Abstract
Objective
To characterize clonidine utilization trends among children.
Design/Methods
Serial cross-sectional analysis of Michigan Medicaid claims data for children aged 6 to 18 years. The authors identified children with ≥1 clonidine prescription; the authors examined their ICD-9 diagnoses categorized as simple and complex attention deficit hyperactivity disorder (ADHD), non-ADHD mental health disorder, hypertension, or others. Also identified were child demographics and prescribing physician specialty.
Results
From 2003 to 2008, the proportion of children receiving clonidine prescription nearly doubled in all demographics. Across years, the majority of clonidine prescription was for simple and complex ADHD and other mental health disorders. Leading prescribers were psychiatrists followed by general pediatricians and adult primary care physicians.
Conclusions
Clonidine was used extensively to treat simple and complex ADHD in children although FDA approval for this indication did not occur until 2010. Further study is warranted to better understand clinical outcomes and costs associated with clonidine use for the treatment of children with ADHD.
Keywords: clonidine, hypertension, ADHD, FDA approval, off-label use
Introduction
Clonidine is an antihypertensive drug, FDA approved since 1974, to treat hypertension (HTN) in both children and adults.1,2 Clonidine was initially a popular drug choice for the treatment of HTN since it was not associated with common side effects seen in other antihypertensive drugs, such as postural and exercise-induced hypotension.1 However, unfavorable side effects—particularly those associated with sudden discontinuation of clonidine, such as rebound HTN and sympathetic overdrive—have resulted in cardiac arrhythmias, hypertensive encephalopathy, and death.1,3 Additionally, complex drug interactions have been demonstrated between clonidine and various other drugs; for example, tricyclic antidepressants block the hypotensive effect of clonidine, but diuretics may enhance clonidine’s hypotensive effect.1,3 Thus, clonidine is not a first-line antihypertensive drug for the treatment of HTN in children.
As with any medication, clonidine can be prescribed “off-label” by physicians for conditions other than its FDA-approved indication of HTN. It is believed that clonidine is used “off-label” to treat non-FDA-approved conditions including neuropathic pain, narcotic withdrawal, sleep disorders,4 and attention deficit hyperactivity disorder (ADHD) in children.5 Previous studies have also shown increasing use of stimulant and nonstimulant medications to treat ADHD in children.6–8
In October 2010, clonidine was approved by the FDA for the treatment of ADHD in children as monotherapy or as adjunctive therapy to traditional stimulant medications. 9 Little is known about clonidine use in children prior to the expanded approval. The purpose of this study was to describe clonidine utilization trends among children over a multiyear period.
Methods
Study Design
We conducted a serial, cross-sectional analysis using Michigan Medicaid outpatient claims and pharmacy data for children between 6 and 18 years of age, enrolled during 2003–2008, and who had at least 1 prescription claim for clonidine (brand name and generic options) for each study year. This study was approved by the institutional review board of University of Michigan Medical School.
Study Population
The sampling frame was children 6 to 18 years of age on 1 January of each study year (2003–2008) who were eligible for Michigan Medicaid. We included both fee for- service and managed care Medicaid coverage and included those with dual Title V eligibility. We excluded children with <11 months of Medicaid coverage and those with other insurance coverage for each study year.
Variable of Interest
We identified children with ≥1 prescription for clonidine (brand name and generic options) for each study year using pharmacy claims that included National Drug Codes and prescriber identifiers. Companion data files were used to link prescriber identification numbers with physician specialty data. Since multiple physicians could have prescribed clonidine for each child in our sample, we considered the first prescriber of clonidine for each child for each year as the prescribing physician.
Independent Variables
Demographic variables included age on 1 January of each study year categorized as children (6–11 years) versus adolescents (12–18 years); race categorized as White, Black, Hispanic, and other/unknown; and gender. Physician specialty was categorized as adult primary care physicians (PCP; family physicians, general practitioners, internists, medicine-pediatrics), pediatric PCPs (general pediatricians), psychiatrists, neurologists, behavior and development specialists, other subspecialists (both adult and pediatric subspecialties including but not limited to cardiology, nephrology, emergency medicine, pain medicine), and unknown.
For each child, we examined and categorized all claims with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for each study year as simple ADHD (ADHD only), complex ADHD (ADHD and another mental health diagnoses without HTN), non-ADHD mental health (mental health diagnoses without ADHD or HTN), any HTN (HTN diagnoses with or without ADHD or another mental health diagnoses), and other. We chose to combine HTN diagnoses with or without ADHD or another mental health diagnoses because of small numbers.
For the subset of children who received at least 1 clonidine prescription and had a ICD-9-CM diagnosis of ADHD (simple or complex), we examined their pharmacy claims for stimulant (short acting and long acting) and nonstimulant medications recommended and used in the treatment of ADHD for each year.10,11 Because the majority of children continued on the same ADHD medication for the duration of the year, we classified children based on the first ADHD prescription filled per year. We considered children who filled more than 1 ADHD medication type on the same date (eg, short-acting stimulant + long-acting stimulant), to have received combination ADHD medication. We also examined the specialty of the prescribing physician of the first ADHD medication and compared with the clonidine prescriber for specialty concordance.
Statistical Analysis
Descriptive analyses included simple counts and proportions. We used χ2 tests to assess associations between receipt of clonidine prescription and demographic characteristics and ICD-9 diagnoses and demographic characteristics.
For the subset of children who received clonidine prescription and had ADHD (simple or complex), we tested the association between ADHD medication categories and diagnoses. P values ≤.05 were considered statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
Study Sample Characteristics
From 2003 to 2008, the proportion of children receiving clonidine prescription nearly doubled in all demographic characteristics, with the largest proportional increases seen in adolescents (12–18 years) and Hispanics (Table 1). Across years, boys were more likely to receive clonidine prescription than girls; children (6–11 years) were more likely to receive clonidine prescription than adolescents (12–18 years); Whites were more likely to receive clonidine prescription than non-Whites (P < .0001).
Table 1.
Demographic Characteristics of Medicaid Children With Clonidine Prescription per Year
| No. of Kids (Percentage of All Kids) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | |
| Gender | ||||||
| Male | 2202 (1.4) | 2865 (1.6) | 3727 (2.0) | 4011 (2.0) | 4534 (2.3) | 4925 (2.4) |
| Female | 717 (0.4) | 977 (0.6) | 1307 (0.7) | 1487 (0.8) | 1654 (0.8) | 1823 (0.9) |
| Age (years) | ||||||
| 6–11 | 1999 (1.1) | 2552 (1.4) | 3241 (1.7) | 3527 (1.8) | 3938 (2.0) | 4220 (2.1) |
| 12–18 | 920 (0.6) | 1290 (0.8) | 1793 (1.0) | 1971 (1.0) | 2250 (1.1) | 2528 (1.3) |
| Race | ||||||
| White | 1966 (1.3) | 2660 (1.6) | 3466 (1.9) | 3767 (2.0) | 4280 (2.2) | 4725 (2.3) |
| Black | 803 (0.5) | 1005 (0.6) | 1323 (0.8) | 1431 (0.9) | 1545 (0.9) | 1594 (1.0) |
| Hispanic | 67 (0.4) | 88 (0.5) | 106 (0.6) | 139 (0.7) | 167 (0.7) | 223 (0.9) |
| Other/unknown | 83 (1.1) | 89 (1.0) | 139 (1.4) | 161 (1.6) | 196 (1.8) | 206 (1.7) |
| Total | 2919 (0.9) | 3842 (1.1) | 5034 (1.3) | 5498 (1.4) | 6188 (1.6) | 6748 (1.7) |
Children With Clonidine Prescription by ICD-9 Diagnoses per Year
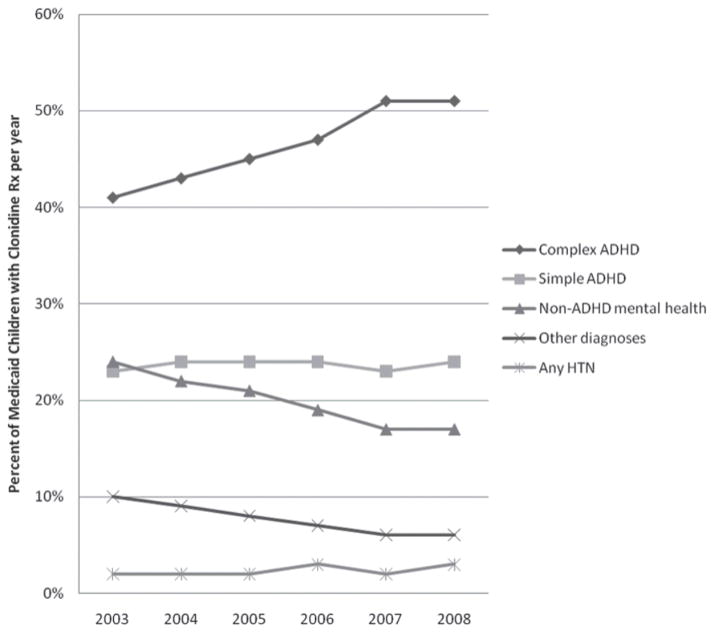
Across years, the majority of clonidine was prescribed for the treatment of complex ADHD, simple ADHD, and non-ADHD mental health disorders; clonidine was rarely prescribed for the treatment of HTN (Figure 1). From 2003 to 2008, an increasing proportion of clonidine prescription was for the treatment of complex ADHD. In contrast, the proportion of clonidine prescription for the treatment of simple ADHD and HTN stayed constant, whereas the proportion of clonidine prescription for the treatment of non-ADHD mental health and other conditions decreased across years (Figure 1).
Figure 1.
Medicaid children with clonidine prescription by diagnoses per year
Abbreviations: Rx, prescription; ADHD, attention deficit hyperactivity disorder; HTN, hypertension.
Demographic Characteristics Associated With ICD-9 Diagnoses Among Children With Clonidine Prescription
Among children with clonidine prescription, boys were significantly more likely to have complex ADHD and less likely to have non-ADHD mental health disorders compared with girls during each study year (2008 data presented in Table 2). A higher proportion of children (6–11 years old) compared with adolescents (12–18 years old) had simple or complex ADHD during each study year (2008 data presented in Table 2). In contrast, more adolescents had non-ADHD mental health disorders and HTN compared with children during each study year (2008 data presented in Table 2).
Table 2.
Demographic Characteristics Associated With Diagnoses for Medicaid Children With Clonidine Prescription in 2008
| Complex ADHD | Simple ADHD | Non-ADHD Mental Health | HTN | Other Diagnoses | χ2 P Value | |
|---|---|---|---|---|---|---|
| Overall (n = 6748) | 51% | 24% | 17% | 3% | 6% | <.0001 |
| Gender | ||||||
| Male (n = 4925) | 52% | 24% | 15% | 3% | 6% | <.0001 |
| Female (n = 1823) | 46% | 24% | 20% | 2% | 8% | |
| Age (years) | ||||||
| 6–11 (n = 4220) | 53% | 26% | 13% | 2% | 5% | <.0001 |
| 12–18 (n = 2528) | 46% | 19% | 23% | 5% | 8% | |
| Race | ||||||
| White (n = 4725) | 53% | 24% | 15% | 2% | 6% | <.0001 |
| Black (n = 1594) | 44% | 25% | 20% | 4% | 8% | |
| Hispanic (n = 223) | 47% | 26% | 19% | 4% | 4% | |
| Other/unknown (n = 206) | 57% | 11% | 20% | 2% | 11% | |
Abbreviations: ADHD, attention deficit hyperactivity disorder; HTN, hypertension.
Among those with clonidine prescription, a higher proportion of Whites and subjects from other/unknown race had complex ADHD compared with Blacks during each study year (2008 data presented in Table 2). Across years, Blacks had a greater proportion of clonidine prescription given for non-ADHD mental health disorders and HTN compared with Whites (2008 data presented in Table 2).
Children With Clonidine Prescription by First Prescribing Physician Specialty per Year
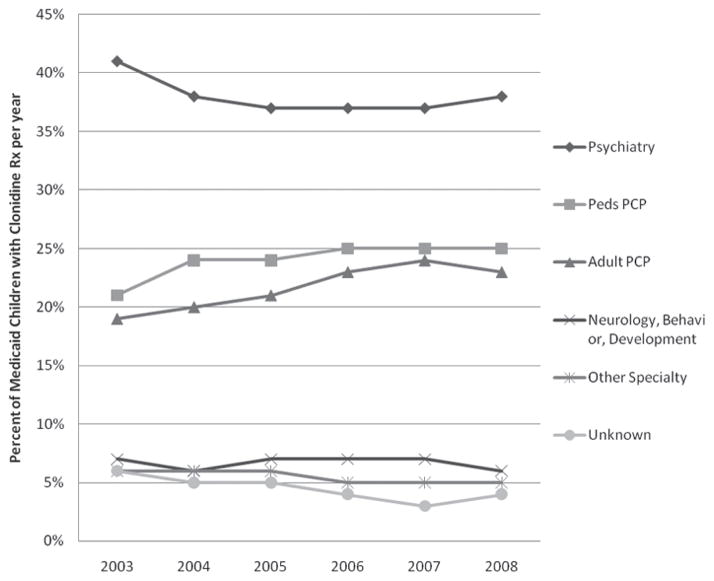
Across years, leading prescribers of clonidine prescription were psychiatrists followed by pediatric PCPs and adult PCPs (Figure 2). Neurologists, behavior and development specialists, and physicians of other or unknown specialties prescribed far fewer clonidine prescriptions. The proportion of clonidine prescription prescribed by pediatric PCPs and adult PCPs increased from 2003–2008 whereas clonidine prescription prescribed by psychiatrists, neurologists, behavior and development specialists, and physicians of other or unknown specialties decreased (Figure 2). Across years, younger children (6–11 years old) were more likely to receive clonidine prescription from general pediatricians than adolescents (12–18 years; 27% vs 21%; P < .0001; 2008 data). In contrast, adolescents were more likely than younger children to receive clonidine prescription from psychiatrists (40% vs 36%; P < .0001; 2008 data).
Figure 2.
Medicaid children with clonidine prescription by the first prescribing physician specialty per year
Abbreviations: Rx, prescription; PCP, primary care physician.
ADHD Medication Use in Children With Clonidine Prescription and ADHD Diagnoses (Simple and Complex)
Among the subset of children who received clonidine prescription and had diagnoses of simple ADHD, 95% (2008 data) were also prescribed stimulant or nonstimulant ADHD medications during the same study year. Similarly, 89% (2008 data) of children who received clonidine prescription and had diagnoses of complex ADHD were also prescribed stimulant or nonstimulant ADHD medications.
Long-acting stimulant medications were the most frequently prescribed ADHD medications followed by short-acting stimulants, nonstimulant medications, and by combination ADHD medications (2008 data presented in Table 3). Nonstimulant medications were more likely prescribed to children with complex ADHD compared with those with simple ADHD (Table 3).
Table 3.
ADHD Medication Use for Children With Clonidine Prescription by ADHD Diagnoses in 2008
| Complex ADHD (n = 3036) | Simple ADHD (n = 1522) | Total (n = 4558) | |
|---|---|---|---|
|
| |||
| Long-acting stimulants | 71% | 75% | 3294 |
| Short-acting stimulants | 11% | 12% | 529 |
| Nonstimulants* | 10% | 6% | 387 |
| Combination | 8% | 7% | 348 |
Abbreviation: ADHD, attention deficit hyperactivity disorder.
P < .0001.
The proportion of stimulants, nonstimulant ADHD medications, and combination ADHD medication use stayed constant across years. Short-acting stimulants alone dropped steadily from 21% in 2003 to 12% in 2008. Additionally, the specialty of first ADHD medication prescribing physician was highly concordant with the specialty of first clonidine prescribing physician (84%; 2008 data) across years.
Discussion
During the 6-year study period, rates of children and adolescents enrolled in Medicaid who received at least 1 clonidine prescription nearly doubled. Our study documents for the first time unexpected and increasing trends of clonidine use by demographic characteristics particularly in adolescents. Poor medication adherence has been well documented in adolescent patients with chronic conditions.12 This is particularly concerning when considering the significant side effects of rebound HTN and sympathetic overdrive associated with sudden discontinuation of clonidine.1,3 Moreover, adverse drug effects of clonidine for adolescents may be profoundly different from adults because of the dynamic changes that occur during this vital period of growth and development but have not been systematically evaluated. Further study to evaluate the interplay of medication adherence, medication use, and adverse effects in pediatric patients treated with clonidine should have particular focus on adolescents given the trends identified in this study.
The majority of clonidine prescriptions in our study were for the treatment of simple and complex ADHD, not for its FDA-approved indication, HTN. Thus, well before FDA’s approval to use clonidine for the treatment of ADHD, it was frequently prescribed “off-label” for children and adolescents for the treatment of ADHD. We have previously demonstrated common off-label use of other antihypertensive medications in children despite the availability of on-label alternatives in the same class of antihypertensive medications.13 Although off-label use of prescription medications for pediatric patients may be common, in the absence of adequate safety and efficacy studies in children and adolescents, off-label prescribing adds an additional layer of complexity with concern for unforeseen potential short-term and longterm adverse drug effects. Thus, a systematic strategy to closely monitor for adverse drug effects in pediatric patients receiving chronic pharmacotherapy prescribed both on-label and off-label should be developed and implemented.
During the 6-year study period, clonidine was increasingly prescribed for children with complex ADHD, whereas use in children with simple ADHD stayed constant across years. This is important when considering that most children in our study with ADHD (simple or complex) who received clonidine prescription were additionally prescribed stimulant or nonstimulant ADHD medications in the same year. Taken together, this concomitant use of clonidine and ADHD medications (stimulant and nonstimulant) in the majority of children in this study raises concerns about polypharmacy.
Adult studies have demonstrated increased probability of adverse drug effects associated with polypharmacy. 14–16 Side effects of clonidine (drowsiness, fatigue, hypotension, and cardiac arrhythmias) and drug interactions have been previously described.2,17,18 Stimulant and nonstimulant ADHD medications have their own side effects in children and adolescents including but not limited to cardiovascular effects such as arrhythmias and mood disorders such as increased suicidal ideation. Patterns of polypharmacy identified in our study need to be further evaluated for potential drug interactions and patient outcomes. Given that the majority of clonidine and ADHD medications were prescribed by the same physician specialty in this study, it seems that adverse drug effect and drug interaction monitoring efforts could be targeted and accomplished within specialties.
Finally, we found that across years the leading prescribers of clonidine for children were psychiatrists. However, we describe unexpected trends in clonidine prescribing where PCPs (adult and pediatric) were increasingly prescribing clonidine whereas clonidine prescribing by specialists such as psychiatrists was decreasing. Given the cross-sectional nature of this analysis, we cannot determine which specialty initiated clonidine prescription for each child. Hence, it is plausible that PCPs are refilling clonidine prescriptions for their pediatric patients and not necessarily initiating treatment decisions. Nevertheless, the pattern of increased role of PCPs in chronic disease management such as ADHD demonstrated in this study is practical, since primary care would be a more reasonable setting for long-term drug monitoring efforts and evaluation of patient outcomes. Yet how this is currently accomplished or evaluated in the primary care setting is unknown and warrants further investigation.
Limitations
Our findings should be interpreted with the following limitations. First, our study population was limited to children enrolled in Michigan Medicaid program for at least 11 consecutive months within a given year, which has potential implications for generalizability of our results. Second, limitations of pharmacy claim analysis suggests that prescription claims that were filled may potentially differ from utilization. Third, we examined prescription claims at the level of drug class not individual drugs.
Conclusion
Although clonidine has long been FDA approved for the treatment of HTN in children, it is rarely prescribed for this indication only. Clonidine was used extensively to treat ADHD in children although FDA approval for this indication did not occur until 2010. Further study is warranted to better understand clinical outcomes and costs associated with clonidine use for the treatment of children with ADHD.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Heart Lung Blood Institute (EYY K23 HL 092060-03).
Footnotes
Dr. Yoon had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Fagan NL, Wargo KA, Malone PM, Malesker MA. The clinical utility of clonidine. [Accessed August 16, 2011.];US Pharm. 2006 5:HS-2–HS-16. http://www.uspharmacist.com/content/s/50/c/11658/ [Google Scholar]
- 2.Highlights of Prescribing Information. [Accessed August 16, 2011.];Kapvay (clonidine hydrochloride) http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022331s001s002lbl.pdf.
- 3.WikiTox. [Accessed August 26, 2011.];Clonidine. http://curriculum.toxicology.wikispaces.net/Clonidine.
- 4.Ming X, Gordon E, Kang N, Wagner GC. Use of clonidine in children with autism spectrum disorders. Brain Dev. 2008;30:454–460. doi: 10.1016/j.braindev.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:1551–1559. doi: 10.1097/00004583-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Cox ER, Halloran DR, Homan SM, Welliver S, Mager DE. Trends in the prevalence of chronic medication use in children: 2002–2005. Pediatrics. 2008;122:e1053–e1061. doi: 10.1542/peds.2008-0214. [DOI] [PubMed] [Google Scholar]
- 7.Castle L, Aubert RE, Verbrugge RR, Khalid M, Epstein RS. Trends in medication treatment for ADHD. J Atten Disord. 2007;10:335–342. doi: 10.1177/1087054707299597. [DOI] [PubMed] [Google Scholar]
- 8.Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA. 2000;283:1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]
- 9.Wikipedia. [Accessed August 26, 2011.];Clonidine. http://en.wikipedia.org/wiki/Clonidine.
- 10.American Academy of Pediatrics, Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033–1044. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- 11.National Initiative for Children’s Healthcare Quality. Stimulant medication management information. [Accessed June 2, 2011.];ADHD toolkit. http://www.nichq.org/resources/adhd_toolkit.html.
- 12.Butow P, Palmer S, Pai A, Goodenough B, Luckett T, King M. Review of adherence-related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4800–4809. doi: 10.1200/JCO.2009.22.2802. [DOI] [PubMed] [Google Scholar]
- 13.Yoon EY, Dombkowski KJ, Rocchini A, Lin JJ, Davis MM. Off-label utilization of antihypertensive medications in children. Ambul Pediatr. 2007;7:299–303. doi: 10.1016/j.ambp.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst E. Herb-drug interactions: potentially important but woefully under-researched. Eur J Clin Pharmacol. 2000;56:523–524. doi: 10.1007/s002280000194. [DOI] [PubMed] [Google Scholar]
- 15.Gidal BE, French JA, Grossman P, Le Teuff G. Assessment of potential drug interactions in patients with epilepsy: impact of age and sex. Neurology. 2009;72:419–425. doi: 10.1212/01.wnl.0000341789.77291.8d. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 17.Ogbru O. [Accessed April 12, 2011];Clonidine, Catapres, Catapres-TTS, Jenloga. http://www.medicinenet.com/clonidine/article.htm.
- 18.Drugs.com. [Accessed August 26, 2011];Clonidine side effects. http://www.drugs.com/sfx/clonidine-side-effects.html.