Abstract
We have developed and characterized a system to analyze light effects on auxin transport independent of photosynthetic effects. Polar transport of [3H]indole-3-acetic acid through hypocotyl segments from etiolated cucumber (Cucumis sativus L.) seedlings was increased in seedlings grown in dim-red light (DRL) (0.5 μmol m−2 s−1) relative to seedlings grown in darkness. Both transport velocity and transport intensity (export rate) were increased by at least a factor of 2. Tissue formed in DRL completely acquired the higher transport capacity within 50 h, but tissue already differentiated in darkness acquired only a partial increase in transport capacity within 50 h of DRL, indicating a developmental window for light induction of commitment to changes in auxin transport. This light-induced change probably manifests itself by alteration of function of the auxin efflux carrier, as revealed using specific transport inhibitors. Relative to dark controls, DRL-grown seedlings were differentially less sensitive to two inhibitors of polar auxin transport, N-(naphth-1-yl) phthalamic acid and 2,3,5-triiodobenzoic acid. On the basis of these data, we propose that the auxin efflux carrier is a key target of light regulation during photomorphogenesis.
Photomorphogenesis is the initiation of an ordered sequence of events on time scales from seconds to weeks, regulating processes from the level of protein and nucleic acid behavior to the differentiation of tissues and organs (Shropshire and Mohr, 1983). In plant stems photomorphogenesis has been described principally in terms of light-induced changes in elongation rate, and the biochemistry underlying these changes. The majority of progress has occurred in the understanding of processes on shorter time scales and limited structural complexity. One mediator of light regulation of stem elongation is the plant hormone auxin (Behringer and Davies, 1992, and refs. therein). A role for polar auxin transport in photomorphogenetic processes can be inferred from the effects of mutations affecting auxin transport, including a variety of defects in photosynthetic, transport, and growth capacities of mature plants (Carland and McHale, 1996; Ruegger et al., 1997). These features of the form and function of mature plants are the central outcomes of development directed by photomorphogenesis.
Polar transport of endogenous and applied auxins is a consistent feature of plant stem and coleoptile tissue, and is closely tied to development (Goldsmith, 1977; Thimann, 1977; Jacobs, 1979; Lomax et al., 1995). Light-induced changes in transport correlate well with rapid changes in stem elongation (Furuya et al., 1969; Jones et al., 1991), and more gradual developmental changes, including vascular differentiation, have been shown to be regulated by auxin transport (Jacobs, 1979; Ruegger et al., 1997; see also introduction in Carland and McHale, 1996). However, auxin transport itself has been described as developmentally regulated (Jacobs, 1979). One example is the decline of auxin transport with increasing tissue maturity in petiole and stem tissue (Jacobs, 1979; Suttle, 1991).
In grass seedlings both rapid effects of light on auxin transport (Jones et al., 1991) and long-term effects of light (Naqvi, 1975) have been reported. When irradiations were given a short interval before the auxin-transport assay, light caused a decrease in the amount of auxin transported through tissue per unit time (transport intensity or export rate), whereas the rate of movement of auxin (transport velocity) was unchanged (Jones et al., 1991). Coleoptiles of corn seedlings grown for long periods in WL exhibit transport velocities that are 50 to 60% of those found in DG seedlings (Naqvi, 1975). There have been few studies on the effects of light on auxin transport in dicot stems, and most of these were confined to effects of light on LG seedlings. An early study indicated that light had no effect on transport in DG pea (Thimann and Wardlaw, 1963). Eliezer and Morris (1980) reported that WL illumination during the transport period caused a 35% increase in the velocity of auxin transport in both DG and LG pea seedings, and that LG plants also showed a 2-fold increase in transport intensity, but only when transport occurred in the light.
One property that characterizes auxin-transport systems, and is diagnostic of their function, is the susceptibility to specific inhibitors of polar auxin transport, all of which act at least in part by blocking auxin efflux (Katekar and Geissler, 1980; Lomax et al., 1995; Delbarre et al., 1996). Developmental changes in responsiveness to these inhibitors have been observed to occur in parallel with changes in polar auxin transport (Suttle, 1991), and altered developmental responses to these inhibitors have been used as screens for mutations affecting auxin transport itself (Ruegger et al., 1997). Chemical and physiological data suggest that synthetic inhibitors of polar auxin transport: (a) have natural analogs that are flavonoid molecules (Jacobs and Rubery, 1988), (b) may have several modes of action (Katekar and Geissler, 1980; Bruun et al., 1992; Delbarre et al., 1996), and (c) act on an efflux carrier target that may consist of multiple components (Morris et al., 1991; Cox and Muday, 1994).
From current understanding of the auxin efflux carrier, the differences between rapid and long-term effects of light on polar auxin transport can be explained in terms of two mechanisms: (a) fast, reversible effects caused by light-induced changes in diffusible regulators of efflux (such as flavonoids; see Jacobs and Rubery, 1988); and (b) slower, less labile modification of the number, position, or function of one or more components of the efflux carrier itself (Morris et al., 1991). The finding that a mutation in a putative phosphatase regulatory subunit gene alters auxin transport (Garbers et al., 1996) demonstrates one possible pathway for efflux carrier regulation. In principle, long-term responses can also be affected by diffusible regulators, and modification of the efflux carrier could come about as a direct, if delayed, regulatory effect of light or as a secondary consequence of earlier light-regulated events influencing cell differentiation.
However, one long-term consequence of photomorphogenesis, the development of the photosynthetic apparatus, causes changes in polar auxin transport related to energy status rather than a developmental change in the auxin-transport system (Thimann and Wardlaw, 1963). Distinguishing between the effects of the input of photosynthetic energy on, and developmental regulation of, the auxin-transport system is inherently difficult. To resolve this difficulty, we studied auxin transport using an intermediate state of photomorphogenesis induced by growth in continuous DRL (0.5 μmol m−2 s−1). Under DRL plants exhibit an altered pattern of stem elongation, but an “etiolated” morphology is preserved (Shinkle et al., 1992). Seedlings grown under DRL have closed hooks, high hypocotyl elongation rates, and cotyledons that expand and green minimally. Elongation of the hypocotyl differs from that of DG seedlings in that the apical-to-basal graded decline in the elongation rate seen in DG seedlings is not as steep in DRL-grown seedlings. Hence, DRL-grown seedlings exhibit a photomorphogenetic change in growth pattern, but do not develop photosynthetic competence, making it possible to detect developmental changes in auxin transport.
We have characterized several important differences between auxin transport in DG and DRL-grown cucumber (Cucumis sativus L.) seedlings. DRL-grown seedlings showed approximately 2-fold increases in transport velocities and intensities relative to DG controls. Transferring seedlings between light environments indicated that the acquisition and loss of the increased transport capabilities occurred through slow developmental processes. The increase in transport was accompanied by a decrease in sensitivity to polar auxin-transport inhibitors, and the effect of light environment on responsiveness to NPA and TIBA was not the same. These findings support the model presented above for long-term changes in auxin transport caused by modification of the auxin efflux carrier.
MATERIALS AND METHODS
Plant Material and Light Treatments
Cucumber (Cucumis sativus L. cv Burpee's Pickler) seeds were germinated in moist vermiculite in 22- × 7- × 5-cm plastic boxes. Boxes were placed in 42- × 27- × 16-cm clear, plastic storage boxes with a 2- to 4-mm layer of distilled water on the bottom and with lids that were not tightly sealed, but that did limit evaporation. Storage boxes were placed in a growth room maintained at 24 ± 1°C in complete darkness or under DRL at 0.5 μmol m−2 s−1, as described by Shinkle et al. (1992), or in similar growth rooms at the University of North Carolina-Chapel Hill. Plants were grown for varying periods and transferred between darkness and DRL as indicated below and in Results.
Growth Measurements
Relative growth rates of specific regions of hypocotyls were determined as described in Shinkle et al. (1992). Regions of hypocotyls were marked at 5-mm intervals with wet charcoal, and relative growth rates (measured over a 2-h interval) were determined for marked regions. Growth rates for the 10-mm regions used for transport analysis described below were determined from combination and averaging relative growth rates from the appropriate pair of 5-mm-marked regions.
Auxin-Transport Assays
Unless otherwise noted, chemicals were obtained from Sigma. NPA was obtained from Chemical Services (West Chester, PA).
Method 1: Continuous Label Application and Collection Using Agar Blocks (Jacobs and Hertel, 1978)
Cylindrical agar-receiving blocks were prepared with 1.5% (w/v) 5 mm KH2PO4, pH 5.5, drawn into 3-mm i.d. glass tubes, and cut to 3-mm lengths. Donor blocks were made by adding 10−7 m 5-[3H]IAA (0.95 TBq mmol−1, Amersham) to partially cooled, but still liquid, agar, which was then mixed by vortexing for 1 min. Donor and receiver tubes were kept at 4°C until use. Transport assays were performed on 5-mm segments from three regions of seedlings with hypocotyls 5 to 6 cm long (taken at 94–98 h after planting for DG seedlings and 106–110 h after planting for DRL-grown seedlings). Apical segments were cut from within 0 to 10 mm from the hook, middle segments were cut from within the region 20 to 30 mm from the hook, and basal segments were cut from within the region 40 to 50 mm from the hook.
For basipetal-transport assays, segments were placed basal end down on receiver blocks on glass slides, and a donor block was placed on the apical end. To stabilize the tissue agar block assembly, a 10- to 15-mm length of plastic drinking straw was then lowered onto the slide such that the assembly was inside of the straw. Slide assemblies were then placed in a humid chamber. For measurement of acropetal transport, the apical end of the cut segment was placed on the receiver block, but was otherwise the same as for basipetal-transport assays. Donor and receiver agar blocks and hypocotyl segments were placed in separate vials of 5 mL of a standard, nonflammable liquid scintillant (such as Scintisafe E1, Fisher Scientific) and allowed to completely extract by diffusion (18 h), and radioactivity was counted by liquid-scintillation spectroscopy. Transport was measured as cpm in the receiver block relative to the total cpm in the receiver block and in the segment, expressed as a percentage. Quenching effects of tissue and agar samples were close to identical, so conversion of cpm to dpm was not routinely done.
Method 2: Pulsed Application of Labeled IAA (Modified from Hasenstein [1987] and Jones et al. [1991])
Straight hypocotyls (5 cm) harvested from between seed attachment point and hook were cut and immediately placed apical end down in a 10-dram scintillation vial containing 250 μL of 3.8 × 10−7 m 5-[3H]IAA (2.5 μCi or 91.6 kBq) in 5 mm KH2PO4 and 30 mm Suc, pH 5.5 (transport buffer), and the vial was capped to maintain high humidity. For time-course studies, after 15 min of incubation, hypocotyls were removed from the vial, rinsed with distilled water, cut to the apical 1 cm, and inserted vertically, apical end down, in a 1% (w/v) agar slab (5 mm KH2PO4 and 30 mm Suc, pH 5.5) kept in a humid chamber. Receiver blocks made of the same agar were placed on the basal cut surface of the hypocotyls and replaced at 30-min intervals. Blocks from three hypocotyls for each time point and 1-cm hypocotyl segments were each extracted in liquid-scintillation solution and counted as described. For distribution of label at fixed times, the 5-cm hypocotyl segments were handled identically, except that they were left intact, and after the indicated time intervals were sectioned by cutting with a razor blade into the following 5 regions, which were counted as described: 0 to 3 mm, 3 to 6 mm, 6 to 10 mm, 10 to 20 mm, and 20 mm and below. Although transport in inverted tissue as used here has been shown to differ from transport in upright tissue (see Jacobs, 1979), this does not affect the validity of the assay for comparisons between tissue types.
Method 3: Continuous Application of Label and Collection in Distal Tissue
Hypocotyls were handled as in method 2, except after 1 h, the apical 1 cm and basal 4 cm were separated and measured by liquid-scintillation spectroscopy as described. When tissue was treated with NPA or TIBA, these substances were added to the transport buffer and were present throughout the transport period. Transport was routinely expressed as the percentage of total cpm in a hypocotyl found in the basal segment. To test the effect of transfer of seedlings between darkness and DRL (Fig. 4), seedlings were marked with charcoal at the hook/hypocotyl boundary at the time of transfer. At 30 or 50 h after transfer, transport assays were done with segments cut from the most apical region of the hypocotyl and with segments cut from below the charcoal mark using the procedure described above. Controls for these transfers were seedlings grown in darkness and in DRL, marked at the same time as transferred seedlings and left in their original light regimes for the same period of time as transferred seedlings, before being used in transport experiments as above.
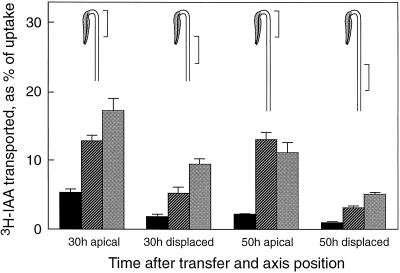
Figure 4.
Effect of transferring seedlings from darkness to DRL for 30 or 50 h on the capacity of hypocotyl segments to transport [3H]IAA through 1 cm in 1 h. Transfers took place when seedlings were 4 to 4.5 d old. For transport assays, apical ends of hypocotyl sections were placed in a thin film of 30 mm Suc buffered to pH 5.5 with 5 mm KH2PO4 containing 2.5 μCi of [3H]IAA, for 1 h. Transport is expressed as percent of total counts taken up found in tissue basal to the first centimeter (with calculation as shown in Table III). Tissue regions tested were the apical 5 cm at the time of the experiment, and the now-displaced region, which was marked as just below the hook at the time of transfer (see drawings on figure). Black bars represent controls marked and maintained in darkness, hatched bars represent transferred tissue, and stippled bars indicate controls marked and maintained in DRL. Results shown are the means for between 18 and 24 replicate sections. Error bars represent se.
Identification of 3H-Labeled Compounds Transported through Tissue
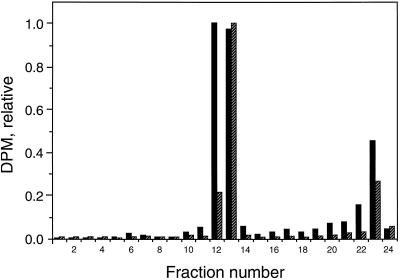
Agar blocks containing transported radioactivity (accumulated over 1 h) from 12 segments were extracted in 500 μL of 100% ethanol for 1 h at room temperature. The extract was concentrated 5-fold in a Speed-Vac (Savant, Farmingdale, NY), then subjected to TLC using Kodak silica gel chromatograms and a solvent system consisting of ethylacetate:isopropanol:ammonium hydroxide (45:35:10, v/v). Chromatograms were cut into 24 sections and counted by liquid scintillation. As a control, 1 μL of 5-[3H]IAA from the original ethanol stock was placed in 25 μL of ethanol for 1 h at room temperature and chromatographed as above. Figure 1 shows that the [3H]IAA transported through the tissue into blocks was not catabolized. The peak in the high fraction numbers (22–23) is an oxidation product formed during TLC (Melhado et al., 1982). The chromatography data shown are for DG hypocotyl segments, but similar results were obtained for DRL-grown tissue (data not shown). Since hypocotyls were allowed to take up [3H]IAA for 1 h, the radioactivity collected is analogous to the radioactivity collected at the first four points in the time course, and the conditions used for all subsequent experiments. This indicates that whatever the final fate of the [3H] label, the counts that crossed the boundary between the apical 1 cm and either an agar block or basal tissue most likely did so as [3H]IAA.
Figure 1.
Distribution of radioactivity from chromatogram of 3H-labeled compounds accumulated in agar receiver blocks placed on 1-cm hypocotyl segments from DG cucumbers incubated in [3H]IAA prior to collection. Apical ends of 5-cm hypocotyl segments were placed in a thin film of 30 mm Suc buffered to pH 5.5 with 5 mm KH2PO4 containing 2.5 μCi of [3H]IAA for 1 h. Segments were then cut to 1 cm, and receiver agar blocks were placed on the basal ends. After 1 h, 12 blocks were removed and extracted in ethanol for 1 h. Extracts were chromatographed on TLC strips in ethylacetate:isopropanol:ammonium hydroxide (45:35:20, v/v). Hatched bars (EtOH) indicate extracts from receivers, black bars (transported) indicate authentic [3H]IAA.
RESULTS
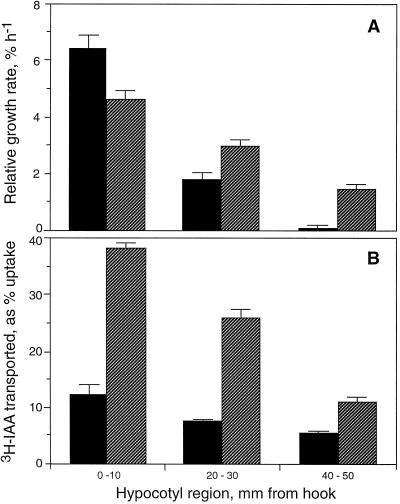
Figure 2A shows the two different growth distributions seen in DG and DRL-grown cucumber seedlings. The decline in growth rate from apical to basal regions was greater in DG seedlings, but as noted previously (Shinkle et al., 1992), the elongation rate of the hypocotyl as a whole was very similar between the two light regimes. Polar transport of [3H]IAA for 3 h through 5-mm segments of hypocotyls taken from the regions identified in Figure 2A also showed an apical-to-basal decline in both types of tissue (Fig. 2B). The results shown extend the well-established finding that stem elongation rate and auxin-transport capacity are not always correlated (see Jacobs, 1979). In both light conditions there was a decline in transport rate along the apical to basal axis of the hypocotyl, as observed previously (Suttle, 1991), but this pattern is clearly not well correlated with the decline in growth rate along the apical-basal axis.
Figure 2.
Differences in regional growth rates and capacity to transport [3H]IAA along the axis of cucumber hypocotyls from seedlings grown in complete darkness or under DRL. Figure 2A shows relative growth rates in the regions indicated determined for 2-h periods. Data shown represent the means for 15 replicate DG seedlings (black bars) and for 12 replicate DRL-grown seedlings (hatched bars). Figure 2B shows transport of [3H]IAA from agar donor blocks through 5-mm segments taken from the indicated regions. Data are represented as percent of counts taken up reaching receiver agar blocks during a 3-h assay (with calculation as shown in Table I), and represent the mean for between 5 and 15 replicate segments for each region. In both figures error bars represent se.
A major difference between DG and DRL-grown material is that the segments from DRL-grown seedlings showed transport levels approximately 3 times greater than those seen in tissue from DG seedlings. Later experiments showed that performing experiments in room light caused an inhibition of transport in DG seedlings (see below); therefore, the difference in transport between DRL-grown and DG seedlings resulted from the combination of growth conditions and conditions during the experiment. Table I shows that the uptake of [3H]IAA was very similar in DG and DRL-grown tissue, so the observed difference in transport was not the result of a difference in uptake. Data are shown for the middle (20–30 mm) segment of the hypocotyl, but similar results were obtained in all three regions tested. Table I also shows that there was some difference in the acropetal movement of [3H]IAA through DG and DRL-grown tissue.
Table I.
Comparison of basipetal and acropetal transport of [3H]IAA from agar blocks through hypocotyl sections from DG and DRL-grown cucumber seedlings
| Seedling | Total cpm | cpm in Section | cpm in Receiver | Uptake Transported |
|---|---|---|---|---|
| % | ||||
| Basipetal | ||||
| DG | 47,482 ± 2,115 | 19,792 ± 1,409 | 1565 ± 108 | 7.5 ± 0.3 |
| DRL-grown | 43,276 ± 1,373 | 15,744 ± 801 | 5418 ± 304 | 26.0 ± 1.4 |
| Acropetal | ||||
| DG | 48,630 ± 393 | 10,972 ± 547 | 87 ± 9 | 0.8 ± 0.1 |
| DRL-grown | 49,949 ± 359 | 12,659 ± 676 | 200 ± 25 | 1.5 ± 0.2 |
Donor agar blocks containing 10−7 m [3H]IAA were prepared in 5 mm KH2PO4 buffer, pH 5.5, and placed on 5-mm hypocotyl sections from the cut within the region from 20 to 30 mm from the hook. Transport took place for 3 h as described in Methods. Results show total cpm (donor plus sections plus receivers), cpm in receivers, and cpm in sections, as well as percent uptake (cpm in receivers plus sections) transported to receivers. Results are the average of 6 to 14 replicate sections, and are shown ± se.
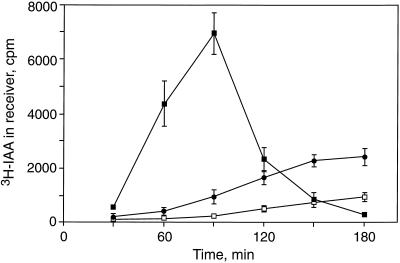
The time course for the movement of a 15-min pulse of [3H]IAA through 1-cm segments from DG and DRL-grown seedlings is shown in Figure 3. It is interesting that the pulse broadened during movement through DG tissue, but remained coherent in DRL-grown tissue. Other differences were that the intensity of movement of IAA was reduced if DG seedling tissue was handled under room light during the experiment, and that the uptake of IAA was also reduced (data not shown). However, tissue from DRL-grown seedlings was insensitive to room light (DRL data are a combination of experiments performed under dim-green light and under room light). The peak of radioactivity transported by DG tissue was 180 min (data not shown), in contrast to 90 min for DRL-grown tissue. These data also provide the timing for the arrival of the radioactivity front for the two treatments (60 min for DG and 30 min for DRL grown, giving minimum transport velocities of 1 and 2 cm h−1, respectively), and the rationale for the design of subsequent experiments.
Figure 3.
Time course for movement of a pulse of [3H]IAA through 1-cm segments of cucumber seedlings grown in darkness or in DRL. Time 0 indicates the end of a 15-min immersion of the apical end of 5-cm hypocotyl segments in a thin film of 30 mm Suc buffered to pH 5.5 with 5 mm KH2PO4 containing 2.5 μCi of [3H]IAA. Segments were then cut to 1 cm and receiver agar blocks were placed on the basal ends. At 30-min intervals, blocks were removed for counting and replaced with fresh blocks. ▪, DRL-grown seedlings; •, DG seedlings, with experiments performed with minimal exposure to dim-green work light; and □, DG seedlings with experiments performed under room light. Data shown are means for 8 replicates for both of the experiments with DG seedlings, and 10 replicates for experiments with DRL-grown seedlings. Error bars represent se.
The spatial distribution of radioactivity was determined at 180 and 90 min for DG and DRL-grown seedlings, respectively. Table II shows that 43% of the counts remained within the first 10 mm of DG tissue, whereas only 24% remained in the same region of DRL-grown tissue. For both tissues, the first 3 mm retained more than one-half of the counts within this first 10 mm. This fraction was considered to be immobilized, since similar values are obtained for 3 and 4 h for DRL-grown and DG seedlings, respectively (data not shown).
Table II.
Distribution 3H label along hypocotyl axes of DG and DRL-grown cucumber seedlings given a 15-min pulse of [3H]IAA
| Seedling | Region
|
|||||
|---|---|---|---|---|---|---|
| 0–3 mm | 3–6 mm | 6–10 mm | 10–20 mm | 20+ mm | Total | |
| DG at 180 min | 1748 ± 44 | 743 ± 32 | 700 ± 35 | 2708 ± 140 | 1542 ± 153 | 7423 ± 240 |
| DRL at 90 min | 1288 ± 48 | 418 ± 26 | 343 ± 23 | 3801 ± 241 | 2751 ± 224 | 8414 ± 458 |
Hypocotyl sections of 5 cm were placed, apical end down, in 200 μL of 1% Suc buffered to pH 5.5 in 5 mm KH2PO4 containing 3.8 × 10−7 m [3H]IAA (26 Ci/mmol) for 15 min, rinsed with distilled water, and inserted, apical end down, in 1% distilled agar containing 1% Suc buffered to pH 5.5 in 5 mm KH2PO4. At 180 or 90 min, for DG and DRL-grown tissue, respectively, sections were removed from agar and cut into regions. Results shown are the average cpm of 28 and 16 replicate sections for DG and DRL-grown tissue, respectively, and are shown ± se.
To characterize aspects of development and mechanism of the DRL-induced changes, a simplified transport assay was developed, which used subtending tissue as the receiver. This method revealed an approximately 10-fold difference in measured transport between DG and DRL-grown seedlings (Table III). At higher IAA levels, the difference between DG and DRL-grown tissue was only slightly reduced. Both IAA concentrations were below the optimal concentration for stimulation of elongation in segments from both tissue types (Cosgrove, 1981; Shinkle and Jones, 1988). The unlabeled IAA caused a decline in the uptake of [3H]IAA in both DG and DRL-grown tissue. Adding excess unlabeled IAA improves transport efficiency in DG tissue, as reported previously (Rayle et al., 1969), but the effect here was a modest one.
Table III.
Effect of IAA concentration on basipetal transport of [3H]IAA from buffered aqueous solution through the first centimeter of 5-cm hypocotyl sections from DG and DRL-grown cucumber seedlings
| Seedling | cpm in Apical cm | cpm in Basal 4 cm | Uptake Transported |
|---|---|---|---|
| % | |||
| Minus cold IAA | |||
| DG | 27,960 ± 1,559 | 361 ± 56 | 1.4 ± 0.2 |
| DRL-grown | 25,278 ± 1,076 | 3239 ± 284 | 12.7 ± 0.8 |
| Plus cold IAA | |||
| DG | 18,407 ± 728 | 556 ± 99 | 2.9 ± 0.5 |
| DRL-grown | 15,379 ± 615 | 1792 ± 142 | 10.5 ± 0.8 |
Sections were placed apical end down in 200 μL of 1% Suc buffered to pH 5.5 in 5 mm KH2PO4 containing 3.8 × 10−7 m [3H]IAA (26 Ci/mmol) minus or plus 3.4 × 10−6 m cold IAA. Transport took place for 1 h. Results shown are cpm in the apical centimeter, cpm in the basal 4 cm, and percent of total cpm taken up transported into the basal 4 cm. Results are the average of 12 to 25 replicate sections, and are shown ± se.
Figure 4 shows the transport capacities of seedlings transferred from darkness to DRL at 4 d after imbibition and allowed to continue to grow for either 30 or 50 h before transport tests were done. Two types of tissue from seedlings at each time point were tested: (a) hypocotyl tissue directly below the hook (referred to as apical), and (b) hypocotyl tissue taken below a charcoal mark that was placed at the hook at the time of transfer and subsequently displaced by further growth (referred to as displaced). The 30-h-after-transfer time point was chosen because this is the time required for DG plants to reestablish a high elongation rate after transfer (Shinkle et al., 1992). At this time, seedlings showed transport capacities intermediate between DG and DRL-grown controls in both the apical and displaced regions. At 50 h after transfer, the apical regions showed the same transport capacity as DRL-grown controls, whereas the displaced region still showed a transport capacity intermediate between DG and DRL-grown controls.
Combining the data in Figures 2 and 4 with those in Table III also indicates that there was an optimum age for maximal transport capacity at 126 h for both DG and DRL-grown seedlings. Maturation and decay of the auxin-transport system with increasing tissue age has also been observed previously (Smith and Jacobs, 1969; Shinkle et al., 1982). Seedlings grown in DRL lost their increased transport capacity slowly as well, and complete loss only occurred in apical tissue (data not shown).
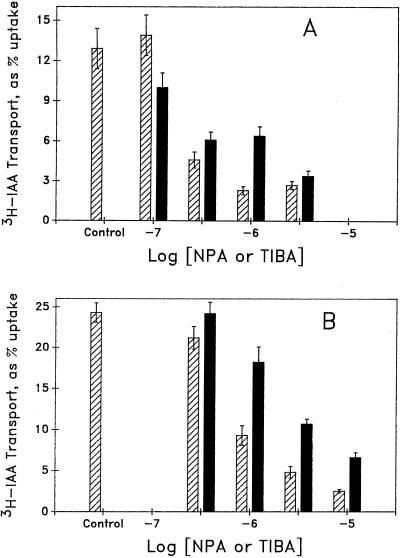
Figure 5 shows a dose-response curve for the effects of NPA and TIBA on transport in DG and DRL-grown tissue. To generate sufficient counts for resolution of the effect of different concentration of inhibitors on transport in DG tissue, a 2-h transport assay was used instead of the 1-h transport period used above. Note that with a 2-h transport period, the difference in [3H]IAA transported between DG and DRL-grown tissue is reduced to a factor of 2. DG tissue (Fig. 5A) was more sensitive to both inhibitors, showing a threshold of sensitivity at 0.3 μm. Saturation of inhibition occurred at 1 μm for NPA and at 3 μm for TIBA (data for 10 μm not shown). The response to TIBA is complex: the discontinuous concentration dependence shown was consistent for three replicate experiments. The response to NPA shows conventional saturation, but at a concentration only 10-fold above the threshold. In contrast, DRL-grown tissue (Fig. 5B) required 1 μm to produce inhibition (0.1 μm had no effect, data not shown), both TIBA and NPA showed a conventional dose-response pattern, and the response to TIBA was always less than the response to the same concentration of NPA. Concentrations higher than 10 μm were not tested because of the potential confusion of specific inhibition with general toxicity (see Katekar and Geissler, 1980) or of the effect of the inhibitors on uptake (Delbarre et al., 1996). Hence, saturation may not have been reached for the effects of inhibitors on DRL-grown tissue.
Figure 5.
Concentration dependence of the effect of TIBA and NPA on the transport of [3H]IAA through hypocotyls from DG (A) and DRL-grown (B) cucumber seedlings. Apical ends of hypocotyl segments were placed in a thin film of 30 mm Suc buffered to pH 5.5 with 5 mm KH2PO4 containing 2.5 μCi of [3H]IAA and the indicated concentrations of NPA or TIBA for 2 h. Transport is expressed as percent of total counts taken up found in tissue basal to the first centimeter (with calculation as shown in Table III). Hatched bars represent the effect of NPA; black bars indicate the effect of TIBA. Data shown are means of 13 to 39 replicate sections. Error bars represent se. In all cases where error bars do not overlap, Student's t tests show samples to be significantly different at P < 0.05 or better.
DISCUSSION
The principal finding from these experiments is that photomorphogenesis evokes a major change in the auxin-transport system in dicots, most likely by altering regulation of auxin efflux. DRL causes cucumber seedlings to develop a 2-fold increase in transport velocity, a 2-fold increase in transport intensity, and altered responses to inhibitors of transport, relative to DG controls. Although Table II shows that hypocotyls from DG seedings immobilize more counts from [3H]IAA than do hypocotyls from DRL-grown seedlings, the data do not distinguish between the possibility that increased metabolism retards transport and the possibility that slower transport allows greater opportunities for the action of pathways metabolizing IAA. The latter explanation is more consistent with the decrease in transport velocity and the change in sensitivity to inhibitors, although these data in no way rule out a contribution of altered auxin metabolism to the overall phenomenon.
The fact that the changes are slow to develop and are acquired more completely in apical tissue that is less differentiated at the time of first irradiation suggests that the developmental process is complex. The timing of the changes in auxin transport is slower than the timing of the DRL-induced change in the growth pattern (Shinkle et al., 1992), indicating that the change in auxin transport is unlikely to cause the change in the growth pattern. Since the pattern of transport capacity is not well correlated with the pattern of growth (Fig. 2), it seems likely that light regulation of these two processes occurs by two independent mechanisms.
Since the efflux carrier establishes the polarity of auxin transport (Goldsmith, 1977; Lomax et al., 1995), and this polarity is preserved in DRL-grown seedlings (Table I), the efflux carrier is of necessity a target for up-regulation or derepression by DRL. Based on the changes in response to inhibitors of auxin transport, the primary effect of which is on the function of the efflux carrier (Lomax et al., 1995; Delbarre et al., 1996), the efflux carrier may be a principal target of the light-induced change in transport.
Our result indicating a decrease in sensitivity to inhibitors coincident with an increase in transport is different from Suttle's (1991) finding that increased tissue maturity caused a decline in polar auxin transport and unchanged sensitivity to NPA and TIBA. Equally novel, the effect of DRL on the response to NPA is different from the effect of the response to TIBA. In DG tissue the highest extent of inhibition of transport is the same for NPA and TIBA, whereas in DRL-grown tissue the inhibition of transport by NPA is always greater than that caused by TIBA. This differential effect was also not observed by Suttle (1991). We believe our results are the first demonstration of an in vivo separation of responses to NPA and TIBA, and thus our results are an important extension of the in vitro evidence that NPA and TIBA do not compete for the same binding site (Bruun et al., 1992). Since the uptake of TIBA may be carrier mediated (Depta and Rubery, 1984), it is conceivable that light differentially affects the uptake of these two inhibitors. It is more likely, since there appear to be several proteins involved in the function of the efflux carrier (Morris et al., 1991; Cox and Muday, 1994), that the effect of DRL could alter one or more of these components in a manner that differentially affects the response of efflux carrier to the two inhibitors.
To our knowledge, the dissipation of coherence of an applied pulse of [3H]IAA seen in DG tissue (Fig. 3) has not previously been reported. Hasenstein's (1987) model of transport combining polar efflux and diffusion may explain our results as an example of a case where diffusion is a greater contributor to IAA movement. Eliezer and Morris (1980), using a method similar to Hasenstein's, did not monitor the movement of a pulse of labeled IAA past a discrete point, and so may have missed this phenomenon. Eliezer and Morris did determine the velocity of transport for DG pea to be in the range of 1 cm h−1. This is consistent with our determination of transport velocity in DG plants from the time at which labeled IAA is first detectable. The relatively low rate of total transport of labeled IAA has been observed previously in DG bean (Smith and Jacobs, 1969) and zucchini (Shinkle et al., 1982). The movement of a coherent pulse of [3H]IAA shown for DRL-grown cucumber hypocotyls has been reported in both DG corn coleoptiles (Goldsmith, 1967) and mesocotyls (Jones et al., 1991) This type of result has also been reported in at least two species of LG dicots (Beyer and Morgan, 1969; Daniel et al., 1989).
Ethylene, a known negative regulator of auxin transport, probably does not mediate the effect of DRL on auxin transport, despite the fact that ethylene production is inhibited by red light (for review, see Burg [1973], although Rohwer and Schierle [1982] found the opposite effect). A principal difficulty is that the ethylene-induced inhibition of auxin transport does not include any alteration of transport velocity (Beyer and Morgan, 1969; Suttle, 1988). In addition, although we grew seedlings in boxes with minimal gas exchange, we did not observe the characteristic effects of ethylene on growth. Further, we grew some batches of DG cucumber seedlings in open containers, and others in our standard containers alongside pea seedlings, plants known to produce physiologically relevant amounts of ethylene (Rohwer and Schierle, 1982). The open-container-grown seedlings did not show any increase in transport, whereas the seedlings grown under presumably increased ethylene levels showed both morphological effects and inhibition of auxin transport (data not shown). The long time required for DG seedlings to acquire increased transport capacity when transferred to DRL (Fig. 4) also mitigates against ethylene as a mediator of the effect.
In general, our results describe responses not previously observed. In corn and oats, light was found to rapidly cause a decline in transport intensity (Naqvi, 1975; Jones et al., 1991), and long-term light treatment was found to cause a decrease in transport velocity (Naqvi, 1975). Our results also differ from those found in the one study of light effects on auxin transport in dicots that we found (Eliezer and Morris, 1980). Light during the transport period caused an increase in transport velocity in DG seedlings of pea and bean, but had no effect on transport intensity. Plants grown for long periods in WL exhibited less than a 10% increase in transport velocity, and a 2-fold increase in transport intensity in pea, but only when transport experiments were also performed in the light. In our study long-term growth in DRL caused at least a 2-fold increase in both transport velocity and transport intensity, and performing transport assays in the light had no effect at all on DRL-grown tissue (Fig. 3). Preliminary experiments indicate that pea and tomato show the same type of response to DRL (data not shown), suggesting that the DRL-induced increase in transport is a consistent feature of dicot photomorphogenesis and that WL-grown plants represent a different developmental state.
In summary, DRL causes an increase in both auxin-transport intensity and auxin-transport velocity in cucumber hypocotyls, and these changes may represent a general theme of photomorphogenesis in dicots. A complex developmental process is suggested by the relatively slow acquisition of the increase and the limitation that complete induction only occurs in hypocotyl tissue differentiating under DRL. Alterations in the response to NPA and TIBA suggest that beyond up-regulation of the activity of the efflux carrier, DRL causes a modification of some component of the efflux carrier complex. The slow, relatively stable nature of these changes is consistent with the roles that auxin transport may play in the development of mature plant form and function.
ACKNOWLEDGMENTS
The technical assistance of Ms. Christiana Rodgers with preliminary experiments is gratefully acknowledged. The majority of the experiments were carried out in the laboratory of Dr. Alan Jones, University of North Carolina at Chapel Hill, while J.R.S. was on sabbatical.
Abbreviations:
- DG
dark-grown
- DRL
dim-red light
- LG
light-grown
- NPA
N-(naphth-1-yl)phthalamic acid
- TIBA
2,3,5-tri-iodobenzoic acid
- WL
white light
Footnotes
This work was supported by the U.S. Department of Agriculture-National Research Inititiative Competitive Grants Program (grant no. 93-37311-9590), a Faculty Development grant from Trinity University to J.R.S., and an undergraduate research fellowship to R.K. from the Pew Charitable Trusts through the Pew Mid-States Consortium for Science and Mathematics.
LITERATURE CITED
- Behringer FJ, Davies PJ. Indole-3-acetic acid levels after phytochrome-mediated changes in stem elongation rate of dark- and light-grown Pisum seedlings. Planta. 1992;188:85–92. doi: 10.1007/BF00198943. [DOI] [PubMed] [Google Scholar]
- Beyer EM, Morgan PW. Ethylene modification of an auxin pulse in cotton stem sections. Plant Physiol. 1969;44:1690–1694. doi: 10.1104/pp.44.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun SA, Muday GK, Haworth P. Auxin transport and the interaction of phytotropins. Plant Physiol. 1992;98:101–107. doi: 10.1104/pp.98.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP. Ethylene and plant growth. Proc Natl Acad Sci USA. 1973;70:591–597. doi: 10.1073/pnas.70.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland FM, McHale NA. LOP1: a gene involved in auxin transport and vascular patterning in Arabidopsis. Development. 1996;122:1811–1819. doi: 10.1242/dev.122.6.1811. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Rapid suppression of growth by blue light. Plant Physiol. 1981;67:584–590. doi: 10.1104/pp.67.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DN, Muday GK. NPA binding activity is peripheral to the plasma membrane and is associated with the cytoskeleton. Plant Cell. 1994;6:1941–1953. doi: 10.1105/tpc.6.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SG, Rayle DL, Cleland RE. Auxin physiology of the tomato mutant diageotropica. Plant Physiol. 1989;91:804–807. doi: 10.1104/pp.91.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Imhoff V, Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, napthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;198:532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- Depta H, Rubery PH. A comparative study of carrier participation in the transport of 2,3,5-triiodobenzoic acid, and 2,4-dichlorophenoxyacetic acid by Cucurbita pepo L. hypocotyl segments. J Plant Physiol. 1984;115:371–387. doi: 10.1016/S0176-1617(84)80036-X. [DOI] [PubMed] [Google Scholar]
- Eleizer J, Morris DA. Cell length, light and 14C-labelled indol-3yl-acetic acid transport in Pisum sativum L. and Phaseolus vulgaris L. Planta. 1980;149:327–331. doi: 10.1007/BF00571165. [DOI] [PubMed] [Google Scholar]
- Furuya M, Pjon CJ, Fujii T, Ito M. Phytochrome action in Oryza sativa L. III. The separation of photoreceptive site and growing zone in coleoptiles, and auxin transport as effector system. Dev Growth Differ. 1969;11:62–76. doi: 10.1111/j.1440-169x.1969.00062.x. [DOI] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruere J, Bernasconi P, Soll D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 1996;15:2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Goldsmith MHM. Movement of pulses of labeled auxin in corn coleoptiles. Plant Physiol. 1967;42:258–263. doi: 10.1104/pp.42.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith MHM. The polar transport of auxin. Annu Rev Plant Physiol. 1977;28:439–478. [Google Scholar]
- Hasenstein K-H. Non-transportable radioactivity in hypocotyls of Helianthus annuus after application of [3H]-IAA: analysis of diffusion parameters. Physiol Plant. 1987;70:139–145. [Google Scholar]
- Jacobs M, Hertel R. Auxin binding to subcellular fractions from Cucurbita hypocotyls: in vitro evidence for an auxin transport carrier. Planta. 1978;142:1–10. doi: 10.1007/BF00385113. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH. Naturally occurring auxin transport regulators. Science. 1988;241:346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jacobs WP (1979) Movements of hormones. In Plant Hormones and Plant Development. Cambridge University Press, Cambridge, UK, pp 195–243
- Jones AM, Cochran DS, Lamerson MA, Evans ML, Cohen JD. Red light-regulated growth. Plant Physiol. 1991;97:352–358. doi: 10.1104/pp.97.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katekar GF, Geissler AE. Auxin transport inhibitors. IV. Evidence of a common mode of action for a proposed class of auxin transport inhibitors: the phytotropins. Plant Physiol. 1980;66:1190–1195. doi: 10.1104/pp.66.6.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Melhado LL, Pearce CJ, D'Alarco M, Leonard NJ. Specifically deuterated and tritiated auxins. Phytochemistry. 1982;21:2879–2885. [Google Scholar]
- Morris DA, Rubery PH, Jarman J, Sabater M. Effects of inhibitors of protein synthesis on transmembrane auxin transport in Cucurbita pepo L. hypocotyl segments. J Exp Bot. 1991;42:773–783. [Google Scholar]
- Naqvi SM. Kinetics of auxin transport in light and in dark grown Zea mays L. coleoptile segments. Z Pflanzenphysiol. 1975;76:379–383. [Google Scholar]
- Rayle DL, Ouitrakul R, Hertel R. Effects of auxins on the auxin transport system in coleoptiles. Planta. 1969;87:49–53. doi: 10.1007/BF00386963. [DOI] [PubMed] [Google Scholar]
- Rohwer F, Schierle J. Effect of light on ethylene production: red light enhancement of 1-aminocyclopropane-carboxylic acid concentration in etiolated pea shoots. Z Pflanzenphysiol. 1982;107:295–300. [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle JR, Jones RL. Inhibition of stem elongation in Cucumis seedlings by blue light requires calcium. Plant Physiol. 1988;86:960–966. doi: 10.1104/pp.86.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle JR, Lomax-Reichert T, Hertel R, Briggs WR. Correlation of in vivo and in vitro auxin transport in developing Cucurbita pepo hypocotyls. Carnegie Inst Wash Year Book. 1982;81:21–23. [Google Scholar]
- Shinkle JR, Sooudi SK, Jones RL. Adaptation to dim-red-light leads to a non-gradient pattern of stem elongation in Cucumis seedlings. Plant Physiol. 1992;99:808–811. doi: 10.1104/pp.99.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire W, Jr, Mohr H. Photomorphogenesis. Encyclopedia of Plant Physiology, New Series, Vol 16A & 16B. Berlin: Springer Verlag; 1983. [Google Scholar]
- Smith CW, Jacobs WP. The movement of IAA-C14 in the hypocotyl of Phaselous vulgaris. Am J Bot. 1969;56:492–497. [Google Scholar]
- Suttle JC. Effects of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol. 1988;88:795–799. doi: 10.1104/pp.88.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle JC. Biochemical bases for the loss of basipetal IAA transport with advancing physiological age in etiolated Helianthus hypocotyls. Plant Physiol. 1991;96:875–880. doi: 10.1104/pp.96.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV (1977) Polarity and transport. In Hormone Action in the Whole Life of Plants. University of Massachusetts Press, Amherst, MA, pp 71–92
- Thimann KV, Wardlaw IF. The effect of light on the uptake and transport of indoleacetic acid in the green stem of the pea. Physiol Plant. 1963;16:368–377. [Google Scholar]