Abstract
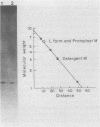
M protein is a major virulence factor of group A streptococci, which provides these organisms with protection against phagocytosis in the absence of specific antibody. To gain insight into the nature of the native M-protein molecule, type 12 M protein was isolated and purified from the extracellular supernatants of a group A streptococcal L form and stabilized protoplasts. The intact purified M protein from both sources had a molecular weight of 58,000, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. This is in contrast to the 32,0000-dalton molecule isolated from the parent type 12 organism by using a nonionic detergent. The purified secretory M protein removed opsonic antibodies from type 12 rabbit immune serum, as demonstrated by a bactericidal assay. Therefore, it appears that either previous nondestructive methods of M-protein isolation have not removed intact M protein from cell walls or part of the molecule is fragmented during its association with cell walls.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLEIWEIS A. S., KARAKAWA W. W., KRAUSE R. M. IMPROVED TECHNIQUE FOR THE PREPARATION OF STREPTOCOCCAL CELL WALLS. J Bacteriol. 1964 Oct;88:1198–1200. doi: 10.1128/jb.88.4.1198-1200.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Campbell G. L., Ofek I. Peptic digestion of streptococcal M protein. II. Extraction of M antigen from group A streptococci with pepsin. Infect Immun. 1974 May;9(5):891–896. doi: 10.1128/iai.9.5.891-896.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Kang A. H. Repeating covalent structure of streptococcal M protein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3163–3167. doi: 10.1073/pnas.75.7.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Walter P., Chang C. N., Goldman B. M., Erickson A. H., Lingappa V. R. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- Cohen J. O., Gross H., Harrell W. K. Immunogenicity and characteristics of M protein released by phage-associated lysin from group-A streptococci types 1 and 23. J Med Microbiol. 1977 May;10(2):179–194. doi: 10.1099/00222615-10-2-179. [DOI] [PubMed] [Google Scholar]
- FREIMER E. H., KRAUSE R. M., McCARTY M. Studies of L forms and protoplasts of group A streptococci. I. Isolation, growth, and bacteriologic characteristics. J Exp Med. 1959 Dec 1;110:853–874. doi: 10.1084/jem.110.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K. New observations on the structure and antigenicity of the M proteins of the group A streptococcus. Immunochemistry. 1969 Jan;6(1):11–24. doi: 10.1016/0019-2791(69)90174-8. [DOI] [PubMed] [Google Scholar]
- KANTOR F. S., COLE R. M. Preparation and antigenicity of M protein released from group A, type 1 streptococcal cell walls by phage-associated lysin. J Exp Med. 1960 Jul 1;112:77–96. doi: 10.1084/jem.112.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARAKAWA W. W., ROTTA J., KRAUSE R. M. DETECTION OF M PROTEIN IN COLONIES OF STREPTOCOCCAL L FORMS BY IMMUNOFLUORESCENCE. Proc Soc Exp Biol Med. 1965 Jan;118:198–201. doi: 10.3181/00379727-118-29796. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M. Studies on the bacteriophages of hemolytic streptococci. II. Antigens released from the streptococcal cell wall by a phage-associated lysin. J Exp Med. 1958 Dec 1;108(6):803–821. doi: 10.1084/jem.108.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med. 1959 Aug 1;110(2):271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancefield R. C. THE ANTIGENIC COMPLEX OF STREPTOCOCCUS HAEMOLYTICUS : I. DEMONSTRATION OF A TYPE-SPECIFIC SUBSTANCE IN EXTRACTS OF STREPTOCOCCUS HAEMOLYTICUS. J Exp Med. 1928 Jan 1;47(1):91–103. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980 Mar 1;151(3):695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Rotta J., Krause R. M., Lancefield R. C., Everly W., Lackland H. New approaches for the laboratory recognition of M types of group A streptococci. J Exp Med. 1971 Nov 1;134(5):1298–1315. doi: 10.1084/jem.134.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell H., Facklam R. R. Guanidine extraction of streptococcal M protein. Infect Immun. 1975 Sep;12(3):679–686. doi: 10.1128/iai.12.3.679-686.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP J. T., HIJMANS W., DIENES L. Examination of the L forms of group A streptococci for the group-specific polysaccharide and M protein. J Exp Med. 1957 Feb 1;105(2):153–159. doi: 10.1084/jem.105.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Processing of streptococcal cell walls by rat macrophages and human monocytes in vitro. Infect Immun. 1977 Sep;17(3):591–598. doi: 10.1128/iai.17.3.591-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Chemical analysis of changes in membrane composition during growth of Streptococcus pyogenes. Infect Immun. 1979 Dec;26(3):883–891. doi: 10.1128/iai.26.3.883-891.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Zabriskie J. B., McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977 Aug 1;146(2):579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]