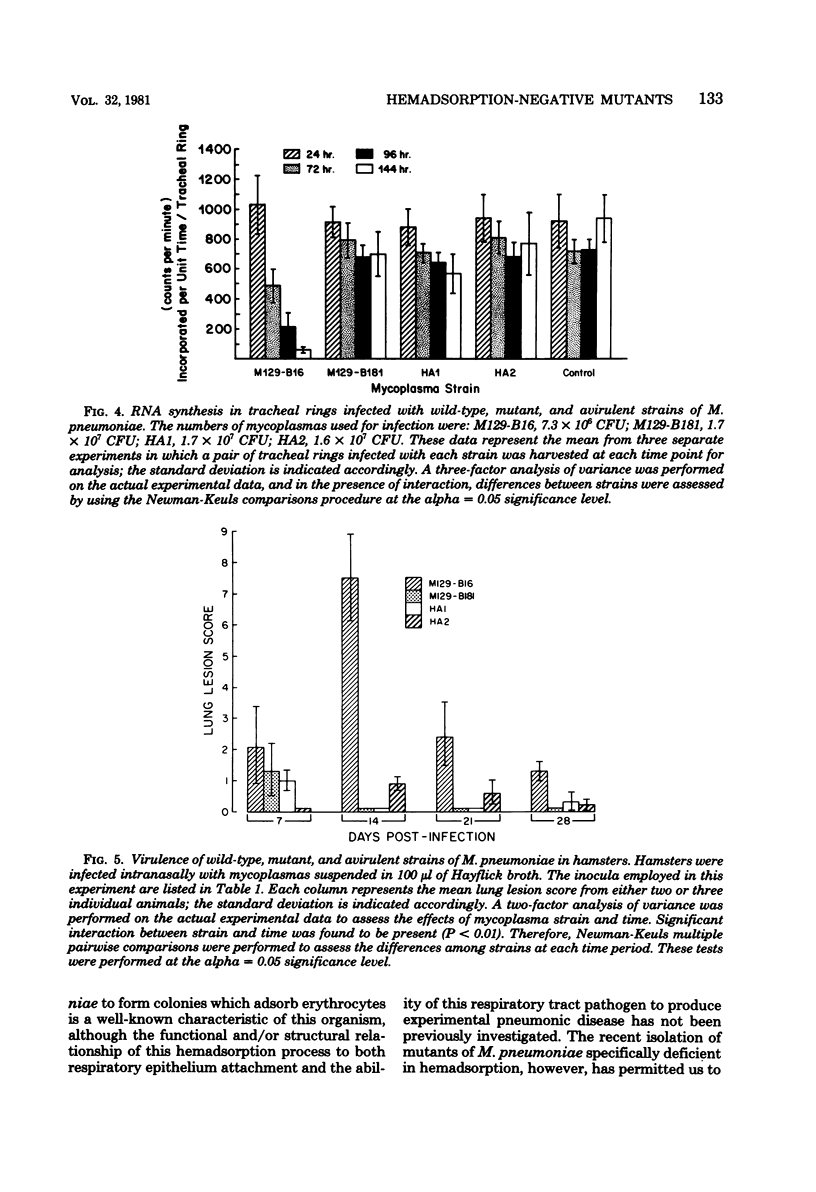
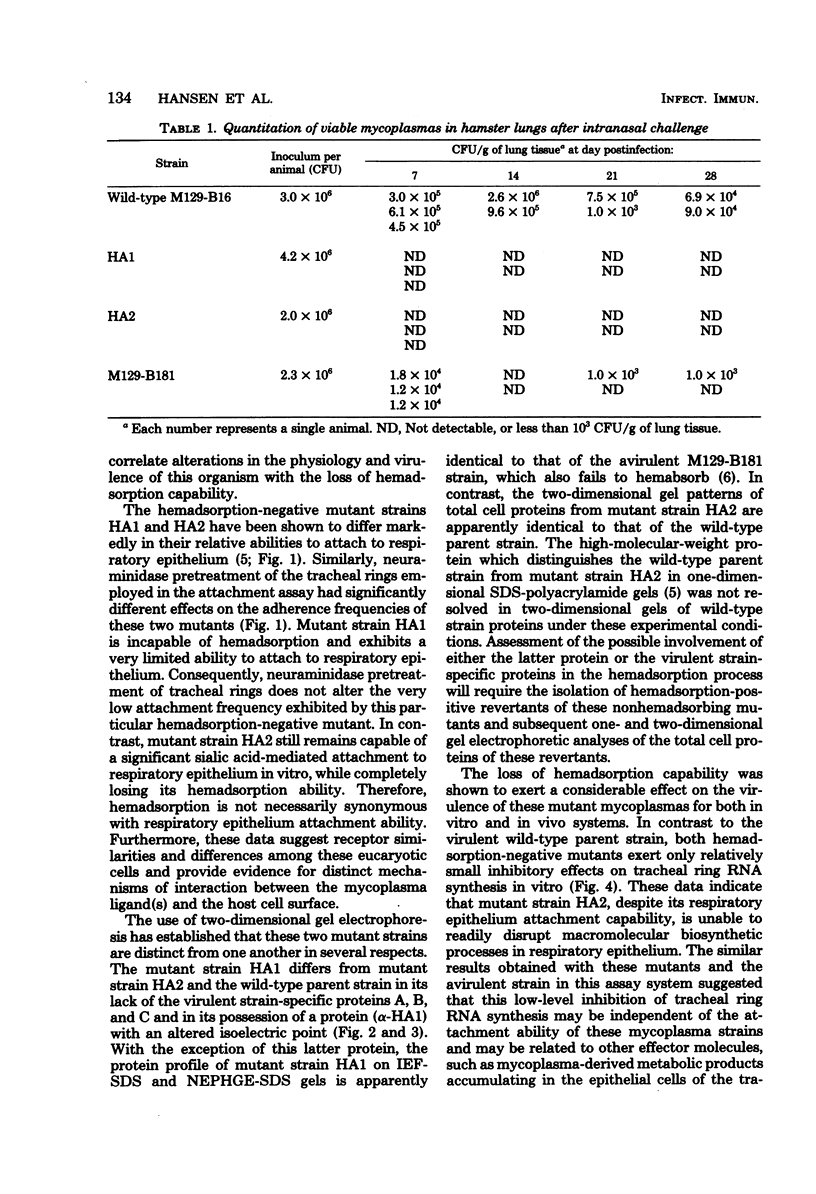
Abstract
Previously isolated mutants of Mycoplasma pneumoniae incapable of hemadsorption were characterized with respect to specific protein content, tracheal ring attachment capability, and virulence for both in vitro and in vivo model systems. Two-dimensional gel electrophoresis revealed both quantitative and qualitative differences between the protein complements of two different mutant strains and that of the virulent parent strain. Studies of mycoplasma attachment to hamster tracheal rings in vitro demonstrated that only one of these mutant strains still possessed the ability to attach to the respiratory epithelium via neuraminidase-sensitive receptors. Measurement of [3H]orotic acid uptake in mycoplasma-infected tracheal rings indicated that infection with the hemadsorption-negative mutants resulted in only slight reductions of ribonucleic acid synthesis, similar to levels observed for tracheal rings infected with an avirulent strain of M. pneumoniae. The virulence potential of the two mutant strains was further investigated by utilizing the hamster model system. Both mutant strains were rapidly cleared from the lungs of infected animals and produced little or no microscopic pneumonia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clyde W. A., Jr Immunopathology of experimental Mycoplasma pneumoniae disease. Infect Immun. 1971 Dec;4(6):757–763. doi: 10.1128/iai.4.6.757-763.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974 Dec;110(6):765–773. doi: 10.1164/arrd.1974.110.6P1.765. [DOI] [PubMed] [Google Scholar]
- DAJANI A. S., CLYDE W. A., Jr, DENNY F. W. EXPERIMENTAL INFECTION WITH MYCOPLASMA PNEUMONIAE (EATON'S AGENT). J Exp Med. 1965 Jun 1;121:1071–1086. doi: 10.1084/jem.121.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Baseman J. B. Isolation of mutants of Mycoplasma pneumoniae defective in hemadsorption. Infect Immun. 1979 Mar;23(3):903–906. doi: 10.1128/iai.23.3.903-906.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Baseman J. B. Two-dimensional gel electrophoretic comparison of proteins from virulent and avirulent strains of Mycoplasma pneumoniae. Infect Immun. 1979 May;24(2):468–475. doi: 10.1128/iai.24.2.468-475.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Alterations in the metabolism of hamster tracheas in organ culture after infection by virulent Mycoplasma pneumoniae. Infect Immun. 1975 Apr;11(4):704–710. doi: 10.1128/iai.11.4.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Interaction of virulent Mycoplasma pneumoniae with hamster tracheal organ cultures. Infect Immun. 1976 Jul;14(1):217–224. doi: 10.1128/iai.14.1.217-224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemski J. V., Hetsko C. M., Helms C. M., Grizzard M. B., Walker J. S., Chanock R. M. Immunoprophylaxis of experimental Mycoplasma pneumoniae disease: effect of aerosol particle size and site of deposition of M. pneumoniae on the pattern of respiratory infection, disease, and immunity in hamsters. Infect Immun. 1977 Apr;16(1):93–98. doi: 10.1128/iai.16.1.93-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr, Denny F. W. Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains. J Bacteriol. 1969 Nov;100(2):1037–1043. doi: 10.1128/jb.100.2.1037-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch S., Gabridge M. G. Role of host cell metabolism in the pathogenesis of Mycoplasma pneumoniae infection. Infect Immun. 1981 Jan;31(1):174–181. doi: 10.1128/iai.31.1.174-181.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]