Abstract
Smallpox is considered a biological threat based upon the possibility of deliberate reintroduction into the population, creating an urgent need for effective antivirals. The antiviral drug cidofovir (Cr) has shown to be effective against poxviruses, although route-specific nephrotoxicity has hampered its development for emergency post-exposure prophylaxis (PEP). In this study, we use a micronized dry powder formulation of pharmaceutical-grade Cr (NanoFOVIR™; Nf) to treat rabbits exposed to aerosolized rabbitpox virus (RPXV) to further evaluate the effectiveness of direct drug delivery to the lung. Naïve rabbits were infected with RPXV by aerosol; three subsets received aerosolized Nf at 0.5, 1.0 or 1.75 mg/kg daily for 3 days post-exposure, positive and negative control groups received intravenous (IV) Cr treatments and no treatment respectively. Nf groups showed an antiviral-dose associated survival of 50% (0.5 mg/kg), 80% (1.0 mg/kg) and 100% (1.75 mg/kg). All animals (100%) from the IV-Cr treatment group and none (0%) from the untreated controls survived. Nf (1.75) protected rabbits from RPX at approximately 10% of the equivalent IV-Cr dose. A dose-related effect was observed in clinical development of RPX disease in Nf groups. Significant reduction of RPX-induced pathological changes was observed in Nf (1.75) and IV-Cr groups. Results suggest that Nf may be a viable antiviral for emergency post-exposure prophylaxis and should be evaluated in other models of poxviral disease.
Keywords: pox virus, rabbitpox, smallpox treatment, aerosol, cidofovir
Variola virus, the etiological agent of smallpox, is naturally transmissible from person to person in aerosols and is the most highly feared agent of bioterrorism (Rotz et al., 2002). There are currently no recommended drugs for the treatment of smallpox patients or for post-exposure prophylaxis of exposed persons. Cidofovir (Cr), an antiviral compound originally developed for treatment of cytomegalovirus infection, has been shown to be active against a number of DNA viruses including poxviruses (Baker et al., 2003; De Clercq, 2002a, b). Since the respiratory system would be the primary and most probable affected tissue in the event of a deliberate release of Variola virus (Bray et al., 2002), drug delivery by aerosol could enhance efficiency of treatment. The effectiveness of direct delivery of cidofovir has already been demonstrated for the prevention of orthopoxvirus infections in a murine model of cowpox infection (Bray et al., 2002; Roy et al., 2003). The aim of this work, therefore, was to evaluate the efficiency of aerosol treatment with a pharmaceutical-grade powder version of Cr (NanoFOVIR™; Nf) on an animal model demonstrating pathological reactions similar to human smallpox. The rabbitpox virus (RPXV) infecting rabbits was chosen as a model for its very similar manifestations when compared to human smallpox (Adams et al., 2007; Westwood et al., 1966). This orthopoxvirus model has already been used to study a new antipoxvirus compound, ST-246 (Nalca et al., 2008) and is ideal for studying antipoxvirus agents (Roy and Voss, 2010). This model of poxviral infection is exceedingly acute and severe in terms of clinical onset of signs (+2d PI) and mean time to death (+6d PI) at the experimental aerosol dose levels of RPXV used in these studies.
Naïve mixed sex New Zealand white rabbits (Oryctolagus cuniculus) weighing between 1.9 and 2.3 kg were purchased from Charles River Laboratories (Austin, TX) and implanted subcutaneously with preprogrammed temperature transponder chips (Bio Medic Data Systems, DE) to determine animal identification and body temperature. This study and all procedures were approved by the Institutional Animal Care and Use Committee at Tulane University, and all animals were handled in accordance with the American Association for Accreditation of Laboratory Animal Care.
RPXV was obtained from ATCC (Manassas, VA) and propagated on African green monkey kidney fibroblast CV-1 (ATCC CCL-70) maintained in Eagle's Minimum Essential Media (EMEM) with 10% Fetal Bovine Serum (FBS) supplemented with 1% Glutamine, 1% L-Glutamine, 1% non-essential amino acids, and 1% Penicillin/Streptomycin solution. Viral stock solutions were prepared as described by Garza et al., 2009. Rabbits were individually challenged by RPXV aerosol (target dose; 250 pfu inhaled) using a muzzle-only dynamic inhalation exposure system set within a class III biosafety cabinet as described elsewhere (Garza et al., 2009; Pitt et al., 2001). The aerosol was generated with a Collison three-jet nebulizer (BGI Inc., Waltham, MA) and characterized with an aerodynamic particle sizer (Model 3321, TSI Instruments, St. Paul, MN); median mass aerodynamic diameter was 1 μm with a geometric standard deviation of 1.4. Air samples were collected from the chamber during exposure in EMEM media supplemented with antifoam using an all-glass impinger (AGI-4). Presented doses were calculated as described by Roy and Pitt (2005) using an estimation of the inhaled volume of air according to Bide's formula (Bide et al., 2000) and the viral concentration in the aerosol as determined by plaque assays of all glass impinger (AGI-4) samples.
After aerosol infection, all animals were treated once daily for three consecutive days starting at time 0 immediately post-exposure. Two groups of animals were treated by intravenous injections (IV) of either Cr (n=9) at 10 mg/kg (as determined by previous experiments; unpublished data) or of PBS (n=9) for untreated controls. Three other groups of animals were treated with the micronized dry powder formulation of Cr, NanoFovir™ (Nf), by aerosol at presented concentrations of 0.5 mg/kg (n=6), 1.0 mg/kg (n=6) and 1.75 mg/kg (n=6). Experiments were performed in three independent studies, each including three animals from each control group and one of three Nf treatment groups. Dry powder aerosol was generated into a six port nose-only aerosol chamber (CH Technologies, NJ) with a RBG 1000 rotating brush powder generator (Palas, Karlsruhe, Germany); three animals were treated simultaneously. Integrated aerosol samples were collected during each treatment with a filter cassette operated at 2 L/min; Nf aerosol concentration was determined by gravimetric analysis. Clinical signs including body temperature and weight were recorded daily until euthanasia or termination of the study (9 to 12 days). Preselected termination of the study was based upon a mean time to death of +6d PI established in our laboratories (Roy and Voss, 2010). All animals were necropsied to determine cause of death and to collect tissue samples for microscopy (hematoxylin/eosin), and qPCR analysis.
Plaque assays on tissue and AGI samples were conducted on CV-1 cells. Total DNA was isolated from infected tissues (DNeasy Tissue kit, Qiagen, Valencia, CA) and analyzed by quantitative PCR (qPCR) with a 7900HT Fast Real-Time PCR System (Applied Biosystems) in 96-well plates. For viral genome quantification, an 83 bp region (positions 167680 to 167762 of the rabbitpox genome; GeneBank accession number is AY484669) of the hemagglutinin (HA) gene (GeneBank accession numbers AF375118 and AF375119) of the rabbitpox virus genome (Li et al., 2005) was targeted using a dual labeled probe (5′-/56-FAM/ATCATACAGTWACAGACACTGTCTCA/3BHQ-1/-3′) and forward (5′-GAGACTCCGGAACCAATTAC-3′) and reverse (5′-CCAGATGATGTACTTACTGTAGTG-3′) primers. Amplification protocol was 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. A 232 bp fragment (positions 167564 to 167795 of the rabbitpox genome; GeneBank accession number is AY484669) of the HA gene (forward primer: 5′-AAATCGCGACTCCGGAAC-3′; reverse primer: 5′-AAATCGCGACTCCGGAAC-3′) containing the target region for viral DNA quantification was cloned into vector pCR2.1 using the TA cloning kit (Invitrogen, Carlsbad, CA), the purified plasmid was used for the constructio of the standard curve for the quantification of viral genome copies. Tissue DNA was quantified using the TaqMan® RNase P Control Reagents Kit (Applied Biosystems, Foster City, CA). Cell copy number was used to normalize the quantification of viral genome copies in tissues. Viral DNA concentration was then expressed as viral genomes per 106 cells (Biagent et al., 2005).
Statistical analysis was performed with the SigmaPlot software (Systat Software, Inc., San Jose, CA). Viral genome concentrations in tissues from treated animals were compared to that of untreated control animals by Mann-Whitney rank sum test. Differences in clinical signs (body weight and temperature) between untreated controls and treated groups were compared by t-test Survival was analyzed by Kaplan-Meier survival analysis (log-rank). All pairwise multiple comparison procedures were done with the Holm-Sidak method. Differences were considered significant for P<0.05.
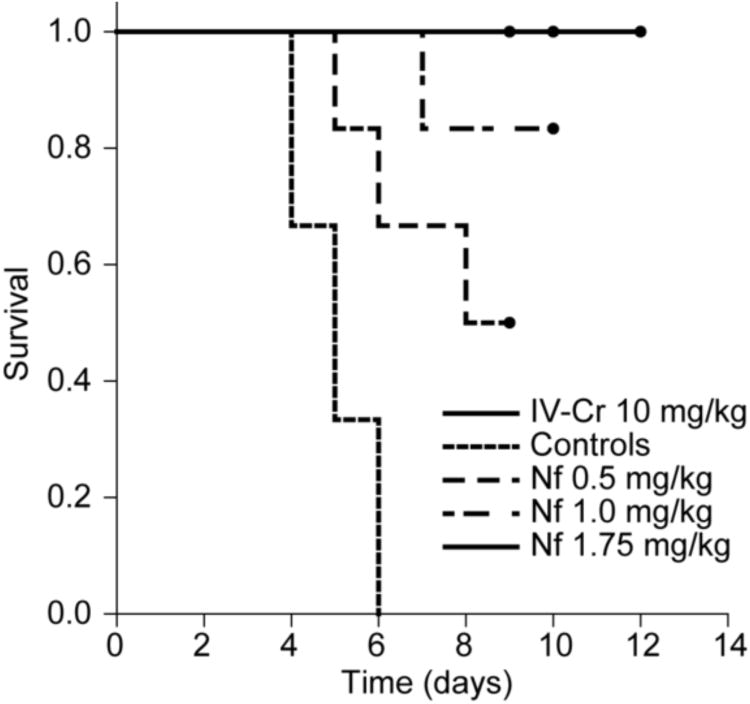
Exposure to RPXV aerosols was lethal to all untreated controls (9/9, 100%) within six days post-exposure (PE) (figure 1). All treatment groups were protected from lethality (P<0.05) in a dose-dependent manner; survival was 50% (3/6), 83% (5/6) and 100% (6/6) for the 0.5 mg/kg, 1.0 mg/kg and 1.75 mg/kg Nf treatment groups respectively and 100% (9/9) for the 10 mg/kg IV-Cr treatment group. A fivefold lower concentration of drug delivered directly to the lung achieved protection against lethality at the same level as the IV-Cr treatment. It has been suggested that the currently accepted dose for the treatment of cytomegalovirus (5 mg/kg) in human patients would necessitate a 5 to 10 fold increase in order to provide the adequate protection for people exposed to Variola virus (McSharry et al., 2009). The nephrotoxic effects of the 5 mg/kg dose have already been documented (Lalezari et al., 1997), increasing dosage could further increase nephrotoxicity. These results show that the delivery of the drug directly to the lung could circumvent the necessity to increase dosage; however, further investigations would be required to confirm this.
Figure 1.
Kaplan-Meier survival analysis of aerosol-treated animals. All animals treated with 10 mg/kg IV-Cidofovir survived until the end of the study; all untreated controls died from RPX. Aerosol treatments with NanoFOVIR™ protected 50% (3/6), 83% (5/6) and 100% (6/6) of animals treated with 0.5, 1.0 and 1.75 mg/kg against lethal RPX infection. Differences are significant (p<0.05) between positive and negative controls and between untreated controls and animals treated with either 0.5, 1.0 or 1.75 mg/kg aerosol. Circles indicate termination of study.
Although Nf treated animals were significantly protected from lethality, clinical observations showed that aerosol treated animals exhibited a similar pattern of fever as control animals; fever resolved by the end of the study in all surviving animals from the Nf 1.0 mg/kg and 1.75 mg/kg groups, whereas protection from variations in body temperature was significant for the IV-Cr 10 mg/kg group on days 2, 3 and 4 (figure 2B). Untreated animals showed significant weight loss on days 5 and 6 PE compared to the 10 mg/kg IV-Cr, 1.0 mg/kg Nf and 1.75 mg/kg Nf groups and on days four and six when compared to the Nf 0.5 mg/kg group (figure 2A). Other notable clinical signs of disease such as accelerated mucus production, and appearance/development of a characteristic poxviral rash were more apparent in untreated control groups than the IV-Cr and Nf-treated groups, although these differences were not quantifiable to the extent that meaningful statistical comparisons could be made.
Figure 2.
Clinical signs after experimental infection with aerosolized RPXV. (A) Variation of body weight. (B) Variation of body temperature. Absent markers denote missing data. Significant differences (P<0.05) from untreated controls are denoted by hollow markers. Error bars are standard error.
Viral concentrations found in the kidney, spleen, brain, bronchial lymph node, liver and lung tissues were not significantly reduced by the 0.5 mg/kg Nf treatment (figure 3). Significant reductions were observed in the 10 mg/kg IV-Cr, 1.0 mg/kg Nf and 1.75 mg/kg Nf groups in all tissues with the exception of the bronchial lymph node tissue for the 1.75 mg/kg treatment group. Comparative histologic analysis of lung tissue showed animals in the group treated with the 1.75 mg/kg Nf with minimal to no resulting pathology from experimental infection (Figure 4C). This contrasted with mild to moderate lung pathology in animals treated with 10 mg/kg IV-Cr (Figure 4A) and relatively severe pathology observed in the controls (figure 4B). The protection of the lungs against RPX-induced pathological changes and the absence of detectable concentrations of viral genomes in the lung suggest that targeting the lungs for antiviral treatment could protect animals from respiratory distress.
Figure 3.
Quantification of rabbitpox virus genomes in tissues obtained after death of animals (qPCR). Data from every sample analyzed is presented in genomes/106 cells of tissue. Treatment groups are separated on the X axis. Tissues are separated on different graphs: kidney; spleen; brain; bronchial lymph node; liver; lung. Fractions over the data sets represent the number of samples over the limit of detection versus the total number of samples tested in duplicate by qPCR. Data sets were compared to untreated controls (Mann-Whitney Rank Sum Test); asterisks (*) are shown when differences are significant (p<0.05). Limit of detection is represented by a dotted line at 104 genomes/106 cells.
Figure 4.
Lungs from animals exposed to aerosolized RPXV. (A) Treated with 10mg/kg IV-Cr: mild to moderate pneumonia with scattered areas of edema and inflammation throughout the lung. (B) Control: severe pneumonia, bronchiolar epithelium is necrotic with severe congestion, edema, and hemorrhage in adjacent alveoli. (C) Treated with 1.75 mg/kg inhaled Nf: tissue is considered normal (Hematoxylin and Eosin).
The results of the clinical observations indicate that the IV-Cr 10 mg/kg and the Nf 1.75 mg/kg group were nearly equivalent in suppressing weight loss and fever, both of which are clinical indicators of suppression of viral replication and infection (Nalca et al., 2009). The primary difference between these two treatment groups is therapeutic dose – the IV-Cr 10 mg/kg group received nearly a log more cidofovir by mass than the Nf 1.75 mg/kg treatment group, yet measured clinical outcome was essentially equivalent. Targeting the organ of entry (the lung) with the antiviral significantly reduced the viral load when compared to the group receivingtreatment by IV administration (Figure 2); the viral load in the lung of the 1.0 mg/kg and 1.75 mg/kg Nf treated groups was undetectable by qPCR, whereas lung tissue in the 10 mg/kg IV-Cr group indicated the presence of high levels of virus at +6-10d PI. This is a significant distinction between the treatment groups and overall an important finding relative to the primary replication of the virus. Poxviruses, when transmitted via the airborne route, have been shown to replicate in the lung and infect virtually every cell type found in lung tissues (Chapman et al., 2010). The secondary systemic spread of the virus is contingent, at least in part on the level of viral titer achieved in the primary infection site which in this case of aerosol infection is the lung. The aerosolized treatment significantly reduced the primary replication of virus in the lung, thus further reducing the systemic spread of the virus into the periphery. The histopathological analysis of lung tissue further demonstrated the reduction of primary viral replication in the target tissue. The 10 mg/kg IV-Cr-treated animals fared much worse in pathological outcome (Figure 4) than animals treated with the 1.75 mg/kg Nf aerosol treatment. Poxviral infection in the lung was apparent in the intravenously-treated animals, with mild to moderate congestive pneumonia, edema, and inflammation noted in the lung tissue. The 1.75 mg/kg Nf-treated animals, conversely, showed minimal histologic changes associated with experimental infection (Figure 4C) when compared to the compromised lung (Figure 4A) or the untreated controls (Figure 4B) which showed pathology that was consistent with fulminant infection.
These results indicate that an inhalable reformulated form of Cr (NanoFOVIR™) is a promising antiviral for emergency post-exposure prophylaxis for aerosol poxviral disease. The RPXV aerosol model that was used to assess the therapeutic potential of a repurposed drug (cidofovir) in an inhalable form showed to be effective in blocking disease at an inhalable dose approximately one log lower than by IV injection (at 10 mg/kg). In addition, the inhaled formulation significantly reduced primary viral spread in the lung, the target organ thought to be the primary point of entry for both naturally-derived and experimental infection (Nalca et al., 2009). These results provide data appropriate for justification of future therapeutic evaluation studies using other relevant disease models of poxviruses, notably aerosolized monkeypox in nonhuman primates.
Research Highlights.
An aerosolized form of cidofovir protects rabbits from aerosol rabbitpox challenge
Aerosolized cidofovir provided comparable protection at a fraction of the IV therapeutic dosage
Aerosolized treatment significantly reduces lung viral load and corresponding pathology associated with RPX disease
Acknowledgments
This research was funded in part by the National Institute of Allergy and Infectious Diseases, the National Institute of Health, and the Department of Health and Human Services under contract number HHSN272200900015C. This work was supported in part by NIH/NCRR grant number P51 RR000164.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Rice AD, Moyer RW. Rabbitpox virus and vaccinia virus infection of rabbits as a model for human smallpox. J Virol. 2007;81:11084–11095. doi: 10.1128/JVI.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent SJ, Smith LP, Currie RJ, Nair VK. Replication kinetics of Marek's disease vaccine virus in feathers and lymphoid tissues using PCR and virus isolation. J Gen Virol. 2005;86:2989–98. doi: 10.1099/vir.0.81299-0. [DOI] [PubMed] [Google Scholar]
- Baker RO, Bray M, Huggins JW. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 2003;57:13–23. doi: 10.1016/S0166-3542(02)00196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bide RW, Armour SJ, Yee E. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Appl Toxicol. 2000;20:273–290. doi: 10.1002/1099-1263(200007/08)20:4<273::aid-jat657>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Bray M, Martinez M, Kefauver D, West M, Roy C. Treatment of aerosolized cowpox virus infection in mice with aerosolized cidofovir. Antiviral Res. 2002;54:129–142. doi: 10.1016/S0166-3542(01)00220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JL, Nichols DK, Martinez MJ, Raymond JW. Animal models of Orthopoxvirus infection. Vet Pathol. 2010;47(5):852–70. doi: 10.1177/0300985810378649. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Cidofovir in the therapy and short-term prophylaxis of poxvirus infections. Trends Pharmacol Sci. 2002a;23:456–458. doi: 10.1016/S0165-6147(02)02091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 2002b;55:1–13. doi: 10.1016/S0166-3542(02)00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza NL, Hatkin JM, Livingston V, Nichols DK, Chaplin PJ, Volkmann A, Fisher D, Nalca A. Evaluation of the efficacy of modified vaccinia Ankara (MVA)/IMVAMUNE against aerosolized rabbitpox virus in a rabbit model. Vaccine. 2009;27:5496–5504. doi: 10.1016/j.vaccine.2009.06.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalezari JP, Stagg RJ, Kuppermann BD, Holland GN, Kramer F, Ives DV, Youle M, Robinson MR, Drew WL, Jaffe HS. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. A randomized, controlled trial. Ann Intern Med. 1997;126:257–263. doi: 10.7326/0003-4819-126-4-199702150-00001. [DOI] [PubMed] [Google Scholar]
- Li G, Chen N, Roper RL, Feng Z, Hunter A, Danila M, Lefkowitz EJ, Buller RM, Upton C. Complete coding sequences of the rabbitpox virus genome. J Gen Virol. 2005;86:2969–2977. doi: 10.1099/vir.0.81331-0. [DOI] [PubMed] [Google Scholar]
- McSharry JJ, Deziel MR, Zager K, Weng Q, Drusano GL. Pharmacodynamics of cidofovir for vaccinia virus infection in an in vitro hollow-fiber infection model system. Antimicrob Agents Chemother. 2009;53:129–135. doi: 10.1128/AAC.00708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalca A, Hatkin JM, Garza NL, Nichols DK, Norris SW, Hruby DE, Jordan R. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antiviral Research. 2008;79:121–127. doi: 10.1016/j.antiviral.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, Gibbs P, Dertzbaugh M, Friedlander AM. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine. 2001;19:4768–4773. doi: 10.1016/s0264-410x(01)00234-1. [DOI] [PubMed] [Google Scholar]
- Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. 2002;8:225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CJ, Baker R, Washburn K, Bray M. Aerosolized cidofovir is retained in the respiratory tract and protects mice against intranasal cowpox virus challenge. Antimicrob Agents Chemother. 2003;47:2933–2937. doi: 10.1128/AAC.47.9.2933-2937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CJ, Pitt L. Infectious disease aerobiology: aerosol challenge methods. In: Swearengen JR, editor. Biodefense: research methodology and animal models. CRC Press Taylor & Francis Group; Boca Raton: 2005. pp. 61–76. [Google Scholar]
- Roy CJ, Voss TG. Use of the Aerosol Rabbitpox Virus Model for Evaluation of Anti-Poxvirus Agents. Viruses. 2010;2:2096–2107. doi: 10.3390/v2092096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood JC, Boulter EA, Bowen ET, Maber HB. Experimental respiratory infection with poxviruses. I. Clinical virological and epidemiological studies. Br J Exp Pathol. 1966;47:453–465. [PMC free article] [PubMed] [Google Scholar]