Abstract
We have previously shown that ceftriaxone, β-lactam antibiotic known to upregulate glutamate transporter 1 (GLT1), reduced ethanol intake in alcohol-preferring (P) rats. GLT1 is a glial glutamate transporter that regulates the majority of extracellular glutamate uptake. We tested in this study the effects of neuroimmunophilin GPI-1046 (3-(3-pyridyl)-1-propyl (2S)-1-(3,3-dimethyl-1,2-dioxopentyl)-2-pyrrolidinecarboxylate), known also to upregulate GLT1 expression, in ethanol intake in P rats. Male P rats had concurrent access to free choice of 15% and 30% ethanol, water, and food for five weeks. On Week 6, P rats continued in this drinking and food regimen and they were administered either 10 or 20 mg/kg GPI-1046 (i.p.), or a vehicle for five consecutive days. Body weight, ethanol intake, and water consumption were measured daily for 8 days starting on Day 1 of GPI-1046 or vehicle i.p. injections. We have also tested the effect of GPI-1046 (20 mg/kg) on daily sucrose (10%) intake. The data revealed significant dose-dependent effects in the reduction of ethanol intake starting 48 h after the first treatment with GPI-1046 throughout treatment and post-treatment periods. There were also dose-dependent increases in water intake. However, GPI-1046 treatment did not affect the body weight of all animals nor sucrose intake. Importantly, GPI-1046 (20 mg/kg) increased GLT1 level compared to all groups in nucleus accumbens core (NAc-core). Alternatively, GPI-1046 (10 mg/kg) upregulated GLT1 level in NAc-core compared to vehicle (ethanol naïve) group. Moreover, both doses of GPI-1046 increased significantly GLT1 level in the prefrontal cortex (PFC) compared to ethanol naïve vehicle group. GPI-1046 (20 mg/kg) increased GLT1 level in PFC compared to naïve control group that was exposed to water and food only. These findings demonstrated that neuroimmunophilin GPI-1046 attenuates ethanol intake in part through the upregulation of GLT1 in PFC and NAc-core.
Keywords: GLT1, EAAT2, glutamate, alcohol dependence, GPI-1046, neuroimmunophilin
INTRODUCTION
Glutamatergic system is considered as one of the neurotransmitter systems involved in drug abuse, including ethanol. Dysfunction of glutamate transmission is found to be associated with alteration of glutamate transport caused by ethanol consumption (Smith, 1997; Smith and Weiss, 1999; Othman et al., 2002). Studies have shown that extracellular glutamate levels are higher during the period of ethanol consumption in some central brain reward regions (Dahchour et al., 2000; Melendez et al., 2005; Kapasova and Szumlinski, 2008). Extracellular glutamate is regulated by glutamate transporters in various brain regions (Gegelashvili and Schousboe, 1997; Seal and Amara, 1999; Anderson and Swanson, 2000). Of these transporters, glutamate transporter 1 (GLT1), also termed excitatory amino acid transporter 2 (EAAT2), is a key player in the removal of the majority of the extracellular glutamate (Rothstein et al., 1995; Danbolt, 2001; Mitani and Tanaka, 2003). The role of GLT1 has been tested in drug abuse models that show dysfunction of glutamate transmission. In a cocaine abuse rat model, we recently reported that ceftriaxone, β-lactam antibiotic known to upregulate GLT1 level (Rothstein et al., 2005; Miller et al., 2008; Sari et al., 2010, 2011), attenuates a cue-induced relapse to cocaine-seeking behavior (Sari et al., 2009). In accordance, Kalivas and colleagues have found similar effects with ceftriaxone in a cocaine relapse-like behavior model (Knackstedt et al., 2010). Furthermore, activation of GLT1 by MS-153, a neuroprotective compound known to enhance glutamate uptake (Shimada et al., 1999), was found to attenuate conditioned rewarding effects of drug abuse, including morphine, methamphetamine and cocaine in mice (Nakagawa et al., 2005). Importantly, we recently reported that alcohol-preferring (P) rats treated with ceftriaxone showed a significant dose-dependent reduction in ethanol consumption compared to vehicle-treated rats. This reduction in ethanol intake persisted for 10 days in a dose-dependent manner (Sari et al., 2011). It is noteworthy that higher doses of ceftriaxone were found associated with long-lasting effect in the reduction of ethanol intake, which was associated in part with the upregulation of GLT1 in prefrontal cortex (PFC) and nucleus accumbens (NAc) (Sari et al., 2011). We focused here in identifying a new compound, GPI-1046 (3-(3-pyridyl)-1-propyl (2S)-1-(3,3-dimethyl-1,2-dioxopentyl)-2-pyrrolidinecarboxylate), that does not have antibiotic action but is known to upregulate GLT1 level (Ganel et al., 2006), for potential attenuation of ethanol intake in male P rats. GPI-1046 is an analog of FK506, which is an immunophilin ligand that has been shown neuroprotective effects in neurodegenerative disease models (Steiner et al., 1997b; Guo et al., 2001; Li et al., 2004). Studies have shown that GPI-1046 also exerts neuroprotective effects in vitro and in vivo animal models (Steiner et al., 1997a, b; Sauer et al., 1999; Moss et al., 2002). GPI-1046-mediated neuroprotection might be due to regulatory effects of several gene products (Steiner et al., 2010). The neuroprotective effect might be also due in part to the upregulation of GLT1 level (Ganel et al., 2006). Thus, our interest in the use of this compound was more toward its upregulatory effect in GLT1. We have also included a water naïve animal group that was exposed to water and food only in order to determine whether chronic ethanol intake would have any effect in GLT1 level as compared to the water naïve group in PFC and the NAc core. We also determined the effect of GPI-1046 in sucrose intake in male P rats.
MATERIAL AND METHODS
Animals
Male P rats were obtained from the Indiana University School of Medicine (Indianapolis, IN) breeding colonies at the age of 21 days. P rats were acclimated to the laboratory animal facility at the University of Toledo. All animals had ad lib access to water and food, and all experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Toledo, Health Science Campus, Toledo, OH. These procedures are in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals. The program is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AAALACI) and is in compliance with municipal, state and federal laws and regulations governing animal research.
Animals were experimented at the age of 3 months and they were individually housed in bedded plastic cages in a temperature (21 °C) and humidity (50%)-controlled vivarium that was maintained on a 12/12 h light/dark cycle (lights off at 1900 h). We tested four groups for ethanol consumption: (1) water naive (exposed to water and food only and received i.p. injections of 2% dimethylsulfoxide [DMSO]) group (n=6); (2) vehicle control group or ethanol naïve group (received i.p. injections of 2% DMSO) (n=7); (3) GPI-1046-treated group at a dose of 10 mg/kg, i.p. (n=6); and (4) GPI-1046-treated group at a dose of 20 mg/kg, i.p. (n=8). Note that both vehicle and GPI-1046 (cat# ES-0040; Key Organics, Cornwall, UK) contained 2% DMSO. Vehicle (2% DMSO) and GPI-1046 (2% DMSO) i.p. injected groups had access to free choice to water, ethanol (15% and 30%) and food throughout the experimental period. This is an established model of ethanol drinking consisting of multiple choice of ethanol (15% and 30%) that is known to increase ethanol intake in P rats (Rodd-Henricks et al., 2001; Sari et al., 2006). We have tested also water naive group that had access to only water and food throughout the experimental period. The naïve (water naïve and ethanol naïve) animals were i.p. injected with vehicle containing 2% DMSO. We have also tested the effects of GPI-1046 at dose of 20 mg/kg, i.p. in sucrose intake.
Ethanol-drinking procedures and measurements
Male P rats were exposed to free choice ethanol (15% and 30%, v/v), water and food at the age of 3 months; these include vehicle (ethanol naïve) and drug-treated groups. P rats were exposed to a continuous, free-choice access to ethanol drinking for five weeks as described recently (Sari et al., 2011). However, water naive group was exposed only to water and food. Animal body weight, water intake, and ethanol intake were measured three times per week during the five-week continuous ethanol-drinking paradigm to ensure that the P rats met the criteria for ethanol dependence as demonstrated previously (Stewart et al., 1991). The measurements of ethanol intake were evaluated to the nearest 10th of gram by subtracting bottle weight from its initial weight containing ethanol. The measurements were converted, using densitometry formula, to the actual grams of ethanol consumed per kilogram of animal body weight per day. Animals that are not consuming ≥4 g/ kg/day of ethanol intake were excluded from the study. We have adopted this criterion (4 g/kg/day) based on previous study reporting the development of dependence to ethanol (Li et al., 1987) and based on our recent work using similar drinking paradigm (Sari et al., 2011). We have averaged ethanol intake across the last two weeks of the 5-week drinking protocol as a baseline. On Week 6, animals were injected 10 and 20 mg/kg GPI-1046 (i.p.) (treatment groups), and i.p. injected vehicle (ethanol naïve group) around 11:00 AM once a day for 5 days. Ethanol and water intakes, and animal body weight were measured daily for 8 days starting from the first day of vehicle or GPI-1046 i.p. injections. Note that water naïve group was i.p. injected of 2% DMSO as a vehicle control solution.
Sucrose-drinking procedures and measurements
We tested the effects of GPI-1046 on sucrose (10%) intake as an appetitive control for drinking-motivated behavior. Two groups of animals experienced continuous access to 10% sucrose for 5 weeks. Stable intake of sucrose was observed throughout this exposure period. On Week 5, P rats received vehicle or 20 mg/kg GPI-1046 (i.p.) for five consecutive days. Sucrose was available 8 days starting with the first day of GPI-1046 and vehicle i.p. injections. Sucrose and water intakes, and animal body weight were measured daily for 8 days starting from the first day of vehicle or GPI-1046 i.p. injections.
Brain tissue harvesting
Three days after the last vehicle or GPI-1046 injections, animals were euthanized around 11:00 AM by exposure to Isoflurane® and decapitated, and the brains were removed and stored at −70 °C. The PFC and the NAc-core were extracted after they have been micropunched stereotaxically using cryostat apparatus that was maintained at −20 °C to keep the tissue frozen. We have followed the stereotaxic coordinates for the rat brain to identify and dissect the PFC and NAc core (Paxinos and Watson, 2007). The NAc was identified with the appearance of anterior commissure. We have used surgical blades to isolate NAc core and the PFC following visualized landmarks. The PFC (medial part) was dissected at the same level of NAc core. These brain regions were extracted according to Paxinos and Watson Atlas of the rat brain and then frozen for Western blot procedure to examine GLT1 protein level.
Western blot for determination of GLT1 expression
Western blot was performed to determine the GLT1 level as described recently (Sari et al., 2009, 2010, 2011). PFC and NAc-core from all groups (n=6 for each group) were homogenized in lysis buffer, and proteins were extracted and quantified using Bio-Rad reagents (Bio-Rad, Hercules, CA). Equal amount of proteins from all groups were separated in 10–20% glycine gel (Invitrogen) at 200 V using electrophoresis gel box. The proteins were then transferred onto a nitrocellulose membrane electrophoretically. The membranes containing proteins were incubated in a blocking buffer containing 3% milk in TBST (50 mM Tris HCl; 150 mM NaCl, pH7.4; 0.1% Tween20) for 30 min at room temperature. The nitrocellulose membranes were then incubated with guinea pig anti-GLT1 antibody in blocking buffer (Millipore Bioscience Research Reagents) at 1:5,000 dilution overnight at 4°C. The membranes were washed and incubated with horseradish peroxidase (HRP)-labeled anti-guinea pig secondary antibody in blocking buffer at 1:5,000 dilution. Protein loading was normalized using β-tubulin immunoblotting as a loading control. Chemiluminescent detection of HRP (SuperSignal West Pico; Pierce) was then performed by exposure of the membranes to a Kodak BioMax MR film (Thermo Fisher Scientific). The films were then developed on a SRX-101A machine. Identified blots showing immunoreactive proteins (GLT1 and β-tubulin) in the films were digitized and quantified using MCID system. The data are reported as ratios of GLT1/β-tubulin.
Statistical analyses
We have used two-way mixed analysis of variance (ANOVA) (repeated measures) to analyze the body weight, ethanol and water data as we have performed recently (Sari et al., 2011). After we found significant main effect of day and interaction effect (Day by Treatment), one-way ANOVA analyses between vehicle control (ethanol naïve) and drug-treated groups were followed by posthoc Dunnett’s (two-sided) multiple comparison test. Sucrose intake data were analyzed by independent samples t-test. Western blot data were analyzed using one-way ANOVA, and Newman–Keuls’s test for comparison between control (water naïve and ethanol naïve groups) and drug-treated groups. All statistical tests were based on p<0.05 level of significance.
RESULTS
Effects of GPI-1046 treatment on ethanol consumption
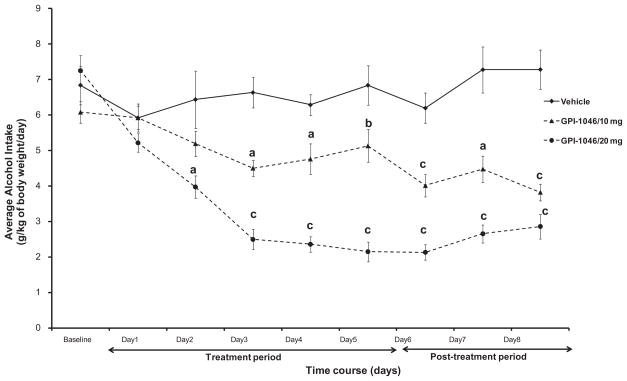
The daily average of ethanol intake (g/kg body weight/ day) was measured for a period of 8 days (starting 24 h after the first injection, Day 1) in P rats treated with vehicle, and/or GPI-1046 (10 or 20 mg/kg). Fig. 1 indicates the average daily ethanol intake across 8 days by the control and treatment groups. Baseline was estimated as an average ethanol intake for the last two weeks prior to vehicle or GPI-1046 i.p. injections.
Fig. 1.
Daily ethanol intake of male P rats treated for 5 days with 10 mg/kg (i.p.) GPI-1046 (n=6), 20 mg/kg (i.p.) GPI-1046 (n=8), or vehicle (ethanol naïve) (n=7). Graph represents average daily ethanol (±SEM) intake during the treatment (Days 1–5) and post-treatment periods (Days 6–8). Baseline was estimated as an average ethanol intake for the last two weeks prior to vehicle or GPI-1046 injections. One-way ANOVA analyses revealed significance difference (F>5.52, p<0.02) among control and treatments groups. Dunnett’s test analyses revealed significant reduction in ethanol intake with the higher dose of GPI-1046 (20 mg/kg, i.p.) starting Day 2 through Day 8 as compared to vehicle (ethanol naïve) groups. Moreover, the lower dose of GPI-1046 (10 mg/kg, i.p.) showed a significant reduction in ethanol intake, relative to vehicle-treated group, starting at Day 3 through Day 8 of treatment. Dunnett’s test analyses revealed significance difference in ethanol intake between both doses of GPI-1046 (10 and 20 mg/kg) from Day 3 through Day 7. (a: p<0.01, b: p<0.05, c: p<0.001.)
A 3 × 8 (Dose by Day) two-way ANOVA performed on ethanol consumption, which was followed by posthoc Dunnett’s test (two-sided), revealed a significant main effect of Day [F(1, 8)=16.27, p<0.001] and a significant Day × Treatment interaction effect [F(2,16)= 9.25, p<0.0001]. One-way ANOVA analyses for each day demonstrated significant difference (F>5.52, p<0.02) among vehicle and the two doses of GPI-1046 from Day 2 through Day 8. Dunnett’s test analyses revealed significant reduction in ethanol intake with the higher dose of GPI-1046 (20 mg/kg, i.p.) at Day 2 (p<0.01), and at Day 3 through 8 (p<0.001) as compared to vehicle control group. Moreover, the lower dose of GPI-1046 (10 mg/kg, i.p.) showed a significant reduction in ethanol intake, relative to vehicle-treated group, starting at Day 3 through Day 8 of treatment (Fig. 1; a: p<0.01, b: p<0.05, c: p<0.001). In addition, Dunnett’s test revealed significance difference in ethanol intake between both doses of GPI-1046 (10 and 20 mg/kg) at Day 3–6 (p<0.001) and Day 7 (p<0.05); however, we did not see any significance difference in ethanol intake on Day 1–2 and Day 8 between both doses of GPI-1046.
Effects of GPI-1046 treatment on water consumption
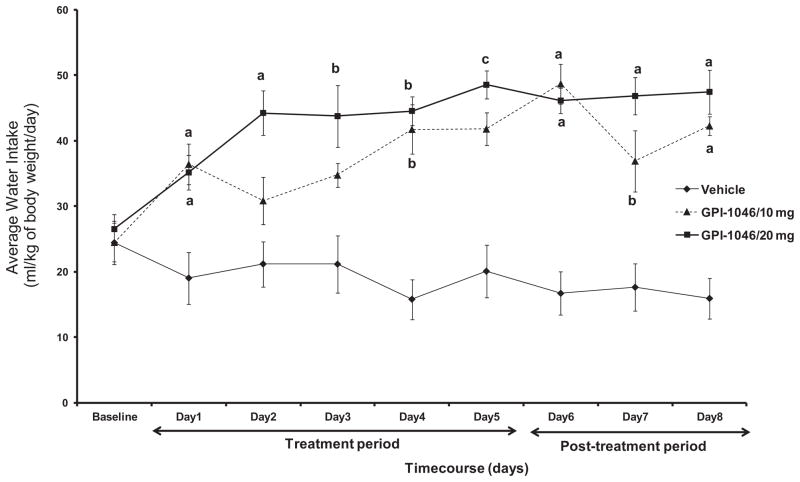
We also have examined water intake in control and drug-treated groups. The daily average of water intake (ml/kg body weight/day) was also measured for a period of 8 days (starting 24 h after the first injection, Day 1) in P rats treated with vehicle, or GPI-1046 (10 or 20 mg/kg). Fig. 2 indicates the average daily water intake across 8 days by the vehicle and treatment groups. A 3 × 8 (Dose by Day) two-way ANOVA performed on water intake, which was followed by Dunnett’s test (two-sided), revealed a significant main effect of Day [F(1, 8)=2.30, p<0.03] and a significant Day × Treatment interaction effect [F(2,16)=2.01, p<0.02]. One-way ANOVA analyses for each day revealed significant difference (F>4.21, p<0.05) in water intake between vehicle and both GPI-1046-treated groups starting Day 1 through Day 8. Dunnett’s test analyses revealed significant increase in water intake starting Day 1 through Day 8 (Fig. 2; a: p<0.005, b: p<0.05, c: p<0.01) at higher dose of GPI-1046 (20 mg/kg), and Day 1 (p<0.005), Day 4 (p<0.05), and Days 6–8 (Fig. 2, a: p<0.005, b: p<0.05) at lower dose of GPI-1046 (10 mg/kg).
Fig. 2.
Daily water intake of male P rats treated for 5 days with 10 mg/kg (i.p.) GPI-1046 (n=6), 20 mg/kg (i.p.) GPI-1046 (n=8), or vehicle (ethanol naïve) (n=7). Graph represents average daily water (±SEM) intake during the treatment (Days 1–5) and post-treatment periods (Days 6–8). One-way ANOVA analyses revealed significance difference (F>4.21, p<0.05) among vehicle (ethanol naïve) and GPI-1046 treated groups starting Day 1 through Day 8. Dunnett’s test analyses revealed significant increase in water intake starting Day 1 through Day 8 at higher dose of GPI-1046 (20 mg/kg), and Day 1, Day 4, and Days 6–8 at lower dose of GPI-1046 (10 mg/kg). (a: p<0.005, b: p<0.05, c: p<0.01.)
Effects of GPI-1046 treatment on body weight
We also have determined the effects of GPI-1046 on body weight (grams). A 3 × 8 (Dose by Day) two-way ANOVA performed on body weight, which was followed by Dunnett’s test (two-sided), revealed a significant main effect of Day [F(1, 8)=38.61, p<0.001], but no significant Day by Treatment interaction effect has been observed [F(2,16)=0.93, p=0.53]. Importantly, one-way ANOVA analyses for each day did not reveal any significant differences between vehicle (ethanol naïve) control (454.46±8.35), and GPI-1046–10 mg (489.14± 23.09) and GPI-1046–20 mg (476.40±16.69) treatment groups (F<1.25, p>0.5) in body weight. These data indicate that GPI-1046 did not alter the body weight of the rats.
Effects of GPI-1046 treatment on sucrose consumption
We next tested the effects of GPI-1046 at higher dose 20 mg/kg on sucrose intake (ml/kg body weight). An independent t-test revealed no significant difference in sucrose intake, across the 5 days of treatment and 3 days post-treatment, between vehicle (sucrose naïve) control (17.8±0.7) and GPI-1046–20 mg (19.8±1.0)-treated group (p>0.05). These results indicate that GPI-1046 did not affect sucrose consumption.
Effects of GPI-1046 treatment on GLT1 expression
We next determined the effects of GPI-1046 treatment on GLT1 expression in PFC using Western blot (Fig. 3A). One-way ANOVA analyses revealed significant main effect between all groups [F(3, 23)=6.46, p<0.01]. Newman-Keuls post hoc test revealed significant increase in PFC GLT1 expression in GPI-1046 (10 mg/kg, i.p.) (p<0.05) and GPI-1046 (20 mg/kg, i.p.) (p<0.01)-treated groups as compared to vehicle (ethanol naïve)-treated group (Fig. 3B). There also was significant increase in PFC GLT1 expression in the GPI-1046 (20 mg/kg) group as compared to the water naïve group. Note that there were no significant differences between water naïve and vehicle (ethanol naïve) groups or between both GPI-1046 doses treated groups.
Fig. 3.
Effects of GPI-1046 at 10 mg/kg (GPI-10, n=6), GPI-1046 at 20 mg/kg (GPI-20, n=6), vehicle (ethanol naïve) (n=6), water naive (water) (n=6) groups on GLT1 expression in PFC. (A) Each panel presents immunoblots for β-tubulin, which was used as a control loading protein, and GLT1. (B) Quantitative analysis revealed a significant increase in the ratio of GLT1/β-tubulin in the GPI-10 and GPI-20 groups as compared to the vehicle (ethanol naïve) group. In addition, a significant increase in GLT1 expression was revealed in GPI-20 group as compared to naive group. Error bars indicate SEM. (*p<0.05; **p<0.01.)
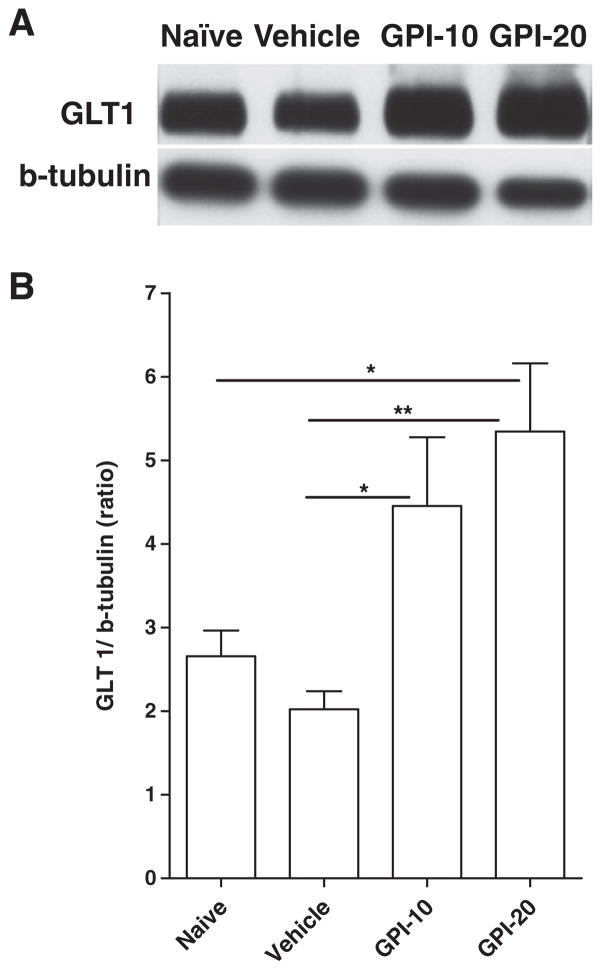
We also determined the effects of GPI-1046 treatment on GLT1 expression in NAc-core using Western blot (Fig. 4A). One-way ANOVA analyses revealed significant main effect between all groups [F(3,23=14.85, p<0.0001]. Newman-Keuls post hoc test revealed significant increase in NAc-core GLT1 expression in GPI-1046 (10 mg/kg, i.p.) (p<0.01) and GPI-1046 (20 mg/kg, i.p.) (p<0.001)-treated groups as compared to vehicle (ethanol naïve)-treated group (Fig. 4B). There also were significant increases in GLT1 expression in GPI-1046 (20 mg/kg, i.p.)-treated compared to GPI-1046 (10 mg/kg, i.p.)-treated (p<0.05) and water naive (p<0.001) groups. Interestingly, statistical analyses revealed significant downregulation of GLT1 expression in the vehicle (ethanol naïve) group (p<0.01) as compared to the water naive group. These later results indicate that chronic ethanol consumption decreased the level of GLT1 in the NAc-core as compared to the water naïve group.
Fig. 4.
Effects of GPI-1046 at 10 mg/kg (GPI-10, n=6), GPI-1046 at 20 mg/kg (GPI-20, n=6), vehicle (ethanol naïve) (n=6), water naive (water) (n=6) groups on GLT1 expression in NAc-core. (A) Each panel presents immunoblots for β-tubulin, which was used as a control loading protein, and GLT1. (B) Quantitative analysis revealed a significant increase in the ratio of GLT1/β-tubulin in GPI-10 and GPI-20-treated groups as compared to vehicle (ethanol naïve). Significant increases in GLT1 expression was revealed in GPI-20 as compared to GPI-10 and water naive (water) groups. Alternatively, statistical analysis revealed significant down-regulation of GLT1 expression in vehicle group as compared to naive group. Error bars indicate SEM. (*p<0.05; **p<0.01; ***p<0.001.)
DISCUSSION
We report here that the administration of GPI-1046 in P male rats reduced ethanol intake in a dose-dependent manner. We found that the higher dose of GPI-1046 (20 mg/kg) reduced dramatically ethanol intake from an average of 7 g/kg of body weight/day to an average of 2 g/kg of body weight/day (70% reduction in ethanol intake). GPI-1046 had no effect in sucrose intake, which suggests that the action of this drug is specific to ethanol intake and does not affect the intake of sucrose as an appetitive control. The attenuation of ethanol intake was associated, at least in part, with the upregulation of GLT1 in PFC and NAc core. These two brain regions involve increases in glutamate transmission that are implicated in drug carving, including ethanol. Alternatively, we have found that GPI-1046 administration induced increase in water intake as compared to vehicle (ethanol naïve)-treated P rats. As we have reported in recent study with ceftriaxone (Sari et al., 2011) that the increase in water consumption is a behavioral mechanism to compensate the decrease in fluid intake, which is ethanol in GPI-1046-treated groups as compared to vehicle-treated groups. Although there was difference in ethanol and water intakes between GPI-1046-and vehicle-(ethanol naïve) treated groups, the overall fluid intake was about the same between these groups. Alternatively, GPI-1046 treatment did not affect the body weight of all animals treated with vehicle or GPI-1046. It is noteworthy that similar to ceftriaxone treatment (Sari et al., 2011), we did not see any side effects of GPI-1046 treatment as compared to vehicle (ethanol naïve) vehicle treatment.
Dysfunction or deficits of glutamate transporters, including GLT1, can lead to the induction and progression of neurodegeneration associated with glutamate neurotoxicity in neurodegenerative diseases (Rothstein et al., 1993, 1996; Tanaka et al., 1997; Watase et al., 1998). It is demonstrated that deafferentation of cortical glutamatergic projections may consequently cause downregulation of GLT1 and the glutamate aspartate transporter (GLAST) termed also excitatory amino acid transporter 1 (EAAT1), but not to the excitatory amino acid carrier 1 (EAAC1), in the striatum and hippocampus (Ginsberg et al., 1995). Extracellular glutamate is regulated by glutamate transporters, including GTL1. It has been demonstrated that GLT1, which regulates the uptake of the majority of extracellular glutamate, plays an important role in protecting neurons from excessive glutamate (Kanner and Schuldiner, 1987; Kanai et al., 1993). Similar to neurodegenerative disease models in which there is dysfunction of the glutamatergic excitatory system, the role of GLT1 has been tested in drug abuse models that show dysfunction of glutamate neurotransmission. Thus, the activation of GLT1 with MS-153 that has been shown to accelerate glutamate uptake through GLT1 (Shimada et al., 1999), attenuated the conditioned place preference in mice that have been conditioned to the rewarding effects of morphine, methamphetamine and cocaine (Nakagawa et al., 2005). Furthermore, ceftriaxone attenuates cue-induced relapse to cocaine-seeking behavior (Sari et al., 2009); this behavioral effect was associated at least in part with upregulation of GLT1 levels in PFC and NAc. Importantly, we have recently found that ceftriaxone administration reduced ethanol intake in male P rats (Sari et al., 2011). The reduction in ethanol intake was also associated at least in part with the upregulation of GLT1 level in PFC and NAc.
Although the neurocircuitry of the glutamatergic system is not fully understood, studies have suggested that brain regions including PFC (Goldstein and Volkow, 2002) and the NAc (Childress et al., 1999) play a critical role in drug reinforcement. These two brain regions of the reward’s system revealing increased glutamate transmission appear to drive drug craving (Kalivas et al., 2009). The importance of these glutamatergic projections from the PFC to the NAc and to the VTA have been revealed using neuroimaging studies that were performed during craving periods, for commonly abused drugs such as ethanol, methamphetamine, cocaine, heroin and nicotine (Childress et al., 1999; Goldstein and Volkow, 2002). Moreover, glutamatergic projections from the PFC to the NAc are also critical in the expression of addictive behaviors [For review see Kalivas, 2004]. Thus, we investigated the effect of GPI-1046 administration in GLT1 level in these two key brain regions, PFC and NAc, since the interactive connections between these two regions are critical in drug reward and drug abuse (Kalivas et al., 2009). It is revealed that these two brain regions receive glutamatergic projections from the amygdala and hippocampus, which are involved in the initiation of drug-seeking behavior (Kalivas et al., 2009). Alternatively, we focused in the NAc core rather than the shell because of previous findings from Kalivas and colleagues demonstrating that the cocaine-induced release of glutamate was more pronounced in the NAc core but not in the shell of animals developing behavioral sensitization (Pierce et al., 1996).
It is noteworthy that GLT1 was found downregulated in NAc core but not in the PFC in animals exposed to ethanol (ethanol naïve animals) as compared to water naïve animals. It is important to note that studies demonstrated also significant downregulation of GLT1 level in the NAc following cocaine-self-administration but not in the PFC (Knackstedt et al., 2010). Although, it is unclear how ethanol and cocaine have similar effect in the downregulation of GLT1 in NAc versus PFC since both drugs act with different mechanisms of action, these drugs may share the same neurocircuitry involving drug reward. We suggest that differences in GLT1 level between PFC and NAc in both ethanol and cocaine models might be due, in part, to the fact that PFC receives and sends glutamatergic projections to and from other brain regions; however, NAc does not send but receives glutamatergic projections. We suggest that neuroadaptations may be occurring due to differences in the anatomical distribution of GLT1 and the level of expression of this transporter in PFC and NAc core, and possibly other brain regions [For review see Danbolt, 2001]. Studies are warranted to explore the differential effect of ethanol in GLT1 level between PFC and NAc core, and other brain regions.
With the use of GPI-1046, we demonstrated the action of this drug in the elevation of GLT1 level, which was associated with the attenuation of ethanol intake in a dose-dependent manner. GPI-1046 is a compound derived from the immunophilin ligand FK506 (tacrolimus). Although both compounds are structurally similar, GPI-1046 does not interact with calcineurin, which is a target of FK506 ligand to induce immunosuppressive action (Steiner et al., 1997b). The mechanisms of action of GPI-1046 are still not clear, it has been studied that the action of FK506 is mediated through the interaction of phosphatase calcineurin and consequently inhibiting FK506-binding protein 12 (FKBP12), which is a rotamase involving inositol triphosphate (IP3) and ryanodine receptor (Brillantes et al., 1994; Cameron et al., 1995). Similarly, GPI-1046 has been shown to attenuate IP3 and ryanodine-sensitive endoplasmic reticulum (ER) calcium release in vitro (Caporello et al., 2006). It is noteworthy that the action of GPI-1046 in lowering ER calcium load might be mediated through different mechanisms of action as compared to those of FK506 since GPI-1046 does not involve calcineurin (Steiner et al., 1997b). It has been suggested that FKBPs are involved in several mechanisms of action including transcription, cell signaling pathways and trafficking of proteins (Harrar et al., 2001). Thus, the mechanisms of action of GPI-1046 are still difficult to understand due to the complexity of the signaling pathways involving FKBPs.
GPI-1046 has been initially studied in several in vitro and in vivo neurodegenerative disease models. Thus, GPI-1046 was found to restore the long-term potentiation of corticostriatal synaptic transmission in 6-hydroxydopamine-lesioned animals (Zhang et al., 2001). In addition, GPI-1046 prevented dopaminergic dysfunction through the activation of striatal glutathione (GSH) levels, which was mediated by the activation of GSH synthesis (Tanaka et al., 2002). In a model of ischemia, GPI-1046 decreased infarct volume and induced neuroprotection, which were mediated through the attenuation of increased rotamase activity, and increased number of cells expressing FKBP12, FKBP52, caspase-8, cytochrome c, and caspase-3 (Li et al., 2004). Moreover, GPI-1046 has been found to increase the level of glial cell line-derived neurotrophic factor (GDNF) in the mouse brain; however, FK506 increases the level of both GDNF and brain-derived neurotrophic factor (Tanaka et al., 2003). Together, these demonstrate the differences of actions of these compounds and their important roles in neuroprotection.
Rothstein and colleagues have investigated whether the neuroprotective action of GPI-1046 is mediated through the upregulation of GLT1 using in vitro and in vivo models of injury (Ganel et al., 2006). Indeed, GPI-1046 treatment upregulated GLT1 level but not GLAST in spinal cord culture (Ganel et al., 2006). Furthermore, oral GPI-1046 administration revealed dramatic upregulation of GLT1 level in adult mice (Ganel et al., 2006). The upregulation of GLT1 level was associated with increase in glutamate uptake. This effect was due directly to the activation of GLT1 action since the administration of dihydrokainate, selective GLT1 inhibitor, attenuated the increase in glutamate uptake. These data provide ample information about the action of GPI-1046 in the activation of GLT1. In the present study, we have found that the administration of GPI-1046 reduced ethanol intake as early as 2 days after the first injection with the higher dose of this drug. It is possible that this reduction in ethanol intake may not be initially associated with the upregulation of GLT1. This suggests that the initial effect of GPI-1046 might be either due to the activation of GLT1 or other unknown pharmacological mechanisms. Findings related to GPI-1046 in neuroprotection demonstrated the involvement of this drug in many cellular pathways involving oxidative stress. For example, GSH is one of the key mechanisms of action that GPI-1046 is involved in inducing neuroprotection or to attenuate oxidative stress as described in previous study (Tanaka et al., 2002). Interestingly, GSH levels were found altered in high alcohol-drinking animal models. Thus, GSH S-transferase-alpha protein expression was found upregulated in the NAc of alcohol non-preferring (NP) rats compared to naïve NP rats (McBride et al., 2009). Another study demonstrated that P rats in ethanol self-administration schedule showed the upregulation of GSH peroxidase 4 gene level in the NAc compared to rats self-administered saccharin (Rodd et al., 2008). Together, these findings suggest that GPI-1046 may overcome increase in GSH level caused by ethanol consumption. Studies are warranted to determine the direct mechanisms of action of GPI-1046 in the regulation of GSH level in P rats consuming ethanol.
We report here for the first time that GPI-1046 administration reduced ethanol intake but not sucrose consumption in male P rats. The reduction in ethanol intake was mediated in dose-dependent manner. The effect of GPI-1046 persisted after 3 days of the last GPI-1046 injection, which suggests that GPI-1046-induced GLT1 upregulation in PFC and NAc core may be at least in part the cause of this lasting effect in the reduction of ethanol intake. Other pharmacological mechanisms of action of GPI-1046 might be also considered critical in its lasting effect in ethanol reduction as well. These data reveal that GPI-1046 might be considered as a therapeutic compound for the treatment of alcohol dependence.
Acknowledgments
The research project described was supported by Award Number R01AA019458 (Y.S.) and R24AA015512 (Lawrence Lumeng) from the National Institutes on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health. The authors would also like to thank Charisse Montgomery for editing this manuscript.
Abbreviations
- ANOVA
analysis of variance
- DMSO
dimethylsulfoxide
- ER
endoplasmic reticulum
- FKBP12
FK506 binding protein 12
- GDNF
glial cell line-derived neurotrophic factor
- GLAST
glutamate aspartate transporter
- GLT1
glutamate transporter 1
- GPI-1046
3-(3-pyridyl)-1-propyl (2S)-1-(3,3-dimethyl-1,2-dioxopentyl)-2-pyrrolidinecarboxylate
- GSH
glutathione
- IP3
inositol triphosphate
- NAc-core
nucleus accumbens core
- NP rat
alcohol nonpreferring rat
- P rat
alcohol-preferring rat
- PFC
prefrontal cortex
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Sabatini DM, Kaplin AI, Walensky LD, Snyder SH. Immunophilin FK506 binding protein associated with inositol 1,4,5-trisphosphate receptor modulates calcium flux. Proc Natl Acad Sci U S A. 1995;92:1784–1788. doi: 10.1073/pnas.92.5.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporello E, Nath A, Slevin J, Galey D, Hamilton G, Williams L, Steiner JP, Haughey NJ. The immunophilin ligand GPI1046 protects neurons from the lethal effects of the HIV-1 proteins gp120 and Tat by modulating endoplasmic reticulum calcium load. J Neurochem. 2006;98:146–155. doi: 10.1111/j.1471-4159.2006.03863.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, Hoffman A, Deitrich R, de Witte P. Effects of ethanol on extracellular amino acid levels in high-and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol. 2000;35:548–553. doi: 10.1093/alcalc/35.6.548. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Ganel R, Ho T, Maragakis NJ, Jackson M, Steiner JP, Rothstein JD. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiology Dis. 2006;21:556–567. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Martin LJ, Rothstein JD. Regional deafferentation down-regulates subtypes of glutamate transporter proteins. J Neurochem. 1995;65:2800–2803. doi: 10.1046/j.1471-4159.1995.65062800.x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Dawson VL, Dawson TM. Neuroimmunophilin ligands exert neuroregeneration and neuroprotection in midbrain dopaminergic neurons. Eur J Neurosci. 2001;13:1683–1693. doi: 10.1046/j.0953-816x.2001.01542.x. [DOI] [PubMed] [Google Scholar]
- Harrar Y, Bellini C, Faure JD. FKBPs: at the crossroads of folding and transduction. Trends Plant Sci. 2001;6:426–431. doi: 10.1016/s1360-1385(01)02044-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Smith CP, Hediger MA. The elusive transporters with a high affinity for glutamate. Trends Neurosci. 1993;16:365–370. doi: 10.1016/0166-2236(93)90094-3. [DOI] [PubMed] [Google Scholar]
- Kanner BI, Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22:1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Omori N, Hayashi T, Jin G, Sato K, Nagano I, Shoji M, Abe K. Protection against ischemic brain damage in rats by immunophilin ligand GPI-1046. J Neurosci Res. 2004;76:383–389. doi: 10.1002/jnr.20067. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl. 1987;1:91–96. [PubMed] [Google Scholar]
- McBride WJ, Schultz JA, Kimpel MW, McClintick JN, Wang M, You J, Rodd ZA. Differential effects of ethanol in the nucleus accumbens shell of alcohol-preferring (P), alcohol-non-preferring (NP) and Wistar rats: a proteomics study. Pharmacol Biochem Behav. 2009;92:304–313. doi: 10.1016/j.pbb.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Birkestrand B, Fowler SC. The neuroimmunophilin GPI-1046 partially protects against 3-acetylpyridine toxicity in the rat. Neurosci Lett. 2002;321:53–56. doi: 10.1016/s0304-3940(01)02571-x. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res. 2005;156:233–239. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Othman T, Sinclair CJ, Haughey N, Geiger JD, Parkinson FE. Ethanol alters glutamate but not adenosine uptake in rat astrocytes: evidence for protein kinase C involvement. Neurochem Res. 2002;27:289–296. doi: 10.1023/a:1014955111742. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. New York: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rodd ZA, Kimpel MW, Edenberg HJ, Bell RL, Strother WN, McClintick JN, Carr LG, Liang T, McBride WJ. Differential gene expression in the nucleus accumbens with ethanol self-administration in inbred alcohol-preferring rats. Pharmacol Biochem Behav. 2008;89:481–498. doi: 10.1016/j.pbb.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Sari Y, Bell RL, Zhou FC. Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-preferring rats. Alcohol Clin Exp Res. 2006;30:46–56. doi: 10.1111/j.1530-0277.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. J Biomed Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Francis JM, Jiang H, Hamilton GS, Steiner JP. Systemic treatment with GPI 1046 improves spatial memory and reverses cholinergic neuron atrophy in the medial septal nucleus of aged mice. Brain Res. 1999;842:109–118. doi: 10.1016/s0006-8993(99)01851-x. [DOI] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Shimada F, Shiga Y, Morikawa M, Kawazura H, Morikawa O, Matsuoka T, Nishizaki T, Saito N. The neuroprotective agent MS-153 stimulates glutamate uptake. Eur J Pharmacol. 1999;386:263–270. doi: 10.1016/s0014-2999(99)00735-9. [DOI] [PubMed] [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus-nonpreferring rats. J Pharmacol Exp Ther. 1999;288:1223–1228. [PubMed] [Google Scholar]
- Smith TL. Regulation of glutamate uptake in astrocytes continuously exposed to ethanol. Life Sci. 1997;61:2499–2505. doi: 10.1016/s0024-3205(97)00985-5. [DOI] [PubMed] [Google Scholar]
- Steiner JP, Connolly MA, Valentine HL, Hamilton GS, Dawson TM, Hester L, Snyder SH. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat Med. 1997a;3:421–428. doi: 10.1038/nm0497-421. [DOI] [PubMed] [Google Scholar]
- Steiner JP, Hamilton GS, Ross DT, Valentine HL, Guo H, Connolly MA, Liang S, Ramsey C, Li JH, Huang W, Howorth P, Soni R, Fuller M, Sauer H, Nowotnik AC, Suzdak PD. Neurotrophic immunophilin ligands stimulate structural and functional recovery in neurodegenerative animal models. Proc Natl Acad Sci U S A. 1997b;94:2019–2024. doi: 10.1073/pnas.94.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JP, Payne KB, Main CD, D’Alfonso S, Jacobsen KX, Hicks TP, Staines WA, Poulter MO. GPI-1046 increases presenilin-1 expression and restores NMDA channel activity. Can J Neurol Sci. 2010;37:457–467. doi: 10.1017/s0317167100010465. [DOI] [PubMed] [Google Scholar]
- Stewart RB, McBride WJ, Lumeng L, Li TK, Murphy JM. Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacology (Berl) 1991;105:530–534. doi: 10.1007/BF02244375. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Fujita N, Ogawa N. Immunosuppressive (FK506) and non-immunosuppressive (GPI1046) immunophilin ligands activate neurotrophic factors in the mouse brain. Brain Res. 2003;970:250–253. doi: 10.1016/s0006-8993(03)02434-x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Yoshioka M, Miyazaki I, Fujita N, Ogawa N. GPI1046 prevents dopaminergic dysfunction by activating glutathione system in the mouse striatum. Neurosci Lett. 2002;321:45–48. doi: 10.1016/s0304-3940(01)02547-2. [DOI] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- Zhang C, Steiner JP, Hamilton GS, Hicks TP, Poulter MO. Regeneration of dopaminergic function in 6-hydroxydopamine-lesioned rats by neuroimmunophilin ligand treatment. J Neurosci. 2001;21:RC156. doi: 10.1523/JNEUROSCI.21-15-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]