases, 451 (53%) were serous, 78 (9%) endometrioid, 38 (4%) mucinous, 27 (3%) clear cell, and 255 (30%) other epithelial carcinomas. The histological subtypes in the “other epithelial cancer” comprised of squamous cell carcinoma, transitional cell carcinoma, mixed cell adenocarcinoma, papillary carcinoma, as well as a high percentage of cases with a diagnosis of unspecified adenocarcinoma or carcinomas. In general, women with serous (mean=62.6 years), mucinous (63.5 years), and other epithelial carcinoma (63.9 years) subtypes were older while endometrioid (61.0 years) and clear cell (59.7 years) cases were younger. Differences were also observed across case groups in the distribution of tumor stage and grade, with endometrioid and mucinous cases more likely to be localized low grade tumors and the three other tumor types more likely to be high grade tumors and to have regional/distant metastases.
Table 1.
Select baseline characteristics of epithelial ovarian cancer cases among 169,391 women in NIH-AARP Diet and Health Study, 1995-2006
| Epithelial Cases (N=849) |
Histological Subtypes |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Study Subjects | Non-Cases (N=168,542) |
Serous (N=451) |
Endometrioid (N=78) |
Mucinous (N=38) |
Clear Cell (N=27) |
Other (N=255) |
||||||||||
| Person-Years | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Race/ethnicity | ||||||||||||||||
| White | 1,488,349 | 90% | 151,329 | 90% | 792 | 93% | 425 | 94% | 71 | 91% | 36 | 95% | 26 | 96% | 234 | 92% |
| Non-White | 169,618 | 10% | 17,213 | 10% | 57 | 7% | 26 | 6% | 7 | 9% | 2 | 5% | 1 | 4% | 21 | 8% |
| Age (years) | ||||||||||||||||
| Mean (standard deviation) | 61.8 (5.4) | 62.8 (5.3) | 62.6 (5.4) | 61.0 (6.2) | 63.5 (5.5) | 59.7 (6.2) | 63.9 (4.8) | |||||||||
| < 55 | 250,797 | 15% | 24,960 | 15% | 90 | 11% | 52 | 12% | 8 | 10% | 4 | 11% | 9 | 33% | 17 | 7% |
| 55-59 | 388,246 | 23% | 39,027 | 23% | 178 | 21% | 92 | 20% | 32 | 41% | 6 | 16% | 6 | 22% | 42 | 16% |
| 60-64 | 460,607 | 28% | 46,791 | 28% | 214 | 25% | 121 | 27% | 15 | 19% | 7 | 18% | 5 | 19% | 66 | 26% |
| 65-69 | 503,078 | 30% | 51,945 | 31% | 326 | 38% | 164 | 36% | 21 | 27% | 18 | 47% | 5 | 19% | 118 | 46% |
| ≥ 70 | 55,239 | 3% | 5,819 | 3% | 41 | 5% | 22 | 5% | 2 | 3% | 3 | 8% | 2 | 7% | 12 | 5% |
| Education | ||||||||||||||||
| ≤ High school | 508,307 | 32% | 51,854 | 32% | 245 | 30% | 116 | 27% | 27 | 35% | 14 | 39% | 10 | 37% | 78 | 32% |
| > High school | 1,099,530 | 68% | 111,531 | 68% | 570 | 70% | 313 | 73% | 50 | 65% | 22 | 61% | 17 | 63% | 168 | 68% |
| Age at menopause (years) | ||||||||||||||||
| Premenopausal | 76,000 | 6% | 7,517 | 6% | 24 | 4% | 17 | 5% | 1 | 2% | 0 | 0% | 3 | 14% | 3 | 2% |
| < 45 | 133,288 | 11% | 13,847 | 11% | 56 | 9% | 34 | 11% | 2 | 4% | 2 | 6% | 1 | 5% | 17 | 9% |
| 45-49 | 314,026 | 26% | 32,073 | 26% | 158 | 26% | 81 | 26% | 13 | 25% | 12 | 38% | 8 | 38% | 44 | 24% |
| 50-54 | 549,816 | 46% | 55,539 | 46% | 293 | 49% | 144 | 46% | 29 | 56% | 15 | 47% | 9 | 43% | 96 | 53% |
| ≥ 55 | 123,882 | 10% | 12,516 | 10% | 67 | 11% | 35 | 11% | 7 | 13% | 3 | 9% | 0 | 0% | 22 | 12% |
| Stage at diagnosisα | ||||||||||||||||
| In situ/Localized | 49 | 10% | 14 | 6% | 18 | 41% | 11 | 42% | 1 | 10% | 5 | 4% | ||||
| Regional/distant metastases | 422 | 90% | 239 | 94% | 26 | 59% | 15 | 58% | 9 | 90% | 133 | 96% | ||||
| Grade at diagnosis | ||||||||||||||||
| Grade I | 44 | 7% | 23 | 6% | 10 | 14% | 7 | 25% | 1 | 5% | 3 | 2% | ||||
| Grade II | 134 | 22% | 79 | 21% | 25 | 36% | 11 | 39% | 2 | 11% | 17 | 14% | ||||
| Grade III-IV | 435 | 71% | 271 | 73% | 34 | 49% | 10 | 36% | 16 | 84% | 104 | 84% | ||||
Numbers may not add up to total because of missing values. Missing values were excluded from percentage calculations.
SEER Summary Staging Manual 2000: in situ/localized, regional/distant metases
Table 2 presents the associations between hormonal and reproductive factors overall and by histologic subtype. Increased risk for epithelial ovarian carcinoma was associated with MHT use (RR=1.33; 95% CI: 1.16, 1.53), and inversely associated with OC use (RR=0.74; 95% CI: 0.63, 0.87) and parity (RR=0.71; 95% CI: 0.61, 0.85). There were significant differences between histologic subtypes for MHT use, OC use, and parity (p-heterogeneity=0.01, 0.03, 0.05, respectively). Compared with never MHT users, ever users were at increased risk for all histologic subtypes and the association was statistically significant for serous carcinoma (RR=1.33; 95% CI: 1.16, 1.53), but mucinous carcinoma showed a significant inverse relation (RR=0.37; 95% CI: 0.18, 0.80). Ever OC users were at significantly decreased risk for serous (RR=0.69; 95% CI: 0.55, 0.85) and other epithelial cancers (RR=0.70; 95% CI: 0.52, 0.94), and there was a suggestion of decreased risk for endometrioid carcinomas; in contrast, OC use was associated with non-significant increase in risk of mucinous and clear cell carcinomas.
Table 2.
Adjusted RR and 95% CI for ovarian cancer in relation to hormonal and reproductive factors in the NIH-AARP Diet and Health Study, 1995-2006
| Histological Subtypes | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-cases | All Epithelial (N=849) | Serous (N=451) | Endometrioid (N=78) | Mucinous (N=38) | Clear Cell (N=27) | Other (N=255) | |||||||||||||
| N | N | RR | 95% CI | N | RR | 95% CI | N | RR | 95% CI | N | RR | 95% CI | N | RR | 95% CI | N | RR | 95% CI | |
| Age at menarche (years) | |||||||||||||||||||
| < 13 | 81,348 | 406 | 1(ref) | 228 | 1(ref) | 38 | 1(ref) | 17 | 1(ref) | 11 | 1(ref) | 112 | 1(ref) | ||||||
| 13-14 | 70,564 | 363 | 1.00 | (0.88-1.16) | 181 | 0.90 | (0.74-1.09) | 34 | 1.05 | (0.66-1.66) | 17 | 1.12 | (0.57-2.20) | 14 | 1.53 | (0.69-3.37) | 117 | 1.15 | (0.89-1.50) |
| ≥ 15 | 16,071 | 77 | 0.94 | (0.74-1.20) | 42 | 0.92 | (0.66-1.28) | 5 | 0.69 | (0.27-1.75) | 4 | 1.14 | (0.38-3.38) | 2 | 1.00 | (0.22-4.53) | 24 | 1.03 | (0.67-1.61) |
| Pheterogenity= | 0.67 | ||||||||||||||||||
| Age at menopause | |||||||||||||||||||
| < 45 | 13,847 | 56 | 1(ref) | 34 | 1(ref) | 2 | 1(ref) | 2 | 1(ref) | 1 | 1(ref) | 17 | 1(ref) | ||||||
| 45-49 | 32,073 | 158 | 1.01 | (0.83-1.24) | 81 | 0.93 | (0.71-1.22) | 13 | 0.90 | (0.46-1.77) | 12 | 2.10 | (0.85-5.19) | 8 | 2.01 | (0.69-5.87) | 44 | 1.00 | (0.69-1.46) |
| 50-54 | 55,559 | 293 | 1.07 | (0.91-1.27) | 144 | 0.94 | (0.74-1.18) | 29 | 1.16 | (0.67-1.99) | 15 | 1.58 | (0.66-3.75) | 9 | 1.39 | (0.49-3.94) | 96 | 1.24 | (0.92-1.68) |
| ≥ 55 | 12,516 | 67 | 1.04 | (0.79-1.36) | 35 | 0.95 | (0.66-1.37) | 7 | 1.31 | (0.56-3.04) | 3 | 1.35 | (0.36-5.11) | 0 | 22 | 1.17 | (0.73-1.88) | ||
| Pheterogenity= | 0.29 | ||||||||||||||||||
| Menopausal hormone | |||||||||||||||||||
| Never user | 91,716 | 420 | 1(ref) | 209 | 1(ref) | 34 | 1(ref) | 29 | 1(ref) | 14 | 1(ref) | 134 | 1(ref) | ||||||
| Ever user | 76,826 | 429 | 1.33 | (1.16-1.53) | 242 | 1.50 | (1.24-1.81) | 44 | 1.57 | (0.99-2.47) | 9 | 0.37 | (0.18-0.80) | 13 | 1.06 | (0.49-2.29) | 121 | 1.25 | (0.98-1.61) |
| Pheterogenity= | 0.01 | ||||||||||||||||||
| Duration of MHT use | |||||||||||||||||||
| Never user | 91,716 | 420 | 1(ref) | 209 | 1(ref) | 34 | 1(ref) | 29 | 1(ref) | 14 | 1(ref) | 134 | 1(ref) | ||||||
| <5 years | 33,286 | 139 | 1.03 | 0.85, 1.25 | 82 | 1.21 | 0.93, 1.56 | 15 | 1.18 | 0.63, 2.19 | 2 | 0.22 | 0.05, 0.92 | 3 | 0.47 | 0.13, 1.67 | 37 | 0.96 | 0.66, 1.39 |
| 5-9 years | 21,061 | 132 | 1.51 | 1.24, 1.84 | 78 | 1.78 | 1.36, 2.32 | 12 | 1.49 | 0.76, 2.90 | 3 | 0.45 | 0.13, 1.49 | 5 | 1.52 | 0.54, 4.30 | 34 | 1.34 | 0.92, 1.97 |
| ≥10 years | 22,260 | 158 | 1.57 | 1.31, 1.89 | 82 | 1.64 | 1.27, 2.13 | 17 | 2.27 | 1.26, 4.09 | 4 | 0.50 | 0.17, 1.42 | 5 | 1.82 | 0.64, 5.17 | 50 | 1.53 | 1.11, 2.13 |
| Pheterogenity= | 0.06 | ||||||||||||||||||
| Oral contraceptive use | |||||||||||||||||||
| Never user | 99,727 | 577 | 1(ref) | 309 | 1(ref) | 51 | 1(ref) | 22 | 1(ref) | 13 | 1(ref) | 182 | 1(ref) | ||||||
| Ever user | 67,605 | 265 | 0.74 | 0.63, 0.87 | 140 | 0.69 | 0.55, 0.85 | 27 | 0.69 | 0.42, 1.14 | 16 | 1.72 | 0.86, 3.42 | 14 | 1.47 | 0.64, 3.40 | 68 | 0.70 | 0.52, 0.94 |
| Pheterogenity= | 0.03 | ||||||||||||||||||
| Duration of oral contraceptive use | |||||||||||||||||||
| Never user | 99,727 | 577 | 1(ref) | 309 | 1(ref) | 51 | 1(ref) | 22 | 1(ref) | 13 | 1(ref) | 182 | 1(ref) | ||||||
| 1-4 years | 29,975 | 128 | 0.82 | 0.67, 1.00 | 66 | 0.75 | 0.57, 0.98 | 12 | 0.72 | 0.38, 1.38 | 7 | 1.65 | 0.68, 4.00 | 9 | 2.19 | 0.87, 5.50 | 34 | 0.79 | 0.54, 1.15 |
| 5-9 years | 21,205 | 87 | 0.78 | 0.62, 0.98 | 48 | 0.75 | 0.55, 1.03 | 10 | 0.83 | 0.41, 1.67 | 4 | 1.42 | 0.48, 4.26 | 4 | 1.36 | 0.42, 4.43 | 21 | 0.68 | 0.43, 1.09 |
| ≥10 years | 16,425 | 50 | 0.56 | 0.42, 0.75 | 26 | 0.51 | 0.34, 0.77 | 5 | 0.52 | 0.20, 1.32 | 5 | 2.30 | 0.84, 6.27 | 1 | 0.43 | 0.05, 3.36 | 13 | 0.52 | 0.29, 0.92 |
| Pheterogenity= | 0.13 | ||||||||||||||||||
| Parous | |||||||||||||||||||
| Nulliparous | 25,785 | 170 | 1(ref) | 82 | 1(ref) | 22 | 1(ref) | 8 | 1(ref) | 11 | 1(ref) | 47 | 1(ref) | ||||||
| Parous | 142,092 | 676 | 0.71 | 0.61, 0.85 | 369 | 0.83 | 0.65, 1.06 | 56 | 0.49 | 0.30, 0.80 | 29 | 0.54 | 0.25, 1.14 | 16 | 0.28 | 0.13, 0.62 | 206 | 0.76 | 0.56, 1.04 |
| Pheterogenity= | 0.05 | ||||||||||||||||||
| Age at first birth (years) | |||||||||||||||||||
| Nulliparous | 25,785 | 170 | 1ref | 82 | 1(ref) | 22 | 1(ref) | 8 | 1(ref) | 11 | 1(ref) | 47 | 1(ref) | ||||||
| <20 | 27,790 | 122 | 0.70 | 0.50, 0.98 | 60 | 0.69 | 0.50, 0.97 | 12 | 0.51 | 0.25, 1.04 | 3 | 0.34 | 0.09, 1.29 | 3 | 0.26 | 0.07, 0.94 | 44 | 0.92 | 0.61, 1.39 |
| 20-<25 | 72,486 | 338 | 0.81 | 0.62, 1.05 | 187 | 0.80 | 0.61, 1.03 | 28 | 0.47 | 0.27, 0.83 | 17 | 0.68 | 0.29, 1.59 | 4 | 0.14 | 0.04, 0.44 | 102 | 0.74 | 0.52, 1.05 |
| 25-<30 | 31,133 | 158 | 0.98 | 0.67, 1.22 | 90 | 0.89 | 0.66, 1.20 | 7 | 0.28 | 0.12, 0.65 | 8 | 0.73 | 0.27, 1.96 | 7 | 0.54 | 0.21, 1.43 | 46 | 0.75 | 0.50, 1.13 |
| >30 | 10,644 | 58 | 0.89 | 0.59, 1.35 | 30 | 0.88 | 0.58, 1.34 | 9 | 1.06 | 0.49, 2.32 | 2 | 0.52 | 0.11, 2.46 | 2 | 0.45 | 0.10, 2.03 | 15 | 0.72 | 0.40, 1.29 |
| Pheterogenity= | 0.36 | ||||||||||||||||||
| Parity (births) | |||||||||||||||||||
| Nulliparous | 25,785 | 170 | 1ref | 82 | 1(ref) | 22 | 1(ref) | 8 | 1(ref) | 11 | 1(ref) | 47 | 1(ref) | ||||||
| 1 | 17,398 | 104 | 0.93 | 0.73, 1.19 | 55 | 1.02 | 0.73, 1.44 | 11 | 0.77 | 0.37, 1.59 | 6 | 1.06 | 0.37, 3.09 | 4 | 0.51 | 0.16, 1.61 | 28 | 0.91 | 0.57, 1.46 |
| 2 | 43,696 | 216 | 0.76 | 0.62, 0.93 | 119 | 0.87 | 0.65, 1.15 | 20 | 0.54 | 0.29, 1.00 | 10 | 0.73 | 0.28, 1.80 | 5 | 0.26 | 0.09, 0.74 | 62 | 0.79 | 0.54, 1.16 |
| ≥ 3 | 81,018 | 356 | 0.64 | 0.53, 0.77 | 195 | 0.74 | 0.57, 0.96 | 25 | 0.38 | 0.21, 0.67 | 13 | 0.44 | 0.18, 1.07 | 7 | 0.23 | 0.09, 0.60 | 116 | 0.72 | 0.51, 1.02 |
| Pheterogenity= | 0.03 | ||||||||||||||||||
† Adjusted for age (continuous), oral contraceptive use (ever/never), parity (yes/no), menopausal hormone therapy (ever/never). Unknow n/missing set as a separate category within each factor. For duration of oral contraceptive and menopausal hormone therapy use, excluded dichotomous ever/never use variable accordingly, as done for parous for categories of parity.
‡ P value from polytomous logistic regression of case-only analysis comparing each covariate, adjusted for factors listed above.
Compared to nulliparous women, parous women were at decreased risk for all subtypes, with the RRs ranging from 0.28 (95% CI: 0.13, 0.62) for clear cell to 0.83 (95% CI: 0.65, 1.06) for serous carcinomas. When MHT and OC use were examined according to years of use and parity by number of live births, the associations were in the same direction as the dichotomous indicators of these variables, although the P-heterogeneity by histologic subtypes was only significant for number of births. We did not observe an association between ovarian cancer risk with the other hormonal and reproductive factors we examined and noted less consistency in subtype-specific risk estimates with respect to ages at menarche, menopause, and first birth (P-heterogeneity ≥ 0.29).
Table 3 presents the associations between demographic and lifestyle factors and ovarian cancer by histologic subtypes. We observed decreased risk among non-White race/ethnicity compared to Whites for epithelial ovarian carcinoma overall and for each histologic subtype, although there was no significant difference in magnitude of association across histologic subtypes (P-heterogeneity = 0.57). We observed some differences in the risks associated with various demographic and lifestyle factors by histologic type, but statistically significant heterogeneity only for BMI (P-heterogeneity=0.03). Compared to normal/overweight (BMI<30kg/m2), being obese (BMI ≥30kg/m2) was associated with statistically significant increased risk in endometrioid (RR=1.64; 95% CI: 1.00, 2.70) and other epithelial (RR=1.53; 95% CI: 1.17, 2.02) carcinomas, while there was some suggestion of decreased risk for serous, mucinous, and clear cell histologic subtypes. We also observed some indication of heterogeneity across histologies in risks associated with education, physical activity, and smoking status, albeit the heterogeneity was not statistically significant (P-heterogeneity = 0.11, 0.18, and 0.19, respectively). Less consistency in subtype-specific associations were noted with respect to amount of alcohol intake (P-heterogeneity = 0.53).
Table 3.
Adjusted RR and 95% CI for ovarian cancer in relation to demographic and lifestyle factors in the NIH-AARP Diet and Health Study, 1995-2006
| Histological Subtypes | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-cases | All Epithelial (N=849) | Serous (N=451) | Endometrioid (N=78) | Mucinous (N^38) | Clear Cell (N=27) | Other (N=255) | |||||||||||||
| N | N | RR | 95% CI | N | RR | 95% CI | N | RR | 95% CI | N | RR | 95% CI | N | RR | 95% CI | N | RR | 95% CI | |
| Race/ethnicity | |||||||||||||||||||
| White | 151,329 | 792 | 1(ref) | 425 | 1(ref) | 71 | 1(ref) | 36 | 1(ref) | 26 | 1(ref) | 234 | 1(ref) | ||||||
| Non-White | 17,213 | 57 | 0.66 | (0.50-0.86) | 26 | 0.56 | (0.38-0.84) | 7 | 0.90 | (0.42-1.97) | 2 | 0.47 | (0.11-1.94) | 1 | 0.35 | (0.05-2.60) | 21 | 0.81 | (0.52-1.26) |
| Pheterogenity= | 0.57 | ||||||||||||||||||
| Education | |||||||||||||||||||
| < High school | 51,854 | 245 | 1(ref) | 116 | 1(ref) | 27 | 1(ref) | 14 | 1(ref) | 10 | 1(ref) | 78 | 1(ref) | ||||||
| ≥ High school | 111,531 | 570 | 1.09 | (0.94-1.27) | 313 | 1.26 | (1.02-1.57) | 50 | 0.75 | (0.46-1.21) | 22 | 0.81 | (0.41-1.61) | 17 | 0.57 | (0.25-1.28) | 168 | 1.08 | (0.82-1.42) |
| Pheterogenity= | 0.11 | ||||||||||||||||||
| Body mass index (kg/m2) | |||||||||||||||||||
| < 30 | 126,173 | 617 | 1(ref) | 340 | 1(ref) | 50 | 1(ref) | 30 | 1(ref) | 21 | 1(ref) | 176 | 1(ref) | ||||||
| ≥ 30 | 37,081 | 197 | 1.15 | (0.98-1.35) | 89 | 0.95 | (0.75-1.20) | 23 | 1.64 | (1.00-2.70) | 7 | 0.76 | (0.33-1.73) | 4 | 0.66 | (0.23-1.94) | 74 | 1.53 | (1.17-2.02) |
| Pheterogenity= | 0.03 | ||||||||||||||||||
| Frequency of vigorous physical activity | |||||||||||||||||||
| Never/Rarely | 37,898 | 188 | 1(ref) | 90 | 1(ref) | 14 | 1(ref) | 14 | 1(ref) | 9 | 1(ref) | 61 | 1(ref) | ||||||
| <2 times/week | 59,319 | 291 | 0.97 | (0.81-1.17) | 157 | 1.08 | (0.83-1.40) | 32 | 1.37 | (0.73-2.56) | 12 | 0.59 | (0.27-1.27) | 10 | 0.64 | (0.26-1.57) | 80 | 0.85 | (0.61-1.19) |
| 3+ times/week | 69,386 | 362 | 0.99 | (0.83-1.18) | 198 | 1.12 | (0.87-1.44) | 31 | 1.13 | (0.60-2.13) | 12 | 0.49 | (0.22-1.06) | 8 | 0.45 | (0.17-1.17) | 113 | 0.96 | (0.70-1.31) |
| Pheterogenity= | 0.18 | ||||||||||||||||||
| Alcohol intake | |||||||||||||||||||
| 0 grams/day | 48,956 | 235 | 1(ref) | 127 | 1(ref) | 16 | 1(ref) | 6 | 1(ref) | 9 | 1(ref) | 77 | 1(ref) | ||||||
| >0 - <12 grams/day | 95,197 | 482 | 1.06 | (0.90-1.24) | 249 | 1.00 | (0.81-1.24) | 48 | 1.47 | (0.83-2.59) | 29 | 2.68 | (1.11-6.46) | 15 | 0.76 | (0.33-1.75) | 141 | 0.98 | (0.74-1.30) |
| 12 - >24 grams/day | 14,857 | 90 | 1.23 | (0.96-1.57) | 54 | 1.36 | (0.99-1.87) | 10 | 1.95 | (0.88-4.30) | 1 | 0.57 | (0.07-4.74) | 3 | 0.94 | (0.25-3.49) | 22 | 0.94 | (0.59-1.52) |
| ≥ 24 grams/day | 9,532 | 42 | 0.93 | (0.67-1.30) | 21 | 0.87 | (0.55-1.38) | 4 | 1.21 | (0.40-3.62) | 2 | 1.76 | (0.35-8.73) | 0 | 15 | 1.06 | (0.61-1.85) | ||
| Pheterogenity= | 0.53 | ||||||||||||||||||
| Smoking status | |||||||||||||||||||
| Never smoker | 73,587 | 407 | 1(ref) | 235 | 1(ref) | 34 | 1(ref) | 14 | 1(ref) | 10 | 1(ref) | 114 | 1(ref) | ||||||
| Ever smoker | 89,961 | 425 | 0.93 | (0.81-1.06) | 207 | 0.78 | (0.65-0.94) | 42 | 1.05 | (0.67-1.64) | 23 | 1.38 | (0.72-2.65) | 17 | 1.48 | (0.68-3.25) | 136 | 1.08 | (0.85-1.39) |
| Pheterogenity= | 0.19 | ||||||||||||||||||
| Former smoker | 65,701 | 327 | 0.93 | (0.81-1.08) | 155 | 0.76 | (0.62-0.93) | 35 | 1.18 | (0.73-1.89) | 18 | 1.49 | (0.74-3.00) | 13 | 1.46 | (0.64-3.35) | 106 | 1.09 | (0.84-1.42) |
| Pheterogenity= | 0.12 | ||||||||||||||||||
| Current smoker | 24,260 | 98 | 0.83 | (0.67-1.04) | 52 | 0.76 | (0.57-1.03) | 7 | 0.67 | (0.30-1.51) | 5 | 1.12 | (0.40-3.13) | 4 | 1.20 | (0.38-3.85) | 30 | 0.96 | (0.64-1.43) |
| Pheterogenity= | 0.87 | ||||||||||||||||||
† Adjusted for age (continuous), oral contraceptive use (ever/never), parity (yes/no), menopausal hormone therapy (ever/never). Unknow n/mssing set as a separate category within each factor.
‡ P value from polytomous logistic regression of case-only analysis comparing each covariate, adjusted for factors listed above.
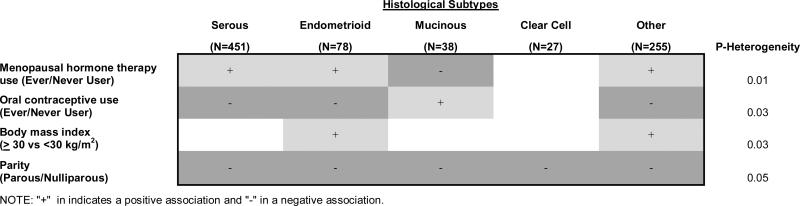
Figure 1 summarizes the risk factor associations for those that differed by histologic types (P-heterogeneity ≤ 0.05), namely menopausal hormone therapy use, oral contraceptive use, BMI and parity. We limited our presentation to dichotomous indicators for each of these variables. This figure illustrates risk factor associations that differed by ovarian carcinoma histologic type in our analysis.
Figure 1.
Attached two versions: (1) color format for online publication (2) b/w for print publication
Summary of risk associations by ovarian cancer histologic subtype in the NIH-AARP Diet and Health Study, 1995-2006
We also calculated P-heterogeneity excluding the other epithelial subtype and found similar results, except for BMI (P-heterogeneity = 0.17). As an additional sensitivity analysis, we applied a model based on competing risk as described by Lunn et al.32 to assess P-heterogeneity among the five subtypes. The competing risk approach gave similar results (data not shown). In all our main analyses, we included all ovarian cancer cases irrespective of whether they had a previous cancer at other sites to maintain sample size. As a sensitivity analysis, we also limited our case definition to 697 primary incident ovarian cancer cases that had no other previous cancers at other sites before the ovarian cancer (data not shown). Of the total 697 cases, 373 (54%) were classified as serous, 66 (9%) as endometrioid, 32 (5%) as mucinous, 24 (3%) as clear cell, and 202 (29%) as other epithelial ovarian cancer. We noted similar heterogeneity in risk estimates for MHT use, OC use, parity, and BMI across subtypes when the analysis was limited to these cases, albeit the heterogeneity was not statistically significant for OC use, parity, and BMI P-heterogeneity = 0.07, 0.19, 0.08). As expected, the associations were in the same direction, with comparable or slightly weaker magnitude of effects, except for BMI, which were based on fewer number of mucinous (n=32 vs. 38) and clear cell (n=24vs. 27) cases.
DISCUSSION
Our analysis of 849 ovarian cancer cases included in the NIH-AARP Diet and Health Study cohort of 169,391 women confirms previously identified risk factor associations for ovarian carcinoma and extends this work to examine potential heterogeneity by histologic subtype. Similar to some prior reports, we found differences in associations by histologic subtypes.
Histologic comparisons in our analysis were hampered by the high percentage of cases with a diagnosis of unspecified adenocarcinoma or carcinomas, but this is an inherent problem of many epidemiological studies assessing ovarian cancer endpoints. For example, in an examination of the histologic subtypes of ovarian carcinomas (n=112,541) from 1995-2004 identified through 24 population-based registries in the United States, serous carcinomas (44%) were the most commonly diagnosed histologic type and approximately 30% were defined as “adenocarcinoma, NOS” or “unspecified”.33
Our results showed that MHT and OC use were associated with increased and decreased risks, respectively, for serous and endometrioid cancers, consistent with previous findings of studies that have focused on ovarian cancer histologic-subtypes.8, 12, 14 In contrast, we observed a statistically significant decreased risk between MHT and mucinous carcinomas similar to other previous studies.7, 12 In terms of number of pregnancies or live births, similar to our results for parity, some studies have shown inverse relationships for all histologic subtypes.7-11 We found the inverse relationship to be strongest for clear cell tumors, consistent with a previous pooled analysis of 10 case-control studies.10 Similar to some previous reports, we also observed no significant variation by ovarian histology for ages at menarche,8, 10, 21 menopause,10, 14 or first birth.10, 16 We were limited in our ability to examine the variability of these variables given that they were only available as categorical variables.
We observed that obesity (BMI<30kg/m2 vs. ≥30kg/m2) was positively associated with endometrioid ovarian cancers in our study. In contrast, we observed no relationship of obesity for serous ovarian cancers. Similarly, a recent analysis in the Nurse's Health Study involving 721 cases reported that BMI was positively associated with endometrioid cancers, but unassociated with serous and mucinous carcinomas.14 A systematic review of obesity and risk of epithelial ovarian cancer reported that only one case-control study and the pooled analysis found BMI linked with increases in risk for endometrioid cancers, leading the authors to cautiously interpret the relationship.18
A unified ovarian cancer progression model has not yet been established and growing evidence suggests that the histologic diversity of epithelial ovarian cancers indicates different etiologic pathways.34 Progress in understanding ovarian carcinogenesis is partly limited by our lack of clarity on the exact tissue of origin of ovarian epithelial carcinomas. Several ovarian cancer subtypes have been described to resemble cancers of other sites, both morphologically and molecularly: fallopian tube (serous), endometrium (endometrioid), gastrointestinal tract (mucinous), and unspecified glycogeneated epithelium (clear cell).35-36 Accordingly, patterns of gene expression of different histotypes of epithelial ovarian cancer have shown to correlate with normal fallopian tube, endometrium, and colon.37-38 We observed several risk factor associations that showed considerable heterogeneity across ovarian cancer subtypes and that were similar to associations observed for the phenotypically similar cancers. Notably, obesity was positively associated only with endometrioid ovarian cancers in our study, consistent with increased risk observed for endometrial cancer.39 In addition, we observed similar risk associations for MHT and OC use with serous and endometrioid ovarian cancer tumors as with studies of cancers of the fallopian tube and endometrium40-42 in parallel with evidence that fallopian tube epithelium and the peritoneal epithelial lining might give rise to some ovarian cancers.43-45 The inverse association between mucinous carcinomas and MHT use well as parity is consistent with the relationship of these hormonal and reproductive factors with colorectal cancer risk.46-47
Given the emerging studies demonstrating variation in molecular, pathological, and clinical characteristics between subtypes of ovarian cancer,3-5, 48 our results add to the evolving hypotheses about the etiology of epithelial ovarian cancer. The similarities between the subtypes with histologic subtypes of other cancers support the likelihood of a common pathogenesis. However, the ovary is a common site of metastases, with secondary carcinomas accounting for approximately 10-30% of all ovarian malignancies.49 In some occasions, the primary carcinoma may remain undetected, leading to misclassification of the ovarian metastasis as a primary carcinoma. Most common sites of origin of metastases to the ovaries are the gastrointestinal tract and the endometrium.50-51 Thus, similar risk factors may be observed because of misclassification of metastases from other cancer sites. Furthermore, the same initial carcinogenic mechanism could give rise to different histologic phenotypes. Molecular analyses of individual lesions, which incorporate assessment of detailed pathologic characterization as well as the epidemiological data, may assist in further clarifying our understanding about ovarian cancer heterogeneity.
The recently updated follow-up data of the NIH-AARP Diet and Health Study provided an opportunity to examine prospectively the association of potential ovarian cancer risk factors by ovarian cancer histologic subtypes in a large cohort of women. Previous analyses of MHT, physical activity, and BMI and ovarian cancer risk in the NIH-AARP dataset were based on smaller number of ovarian cancer cases, limiting the feasibility to examine these potential ovarian cancer risk factors by histological subtype. Additionally, our analysis was based on a single cohort with risk factors assessed in the same manner and with cases accrued over a relatively short period of time (approximately 5 years), limiting secular trends of histology terminology and prevalence of risk factors.
Despite the large number of ovarian cancers, only a few of the case-case comparisons with different directions of the associations and different effect size estimates were statistically significant because several subtypes, particularly mucinous and clear cell, were rare. Another limitation of the study is that we relied on pathology information recorded by local pathologists and did not perform centralized pathology reviews, which may have led to misclassification bias. A substantial proportion of our cases were categorized as epithelial cancer without further specification. While these unclassified epithelial cancers are frequently thought to be serous primarily, the associations observed in our study did not reveal a clear pattern as to which subtype this group most resembled. In addition, we may be missing ovarian cancer at young age given the age of the study population of AARP members. Our exposure information was collected at baseline, and some of our factors of interest, such as lifestyle factors, may have changed, potentially leading to misclassification of exposures in some women. However, this would not be expected to give rise to spurious differences in relationships according to carcinoma histology.
In conclusion, our findings suggest that phenotypic heterogeneity of ovarian cancers might reflect etiologic heterogeneity. To attain adequate power to establish or rule out putative associations specific to ovarian cancer subtypes, additional evaluation of potential risk factors will likely need to depend on consortial efforts. Additionally, molecular analyses of ovarian cancers and the tissue at risk integrated with detailed clinicopathologic and epidemiologic characterization may reveal molecular subtypes of ovarian cancer that can more precisely clarify our understanding of ovarian cancer heterogeneity.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System under contract to the Department of Health (DOH). The views expressed herein are solely those of the authors and do not necessarily reflect those of the contractor or DOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services.
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis.
Megan A Murphy is supported in part by training grant NIH 5 T32 CA09001-35.
In memory of Dr. Arthur Schatzkin, visionary investigator who founded the NIH-AARP Diet and Health Study.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Fleming JS, Beaugie CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Mol Cell Endocrinol. 2006;247:4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Kobel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, Prentice LM, Miller D, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Medicine. 2008;5:e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soslow RA. Histologic subtypes of ovarian carcinoma: an overview. International Journal of Gynecological Pathology. 2008;27:161–74. doi: 10.1097/PGP.0b013e31815ea812. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Raposo C, Mendiola M, Barriuso J, Hardisson D, Redondo A. Molecular characterization of ovarian cancer by gene-expression profiling. Gynecologic Oncology. 118:88–92. doi: 10.1016/j.ygyno.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Kaku T, Ogawa S, Kawano Y, Ohishi Y, Kobayashi H, Hirakawa T, Nakano H. Histological classification of ovarian cancer. Med Electron Microsc. 2003;36:9–17. doi: 10.1007/s007950300002. [DOI] [PubMed] [Google Scholar]
- 7.Modugno F, Ness RB, Wheeler JE. Reproductive risk factors for epithelial ovarian cancer according to histologic type and invasiveness. Annals of Epidemiology. 2001;11:568–74. doi: 10.1016/s1047-2797(01)00213-7. [DOI] [PubMed] [Google Scholar]
- 8.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, Persson IR. Risk factors for invasive epithelial ovarian cancer: results from a Swedish case-control study. Am J Epidemiol. 2002;156:363–73. doi: 10.1093/aje/kwf048. [DOI] [PubMed] [Google Scholar]
- 9.Tung KH, Goodman MT, Wu AH, McDuffie K, Wilkens LR, Kolonel LN, Nomura AM, Terada KY, Carney ME, Sobin LH. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. American Journal of Epidemiology. 2003;158:629–38. doi: 10.1093/aje/kwg177. [DOI] [PubMed] [Google Scholar]
- 10.Kurian AW, Balise RR, McGuire V, Whittemore AS. Histologic types of epithelial ovarian cancer: have they different risk factors? Gynecologic Oncology. 2005;96:520–30. doi: 10.1016/j.ygyno.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 11.Nagle CM, Olsen CM, Webb PM, Jordan SJ, Whiteman DC, Green AC. Endometrioid and clear cell ovarian cancers: a comparative analysis of risk factors. European Journal of Cancer. 2008;44:2477–84. doi: 10.1016/j.ejca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Risch HA, Marrett LD, Jain M, Howe GR. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. American Journal of Epidemiology. 1996;144:363–72. doi: 10.1093/oxfordjournals.aje.a008937. [DOI] [PubMed] [Google Scholar]
- 13.Parazzini F, Chiaffarino F, Negri E, Surace M, Benzi G, Franceschi S, Fedele L, La Vecchia C. Risk factors for different histological types of ovarian cancer. International Journal of Gynecological Cancer. 2004;14:431–6. doi: 10.1111/j.1048-891x.2004.14302.x. [DOI] [PubMed] [Google Scholar]
- 14.Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. American Journal of Epidemiology. 171:45–53. doi: 10.1093/aje/kwp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. A prospective study of postmenopausal hormone use and ovarian cancer risk. Br J Cancer. 2007;96:151–6. doi: 10.1038/sj.bjc.6603527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riman T, Dickman PW, Nilsson S, Nordlinder H, Magnusson CM, Persson IR. Some life-style factors and the risk of invasive epithelial ovarian cancer in Swedish women. Eur J Epidemiol. 2004;19:1011–9. doi: 10.1007/s10654-004-1633-8. [DOI] [PubMed] [Google Scholar]
- 17.Lacey JV, Jr., Leitzmann M, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Weight, height, and body mass index and risk for ovarian cancer in a cohort study. Ann Epidemiol. 2006;16:869–76. doi: 10.1016/j.annepidem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. 2007;43:690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Schouten LJ, Rivera C, Hunter DJ, Spiegelman D, Adami HO, Arslan A, Beeson WL, van den Brandt PA, Buring JE, Folsom AR, Fraser GE, Freudenheim JL, et al. Height, body mass index, and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2008;17:902–12. doi: 10.1158/1055-9965.EPI-07-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahmann PH, Cust AE, Friedenreich CM, Schulz M, Lukanova A, Kaaks R, Lundin E, Tjonneland A, Halkjaer J, Severinsen MT, Overvad K, Fournier A, et al. Anthropometric measures and epithelial ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 126:2404–15. doi: 10.1002/ijc.24952. [DOI] [PubMed] [Google Scholar]
- 21.Chiaffarino F, Parazzini F, Bosetti C, Franceschi S, Talamini R, Canzonieri V, Montella M, Ramazzotti V, La Vecchia C. Risk factors for ovarian cancer histotypes. European Journal of Cancer. 2007;43:1208–13. doi: 10.1016/j.ejca.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Olsen CM, Bain CJ, Jordan SJ, Nagle CM, Green AC, Whiteman DC, Webb PM. Recreational physical activity and epithelial ovarian cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2007;16:2321–30. doi: 10.1158/1055-9965.EPI-07-0566. [DOI] [PubMed] [Google Scholar]
- 23.Genkinger JM, Hunter DJ, Spiegelman D, Anderson KE, Buring JE, Freudenheim JL, Goldbohm RA, Harnack L, Hankinson SE, Larsson SC, Leitzmann M, McCullough ML, et al. Alcohol intake and ovarian cancer risk: a pooled analysis of 10 cohort studies. Br J Cancer. 2006;94:757–62. doi: 10.1038/sj.bjc.6603020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson NB, Trentham-Dietz A, Newcomb PA, Chen Z, Hampton JM, Willett WC, Egan KM. Alcohol consumption and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2006;119:2423–7. doi: 10.1002/ijc.22137. [DOI] [PubMed] [Google Scholar]
- 25.Marchbanks PA, Wilson H, Bastos E, Cramer DW, Schildkraut JM, Peterson HB. Cigarette smoking and epithelial ovarian cancer by histologic type. Obstet Gynecol. 2000;95:255–60. doi: 10.1016/s0029-7844(99)00531-1. [DOI] [PubMed] [Google Scholar]
- 26.Pan SY, Ugnat AM, Mao Y, Wen SW, Johnson KC. Association of cigarette smoking with the risk of ovarian cancer. Int J Cancer. 2004;111:124–30. doi: 10.1002/ijc.20242. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. Cigarette smoking and increased risk of mucinous epithelial ovarian cancer. Am J Epidemiol. 2004;159:133–9. doi: 10.1093/aje/kwh015. [DOI] [PubMed] [Google Scholar]
- 28.Gram IT, Braaten T, Adami HO, Lund E, Weiderpass E. Cigarette smoking and risk of borderline and invasive epithelial ovarian cancer. Int J Cancer. 2008;122:647–52. doi: 10.1002/ijc.23108. [DOI] [PubMed] [Google Scholar]
- 29.Tworoger SS, Gertig DM, Gates MA, Hecht JL, Hankinson SE. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer. 2008;112:1169–77. doi: 10.1002/cncr.23275. [DOI] [PubMed] [Google Scholar]
- 30.Gram IT, Lukanova A, Brill I, Braaten T, Lund E, Lundin E, Overvad K, Tjonneland A, Clavel-Chapelon F, Chabbert-Buffet N, Bamia C, Trichopoulou A, et al. Cigarette smoking and risk of histological subtypes of epithelial ovarian cancer in the EPIC cohort study. Int J Cancer. 2011 doi: 10.1002/ijc.26235. [DOI] [PubMed] [Google Scholar]
- 31.Schatzkin A, Midthune D, Subar A, Thompson F, Kipnis V. The National Institutes of Health-American association of retired persons (NIH-AARP) Diet and Health Study: Power to detect diet-cancer associations after adjusting for measurement error. American Journal of Epidemiology. 2001;153:S259–S. [Google Scholar]
- 32.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–32. [PubMed] [Google Scholar]
- 33.Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiol Biomarkers Prev. 2009;18:132–9. doi: 10.1158/1055-9965.EPI-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–37. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- 35.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlson J, Roh MH, Chang MC, Crum CP. Recent advances in the understanding of the pathogenesis of serous carcinoma: the concept of low- and high-grade disease and the role of the fallopian tube. Diagn Histopathol (Oxf) 2008;14:352–65. doi: 10.1016/j.mpdhp.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, Atkinson EN, Smith DI, Hartmann L, Fishman D, Berchuck A, Whitaker R, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–26. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 38.Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, Barrett JC, Boyd J, Birrer MJ. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–30. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 39.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:1531–43. [PubMed] [Google Scholar]
- 40.Vicus D, Finch A, Rosen B, Fan I, Bradley L, Cass I, Sun P, Karlan B, McLaughlin J, Narod SA. Risk factors for carcinoma of the fallopian tube in women with and without a germline BRCA mutation. Gynecol Oncol. 118:155–9. doi: 10.1016/j.ygyno.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Allen NE, Tsilidis KK, Key TJ, Dossus L, Kaaks R, Lund E, Bakken K, Gavrilyuk O, Overvad K, Tjonneland A, Olsen A, Fournier A, et al. Menopausal hormone therapy and risk of endometrial carcinoma among postmenopausal women in the European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol. 2010;172:1394–403. doi: 10.1093/aje/kwq300. [DOI] [PubMed] [Google Scholar]
- 42.Mueck AO, Seeger H, Rabe T. Hormonal contraception and risk of endometrial cancer: a systematic review. Endocr Relat Cancer. 2010;17:R263–71. doi: 10.1677/ERC-10-0076. [DOI] [PubMed] [Google Scholar]
- 43.Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, Lee Y, Crum CP. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 44.Przybycin CG, Kurman RJ, Ronnett BM, Shih Ie M, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–16. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 45.Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tube-peritoneal junction: a potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30:4–11. doi: 10.1097/PGP.0b013e3181f29d2a. [DOI] [PubMed] [Google Scholar]
- 46.Wernli KJ, Wang Y, Zheng Y, Potter JD, Newcomb PA. The relationship between gravidity and parity and colorectal cancer risk. J Womens Health (Larchmt) 2009;18:995–1001. doi: 10.1089/jwh.2008.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zervoudakis A, Strickler HD, Park Y, Xue X, Hollenbeck A, Schatzkin A, Gunter MJ. Reproductive History and Risk of Colorectal Cancer in Postmenopausal Women. J Natl Cancer Inst. 2011 doi: 10.1093/jnci/djr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(Suppl 2):S19–32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 49.Kir G, Gurbuz A, Karateke A, Kir M. Clinicopathologic and immunohistochemical profile of ovarian metastases from colorectal carcinoma. World J Gastrointest Surg. 2010;2:109–16. doi: 10.4240/wjgs.v2.i4.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prat J. Ovarian carcinomas, including secondary tumors: diagnostically challenging areas. Mod Pathol. 2005;18(Suppl 2):S99–111. doi: 10.1038/modpathol.3800312. [DOI] [PubMed] [Google Scholar]
- 51.Young RH. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary. Part II. Adv Anat Pathol. 2007;14:149–77. doi: 10.1097/PAP.0b013e3180504abf. [DOI] [PubMed] [Google Scholar]