Abstract
We assessed the involvement of the amygdala in a task in which object choices were guided by internal context. Rhesus monkeys were trained on a biconditional discrimination whereby objects associated with food (but not water) were baited when the monkey was hungry, and objects associated with water (but not food) were baited when the monkey was thirsty. To solve this task monkeys were required to choose objects yielding the reward congruent with their internal motivational state. Lesions of the amygdala did not disrupt learning or performance of this task. We conclude that the involvement of the amygdala in selective-satiation tasks, which depends in part on a change in internal context, is not due to the amygdala playing a general role in representing, or using, internal context.
Keywords: satiety, reward, internal state, motivation, hunger, thirst
Introduction
Previous studies in monkeys have shown that the amygdala is essential for making choices among objects based on current biological needs, a function that is demonstrated in selective-satiation tasks. The task is typically carried out in two phases. In the training phase monkeys learn that different objects predict the future availability of different foods. For example, they learn that Object A predicts Food 1 and that Object B predicts Food 2. In the test phase, monkeys consume one of these foods to satiety, say Food 1, and then face a choice between Object A and Object B. Regardless of their initial preference between the two foods, intact monkeys show a selective-satiation effect; in our example, they tend to choose Object B because it predicts the availability of Food 2, which has not been consumed recently. Monkeys with amygdala lesions do not show this selective-satiation effect; they choose objects without showing the normal influence of having recently consumed one of the foods to satiety (Izquierdo & Murray, 2007; Machado & Bachevalier, 2007; Malkova, Gaffan, & Murray, 1997). Similarly, in rats trained with stimuli such as tones paired with food, lesions of the basolateral portion of the amygdala disrupt this selective-satiation effect (e.g., Blundell, Hall, & Killcross, 2003; Johnson, Gallagher, & Holland, 2009).
The standard interpretation of these findings is that consuming a food to satiety will result in a change in internal cues (internal context) such as blood sugar levels, hormone levels and gut distension. This change in internal context can in turn be used to update (in this case, decrease) the value assigned to the consumed food. Because, through learning, specific objects come to predict specific foods, the temporary reduction in the value of the consumed food can influence object choices. On this view, eating Food 1 decreases the value of Food 1 relative to Food 2. Therefore, if monkeys are given a choice between an object associated with Food 1 and another associated with Food 2, they are more likely to choose the object associated with Food 2, which is currently of higher value. The critical role of the amygdala in the selective-satiation task is held to be in some aspect of updating the representation of food value.
An alternative possibility, however, is that the amygdala is critical for either representing internal context or using internal context to guide behavior. On this view, amygdala damage disrupts the selective-satiation effect because of its effect on processing of internal context, which is needed for the ‘value-updating’ function. The selective-satiation task does not allow us to discriminate between these two possible roles for the amygdala. If the amygdala plays a general role in guiding choice behavior with respect to internal context, then amygdala damage should cause an impairment in other situations in which object choices are based on internal context, and not just when the choices are based on updating of the value of food associated with the objects.
To test this idea, we trained monkeys with amygdala lesions and unoperated controls on a task that required them to make object choices based on internal context but did not require the updating of reward value. These were the same monkeys that were previously tested on the object-based selective-satiation task described above and found to be impaired (Izquierdo & Murray, 2007). Monkeys were trained to associate three classes of objects with three different outcomes: food, water and nonreward. During acquisition, monkeys were on food- and water-control schedules intended to yield one of two motivational states: hunger and thirst. These motivational states and the different sets of internal cues associated with them provide two different internal contexts. Monkeys were required to choose objects yielding the reward congruent with their internal state. Importantly, the food objects were only rewarded on food-control sessions and water objects were only rewarded on water-control sessions. Monkeys were in effect required to learn a biconditional discrimination of the form ‘If hungry, food object is rewarded and water object is not rewarded; if thirsty, water object is rewarded and food object is not rewarded’. Therefore, to learn this discrimination, monkeys had to choose objects based on their internal state (i.e., hunger or thirst). A conceptually similar task has been used to investigate the neural substrates of choice behavior based on internal context in rats (Kennedy & Shapiro, 2004).
If the amygdala is required to use internal context to guide choices of objects, then monkeys with amygdala lesions would be impaired on this task, just as they were impaired on the selective-satiation task. If, however, the amygdala is necessary specifically for the value-updating function that occurs with selective satiation, then amygdala lesions should fail to disrupt object choices in the current task.
Materials and methods
Subjects
A total of nine male rhesus monkeys (Macaca mulatta) were used in the current study. These monkeys were experimentally sophisticated, having undergone a variety of cognitive tests. Five of the monkeys had previously undergone surgery to receive excitotoxic amygdala lesions and four were unoperated controls. All monkeys were housed individually in a temperature and humidity-controlled room on a 12- hr light/dark cycle (with lights on at 7:00 A.M.) and testing occurred during the light period. At the outset of the experiment, the monkeys weighed 8.7–12.4 kg. During the study, the monkeys were given controlled access to water and primate chow to ensure sufficient motivation to respond in the test apparatus.
Surgery
All five monkeys that made up the amygdala group had previously undergone surgery in two stages to produce bilateral excitotoxic lesions of the entire amygdala. During the first surgery, injections of the excitotoxin ibotenic acid were made in the left amygdala in two monkeys (AMG 1, AMG 3), and in the right in the remaining three monkeys (AMG 2, AMG 4, AMG 5). During the second stage surgery, monkeys received injections of excitotoxin in the amygdala of the other hemisphere. One monkey (AMG 3) received a third operation to carry out additional injections of excitotoxin because postoperative magnetic resonance imaging (MRI) scans revealed the lesion was incomplete. During surgery, aseptic procedures were used. Anesthesia was induced with ketamine hydrochloride (10 mg/kg, i.m.) and maintained with isoflurane (1.0–3.0%, to effect). Heart rate, respiration rate, blood pressure, expired CO2, and body temperature were monitored during surgery, and isotonic fluids were given throughout. The pre- and postoperative treatment regimen consisted of dexamethasone sodium phosphate (0.4 mg/kg, i.m.) and Cefazolin antibiotic (15 mg/kg, i.m.) for one day before surgery, and one week after surgery, to reduce swelling and prevent infection, respectively. At the end of surgery, and for two additional days, the monkeys received the analgesic ketoprofen (10–15 mg, i.m.) followed by ibuprofen (100 mg) for the five following days.
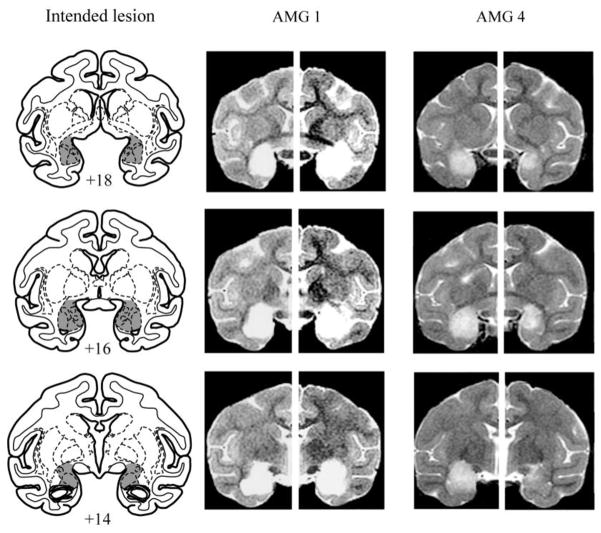
Injection procedures have been described previously (see Izquierdo & Murray, 2005 for a more complete description of the methods). In brief, the injection sites were calculated based on landmarks that were visible on MRI scans obtained prior to the surgery. The sagittal sinus served as a landmark for the mediolateral coordinates and the interaural plane (earbars) served as a landmark for the anteroposterior and dorsoventral coordinates. The monkeys received between 18 and 25 injections located approximately 2 mm apart in each plane. At each injection site 0.6–1.0 μl of ibotenic acid (10–15 μg/μl; 0.2 μl/min; Biosearch Technologies, Novato, CA) was injected via a 30-gauge Hamilton syringe needle. The needle remained in place 2–3 min after each injection to limit diffusion of the toxin up the needle track. The intended lesion (Figure 1) encompassed the entire amygdala, including both basolateral and centromedial nuclear groups.
Figure 1.
The left column shows coronal sections from a standard rhesus monkey brain depicting the location and extent of the intended bilateral amygdala lesion. The numerals indicate the distance (mm) of the sections from the interaural plane (0). The middle and right columns show T2-weighted MR images from two monkeys that received injections of ibotenic acid into the amygdala (AMG 1 and AMG 4) at levels matching the sections in the left column. The white areas are regions of hypersignal due to edema resulting from the injection of excitotoxin and are taken to reflect the location and extent of the amygdala lesion.
Assessment of lesions
The extent of the amygdala lesions sustained by the five operated monkeys was estimated by examination of T2-weighted MRI scans that were obtained within 12 days of surgery. For each of the five operated monkeys, the region of hypersignal present in the scan was plotted onto standard coronal sections spaced at 1 mm intervals (see Izquierdo & Murray, 2004 for more details on the lesion assessment methods). Only one hemisphere could be evaluated in monkey AMG 2 because we were unable to obtain a postoperative MRI scan following surgery to the left hemisphere. Figure 1 shows representative postoperative MR images from two of the monkeys that received injections of excitotoxin into the amygdala. The volume of the amygdala lesion was calculated as a percentage of the volume of the amygdala in a standard brain. The five monkeys were estimated to have complete cell loss in 93.4% (range: 85.2% to 100%) of the amygdala. Each of the monkeys with amygdala lesions sustained some inadvertent damage to adjacent structures. AMG 1 and 5 sustained slight bilateral damage to anterior portions of the entorhinal cortex and hippocampus and to portions of the ventral claustrum, substantia innominata, and piriform cortex whereas the other three monkeys sustained only minor and unilateral damage to a subset of these regions.
Apparatus
Training took place in a modified Wisconsin General Test Apparatus (WGTA) located in a dark room. The WGTA consisted of a large compartment that held the monkey test cage and a small test compartment which was illuminated with two 60-W bulbs and contained a test tray. The two compartments were separated by an opaque screen. When the screen was drawn up via a rope and pulley assembly, the monkeys could gain access to the test compartment. A strategically placed microswitch connected to a timer provided automated timing of the intertrial interval (ITI). A second screen, this one a one-way-vision screen, was located between the experimenter and the test compartment. When the screen was lowered, the experimenter could view the monkey’s responses during trials while remaining unseen by the monkey; when the screen was drawn up via a rope and pulley assembly, the experimenter could gain access to the test compartment. Two test trays measuring 19.2 cm (width) × 72.7 cm (length) × 1.9 cm (height) were used. The tray used during WGTA re-exposure and pretraining contained two food wells spaced 290 mm apart, center to center, on the midline of the tray. The tray used during the main task contained three food wells spaced 180 mm apart, center to center, on the midline of the tray. The wells were 38 mm in diameter and 6 mm deep. The food reward was half a peanut and the water reward was one ice cube, approximately 2.5 ml in volume. During the re-exposure, pretraining, and the main task, one, six and 60 junk objects were used respectively. All objects were novel at the beginning of testing and varied in color, shape and size.
Behavioral testing procedures
Prior to the onset of testing, monkeys were provided with both peanuts (food reward) and ice cubes (water reward) in their home cages to acclimatize them to the rewards and to ensure that they would accept them. Following this, monkeys underwent three phases of training (WGTA re-exposure, pretraining and the main task).
WGTA re-exposure
All monkeys had similar levels of prior experience in the WGTA. To reacquaint them with the WGTA and familiarize them with the experimenter, monkeys were given one re-exposure session consisting of 20 trials. On each trial, monkeys were presented with a single object covering the baited well of the two-well tray. The monkey was allowed to displace the object in order to retrieve the food reward. The location (right or left) of the object on each trial was determined based on a Gellerman schedule. Each trial began when the experimenter raised the screen between the monkey compartment and the test compartment and ended once the monkey had retrieved the food. As the trial ended, the experimenter lowered the screen. Trials were separated by a 10-sec ITI.
Pretraining
The three objects dedicated to this phase were randomly assigned to one of three outcomes: water reward, food reward, or nonreward (i.e., nothing). This object-outcome assignment remained constant across all pretraining sessions and the three objects are therefore referred to as W (water), F (food), and N (nonreward) depending on the outcome with which they were associated. Prior to each session monkey’s diets were controlled to achieve one of two states: hunger or thirst. The schedule was conducted in a pseudorandom order to ensure against monkeys learning alternation strategies as a solution to the task. On each trial the monkey was presented with two of the three objects set covering the wells on a two-well tray. On water-control sessions, the W and N objects were present on every trial, and only the W object was baited (i.e., a small ice cube was present in the well covered by the W object). On food-control sessions, the F and N objects were present on every trial, and only the F object was baited. Thus, to obtain food or water, monkeys were required to displace the object associated with the reward that was congruent with their current motivational state. The location (i.e., the right or left well) of the objects on each trial was determined based on a Gellerman schedule. Monkeys were tested at the rate of 20 trials per session, one session per day.
Each trial began when the experimenter raised the screen between the monkey and the test compartment and ended once the monkey displaced an object. Only one object could be displaced per trial; lowering the screen signaled termination of the trial and commencement of the ITI. If the baited (W or F) object was displaced, the monkey was allowed to retrieve the food or water reward located beneath the object and the trial was scored as being correct. Correctly performed trials were followed by a 20-sec ITI whereas incorrectly performed trials (i.e., if the N object was selected) were followed by a 30-sec ITI to act as a punishment to aid acquisition of the task. No correction trials were given. Pretraining was conducted as described until monkeys reached the criterion for both motivational states. The criterion for each motivational state was set at 18 correct responses in 20 trials for three consecutive sessions. When the monkeys attained criterion they were trained with a new set of three objects according to the same procedure.
Main Task
For the main experimental stage, the 60 objects were randomly assigned to 20 triplets. The triplets remained fixed across the course of the experiment. As was the case for pretraining, each triplet comprised a W, F and a N object. Unlike pretraining, however, monkeys were presented with all three of the objects of a triplet on each trial, set out on a three-well tray. Each triplet appeared once each in the 20 trials that comprised a daily test session. Diets were controlled to manipulate motivational state. The control schedule was conducted in a pseudorandom order to ensure against monkeys learning alternation strategies as a solution to the task. On food-control sessions only the F object was baited and on water-control sessions only the W object was baited. Both the order of triplet presentation across sessions and the location of each object of a triplet on the test tray followed a pseudorandom order.
Trials progressed in a similar fashion to those during pretraining; if a monkey displaced the object congruent with its motivational state (thirst or hunger), it was allowed to retrieve the reward. Trials were separated by 40 sec. Training continued until monkeys reached the criterion of 18 correct responses in 20 trials for three consecutive sessions, for both food- and water-control sessions calculated separately.
Food and water control procedures
Monkeys were given controlled access to food and water to manipulate their motivational state. On some days monkeys were motivated to obtain food and on other days they were motivated to obtain water. Daily provision of food and water was tailored to each individual to achieve the desired state of hunger or thirst. Prior to each day’s test session, we assessed whether the monkey’s motivational state matched the experimenter’s assumptions. Once placed in the WGTA monkeys were given two free trials in which they could choose between a half peanut and a small ice cube. If on both trials they chose the reward that was congruent with their designated motivational state, they were tested on the main task. If they chose the alternate reward on one or both trials, they were returned to their home cage and the same test, using the same motivational state, was run the next day.
Results
Several analyses provided convergent evidence that monkeys with bilateral, selective amygdala lesions learned the biconditional discriminations at the same rate, and in the same manner, as controls.
Trials and Errors to Criterion
The number of trials and errors to criterion are presented in Table 1. Monkeys learned to displace the food-associated objects in a mean of 904.4 trials (controls=905, amygdala=904), and the water-associated objects in a mean of 826.7 trials (controls=825, amygdala=828). A 2×2 mixed-ANOVA on trials to criterion with factors of group (control and amygdala lesion) and motivational state (hunger, thirst) did not produce any main effects or interactions [maximum F(1,7)=2.84, p>0.05] indicating that there was no effect of amygdala lesions nor of motivational state on the number of trials to criterion. The two groups also scored roughly equal numbers of errors in attaining criterion on both the food-associated objects (mean errors to criterion=328.8: controls=325.7, amygdala=331.2), and the water-associated objects (mean errors to criterion=273: controls=274.5, amygdala=271.8). An ANOVA on errors to criterion produced no main effects or interactions [maximum F(1,7)=3.22, p>0.05]. CON 4 did not reach criterion on either food-controlled or water-controlled sessions within the training limit of 1500 trials. It was difficult to achieve the desired motivational state for this monkey and test days were frequently aborted (see section on aborted trials below). Because CON 4 performed above chance, however, we included its scores in this analysis and in the learning rate analysis (below). In this analysis the total number of trials and errors scored within the training limit were used for CON 4. The outcome of the above analyses do not change if CON 4 is removed from consideration.
Table 1.
Trials (T) and errors (E) to criterion for individual monkeys during both food-control and water-control sessions.
| Food-Control Sessions | Water-Control Sessions | |||
|---|---|---|---|---|
| T | E | T | E | |
| AMG 1 | 520 | 234 | 660 | 200 |
| AMG 2 | 1220 | 470 | 980 | 314 |
| AMG 3 | 1500 | 519 | 1460 | 446 |
| AMG 4 | 640 | 233 | 660 | 279 |
| AMG 5 | 640 | 200 | 380 | 120 |
| 904 | 331.2 | 828 | 271.8 | |
| CON 1 | 840 | 251 | 700 | 291 |
| CON 2 | 840 | 383 | 700 | 240 |
| CON 3 | 440 | 135 | 400 | 186 |
| CON 4 | 1500* | 534* | 1500* | 381* |
| 905 | 325.7 | 825 | 274.5 | |
AMG 1–5: monkeys with bilateral excitotoxic lesions of the amygdala. CON 1–4: unoperated control monkeys.
indicates the number of trials and errors scored within the training limit of 1500 trials for CON 4, a monkey that did not reach criterion within the training limit.
Learning rate
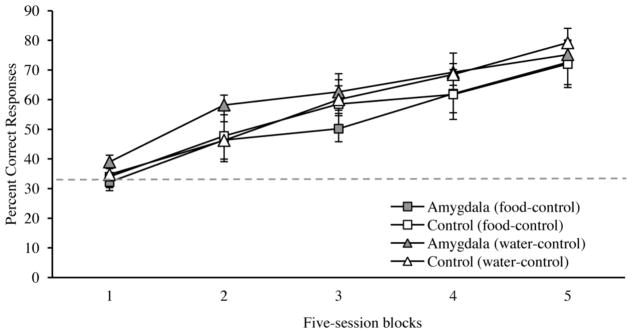
We assessed learning rates across 5-trial blocks for food- and water-controlled sessions separately. Figure 2 shows the mean percent correct responses for the first 25 sessions – the only sessions common to all monkeys – during training on the main task. A 2×2×5 mixed-ANOVA with factors of group (control and amygdala lesion), motivational state (hunger, thirst) and block (1–5) produced a main effect of block [F(4, 28)=67.39, p<0.001] due to the increase in correct choices across blocks by both groups as they learned the task. However, there were no main effects or interactions involving group or motivational state [maximum F(1,7)=1.77, p>0.05], indicating that amygdala lesions did not affect the rate of learning and that performance did not differ across food- and water-control sessions. Again, statistical outcomes do not change if CON 4 is removed from the analysis.
Figure 2.
Mean percent correct responses (± S.E.M) across blocks of 5 sessions during training on the main task on food-controlled and water-controlled sessions. The dashed grey line depicts chance performance. Amygdala: monkeys with bilateral excitotoxic lesions of the amygdala (N=5); Control: unoperated control monkeys (N=4).
Confirming the impression given by Figure 2, there was no group difference in scores on session one, which was either a food- or water-control session depending on the monkey’s schedule (mean percent correct responses: controls=29.0, amygdala=28.7; [t(7)=0.05 p>0.05]), nor did the scores differ from chance (33.3%) [t(8)=1.80, p>0.05].
Error Types
In instances when monkeys made an incorrect selection, the choice between the two incorrect objects – alternately rewarded (e.g., F object on a water-control session) and nonrewarded – was considered. On the first session (either food- or water-control depending on the monkey’s schedule), there was no difference in performance between monkeys with amygdala lesions and controls or between the two types of incorrect response. This was demonstrated by a 2×2 mixed-ANOVA with factors of group (control and amygdala lesion) and error type (alternately rewarded, nonrewarded) which produced no main effects or interactions [maximum F(1,7)=2.99, p>0.05]. Overall, performance on the first session did not differ from chance (66.7% error) [t(8)=1.78, p>0.05].
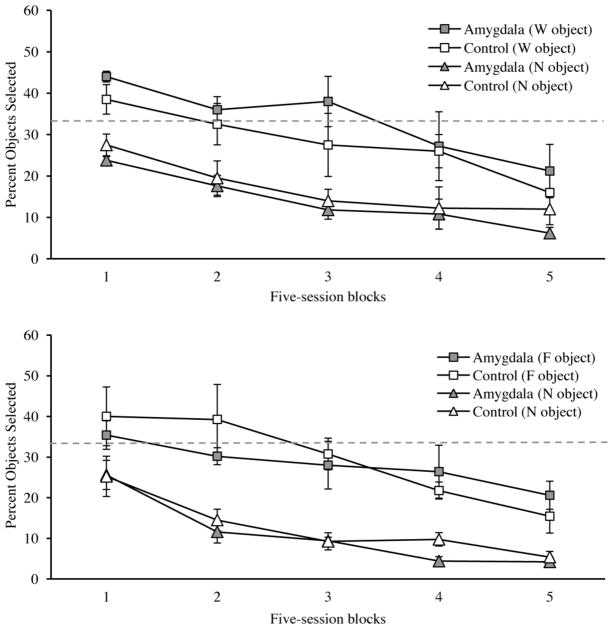
We also examined the types of errors made across the initial 25 sessions (Figure 3). The distribution of errors between the two types of objects presented a similar pattern during food-control and water-control sessions and performance did not differ between groups. As can be appreciated in Figure 3, within the first block of trials, monkeys responded to the F and W objects more frequently than N objects. Across successive 5-session blocks, reduction in the types of errors occurred at the same rate. A 2×2×2×5 mixed ANOVA with factors of group (control and amygdala lesion), motivational state (hunger, thirst), error type (alternately rewarded, N) and session block (1–5) produced a main effect of block [F(4,28)=67.31, p<0.001] due to the decline in the selection of both types of incorrect object choices across blocks by both groups. There was also a main effect of error type [F(1,7)=39.96, p<0.001] reflecting the monkeys preference for selecting the object associated with the alternate reward over the nonrewarded object, but no error type × block interaction [F(4,28)=1.38, p>0.05], indicating that, across blocks, the two types of errors declined at a similar rate. There were no main effects or interactions involving group [maximum F(4,28)=1.54, p>0.05] indicating that amygdala lesions did not affect the type of errors made or the rate at which they were reduced. In addition, there were no main effects or interactions involving motivational state [maximum F(1,7)=1.77, p>0.05] indicating that performance did not differ across food- and water-control sessions.
Figure 3.
Mean percent of trials (± S.E.M) on which each of the two types of incorrect objects were selected by monkeys across blocks of 5 sessions during training on the main task. Performance during food-controlled sessions (top) and water-controlled (bottom) sessions are shown separately. F, W and N refer to food, water and nonreward respectively. The dashed grey line depicts chance performance. Amygdala: monkeys with bilateral excitotoxic lesions of the amygdala (N=5); Control: unoperated control monkeys (N=4).
Performance on First Trial in a Session
As each session progressed, contextual cues other than motivational state (e.g., the presence or absence of particular rewards) became available, and might have been used to guide behavior. Therefore, we cannot rule out the possibility that monkeys were using contextual cues other than their motivational state to perform the task. For example, monkeys could have used a win-stay/lose-shift strategy together with the knowledge of the classes of objects (F, W, N). If so, the presence/absence of a reward on the first trial in a session would be sufficient to guide object choices thereafter. To test whether monkeys used such a strategy, performance on the first trial of the final six food- and water-control sessions (including the three criterion sessions) was assessed in all monkeys who reached criterion. (Note that CON 4 did not reach criterion on either food- or water-control sessions and therefore was not included in the analysis). As depicted in Figure 4, by the end of training, both controls and monkeys with amygdala lesions were selecting the correct object most of the time on the first trial of a session. A 2×2 mixed-ANOVA with factors of group (control and amygdala lesion) and motivational state (hunger, thirst) revealed no significant main effects or interactions [maximum F(1,6)=2.80, p>0.05] indicating that there was no difference between groups on Trial 1 choices, nor was there an effect of motivational state on this measure. Because there was no main effect of group or motivational state, the data for food- and water-control sessions were combined and compared across all monkeys irrespective of group. A one sample t-test indicated that first trial performance at the end of training did not differ from perfect (100% correct) performance [t(7)=2.30, p>0.05]. Thus, with the possible exception of the one subject that failed to make a correct selection on the first trial of each of the final six food-control sessions (CON 2), it appears extremely unlikely that monkeys were using a win-stay/lose-shift strategy to perform the current task.
Figure 4.
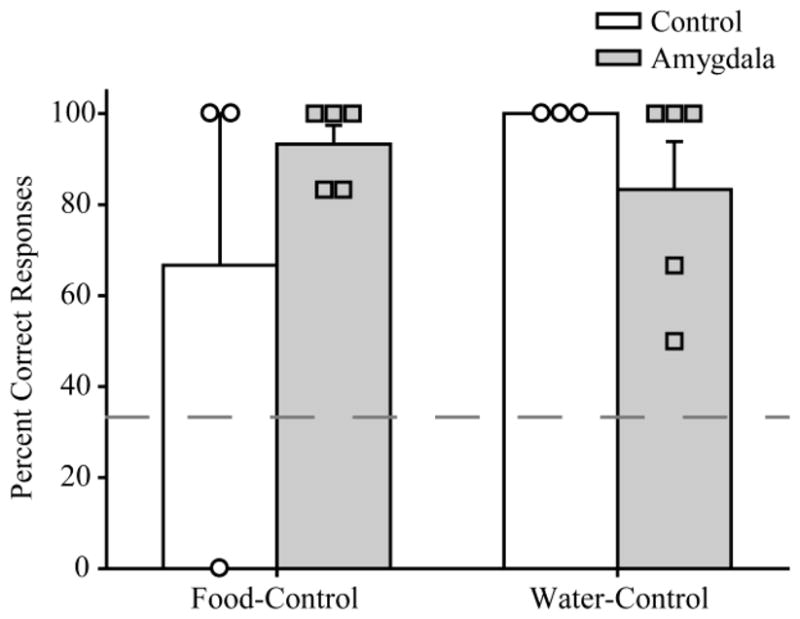
Mean percent correct responses on first trial of (± S.E.M) of the last 6 sessions for those monkeys who reached criterion performance. Symbols represent scores of individual monkeys. Amygdala: monkeys with bilateral excitotoxic lesions of the amygdala; Control: unoperated control monkeys.
Aborted Sessions
Prior to testing on either food- or water-control days, monkeys were given a choice between food and water rewards to ascertain whether the motivational state of the monkey (hunger or thirst) matched the experimenter’s expectations. On average, monkeys selected the reward congruent with the expected motivational state on over 85% of trials (mean % trials incongruent reward was chosen on food-control sessions: controls=0, amygdala=6.1 and on water-control sessions: controls=15.6, amygdala=11.4). A 2×2 mixed-ANOVA with factors of group (control and amygdala lesion) and motivational state (hunger, thirst) indicated that there was no main effect or interaction involving motivational state or group [maximum F(1,7)=4.52, p>0.05]. Importantly, this indicates that the groups did not differ in the extent to which they “reported” the appropriate motivational state, a finding consistent with results from the main task. One control monkey (CON 4) failed the free reward choice test on 40% of water-control days by selecting the food reward. This is the same monkey that failed to attain criterion.
Discussion
We investigated the effect of amygdala lesions on monkeys’ abilities to choose objects based on internal context, in this case their current motivational state (hunger or thirst). Objects associated with food and water were present on every trial, but only the object associated with the reward that was congruent with internal context was baited (e.g., food objects baited when the monkey was hungry). Therefore, to solve the task monkeys had to use their motivational state to guide object choices. We found that unoperated controls were able to learn this biconditional discrimination and that monkeys with amygdala lesions learned at the same rate, and in the same manner. Analysis of the first trial in a session confirmed that monkeys were not simply using within-session cues (e.g., the presence or absence of particular rewards) to guide their choices. Instead, their behavior was guided by the memory of the objects that were rewarded in the current motivational state. Although motivational state was clearly used to guide object choices, there was some generalization in learning across motivational states; when committing errors, monkeys in both groups showed a consistent preference for the alternately rewarded object over the nonrewarded object (e.g., choosing water objects over nonrewarded objects when the monkey was hungry).
The finding that monkeys with selective, excitotoxic lesions of the amygdala were able to use internal context to guide object choices demonstrates that the amygdala is not necessary for representing, discriminating between, or using internal context to guide behavior – at least not in the case of motivational states. The current results are of particular interest when considered in conjunction with two previous sets of findings in monkeys. First, as indicated at the outset, amygdala lesions in monkeys disrupt the normal shift in object choices during a probe test following selective satiation both in the same group of monkeys studied here (Izquierdo & Murray, 2007), and in other monkeys with amygdala lesions (Machado & Bachevalier, 2007; Malkova et al., 1997). This pattern of results is consistent with the notion that the amygdala is necessary for the process of updating the value of a particular food in accordance with the current motivational state, using the updated value to guide choices, or both. Second, amygdala inactivation during satiation, but not during object choice probe tests, disrupts selective-satiation effects in monkeys (Wellman, Gale, & Malkova, 2005). Thus, the amygdala is required for the value-updating function, but is not required to represent or use the value of the reward once the updating process is completed. The foregoing results, taken together with the findings of the current study, indicate that although the amygdala is essential for value updating based on internal context, this is unlikely to be a consequence of the amygdala playing a general role in either representing or using information about internal context.
Why do monkeys with amygdala lesions succeed at the current task but fail at the test phase of the selective-satiation task? One important consideration is the process by which internal context is used to guide object choices in the two tasks. In the current task, there is an opportunity to learn about internal context as a cue across the numerous acquisition sessions. This would have provided the opportunity for internal context to serve as an occasion setter, as it literally ‘sets the occasion’, by providing information about the occurrence of reward in different scenarios. Considerable evidence indicates that occasion setters operate hierarchically by modulating the activation of associations between different stimuli or responses and reward, rather than through direct interaction with the reward itself via binary associations, such as those associations that stimuli are typically described as forming during Pavlovian and instrumental conditioning (for a detailed description see: Bouton, 2007; Rescorla, 1992). Thus, the monkey’s motivational state (hunger or thirst) can be thought of as modulating the associations that guide the monkey’s object choices.
In the test phase of the selective-satiation task, the level of satiety could also in theory be used as an occasion-setter to differentially guide responding when a particular food is rewarding (i.e., when hungry) and when not rewarding/devalued (i.e., when sated). Due to the design of the selective-satiation test, however, there is relatively little opportunity for the internal state of satiation to build up occasion-setting properties governing object selection. This is because there are typically few sessions administered while the monkey is sated and, within each of these probe test sessions, each object pair is encountered only once. As described above, the most likely mechanism through which internal context influences choices during the selective-satiation test is via its direct impact on the current value attached to the reward representation. However, we would predict that, had a greater number of trials been run with individual object pairs, this repeated exposure would allow the internal context to build up occasion-setting properties. This in turn would mean that monkeys with amygdala lesions might have been able to learn to choose adaptively. This idea is open to empirical investigation.
In addition, we note that in the training phase of the selective-satiation task, unlike the current task, objects are consistently paired with the same food outcome; this difference between the tasks may also contribute to the use of different learning mechanisms. There is, however, an aspect of results from selective-satiation tasks that might be explained by occasion-setting mechanisms. In our selective-satiation tasks, to verify that satiety is achieved, a food preference test is typically carried out (Izquierdo & Murray, 2007; Malkova et al., 1997); monkeys are allowed to choose between the sated and nonsated foods in a series of trials. Monkeys with amygdala lesions and controls alike reliably choose the nonsated food. Thus, in the food preference tests, the same amygdalectomized monkeys that are impaired in making adaptive object choices show adaptive food choices, even though both are visual choices. As explained above, there is little opportunity for occasion-setting mechanisms to develop for object choices; however, this is not the case for food choices. During the selective-satiation procedure, monkeys had considerable exposure to the food in a sated state. Therefore, one might speculate that satiety became an occasion setter, modulating food choices in both controls and monkeys with amygdala lesions, as we propose occurs with hunger/thirst and object choices in the current study.
Although the results of the current task did not identify a role for the monkey amygdala in using internal context to guide object choices, this task could be used to identify other brain regions important for this function. Kennedy and Shapiro (2004) showed that rats with bilateral lesions of the hippocampus were impaired on a task that is conceptually similar to the one used in the current study. This finding is consistent with a role for the hippocampus in contextual learning (for reviews see Holland & Bouton, 1999; Maren, 2001; Myers & Gluck, 1994), and provides evidence that the hippocampus in rats plays an essential role in the use of internal context to guide object choices. This suggests that the hippocampus in monkeys would likewise be necessary for performance of the current task. If one were to undertake such a study in monkeys, there are several factors that need to be considered. First, Kennedy and Shapiro tested the effects of post-training lesions on task performance. A more complete understanding of the role of the hippocampus in this task would be gained from an examination of the effects of pre-training lesions as well. Second, although the authors interpreted the effects of the hippocampal lesion in terms of contextual retrieval of nonspatial memory, we note that there are instances in which hippocampal lesions do not disrupt tasks in which internal context guides behavior. For example, hippocampal lesions in rats (Corbit & Balleine, 2000) and monkeys (Chudasama, Wright, & Murray, 2008) do not disrupt performance on selective-satiation tasks, indicating that the hippocampus is not required for using internal context in this setting. It would appear, therefore, that not all internal context-related functions depend on the hippocampus (for a review of the role of the hippocampus in memory see White & McDonald, 2002). Finally, the selective-satiation task and current task involve different types of internal context manipulations; it is possible that the hippocampus plays a greater role in guiding behavior based on hunger relative to satiety.
One problem that arises when using the level of hunger or thirst as an internal contextual cue is that it is difficult to dissociate direct learning about these cues (i.e., the build-up of associations involving internal context representations) from the motivational impact (via reward value updating) of these states on both learning and behavior (Davidson, Flynn, & Jarrard, 1992). The design of the current study ensured that the monkey’s motivational state was concordant with the reward that it predicted, so for example hunger predicted that food would be received as a reward. This means that ideally there would be no conflict between the behavior that internal context drives as a cue and as a motivator so that one would not negatively impact on the other. In practice, however, hunger and thirst are not entirely independent of one another. For instance, when animals are thirsty they often eat less, and might be motivated to work for food as well as water. Indeed, this challenge surrounding the maintenance of thirst may have led to the poor performance observed in one of the monkeys (CON 4).
In summary, using the motivational states of hunger and thirst as internal contexts we have found that lesions of the amygdala do not disrupt learning or performance of an internal context cued biconditional discrimination in monkeys. This demonstrates that the amygdala is not necessary for the representation of internal states such as hunger and thirst. Therefore, it follows that the previously observed disruption of selective-satiation effects in monkeys following amygdala lesions is not due to disruption of internal context representations, or a general deficit in the use of internal context representations to guide behavior.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health. We thank Alicia Izquierdo for assisting with surgical procedures and Luke J. Humphrey for help testing monkeys.
References
- Blundell P, Hall G, Killcross AS. Preserved sensitivity to outcome value after lesions of the basolateral amygdala. Journal of Neuroscience. 2003;23(20):7702–7709. doi: 10.1523/JNEUROSCI.23-20-07702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Learning and behavior: A contemporary synthesis. Sunderland, MA: Sinauer; 2007. [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biological Psychiatry. 2008;63(11):1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of the hippocampus in instrumental conditioning. Journal of Neuroscience. 2000;20(11):4233–4239. doi: 10.1523/JNEUROSCI.20-11-04233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Flynn FW, Jarrard LE. Potency of food deprivation intensity cues as discriminative stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18(2):174–181. doi: 10.1037//0097-7403.18.2.174. [DOI] [PubMed] [Google Scholar]
- Holland P, Bouton ME. Hippocampus and context in classical conditioning. Current Opinion in Neurobiology. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. Journal of Neurophysiology. 2004;91(5):2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. European Journal of Neuroscience. 2005;22(9):2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. Journal of Neuroscience. 2007;27(5):1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, Holland PC. The basolateral amygdala is critical to the expression of Pavlovian and instrumental outcome-specific reinforcer devaluation effects. Journal of Neuroscience. 2009;29(3):696–704. doi: 10.1523/JNEUROSCI.3758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. Journal of Neuroscience. 2004;24(31):6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. European Journal of Neuroscience. 2007;25(9):2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. Journal of Neuroscience. 1997;17(15):6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Myers CE, Gluck MA. Context, conditioning, and hippocampal representation in animal learning. Behavioral Neuroscience. 1994;108:835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Hierarchical associative relations in Pavlovian conditioning and instrumental training. Current Directions in Psychological Science. 1992;1(2):66–70. [Google Scholar]
- Wellman LL, Gale K, Malkova L. GABA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. Journal of Neuroscience. 2005;25(18):4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiology of Learning and Memory. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]