Abstract
Objective
To determine the safety and efficacy of propranolol given for 1 year on cardiac function, resting energy expenditure, and body composition in a prospective randomized single-center controlled study in pediatric patients with large burns.
Summary Background Data
Severe burns trigger a hypermetabolic response that persists for up to 2 years after burn. Propranolol given for 1 month post burn blunts this response. Whether propranolol administration for 1 year after injury provides a continued benefit is currently unclear.
Methods
One-hundred seventy nine pediatric patients with >30% total body surface area burns were randomized to receive control (n = 89) or 4 mg/kg/d propranolol (n = 90) for 12 months after burn. Changes in resting energy expenditure, cardiac function, and body composition were measured acutely at 3, 6, 9, and 12 months postburn. Statistical analyses included techniques that adjust for non-normality, repeated measures, and regression analyses. P <0.05 was considered significant.
Results
Long-term propranolol treatment significantly reduced the percent of the predicted heart rate and percent of the predicted resting energy expenditure, decreased accumulation of central mass and central fat, prevented bone loss, and improved lean body mass accretion. There were very few adverse effects from the dose of propranolol used.
Conclusions
Propranolol treatment for 12 months, following thermal injury, ameliorates the hyperdynamic, hypermetabolic, hypercatabolic, and osteopenic responses in pediatric patients. This study is registered at clinicaltrials.gov, NCT00675714.
INTRODUCTION
Severe burns induce a hypermetabolic response characterized by increased heart rate (HR), cardiac work, metabolism, lipolysis, and protein catabolism, resulting in loss of muscle mass and a subsequent increase in the risk of organ failure or death.1 These prolonged hypermetabolic changes can last for at least 1 to 2 years after the injury.2, 3 Catecholamines, which are elevated for 2 years after burn, are primary mediators of the hypermetabolic response.4, 5 Catecholamines potentiate cardiovascular activity such as increased myocardial oxygen consumption, local myocardial hypoxia, tachycardia, and increased myocardial contractility.6 Elevated levels of catecholamines have further been associated with cardiotoxicity, as evidenced by myocardial necrosis and myocarditis.7 Propagation of catecholamine signaling is mainly through the β-adrenergic receptors.
Propranolol, a nonselective β-adrenergic receptor antagonist, mitigates the actions of plasma catecholamines and significantly reduces the hyperdynamic and hypermetabolic state in acutely burned patients.5 Administration of this drug for 2 weeks to decrease admission HR by 15% augments net protein balance in muscle, decreasing loss of lean mass and lowers resting energy expenditure (REE).8 Cardiac function is improved by propranolol administration due to reductions in cardiac work as measured by the rate pressure product [RPP = HR × mean arterial pressure (MAP)].9 The beneficial effects of β-blockade are not, however, limited to burn patients, as patients undergoing major elective surgery experience fewer cardiac complications and overall mortality when treated with β blocking agents.10 Although propranolol appears to be beneficial in critical care, concerns remain regarding whether propranolol will increase morbidity in burn patients by blunting the stress and hyperdynamic responses. Animal studies suggest that propranolol may decrease some immune functions and is detrimental during infectious episodes or septicemia.11 Deleterious effects on immune function or inflammation were not noted in a study of severely burned children given propranolol acutely.12 Moreover, a large randomized study of adult surgical patients who had atherosclerotic disease and were undergoing non-cardiac surgery suggests that β-blockade with metoprolol is associated with increased mortality.13
This prospective randomized single-center controlled study was conducted to determine whether long-term propranolol administration improves cardiac function, REE, and body composition. This study presents an interim analysis of the aforementioned parameters including adverse events.
METHODS
Patients
Between 2002 and 2011, 2,468 pediatric patients were admitted to Shriners Hospitals for Children—Galveston (Galveston, TX). Four hundred forty-three patients with >30%of their total body surface area (TBSA) burned were prospectively randomized to clinical studies. Among these patients, 179 were randomly assigned to control (n = 89) or to receive propranolol (n = 90) administered at 4 mg/kg/d (Fig. 1).
FIGURE 1.
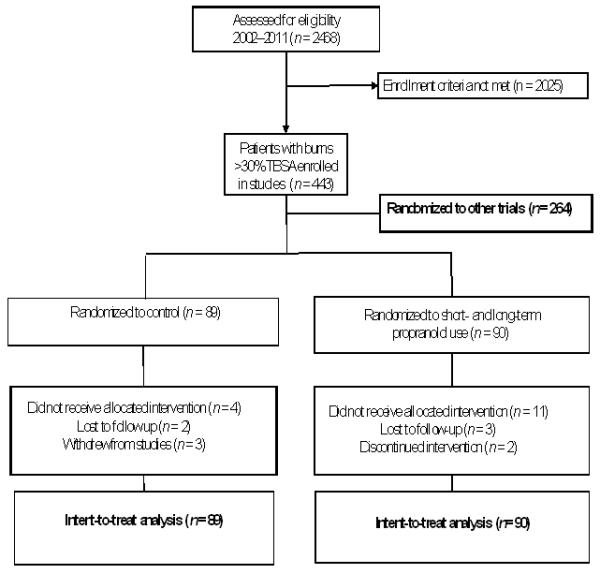
Patient enrollment diagram.
Study patients, 0.3 – 18 years of age at the time of injury, had >30% TBSA burns and required one or more surgical interventions. Patients were excluded from study participation if they met one of the following criteria: burn injury deemed so severe as to be futile to treat; anoxic brain injury; the presence of pre-existing conditions such as HIV or AIDS; 5-year history of malignancy, severe diabetes, or asthma; or an inability to obtain informed consent. Propranolol treatment was started within 3±2 days following admission. Patients received doses of propranolol intended to lower their HR by 15% (mean dose, 4 mg/kg/d). When bradycardia occurred, a single dose of propranolol was held and administration recommenced after 16 hours with one half of the original dose. The dose was then escalated back to the 4mg/kg/d dose over the following 48 hours. Assessments were performed at admission, during the hospital stay, at discharge, and at 3, 6, 9, and 12 months after injury. Data from patients withdrawing from the study were included in the analysis up to the time of withdrawal. This study was part of a larger trial (www.clinicaltrials.gov, NCT00675714) designed to determine burn outcomes after administration of anabolic agents including oxandrolone, growth hormone, and insulin. Legal guardians provided consent by signing a written informed consent form approved by the Institutional Review Board of the University of Texas Medical Branch (Galveston, TX). Assent was obtained from children >7 years of age before study enrollment.
Standard Burn Care
Fluid resuscitation was given according to the Galveston formula (a total of 5,000 mL/m2 TBSA burned + 2,000 mL/m2 TBSA lactated Ringer’s solution given during the first 24 hours). For all patients, burn wound excision and placement of autograft or allograft was performed within 48 hours of admission. Following 4 days of bed rest, patients ambulated every day until the next surgery. Excision and grafting procedures were repeated every 6 to 7 days until all burned sites were 95% healed.
Patients were fed Vivonex TEN® enteral nutrition (6% fat, 15% protein, 82% carbohydrate) via a nasoduodenal or nasogastric tube. Initially, intake was determined using the formula 1,500 kcal/m2 TBSA + 1,500 kcal/m2 TBSA burned. After the first week of hospitalization and throughout the remainder of acute hospitalization, this intake was modified to 1.4 times the weekly measured REE (see Indirect Calorimetry for more details). Nutritional status was monitored in all hospitalized patients by measuring levels of retinol-binding protein, pre-albumin, and albumin. After discharge from the hospital, patients’ diets were supplemented three times per day with Boost® (Nestle Health Care Nutrition, Nestlé S.A., Vevey, Switzerland; 41 g of carbohydrate, 10 g of protein, and 4 g of fat). Supplementation continued until the nutritionist confirmed that the regular diet met the patient’s caloric requirements of 1.4times the REE. Dietary intake was determined through interviews with caretakers, which were conducted daily when patients visited the tub room, weekly while residing close to the hospital, and at long-term clinical follow-up visits by recall questionnaires.
Patient Characteristics
At admission, patient demographics (e.g., age and gender) and injury characteristics (e.g., burn depth and size) were recorded. Burn size was determined using age-appropriate diagrams.14 During the remainder of hospitalization, mortality, morbidity, sepsis, and inhalation injury were also documented.15 Fiber-optic bronchoscopy was performed 24 hours after admission to identify inhalation injury, which was confirmed by the presence of mucosal necrosis, charring, soot, inflammation, or airway edema.
Cardiac and Blood Pressure Measurements
Resting HRs and blood pressures were measured by continuous arterial monitoring or noninvasive cuff measurements. Recordings were made continuously during acute hospitalization and at each follow-up visit. Patients and families were issued blood pressure cuffs, taught how to use them, and asked to measure and record HR and blood pressure four times per day. They were instructed to call a nurse or physician if the patient’s HR was less than 60 bpm or blood pressure was less than normal for age. HR values were compared with accepted normograms for age-matched, healthy non-burned children and were analyzed as percent of predicted HR to control for age-related differences.16, 17 The percent of predicted HR was calculated by dividing actual HR by the age-adjusted norm. The RPP was calculated as the product of MAP and HR.17
HR and blood pressure data collected by the parents was reviewed by a pediatrician at least every week initially then less often as indicated. The propranolol dose was titrated up to provide systolic blood pressure and pulse within 15% of the mean values of the normal population of that age. The parameters to be met for receiving the next dose of medication were a systolic blood pressure of 90 mmHg for teens down to 80 mmHg for younger children and a HR ranging from 70 bpm for teenagers up to 90 bpm for younger children. If the parameter was not met at the time of the next dose, the dose was not given until the time of the following dose. If several doses were not given in a week because the parameters were not met, then the dose was adjusted to a lower level so that the parameters were being met.
Indirect Calorimetry
A Sensor-Medics Vmax 29 metabolic cart (Yorba Linda, CA) was used to measure REE at weekly intervals during the acute hospital stay. Measurements were obtained from resting patients between 12 AM and 5 AM. Inspired and expired gas analysis was made every minute. When CO2 production and O2 consumption reached a steady state and was maintained for 5 min, the values were recorded. Measured values were compared with normal values, which were predicted using body mass index and the Harris-Benedict equation.14, 18, 19
Body Composition
Central mass, central fat, peripheral lean body mass (PLBM), total bone mineral content (TBMC), and total lumbar bone mineral content (TLBMC) were measured by dual energy x-ray absorptiometry (DEXA) (QDR-4500W Hologic, Waltham, MA). Systematic deviations were minimized by performing daily calibrations with a spinal phantom in the single-beam, lateral, and anteroposterior modes. Individual pixels were calibrated using a tissue bar phantom to correctly identify air, bone, fat, or lean mass.20 Feedings and intravenous fluids were discontinued prior to the exam and reinitiated afterward. Results were expressed as percent change from patient baseline values within 3 ± 2 weeks postburn. Bone loss was determined by calculating the percent change from baseline at each time point and stratifying patients based on a >5% loss in two parameters obtained from DEXA measurements: total body bone mineral content less head per total body mass (TBMC/TBM) and TLBMC. The proportion of propranolol and control patients in each strata was determined and the likelihood (odds ratios ± 95% CI) of presenting a clinically significant bone loss of at least 5% in both of these parameters was estimated.
Measurement of Hormones
Blood and urine were collected at admission, during acute hospitalization, and at follow-up visits. Blood was collected in serum-separator tubes and centrifuged at 1,320 rpm for 10 min. The resulting serum was stored at −80°C until further analysis. Serum levels of parathyroid hormone (PTH), IGF-I, IGFBP-3, osteocalcin, testosterone, albumin, and total protein were determined using HPLC and ELISA, as described elsewhere.18-20 Urinary catecholamine levels were determined as previously described.18-20
Statistical Analysis
The data distribution was evaluated using the Kolmogorov-Smirnov normality test and QQ plots. Two-sided equal-variance t-tests were used to compare normally distributed continuous data. Two-sided Wilcoxon exact tests were used to compare non-normally distributed data. Normally distributed data are presented as the means ± standard deviation (SD) or standard error of the mean (SEM). Frequency data are expressed as percentages or counts. All trends in the data were estimated using ANCOVA models, which adjusted the post-treatment measurement with the baseline measurement as a covariate and are presented as the expected value ± SEM. Testing of differences between control and propranolol were done using standard t-tests on the coefficients of treatment effect within each of the models. Each time point was modeled and tested separately. To control the overall family-wise error rate across time, we used a step-down procedure based on the permutation distribution.21 To stabilize the variance and keep the data distribution approximately normal, we transformed data, when necessary, using the Box-Cox family of transformations. Confidence intervals for odds ratios were calculated based on Fisher’s exact test calculations. A permutation test corrected by a step-down procedure was used to correct for elevated overall Type I error rates across time.21 SAS (version 9.2) and R (version 13.2) were used for data analysis and hypothesis testing. Error rates (overall and individual P values) less than 0.05 were accepted as significant.
RESULTS
Patient Disposition and Demographics
Of the 179 patients enrolled in this study, 5 were lost to follow-up (control, n = 2; propranolol, n = 3), and 5 withdrew from study participation (control, n = 3; propranolol, n = 2). Demographics did not differ significantly between groups (Table 1). Two-thirds of the patients were male, which is typical of the overall patient representation at our institution. The severity of the injury was comparable in both groups (as assessed by percent TBSA burned and percent third degree burned). One-third of the patients in each group had an inhalation injury (P = 0.49). The length of hospital stay was approximately one half day per percent of TBSA burned. Mortality was low with no significant differences between the control and the propranolol groups (P = 0.72); the deaths were due to sepsis (n=6). Assessments of infections, pneumonias, and acute respiratory distress syndrome demonstrated no significant differences between groups. Nutritional status on admission to the burn unit did not differ between groups. Dietary intake was comparable between groups throughout the 12-month study.
TABLE 1.
Patient Characteristics
| Variable | Control (n=89) |
Propranolol (n=90) |
P Value |
|---|---|---|---|
| Age (Years) | 7 ± 5 | 7 ± 5 | NS |
| Gender (M:F) | 56:33 | 67:23 | NS |
| Ethnicity | |||
| Hispanic | 97% | 91% | NS |
| Non-Hispanic | 3% | 9% | NS |
| Etiology | |||
| Flame | 72% | 66% | NS |
| Scald | 20% | 29% | NS |
| Electrical | 7% | 6% | NS |
| Chemical | 1% | 0% | NS |
| Burn to Admission, Median (Q1, Q3) | 2.0(1.0, 3.0) | 2.0(1.0, 4.0) | NS |
| Delayed admissions | 10% | 17% | NS |
| % TBSA burned | 57.5 ± 13.5 | 55.7 ± 16.5 | NS |
| % Third degree | 45.6 ± 22.7 | 42.9 ± 24.0 | NS |
| Inhalation Injury | 36% | 31% | NS |
| Survivors Length of stay, (Days) | 26.8 ± 16.4 | 31.2 ± 22.1 | NS |
| Non-Survivors Length of stay (Days) | 57.1 ± 15.4 | 65.2 ± 19.3 | NS |
| Operations | 4.2 ± 3.2 | 5.1 ± 3.6 | NS |
| Days between Operations | 6.5 ± 1.8 | 6.6 ± 2.3 | NS |
| Mortality | 6% | 4% | NS |
Data are expressed as mean ± SD, median (Q1,Q3), counts, or percentages.
TBSA = Total Body Surface Area. NS = Not significant.
Z scores were calculated on admission according to World Health Organization growth charts.
Cardiac Function
To evaluate the efficacy of β-blockade with propranolol on cardiac function, we calculated the percent of predicted HR16, 17 (Table 2). Measurements were recorded continuously during hospitalization and at each follow-up visit. Burn-related elevation in HR was ~1.7 fold above normal-for-age in both groups at the time of admission (control, 169±34% predicted vs. propranolol, 163±33% predicted; P=0.19). The RPP (MAP × HR) was obtained as a correlate of myocardial oxygen consumption.17 The RPP increased by ~1.6 fold in both groups (control, 11,009±280 bpm vs. propranolol, 11,435±304 bpm × mmHg, P=0.66). Propranolol significantly lowered the percent of the predicted HR (Fig. 2a), an effect that persisted up to 1 year postburn (119±2% vs. 110±2%, P=0.01). Propranolol reduced the percent of the predicted HR at 1 week postburn by approximately 15%. Similarly, the RPP was significantly lower in the propranolol group than in the control group between 2 weeks and 6 months postburn (by approximately 15%) (Fig.2b). Arterial pressure was monitored by MAP recordings as a continuous variable and alternatively, by comparing the probabilities of developing hypotension (MAP<65mm Hg) to determine whether propranolol adversely affects arterial pressure. Propranolol-treated patients showed subtle decreases in MAP as compared to control patients at 2 weeks, 4 weeks, and 2 months. However, these decreases did not reach significance after adjusting for multiple testing nor did they represent an increased number of instances of hypotension (MAP <65 mmHg, n = 0).
TABLE 2.
Cardiac, Metabolic, and Body Composition Comparisons from Admission to 12 Months Postburn In Control and Propranolol-Treated Patients
| Variable | Control (n = 89) |
Propranolol (n = 90) |
P value |
|---|---|---|---|
| Percent Predicted Heart Rate | |||
| Admit | 169 ± 29 | 163 ± 28 | NS |
| 1 week | 167 ± 16 | 153 ± 16 | <0.001 |
| 2 weeks | 168 ± 14 | 152 ± 14 | <0.001 |
| 4 weeks | 162 ± 17 | 150 ± 17 | <0.001 |
| 2 months | 153 ± 23 | 138 ± 23 | <0.001 |
| 3 months | 150 ± 20 | 133 ± 20 | <0.001 |
| 6 months | 127 ± 19 | 116 ± 19 | <0.01 |
| 12 months | 119 ± 16 | 110 ± 16 | <0.01 |
| Mean Arterial Pressure (mmHg) | |||
| Admit | 75 ± 15 | 74 ± 15 | NS |
| 1 week | 75 ± 10 | 74 ± 10 | NS |
| 2 weeks | 78 ± 8 | 73 ± 8 | 0.01 |
| 4 weeks | 79 ± 8 | 74 ± 8 | 0.01 |
| 2 months | 80 ± 8 | 77 ± 8 | 0.03 |
| 3 months | 83 ± 9 | 80 ± 9 | NS |
| 6 months | 81 ± 9 | 77 ± 9 | NS |
| 12 months | 78 ± 8 | 77 ± 8 | NS |
| Rate Pressure Product (bpm × mmHg) | |||
| Admit | 11,009 ± 2,219 | 11,435 ± 2,407 | NS |
| 1 week | 11,571 ± 1,744 | 11,138 ± 1,753 | NS |
| 2 weeks | 12,009 ± 1,712 | 10,303 ± 1,721 | <0.001 |
| 4 weeks | 11,919 ± 1,835 | 10,183 ± 1,839 | <0.001 |
| 2 months | 11,696 ± 1,831 | 10,220 ± 1,838 | <0.001 |
| 3 months | 11,403 ± 2,097 | 9,992 ± 2,096 | <0.001 |
| 6 months | 9,611 ± 1,788 | 8,362 ± 1,782 | <0.01 |
| 12 months | 8,140 ± 1,596 | 7,771 ± 1,595 | NS |
| Percent Predicted REE | |||
| Admit | 113 ± 36 | 112 ± 32 | NS |
| 2 weeks | 145 ± 33 | 127 ± 33 | <0.01 |
| 4 weeks | 140 ± 36 | 119 ± 36 | <0.01 |
| 3 months | 134 ± 29 | 114 ± 29 | <0.01 |
| 6 months | 119 ± 24 | 106 ± 24 | 0.01 |
| 12 months | 108 ± 22 | 104 ± 23 | NS |
| Central Mass (g) | |||
| Admit | 11,920 ± 8,652 | 13,487 ± 9,590 | NS |
| 3 months | 14,210 ± 3,471 | 12,098 ± 3,467 | <0.001 |
| 6 months | 14,841 ± 3,870 | 12,252 ± 3,866 | <0.001 |
| 9 months | 15,924 ± 4,822 | 12,790 ± 4,821 | <0.001 |
| 12 months | 16,442 ± 4,684 | 12,901 ± 4,679 | <0.001 |
| Truncal Fat (g) | |||
| Admit | 2,625 ± 2,665 | 2,780 ± 2,524 | NS |
| 3 months | 3,012 ± 983 | 2,622 ± 982 | 0.02 |
| 6 months | 3,082 ± 1,289 | 2,713 ± 1,287 | NS |
| 9 months | 3,497 ± 1,638 | 2,919 ± 1,637 | NS |
| 12 months | 3,737 ± 1,703 | 2,970 ± 1,701 | 0.01 |
| Peripheral Mass (g) | |||
| Admit | 16,055 ± 10,979 | 13,782 ± 9,929 | NS |
| 3 months | 14,473 ± 3,184 | 15,249 ± 3,180 | 0.03 |
| 6 months | 14,977 ± 3,695 | 16,262 ± 3,691 | 0.02 |
| 9 months | 16,021 ± 4,350 | 17,128 ± 4,348 | NS |
| 12 months | 16,718 ± 4,389 | 17,549 ± 4,384 | NS |
| Peripheral Lean Mass (g) | |||
| Admit | 10,997 ± 7,599 | 9,315 ± 6,601 | NS |
| 3 months | 9,693 ± 2,328 | 10,357 ± 2,325 | 0.02 |
| 6 months | 10,231 ± 2,547 | 11,187 ± 2,544 | 0.02 |
| 9 months | 10,858 ± 2,900 | 11,680 ± 2,899 | NS |
| 12 months | 11,234 ± 2,997 | 11,951 ± 2,994 | NS |
Data are expressed as the mean ± SD.
NS = Not significant
FIGURE 2.
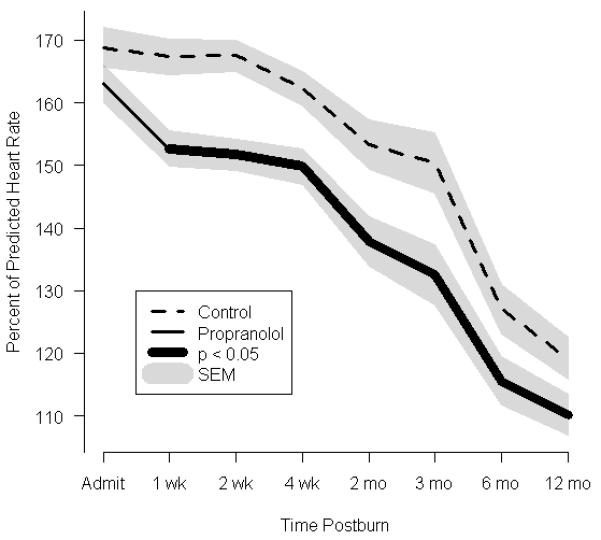
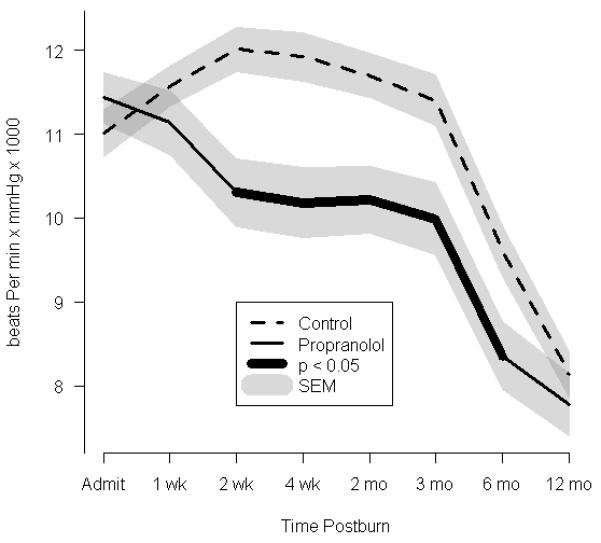
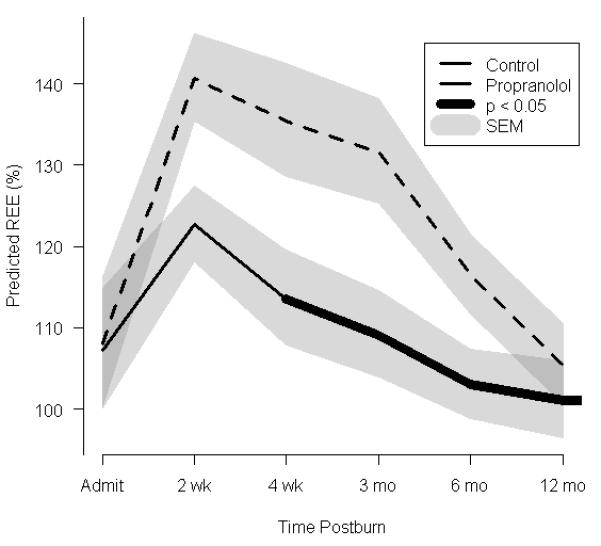
Effect of propranolol on cardiac and metabolic indices. (a) Percent predicted heart rate. (b) Rate pressure product. (c)Resting energy expenditure (REE), expressed as the percentage of energy expenditure predicted by the Harris Benedict equation. In a-c, data are shown as the Loess-smoothed trend with shading indicating SEM.
Hypermetabolism
The percent of predicted REE changed over time (Fig. 2c). All patients were hypermetabolic by the initial study period, as seen by comparison to the basal metabolic rate predicted by the Harris-Benedict equation.22, 23 In both the control and propranolol groups, the percent of predicted REE peaked at140% and 120%, respectively. Both groups remained hypermetabolic throughout the 12-month time frame, as determined by elevated percent predicted REE measurements. The percent of predicted REE significantly decreased beginning with the acute studies and continuing throughout 6, 9, and 12 months postburn. This decrease was more pronounced in the propranolol group than in the control group between 2 weeks and 6 months after burn.
Body Composition
Central mass, central fat mass, PLBM, TBMC, and TLBMC were measured by DEXA (Fig. 3). In both groups, total body mass increased between baseline and 12 months after burn (Table 2). Despite both groups having similar nutritional intake, central mass accretion was significantly higher in the control group, while there was a 17% decrement in central mass as early as 3 months postburn following propranolol administration. These differences between the groups were significant throughout 12 months postburn (Fig. 3a). Central fat mass was significantly lower in the propranolol than in the control group, reaching a maximal decrease of 23%at 12 months postburn (Fig.3b).An 11% increase in PLBM was seen in the propranolol group when compared to control at 6 months after burn (Fig. 3c).
FIGURE 3.
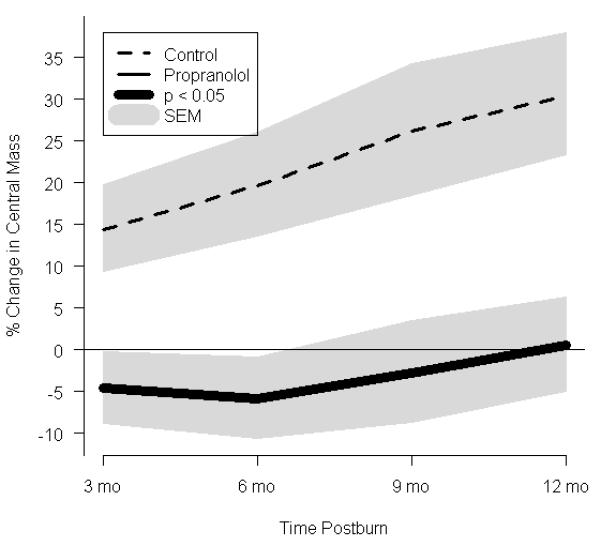
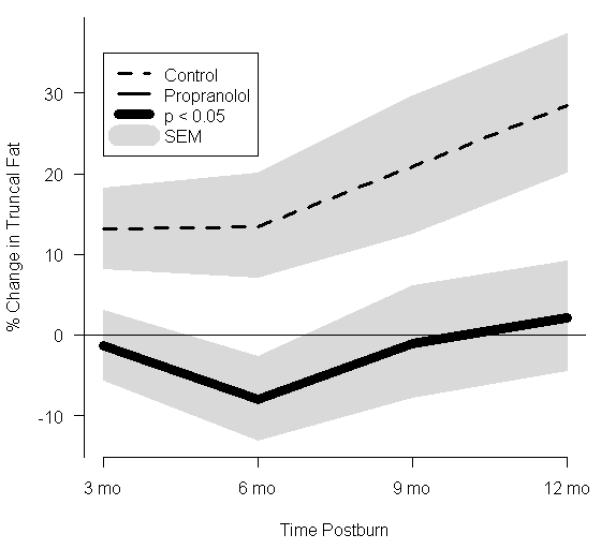
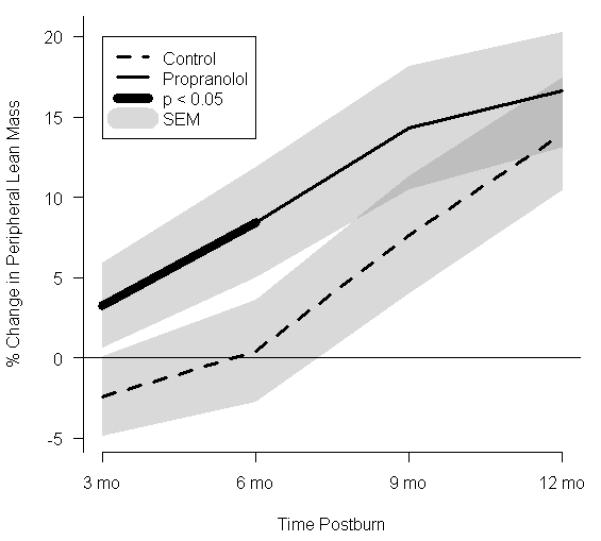
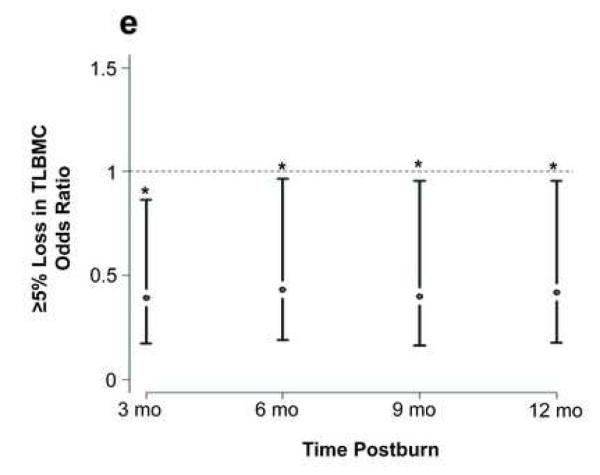
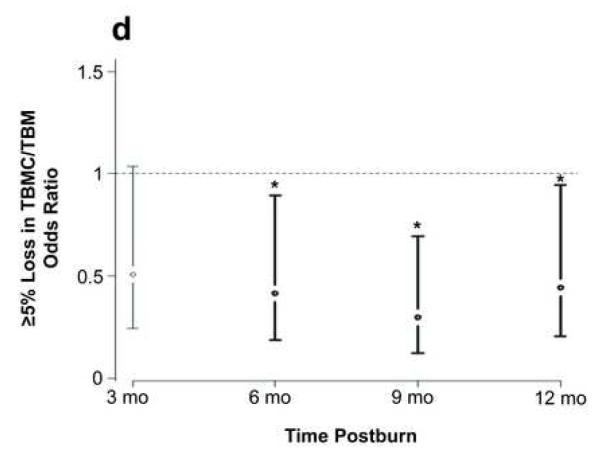
Effect of propranolol on body composition. (a) Percent change in central mass, (b) percent change in truncal fat, and (c) percent change in peripheral lean mass. In a-c, data are expressed as percent change from patient baseline and are shown as the Loess-smoothed trend with shading indicating SEM. (d, e) Comparison of the likelihood of losing ≥5% of (d) total bone mineral content/total body mass (TBMC/TBM) and (e) total lumbar bone mineral content (TLBMC) in control and propranolol-treated patients. Data are expressed as the odds ratios. *Significant difference at P< 0.05.
Approximately 70% of control and 50% of propranolol-treated patients lost more than 5% of TBMC/TBM by 6 months after burn (P = 0.01). Propranolol decreased the likelihood of TBMC/TBM loss at 6 months (OR = 0.5, 95% CI: 0.25 to 0.75) when compared with control; this effect remained significant throughout the rest of the study period (Fig. 3d, Table 3).
TABLE 3.
Odds of ≥5% Skeleton Loss in Control (n=89) and Propranolol- (n = 90) Treated Patients
| Variable | Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| ≥5% Loss TBMC/TBM | |||
| 3 months | 0.51 | (0.24 – 1.03) | NS |
| 6 months | 0.41 | (0.19 – 0.89) | 0.02 |
| 9 months | 0.30 | (0.12 – 0.69) | <0.01 |
| 12 months | 0.44 | (0.20 – 0.94) | 0.02 |
| ≥5% Loss LBMC | |||
| 3 months | 0.39 | (0.18 – 0.86) | 0.02 |
| 6 months | 0.43 | (0.19 – 0.96) | 0.03 |
| 9 months | 0.40 | (0.16 – 0.96) | 0.03 |
| 12 months | 0.42 | (0.18 – 0.96) | 0.03 |
TBMC/TBM = Total bone mineral content/Total body mass. LBMC = Lumbar bone mineral content. NS = Not significant.
The propranolol group also had a lower likelihood of experiencing a ≥5% loss of TLBMC than the control group during the study duration (Fig. 3e). Propranolol significantly preserved LBMC at 3, 6, 9, and 12 months (P = 0.02).
Adverse Events
Incidences of hypotension, bradycardia, hypoglycemia, cardiac arrhythmia, respiratory arrest, and death were recorded. In the control group, there were five deaths. In the propranolol group, there were no occurrences of hypotension with few incidences of bradycardia (n=2), hypoglycemia (n=1), cardiac arrhythmia (n=1), respiratory compromise (n=2), and death (n = 4). In all cases of adverse events, propranolol treatment was halted and reinitiated according to the standard operating procedure delineated in the methods section. Autopsies of five control patients and four propranolol-treated patients revealed the cause of death to be sepsis in all cases.
DISCUSSION
Three hundred patients in each group had been planned for this large randomized single center controlled study, which was designed to test the efficacy of propranolol in children with >30% TBSA burns when given at an approximate dose of 4mg/kg/d from 96 hours after burn injury until one year later. An interim analysis was planned after enrollment of 90 patients per group. In this study, the 4mg/kg/d dose of propranolol was extremely well tolerated in the pediatric age group used, with few episodes of bradycardia, hypoglycemia, morbidity, or major adverse effects related to the use of this drug.
The primary aim of the study was to determine whether this dose of propranolol decreased HR by approximately 15%. For the duration of drug administration, there was a persistent decrease in HR. Although the RPP was decreased by approximately 15% at 4 weeks postburn, all patients, whether in the control or propranolol group, still exhibited massive tachycardia of 120 – 140% of predicted normal for 1 – 3 months after injury. HR then decreased to 110% at 6 months post injury with propranolol administration. Larger doses of propranolol could potentially decrease HR and cardiac work even more than demonstrated here, since very few episodes of bradycardia and no significant decreases in blood pressure were seen with the 4 mg/kg/d dose. Elevations in cardiac work caused by persistent tachycardia could then be reduced even further. Patients need to be studied well into adulthood to see if these prolonged episodes of tachycardia and increased cardiac work have long-term effects on cardiovascular morbidity as well as to determine whether decreasing HR with propranolol reduces this morbidity.
REE was a secondary endpoint of this study, and the administration of propranolol did decrease REE by 20% during the first 6 months after injury. Understanding whether a larger dose would have a greater affect and whether long-term benefits of this diminution of REE in this patient population will require following children to full growth and examining strength, growth, height and weight velocities, final stature, and their return to work and school.
Another end point of the study was central mass. Propranolol was found to decrease accrual of central mass by 15% at 3 months after burn and for as long as the drug was being administered relative to control patients, who exhibited a 25% increase in percent change of central mass from baseline. In the propranolol group, central mass remained at or below base line. Central mass is composed of organs (liver, spleen, kidney) and mesenteric fat. DEXA revealed that, in the propranolol group, truncal fat was reduced by approximately 15%, with truncal fat remaining at or below or baseline levels throughout the hospital course. In contrast, controls exhibited a 20% increase from baseline over 1 year after injury.
Taken together, these findings indicate that propranolol decreases the central deposition of fat, a finding that is in keeping with previous studies by Wolf and Herndon showing that propranolol decreases mesenteric blood flow24 and reduces peripheral lipolysis.25 Burn-induced lipolysis typically results in the hydrolysis of triglycerides into free fatty acids, which are then released into the circulation and deposited in tissues or vital organs. A reduction in mesenteric fat may therefore be accompanied by fatty liver and a subsequent increase in liver size and may result in liver dysfunction. Steatosis of vital organs aside, lipolysis and free fatty acids contribute to post-burn morbidity and mortality by mediating insulin resistance.26 Insulin-stimulated glucose uptake is impaired by free fatty acids and triglygerides27, 28 with insulin resistance occurring as a direct consequence of the inhibition of glucose transport activity.29 Recent evidence suggests a strong relationship between fat and glucose metabolism.30 Additionally, catecholamines increase lypolysis, further stimulating insulin resistance.
Zimmers et al. have suggested that chronic elevation of IL-6 and inflammatory mediators results in organ hypertrophy after a thermal injury.31 This study has also suggested that the use of β-blocking agents may decrease the inflammatory response by decreasing levels of IL-6, MCP-1, and other cytokines.12
Another secondary endpoint of the study was PLBM, which showed a 10% improvement in the propranolol group compared to the control group. This finding is in keeping with previous studies showing an improvement in protein to find out whether it was protein synthesis or protein degradation.8
In the current study, the beneficial effects of propranolol could also be extended to bone. In previous animal experiments, propranolol was shown to be beneficial in the partial preservation against post-ovariectomy bone loss.32 It has been previously shown that propranolol reduced bone mineral loss after burns.33 The direct or indirect effects that beta-blocking agents provide on bone have yet to be elucidated. The present study shows that the odds ratio for more than 5% loss of TBMC is reduced by propranolol. Improvement in bone strength may indirectly improve lean body mass. In consideration of the changes in bone mass and density, bone growth spurts were examined. The number of individuals in growth spurts in each group was similar. Data predict that continuation of this study to meet the full sample size of 300 patients per group will provide sufficient power to determine whether propranolol affects infections and whether there will be a long-term effect on growth (as seen by height weight percentiles in this patient population over time).
In this study, all patients who were older than 7 years of age were offered the opportunity to participate in a 3-month exercise program in which children exercised 5 days a week at 75% of their individual VO2 maximum and used progressive resistance exercise, lifting 8 to 12 times their maximum capacity for 3 sets, 3 times weekly. An equal number of individuals in each group (control or propranolol) participated in the exercise program. Patients who did not participate in the exercise program were exercise-tested and given physical and occupational therapy routines that involved exercise. An equivalent number of patients declined exercise therapy in each group. All patients were tested when they returned to the clinic at 6, 9, and 12 months after burn. There were no differences in habitual physical activity reported by the patient parents or evaluated by exercise testing capabilities at discharge or at the 6 or 12 month intervals.
Nutrition was controlled as it affects all of the variables that were studied. As described in the methods section, all patients admitted to the hospital, were administered 1,500 kcal/m2 TBSA + 1,500 kcal/m2 TBSA burned enteral nutrition via continuous drip. Patients in both cohorts tolerated the nutritional diet equally well. Upon discharge, each patient was advised by a dietician to eat 1.4 to 1.6 times their resting energy expenditure with detailed diary training performed on at least two different occasions for all patients in the study. Dietary recall was obtained when patients returned at 6 and 12 month intervals. No differences in dietary intake between the two groups could be detected, probably due to the large number of individuals from similar social economic and cultural backgrounds.
The patients enrolled in this study were young, with an average age of 7 ± 5 years in each group. Four of the patients underwent menarche (two in each group). Although the number of males allocated to propranolol was greater (74% versus 63%, propranolol and control, respectively), the differences in gender between the two groups were not statistically significant. There was no statistical difference in gender between the two groups. Thus it is unlikely that this is a type 2 error as this was randomized study. Larger numbers will be enrolled in outcome studies to validate this assumption.
The initial analyses of patients administered short-term or long-term propranolol have shown that there is no effect on acute stress disorder or post-traumatic stress disorder. Analyses of larger groups at 5 and 10 years after burn will be conducted to determine whether propranolol has an effect on psychological outcomes such as those measured by the Shriners outcome instruments. Larger numbers and longer follow up will be required to achieve significance given the limits of these measures.
Future studies will investigate cardio-pulmonary and muscle endurance by assessing patients’ aerobic capacity as well as long-term outcomes of these individuals by looking at time to return to school, acute distress disorder, post-traumatic stress disorder, and anxiety over all, as examined by scaled psychological tests.
In conclusion, analysis of long-term administration of propranolol in children with >30% TBSA burns indicates that this treatment is safe and markedly decreases HR and cardiac work. It also shows that this treatment decreases central body mass, reduces truncal fat, and improves lean body mass and bone mineral density. This study suggests that a larger study population will show long-term health benefits in terms of growth, cardiac health, and improvement of the metabolic syndrome.
DISCUSSANT
DR. BASIL PRUITT, JR. (San Antonio, TX)
Over the past four decades, Dr. Herndon and his colleagues have characterized the metabolic responses to severe injury and now present these intriguing data, suggesting that those responses can be safely modified to the patient’s benefit.
You note that propranolol reduced central body mass and truncal fat. Were those changes accompanied by alteration of fat as an energy source? Specifically, did you calculate the respiratory quotient when you measured resting energy expenditure, and was it lower in the propranolol-treated patients? If it was not, by what mechanism do you propose propranolol decreases central fat accumulation?
Since lean body mass is strongly influenced by exercise, how comparable was the physical activity in the two groups, and was there any difference between males and females in lean body mass accretion?
In that same vein, was there a difference in the number of children in the two groups who participated in the special on-site exercise programs that you have reported to be so beneficial? You note that after discharge from the hospital, the patients received dietary supplements until the “nutritionist confirmed that the regular diet met the caloric goal.” Was the duration of such support and such supplementation comparable in the two groups?
Recently, you and your colleagues reported that the adipose tissue transcriptome is markedly altered following burn injury, with an upregulation of genes involved in inflammation pathways. Were those gene alterations reduced by propranolol? And, if so, are those patients more susceptible to infection?
Also, if 4 milligrams per kilogram per day evokes all of these good effects, would 8 milligrams per kilogram per day be twice as good?
Finally, if we are to apply this treatment to other patients should the dose be proportional to the severity of injury as defined by some score or perhaps, more specifically, the magnitude of hypermetabolism?
The possible application of these findings to other surgical populations makes this a paper of potentially great importance.
DR. DAVID N. HERNDON
We performed RQs, and they do go along with the results that we demonstrated. Lipolysis is decreased by propranolol. We intend to do studies with stable isotopically labeled palmitate to confirm the mechanism, but peripheral lipolysis is prevented, and central deposition of fat in the liver is diminished. There is a decrease in blood flow to the liver and a decrease in central deposition of fat, which is a presumed mechanism for that. Physical activity is similar in both cases. We do advocate for an exercise program in which patients exercise aerobically three days a week, to 75% VO2 max, and two days a week to three repetitions of 75% max of their lifting and pushing capabilities. There were equal numbers of patients who participated in this activity in both groups. Propranolol seems to have improved aerobic capacity, and we will be analyzing those data in more detail in the future. The dietary supplementation in these individuals was equal between the groups. We keep patients in the hospital for two months after discharge, and they are seen by a dietitian on a daily basis, with dietary recall five days per week, and supplementation administered to meet 1.4 times the resting energy expenditure requirements.
These supplementations are continued in those patients who need it after the first two months. Dietary recall was performed at three months, six months, nine months, and 12 months in all cases. There were no differences in dietary intake that were recordable between the two groups.
Adipose tissue transcriptome was examined in these individuals, and, in fact, there is a marked upregulation of inflammation in fat. Fat appears to be a very highly active organ after burn injury, perhaps secreting cytokines as well as serving as the source of lipolysis. The changes in the transcriptome in the fat are very similar to those that I showed briefly in muscle, with a decrease in beta and alpha adrenergic signaling transduction pathways and cyclic A and P transduction pathways, a decrease in leptin.
As for the inflammatory responses and increases and decreases, infection may or may not be present in this group, and this will be studied in the full trial, where the power to distinguish infections will be achieved.
The dose of propranolol should probably be more than 4 milligrams per kilogram per day, which is a very high dose; one that would perhaps strike fear into a normal surgeon’s heart, in fact, but we started with this beginning dose and we think we may be able to push it even higher. We were able to decrease heart rate by only 15%, yet these patients remain massively tachycardic throughout the first year postinjury. Cardiac work could be decreased more by increasing the dose, and we will do that according to injury and according to metabolic response in future studies.
DISCUSSANT
DR. WILLIAM G. CIOFFI (Providence, RI)
Almost 200 patients were randomized in this one-year propranolol trial, with laboratory, metabolic, and body composition studies performed. The treatment was safe, with few untoward effects, and did show significant effects on reducing cardiac work and resting energy expenditure.
Your results are statistically significant, but are they clinically relevant? You have over three decades of burn experience. Do you know if severely burned children truly are at risk later in life for cardiac events and does propranolol supplementation for one year reduce this risk? More important are the metabolic effects that you measured in your patients. Do the treated children really show improved muscle strength and exercise tolerance?
In order for this to really make a difference, these kids should show increased muscle mass, increased muscle strength, and exercise tolerance.
DR. DAVID HERNDON
How propranolol will decrease future cardiac events in these patients remains to be determined by following them for five and ten years and conducting studies with a larger number of patients.
Similarly, strength is improved and aerobic capacity improved in patients receiving propranolol, but to determine effects on growth, stature, and whether these children attain full growth, which their predecessors have not, will require five- and ten-year follow-up and an N of 150 to 300 in each group, and we intend to do that.
DISCUSSANT
DR. ANTHONY A. MEYER (Chapel Hill, NC)
We have an N of 1, and that is a pair of identical twins, one of whom was burned as a child; the other one was not. We saw the significant difference in growth.
Given the studies that you are doing, are you considering using echocardiography to measure ventricular wall thickness, chamber volume, and ejection fraction to see if any of the parameters of long-term effects of the propranolol are helpful in those patients by changing those characteristics in your burn victims?
DR. DAVID HERNDON
That’s an excellent suggestion, and we do intend to use transesophageal echocardiography to measure the parameters you suggest. Preliminary data indicate that there are salutory effects. We will follow that up and report back to you personally and publicly.
DISCUSSANT
DR. STANLEY DUDRICK (Waterbury, CT)
I would just like to ask what are your thoughts about the intangible explanation of why we do better, at least clinically, and yet the outcomes, meaning the ultimate outcome, death or survival, do not reward us. Are there any other things that you have measured or can measure to indicate that the expenditure of so much effort and resources on your part and that of your team and on ours is merited by something better for the patient?
I know it is more of a philosophical, ethical question than an objective one; but it is one that has been bugging me for more than 45 years. The critics say that you’re not really doing any good, and I don’t mean to imply that at all. I’m entirely in favor of what David has done, and in awe of the results. I thank him from the bottom of my heart.
DR. DAVID HERNDON
We hope that by giving propranolol to a very large number of patients over time, we will decrease stress, and we will decrease anxiety. We hope to decrease acute stress disorder, or post-traumatic stress disorder. We hope that this agent will improve strength and will allow patients to return to work and school earlier.
We have to look at long-term outcomes to see, as Dr. Cioffi indicated, whether giving this drug really will make a difference.
Importantly, this patient population is not cured when they go home. They are massively ill for a very, very long time. Many of our trauma patients and many of our surgical patients are weakened. They suffer ravages that persist for months and years after we work with them and on them. We need to treat those ravages with agents like propranolol, pain medicines, antianxiety medicines, and mood-altering medicines, to make their lives better.
I think we should be committed to improving long-term outcomes and not just focusing on physiologic and metabolic effects.
ACKNOWLEDGEMENTS
The authors thank Deborah Benjamin, Wes Benjamin, Maria Cantu, Mario Celis, Tabatha Elliot, Kathryn Epperson, Eric Henry, Holly Goode, Kara Hougen, Joanna Huddleston, Mary Kelly, Xuyang Liang, Maria Magno, Liz Montemayor, Marc Nicolai, Sylvia Ojeda, Maricela Pantoja, Cathy Reed, Lisa Richardson, Lucile Robles, Pam Stevens, Sierra Tinney, Judith Underbrink, Becky Whitlock, the nutrition department, and the respiratory therapy team for their assistance in obtaining the study measurements. Finally, we thank Kasie Cole-Edwards for editing and proofreading the manuscript. This study was supported by grants from the National Institute for Disabilities and Rehabilitation Research (H133A070026 and H133A70019), the National Institutes of Health (P50-GM60338, R01-HD049471, R01-GM56687-11S1, and T32-GM8256), and Shriners Hospitals for Children (84080, 8660, 9145, and 8760). CCF is an ITS Career Development Scholar supported, in part, by NIH KL2RR029875 and NIH UL1RR029876. This study is registered at clinicaltrials.gov, NCT00675714.
This study was supported by grants from the National Institute for Disabilities and Rehabilitation Research (H133A070026 and H133A70019), the National Institutes of Health (P50-GM60338, R01-HD049471, R01-GM56687-11S1, and T32-GM8256), and Shriners Hospitals for Children (84080, 84309, 8660, 9145, and 8760). CCF is an ITS Career Development Scholar supported, in part, by NIH KL2RR029875 and NIH UL1RR029876.
Footnotes
Disclosure: The authors declare no conflicts of interest.
This study is registered at clinicaltrials.gov, NCT00675714.
AUTHOR CONTRIBUTIONS
Concept/Design: David N. Herndon. Walter Meyer Oscar E. Suman Robert E. Barrow Marc G. Jeschke Celeste C. Finnerty
Data Acquisition: David N. Herndon Ronald P. Mlcak Jong O. Lee Felicia N. Williams Walter Meyer Oscar E. Suman Marc G. Jeschke Celeste C. Finnerty
Data Analysis/Interpretation: David N. Herndon Noe A. Rodriguez Eva C. Diaz Sachin Hegde Kristofer Jennings Ronald P. Mlcak Jaipreet Suri Jong O. Lee Walter Meyer Oscar E. Suman Robert E. Barrow Marc G. Jeschke Celeste C. Finnerty
Drafting/Revising Manuscript: David N. Herndon Noe A. Rodriguez Eva C. Diaz Sachin Hegde Kristofer Jennings Ronald P. Mlcak Jaipreet Suri Jong O. Lee Felicia N. Williams Walter Meyer Oscar E. Suman Robert E. Barrow Marc G. Jeschke Celeste C. Finnerty
Approval: David N. Herndon Noe A. Rodriguez Eva C. Diaz Sachin Hegde Kristofer Jennings Ronald P. Mlcak Jaipreet Suri Jong O. Lee Felicia N. Williams Walter Meyer Oscar E. Suman Robert E. Barrow Marc G. Jeschke Celeste C. Finnerty
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pereira CT, Murphy KD, Herndon DN. Altering metabolism. J Burn Care Rehabil. 2005;26(3):194–9. [PubMed] [Google Scholar]
- 2.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 3.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am. 1978;58(6):1173–87. doi: 10.1016/s0039-6109(16)41685-3. [DOI] [PubMed] [Google Scholar]
- 5.Wilmore DW, Long JM, Mason AD, Jr., et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180(4):653–69. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minifee PK, Barrow RE, Abston S, et al. Improved myocardial oxygen utilization following propranolol infusion in adolescents with postburn hypermetabolism. J Pediatr Surg. 1989;24(8):806–11. doi: 10.1016/s0022-3468(89)80541-x. [DOI] [PubMed] [Google Scholar]
- 7.Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985;17(4):291–306. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- 8.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345(17):1223–9. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 9.Baron PW, Barrow RE, Pierre EJ, et al. Prolonged use of propranolol safely decreases cardiac work in burned children. J Burn Care Rehabil. 1997;18(3):223–7. doi: 10.1097/00004630-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335(23):1713–20. doi: 10.1056/NEJM199612053352301. [DOI] [PubMed] [Google Scholar]
- 11.Oberbeck R, Schmitz D, Wilsenack K, et al. Adrenergic modulation of survival and cellular immune functions during polymicrobial sepsis. Neuroimmunomodulation. 2004;11(4):214–23. doi: 10.1159/000078439. [DOI] [PubMed] [Google Scholar]
- 12.Jeschke MG, Norbury WB, Finnerty CC, et al. Propranolol does not increase inflammation, sepsis, or infectious episodes in severely burned children. J Trauma. 2007;62(3):676–81. doi: 10.1097/TA.0b013e318031afd3. [DOI] [PubMed] [Google Scholar]
- 13.Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–47. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 14.Mlcak RP, Jeschke MG, Barrow RE, et al. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244(1):121–30. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mlcak RP, Buffalo MC. Pre-hospital management, transport, and emergency care. In: Herndon DH, editor. Total Burn Care. 3rd ed Saunders; Philadelphia: 2007. pp. 81–92. [Google Scholar]
- 16.Hazinski MF. Cardiovascular Disorders. In: Hazinski MF, editor. Manual of Pediatric Critical Care. Mosby, Inc.; 1999. pp. 84–288. [Google Scholar]
- 17.Voors AW, Webber LS, Berenson GS. Resting heart rate and pressure-rate product of children in a total biracial community: the Bogalusa Heart Study. Am J Epidemiol. 1982;116(2):276–86. doi: 10.1093/oxfordjournals.aje.a113412. [DOI] [PubMed] [Google Scholar]
- 18.Hart DW, Wolf SE, Herndon DN, et al. Energy expenditure and caloric balance after burn: increased feeding leads to fat rather than lean mass accretion. Ann Surg. 2002;235(1):152–61. doi: 10.1097/00000658-200201000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suman OE, Mlcak RP, Chinkes DL, et al. Resting energy expenditure in severely burned children: analysis of agreement between indirect calorimetry and prediction equations using the Bland-Altman method. Burns. 2006;32(3):335–42. doi: 10.1016/j.burns.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Przkora R, Barrow RE, Jeschke MG, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60(5):968–71. doi: 10.1097/01.ta.0000214580.27501.19. [DOI] [PubMed] [Google Scholar]
- 21.Romano JP, Wolf M. Exact and approximate stepdown methods for multiple hypothesis testing. Journal of the American Statistical Association. 2004;100:94–108. [Google Scholar]
- 22.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Carnegie Institution of Washington; Washington: 1919. [Google Scholar]
- 23.Porro LJ, Herndon DN, Rodriguez NA, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012;214(4):489–502. doi: 10.1016/j.jamcollsurg.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gore DC, Honeycutt D, Jahoor F, et al. Propranolol diminishes extremity blood flow in burned patients. Ann Surg. 1991;213(6):568–74. doi: 10.1097/00000658-199106000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aarsland A, Chinkes D, Wolfe RR, et al. Beta-blockade lowers peripheral lipolysis in burn patients receiving growth hormone. Rate of hepatic very low density lipoprotein triglyceride secretion remains unchanged. Ann Surg. 1996;223(6):777–87. doi: 10.1097/00000658-199606000-00016. discussion 787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randle PJ, Garland PB, Hales CN, et al. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–9. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 27.Boden G, Chen X, Ruiz J, et al. Insulin receptor down-regulation and impaired antilipolytic action of insulin in diabetic patients after pancreas/kidney transplantation. J Clin Endocrinol Metab. 1994;78(3):657–63. doi: 10.1210/jcem.78.3.8126138. [DOI] [PubMed] [Google Scholar]
- 28.Shah P, Vella A, Basu A, et al. Effects of free fatty acids and glycerol on splanchnic glucose metabolism and insulin extraction in nondiabetic humans. Diabetes. 2002;51(2):301–10. doi: 10.2337/diabetes.51.2.301. [DOI] [PubMed] [Google Scholar]
- 29.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103(2):253–9. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin X, Zimmers TA, Perez EA, et al. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006;43(3):474–84. doi: 10.1002/hep.21087. [DOI] [PubMed] [Google Scholar]
- 32.Bouxsein ML, Devlin MJ, Glatt V, et al. Mice lacking beta-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology. 2009;150(1):144–52. doi: 10.1210/en.2008-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein GL, Herndon DN, Langman CB, et al. Long-term reduction in bone mass after severe burn injury in children. J Pediatr. 1995;126(2):252–6. doi: 10.1016/s0022-3476(95)70553-8. [DOI] [PubMed] [Google Scholar]